Abstract
Purpose
Human corneal endothelial cell (HCEC) proliferation is controlled by their cell junctions, of which the mechanism remains unknown. We sought to characterize adherent junction components of in vivo HCECs, and compare their gene expression and their proliferative potential to those of in vitro counterparts.
Methods
Stripped human Descemet’s membranes were digested with collagenase A, and the resultant HCEC aggregates were cultured for 7, 14, and 21 days in supplemented hormonal epithelial medium (SHEM). Growth of HCEC monolayers was monitored by BrdU labeling performed 24 h before termination. Both in vivo and in vitro HCECs were subjected to immunostaining to FITC-phalloidin and antibodies to different junction components and BrdU. Their mRNA expressions were determined by RT-PCR.
Results
In vivo HCECs expressed transcripts of N-, VE-, E-, and P-cadherins, α-, β-, γ-, and p120-catenins, and p190. In vitro HCEC counterparts also expressed all these mRNAs except P-cadherin. In vivo HCECs displayed continuous circular F-actin, N-cadherin, β- and p120-catenins, and p190, discontinuous circular VE-cadherin bands at/close to cell junctions, and E-cadherin in the cytoplasm. Such an in vivo pattern was gradually achieved by in vitro HCECs at day 21 and was correlated with a progressive decline of BrdU labeling.
Conclusions
Both in vivo and in vitro HCECs displayed distinct protein cytolocalization of N-, VE-, and E-cadherins, β- and p120-catenins, and p190. Progressive maturation of adherent junctions was associated with a decline of the proliferative potential. This information allows us to devise new strategies to engineer in vitro HCECs by targeting these components.
Keywords: adherent and tight junctions, human cornea endothelial cells, in vivo, in vitro, cadherins, catenins, proliferation, p120, p190
INTRODUCTION
A monolayer of human corneal endothelial cells (HCECs) located at the posterior surface of the cornea plays a pivotal role in regulating corneal stromal hydration and transparency. Unlike those of other species such as rabbit and bovine, HCECs are notorious for their limited proliferative capacity in vivo1 but do retain the proliferative capacity especially upon cultivation2 (for review, see3). We have recently developed a novel method of isolating, preserving and expanding HCECs based on collagenase digestion of stripped Descemet’s membrane containing HCECs with or without a brief treatment of trypsin/EDTA to disrupt cell junctions followed by cultivation in the supplemented hormonal epithelial medium (SHEM).4 This method resulted in a monolayer of hexagonal cells that express both gap junction protein of connexin 43 and tight junction protein of ZO-1. However, expression and cytolocalization of adherent junction proteins in this model system remains unclear.
Intercellular junctions include gap junction, adherent junction and tight junction, and play an important role in controlling cell adhesion, proliferation, communication, movement, diffusion of small molecules at the junction and maintenance of the corneal endothelial barrier function.5-9 Adherent junction is actin-based small punctuate intercellular junction, mediated by cadherins and catenins. Cadherins include N (neuronal, type I and II)-, VE (vascular endothelial)-, E (epithelial, type I and II)- and P (placental)-cadherins10 that mediate calcium-dependent cell proliferation and adhesion.11,12 N-cadherin (type I) from neurons, heart, skeletal muscle, lens and fibroblasts, regulates transcription13 and migration.14 VE-cadherin in vascular endothelial cells modulates cell growth and barrier function.15,16 E-cadherin (type I) from epithelial cells inhibits cell cycle progression by downregulating β-catenin-mediated signaling activity.17-19
Catenins includes α-, β-, γ- and p120-catenins. α-catenin plays an integral role in remodeling actin cytoskeletons into radial cables.20 β-catenin plays a central role in the Wnt signal transduction cascade.21-23 γ-catenin, highly homologous to β-catenin,24,25 also plays a unique role in the Wnt signaling pathway.26,27 p120-catenin (hereafter abbreviated as p120) helps stabilize cadherin-mediated adherent junctions at the plasma membrane.28-30 p190RhoGAP (hereafter abbreviated as p190), a RhoA family GTPase-activating protein, plays a crucial role in regulating cytoskeletal dynamics by inhibiting focal adhesion and myosin-mediated contraction of F-actin cables,31-33 and mediates the cross talk between a wide variety of receptors in order to coordinate cadherin functions that direct cell adhesion, motility and proliferation.34
Earlier works by immunostaining based on a pan-cadherin antibody identifies the presence but not the specific type of cadherins in HCECs in vivo.35,36 Later, E-cadherin (type I)37 and N-cadherin (type I)38 mRNAs were identified by microarray and PCR, respectively, are expressed by in vivo HCECs. It remains unclear whether HCECs express other adherent junction components in a unique cytolocalization pattern, and whether such expression is correlated with the loss of proliferative potential. Herein, we performed detailed characterization of adherent junctions expressed by both in vivo and in vitro HCECs and demonstrated that gradual maturation of adherent junctions in HCEC monolayers generated by our cultivation method is associated with a marked decline of the proliferative potential. This baseline characterization is important for devising a strategy to target these components for tissue engineering of HCECs in the future.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Ham’s/F12 medium, human epidermal growth factor (hEGF), HEPES buffer, Hanks’ balanced salt solution (HBSS), phosphate-buffered saline (PBS), amphotericin B, gentamicin, fetal bovine serum (FBS), 0.25% trypsin/0.53 mM EDTA (trypsin/EDTA) and fluorescein phalloidin were purchased from Invitrogen (Carlsbad, CA). Collagenase A was obtained from Roche Applied Science (Indianapolis, IN). Hydrocortisone, dimethyl sulfoxide, cholera toxin, insulin-transferrin-sodium selenite media supplement, bovine serum albumin, agarose, PCR marker, paraformaldehyde, Triton X-100, Hoechst 33342 dye, and FITC-conjugated goat anti-mouse and anti-rabbit IgG were purchased from Sigma-Aldrich (St. Louis, MO). Texas red dye-conjugated donkey anti-mouse IgG was obtained from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Specific monoclonal anti-VE-cadherin, -p190, -β-catenin, -ZO-1, -BrdU and polyclonal anti-N-cadherin (type I), -E-cadherin (type I) and -p120-catenin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) or BD Biosciences (San Jose, CA). Vectashield mounting medium was obtained from Vector laboratories Inc. (Burlingame, CA). RNeasy Mini Kits were purchased from Qiagen (Valencia, CA). High Capacity Reverse Transcription and TaqMan Universal PCR Mater Mix kits were obtained from Applied Biosystems (Foster City, CA).
HCECs Isolation and Culture
Human corneas were handled according to the declaration of Helsinki. The isolation and culturing of HCECs followed what has recently been described.4 In short, human donor corneoscleral tissues, from which their central corneal buttons had been used for corneal transplantation, were obtained from the Florida Lions Eye Bank (Miami, FL). All tissues from donors aging between 21 and 72 years were maintained at 4°C in Optisol (Chiron Vision, Irvine, CA) for less than 5 days before being rinsed three times with DMEM containing 50 mg/mL gentamicin and 1.25 mg/mL amphotericin B. The trabecular meshwork was cleaned under microscopy and the rim was trephined within Schwalbe’s line. Descemet’s membranes containing HCECs were stripped from the posterior surface of the peripheral corneoscleral tissue under a dissecting microscope and digested at 37°C for 16 hours with 1 mg/mL collagenase A in the supplemented hormonal epithelial medium (SHEM), which was made of an equal volume of HEPES-buffered DMEM and Ham’s F12 supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/mL hEGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, 1 nM cholera toxin, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. After digestion, HCECs formed aggregates, which were collected by centrifugation at 2000 rpm for 3 minutes to remove the digestion solution. The aggregates were directly cultured in 24-well dishes coated with Collagen IV for up to 21 days in SHEM. Some of the aggregates were pretreated with trypsin/EDTA at 37°C for 5 minutes before the aforementioned cultivation.
Proliferative activity of HCEC Monolayers
As noted during our experiments, without trypsin/EDTA treatment, each HCEC aggregate attached and expanded from the center of the aggregate to form a monolayer in 3 to 5 days on collagen IV-coated dishes in SHEM. To measure the proliferative activity, BrdU was added at a final concentration of 10 μM in the culture medium for 24 hours before termination for each cultivation time point using cultures derived from three different donors at age 44, 53 and 55. For each culture, at least 2000 total nuclei were counted and the BrdU labeling index, defined as the number of BrdU-labeled nuclei divided by the total number of labeled and unlabeled nuclei, was calculated.
PCR Primer Design, RNA Extraction, Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Stripped Descemet’s membranes and HCEC monolayers cultured at day 2 or day 14 from three donors aged at 22, 67 and 72 were used for this experiment. Some of the above cultures were briefly treated with or without trypsin/EDTA. Total RNA from the above samples were extracted using RNeasy Mini Kits. Extracted RNAs were reversetranscribed using High Capacity Reverse Transcription Kits, and respective cDNAs of each junction component were amplified by PCR using specific primers and DNA polymerase in 7000 Real-time PCR System (Applied Biosystems, Foster City, CA). PCR primers for the cDNA from a particular gene, listed in Table 1, were designed by blasting the entire gene bank to ensure the specificity of each primer pair so that they did not overlap with other types or isoforms of cadherins, catenins or any other cDNAs. To avoid false positive reactions potentially from contaminated DNA, every primer pair was par intron. We used cDNAs reverse-transcribed from mRNAs extracted from ARPE-19 cells known to express most known adherent junction components as the positive control and water as the negative control. The PCR profile consisted of 6 minutes of initial activation at 95 °C followed by 35 cycles of 30 second denaturation at 95 °C, 1 minute annealing at 60 °C and 1 minute extension at 72 °C. We confirmed the genuine identity of each PCR product by the correct size determined by 2% agarose gels using ethidium bromide staining and PCR marker using EC3 Imaging System (BioImaging System, Upland, CA).
Table 1.
Nucleotide Sequences of Primers Used in PCR
| Human Gene Name |
Primer Sequence | Product Size |
GenBank Number |
|---|---|---|---|
| N-cadherin, type I | F-CACTGCTCAGGACCCAGAT R-TAAGCCGAGTGATGGTCC |
416 | NM_001792 |
| N-cadherin, type II | F-TTCCAACCTTCACCTTGACC R-CCAGGTCTTGAGCAGTGACA |
211 | BC047608 |
| VE-cadherin | F- ACTCACCCCTTGCAATAA CG R-ACAGAGCAGCCATCAGAGGT |
250 | AF240635 |
| E-cadherin, type I |
F- CGACCCAACCCAAGAATC TA R-AGGCTGTGCCTTCCTACAGA |
172 | NM_004360 |
| E-cadherin, type II |
F- GCCAGGTATGAGATCGTG GT R-GTGTCTTCAGGCACGACAAA |
152 | BC096365 |
| P-cadherin | F-AACCTCCACAGCCACCATAG R-GTCTCTCAGGATGCGGTAGC |
181 | BC014462 |
| α-catenin | F-GTCATCAGTGCTGCCAAGAA R-TGTTCAGCTGGTGGCAGTAG |
151 | D13866 |
| β-catenin | F-GAAACGGCTTTCAGTTGAGC R-CTGGCCATATCCACCAGAGT |
166 | BC058926 |
| γ-catenin | F-AAGGTGCTATCCGTGTGTCC R-GACGTTGACGTCATCCACAC |
216 | M23410 |
| p120 | F-GATGCTGTCAAGTCCAATGCAG R-AGTACTGGGATGCCCTTGAGC |
101 | NM_001331 |
| P190 | F-CAAGACCACAACCTGGACCT R-CTTCGTGGTTTTCCTTTGGA |
211 | NM_004491 |
| ZO-1 | F-GAACGAGGCATCATCCCTAA R-CCAGCTTCTCGAAGAACCAC |
218 | BC111712 |
| GAPDH | F-GAGTCAACGGATTTGGTCGT R-TTGATTTTGGAGGGATCTCG |
238 | M33197 |
Immunostaining
Flat mounts of stripped human Descement’s membranes containing HCECs from three donors at age 55, 61 and 66 and HCEC sphere cultures from three donors aged 33, 58 and 64 were prepared by brief air-drying and fixation in 4% formaldehyde, pH 7.0, for 15 minutes at room temperature. They were rehydrated in PBS, incubated with 0.2% Triton X-100 for 15 minutes, and rinsed three times with PBS for 5 minutes each. After incubation with 2% BSA to block nonspecific staining for 30 minutes, they were incubated with monoclonal anti-VE-cadherin, -p190, -β-catenin, -ZO-1 and polyclonal anti-N-cadherin (type I), -E-cadherin (type I) and -p120 antibodies (all at 1:50 dilution) for 16 hours at 4 °C. After three washes with PBS, they were incubated with a FITC-conjugated goat anti-mouse or anti-rabbit IgG for 60 minutes, followed by counterstaining with 10 μg/ml Hoechst 33342 (blue color) for 10 minutes. The specimens were then mounted with the Vectashield mounting medium and analyzed with fluorescence Nikon Eclipse TE2000-U microscope. For BrdU labeling, samples were fixed with 75% methanol plus 25% acetic acid, denatured by 2 M HCl and neutralized by 0.1 M borate buffer, pH 8.5. Monoclonal anti-BrdU antibody and Texas red dye-conjugated donkey anti-mouse IgG were used for immunostaining of BrdU positive nuclei. The samples were then counterstained with Hoechst 33342 and analyzed with fluorescence Nikon Eclipse TE2000-U microscope. Corresponding mouse and rat sera were used as negative controls for primary monoclonal and polyclonal antibodies, respectively.
Statistics
All summary data were reported as means ± S.D. calculated for each group and compared using Student’s unpaired t-test by MicroSoft Excel™ (MicroSoft, Redmont, WA). Test results were reported as two-tailed p values, where p < 0.05 was considered statistically significant.
RESULTS
Growth Characteristics of Cultured HCEC Monolayers
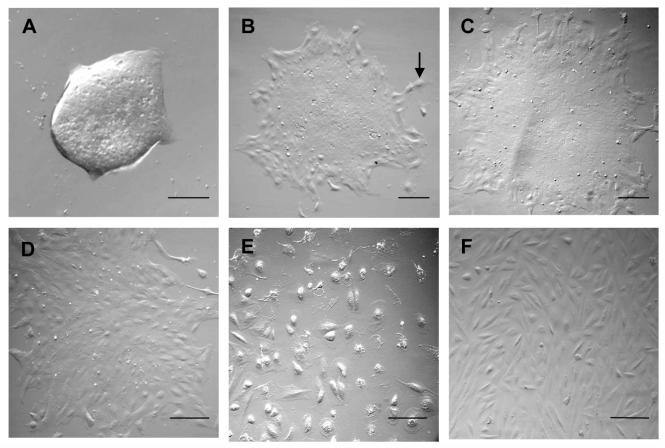
After overnight digestion with collagenase A, an intact stripped Descemet’s membrane of each donor peripheral cornea typically yielded a total of 10 to 12 aggregates. As reported previously,4 without a brief trypsin-EDTA treatment, approximately 80% of these aggregates attached to collagen IV-coated dished and started to migrate out as a monolayer by day 3 when cultured in SHEM (Fig. 1A). Phase contrast microscopy revealed that these monolayers, once continuing to grow, might or might not have a compacted center corresponding to where the aggregate first attached (Fig. 1B). Monolayers with compact centers continued to expand in size with nearly all cells tightly associated with one another, adopting a hexagonal shape; cells in the periphery tended to be more spindle and migratory (Fig. 1B). These monolayers slowly expanded until day 14 (Fig. 1C) and throughout the entire cultivation time up to day 21 (data not shown). Monolayers without compacted centers tended to be more spindle-like cells cultured at day 7 (Fig. 1D), eventually died at day 21 (Fig. 1E) or to be fibroblastic-like cells at day 7 (Fig.1F). The proportion of monolayers with a compact center to those without was approximately 2:1.
Figure 1. Growth Characteristics of Cultured HCEC Monolayers.
A representative culture from a 35 year-old donor showed how HCECs migrated from an isolated aggregate on a collagen IV-coated dish in SHEM on day 3 (A), generated a monolayer with a compact center on day 7 (B, migratory cells are marked in the periphery), and size expansion on day 14 (C). HCEC aggregates without a center expanded at day 7 (D) and eventually died at day 21 (E). Some aggregates became fibroblast-like cells at day 7 (F), grew like fibroblasts (data not shown). Bars represent 100 μm.
Transcript Expression of Adherent Junction Components by in vivo and in vitro HCECs
To know which adherent junction components might be expressed by in vivo HCECs, we first performed RT-PCR using total RNAs extracted from the peripheral corneal endothelia of 22, 58 and 67 year-old donors using specific primers listed in Table 1. Using GAPDH as a loading control and the results from 22-year old donor as a representative (Fig. 2), we found that in vivo HCECs from all three donors expressed transcripts of all adherent junction components such as N- (type I and II), VE-, E- (type I and II) and P-cadherins, α-, β-, γ-, and p120-catenins, and p190 (Fig. 2, Lane 1). Consistent with previous studies,3,4,36 the ZO-1 transcript was also expressed by in vivo HCECs. Following a brief treatment of trypsin/EDTA to disrupt intercellular junctions, HCEC aggregates cultured for 2 days lost expression of all transcripts except for p120 (Fig. 2, Lane 2). In contrast, without a brief trypsin/EDTA treatment, HCEC aggregates still retained expression of VE-cadherin, N-cadherin (type II), β-catenin, and p120 after two days of culture (Fig. 2, Lane 3), and expressed all transcripts of these adherent junction components except E- (type I and II) and P-cadherins when HCEC monolayers were cultured up to day 14 (Fig. 2, Lane 4). When cultures were extended to day 21, transcripts of E-cadherin (type I and II) but not P-cadherin were detected by RT-PCR (data not shown). These results collectively indicated that in vivo HCECs indeed expressed all these adherent junction components, and that such expression was largely abolished shortly when adherent junctions were disrupted by a brief trypsin/EDTA treatment. When adherent junctions were not intentionally disrupted by trypsin/EDTA, expression of some of these transcripts was retained during the early stage of monolayer growth.
Figure 2. Transcript Expression of Adherent Junction Components by HCECs In Vivo and In Vitro.
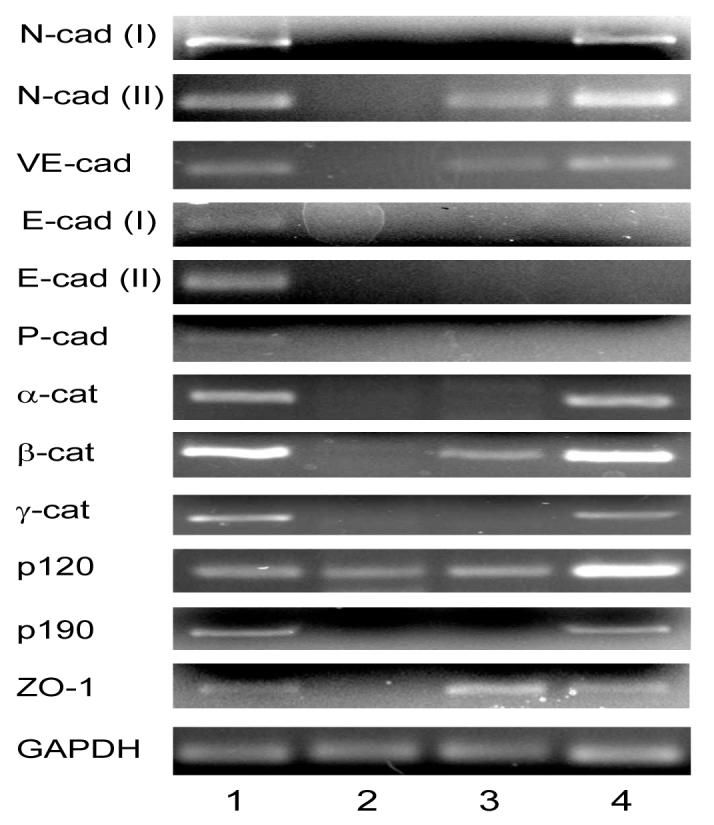
The PCR fragments were of the expected sizes (not shown) and expressed by in vivo HCECs (Lane 1), by HCEC aggregates treated by a brief trypsin/EDTA treatment and cultured for 2 days (Lane 2), and by those without trypsin/EDTA treatment cultured day 2 (Lane 3) or day 14 (Lane 4). The band shown in Lane 2 for E-cadherin (type I) was an artifact.
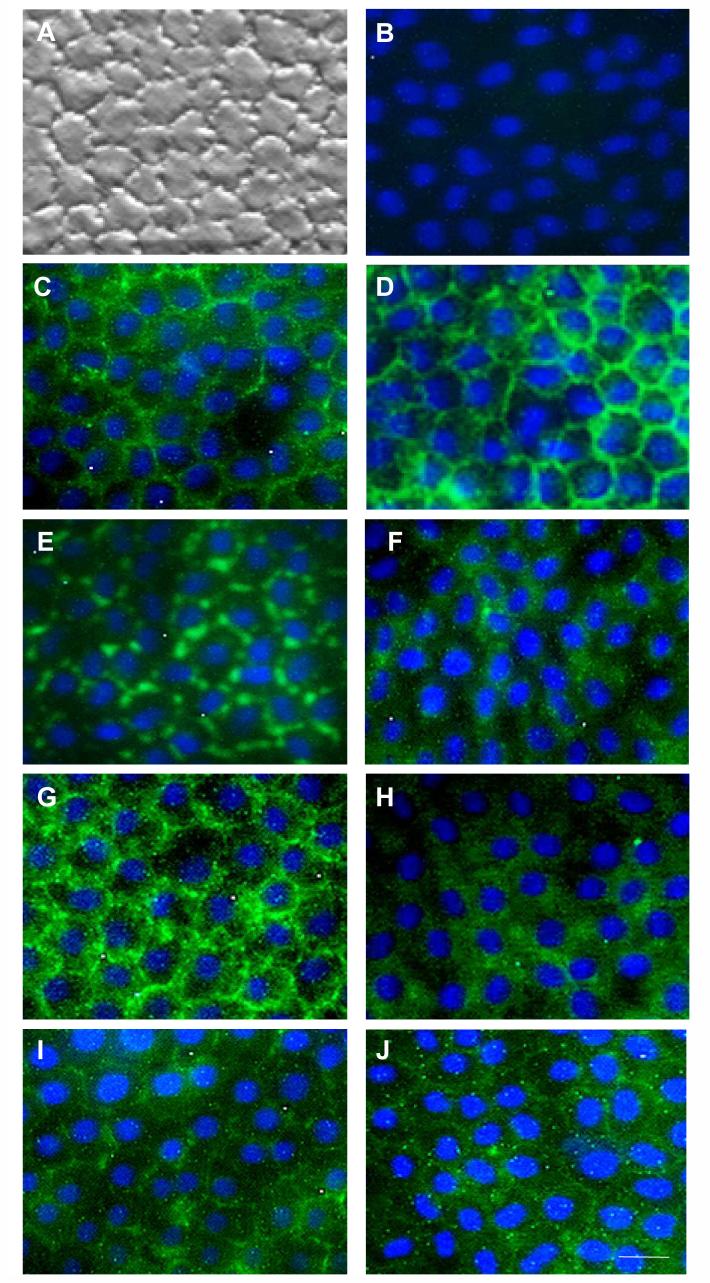
Cytolocalization of Adherent Junction Components Expressed by In Vivo HCECs
To verify that proteins of the aforementioned junction components were indeed expressed by HCECs in vivo, their cytolocalication in reference to the intercellular junction was characterized by immunofluorescence staining. As compared to the characteristic hexagonal configuration of in vivo HCECs (Fig. 3A), phalloidin staining helped localize a preferential circular band of cytoskeletons formed by F-actins close to the intercellular junction (Fig. 3C). Immunostaining to N-cadherin (type I) also showed a continuous circular band (Fig. 3D), but that to VE-cadherin showed a discontinuous circular band (Fig. 3E) distributed similarly at the intercellular junction. In contrast, immunostaining to E-cadherin (type I) was diffuse in the cytoplasm (Fig. 3F). There was no immunostaining to N-cadherin (type II), E-cadherin (type II) and P-cadherin (data not shown), indicating N-cadherin (type II), E-cadherin (type II) and P-cadherin do not play an important role in the adherent junction of HCECs. Immunostaining to β-catenin and p190 also revealed preferential distribution along the intercellular junction (Fig. 3G, 3I), while p120 was distributed equally in the intercellular junction and cytoplasma (Fig. 3H). As reported,3,4,36 ZO-1 was primarily distributed to the intercellular junction (Fig. 3J). The same results were obtained in three different donors, and from the central cornea of two donors (data not shown). The findings disclosed unique cytolocalization of different adherent junction components at intercellular junctions.
Figure 3. Cytolocalization of Junction Components Expressed by In Vivo HCECs.
As compared to hexagonal HCECs in vivo (A), cytolocalization was detected by immunofluorescence staining to phalloidin (C), N-cadherin (type I) (D), VE-cadherin (E), E-cadherin (type I) (F), β-catenin (G), p120 (H), p190 (I) and ZO-1 (J). Negative control using normal mouse serum or rat serum showed the same result as shown in (B). The color of nuclear counterstaining is blue. Magnification bar represents 100 μm.
Gradual Maturation of Adherent Junction Components by in Vitro HCEC Monolayers
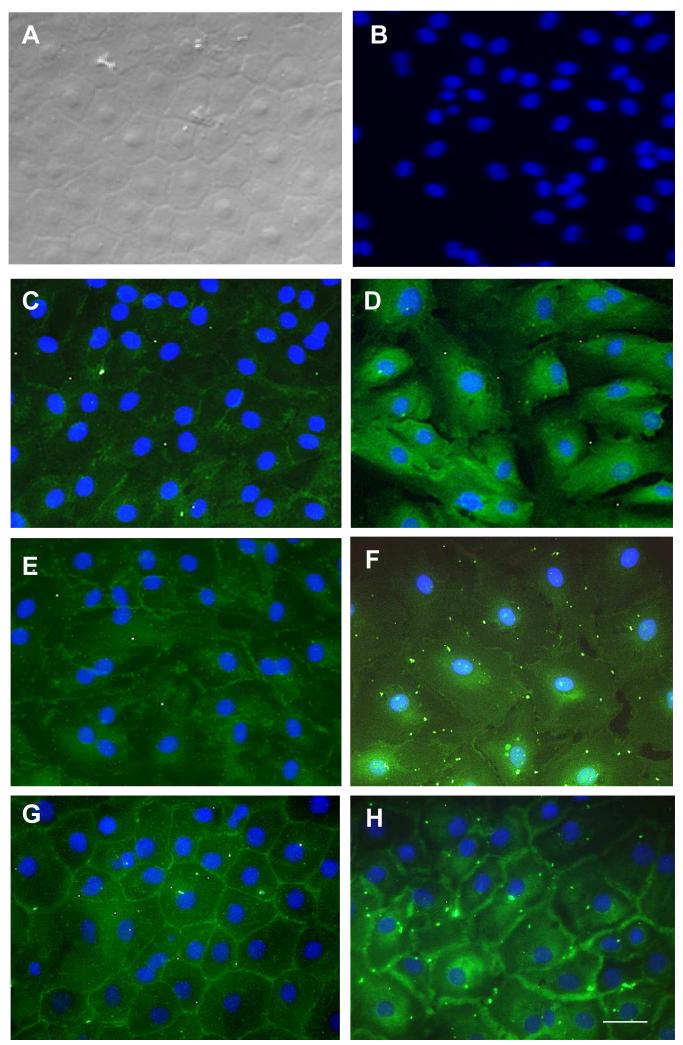
Because transcript expression of several adherent junction components showed gradual maturation toward the in vivo pattern (Fig. 2), we would like to determine whether their protein expression and cytolocalization were also gradually restored to the in vivo pattern (Fig. 3). To do so, we first performed immunostaining to N-cadherin and p120. Phase contrast micrographs indicated that HCECs monolayers cultured on collagen IV-coated dishes in SHEM at day 7, day 14 and day 21 adopted similar characteristic hexagonal shapes (Fig. 4, left column). Their intercellular junctions were more visible at day 7 and day 14 than at day 21. The linear staining of N-cadherin at the intercellular junction was incomplete at day 7 and day 14, but became complete at day 21 (Fig. 4, left column). The staining to p120 was mainly in the cytoplasm at day 7, in both the cytoplasm and the cell junction at day 14, but predominantly in the cell junction at day 21 (Fig. 4, right column). These results suggested that expression and cytolocalization of N-cadherin by in vitro HCEC monolayers gradually reached the in vivo pattern at day 21. Interestingly, p120 in HCECs cultured at day 21 was preferentially distributed in the cell junction rather than equally distributed in the cell junction and cytoplasma, compared to the staining pattern of in vivo HCECs. The significance of this p120 distribution change during in vitro culture of HCECs remained to be explored in the future.
Figure 4. Gradual Maturation of N-cadherin and p-120 by In Vitro HCEC Monolayers.
As compared to the phase contrast micrographs of HCEC monolayers at day 21(A), cytolocalization was examined by immunofluorescence staining to N-cadherin showed gradual maturation from day 7 (C) to day 14 (E) and day 21 (G). Similar gradual maturation was also noted by immunofluorescence staining to p120 from day 7 (D) to day 14 (F) and day 21 (H). Negative control was shown (B). The nuclear counterstaining is blue. The magnification bar represents 100 μm.
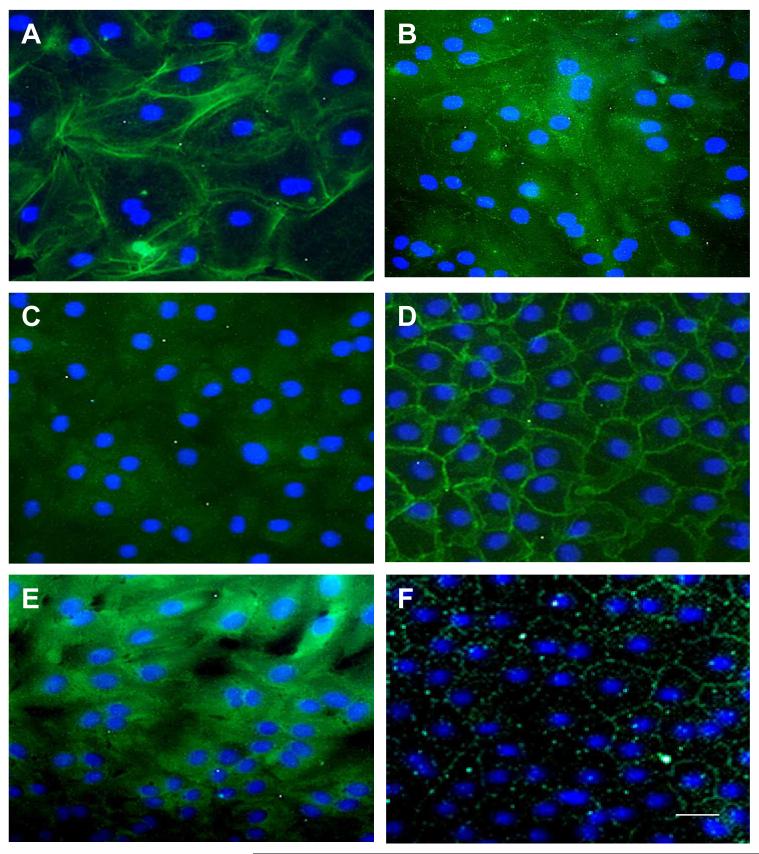
To further verify whether this was the case for other components, we performed immunofluorescence staining to VE- and E-cadherins, β-catenins, p190, and compared to that to F-actin (by phalloidin) and ZO-1 in HCEC monolayers cultured at day 21. Similar to what has been reported,39 F-actin-containing cytoskeletons were distributed as continuous circular bands close to the intercellular junction of each cell (Fig. 5A). The staining to VE-cadherin was noted in the junction and the cytoplasm (Fig. 5B), that of E-cadherin predominantly remained in the cytoplasm, similar to that in vivo (Fig. 5C), that of β-catenin was exclusively in the intercellular junction (Fig. 5D), and that of p190 spread from the junction to the cytoplasm (Fig. 5E). As reported,3,4,36 immunostaining to ZO-1 clearly outlined mostly at the intercellular junction (Fig. 5F). These results indicated that HCEC monolayers cultured up to day 21 indeed achieved a pattern of expression and cytolocalization of all adherent junction components similar to what was observed in vivo.
Figure 5. Maturaturation of Adherent Junction Components by In Vitro HCECs at Day 21.
Immunofluorescence staining of F-actin (by phalloidin) (A), VE-cadherin (B), E-cadherin (C), β-catenin (D), p190 (E), and ZO-1 (F) also showed gradual maturation. Negative control was similar to Fig. 4B (data not shown). The nuclear counterstaining is blue. The magnification bar represents 100 μm.
Decline of the Proliferative Potential in HCECs in Vitro
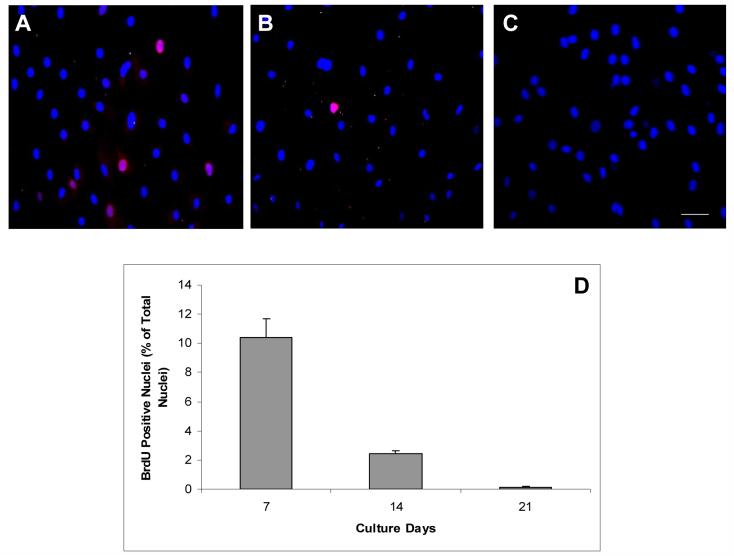
To determine whether the maturation of the cell junction was correlated to the loss of the proliferative activity in HCEC monolayers in vitro, we performed the BrdU labeling experiment at days 7, 14 and 21. The results showed that there were a number of BrdU-labeled nuclei (red) in HCEC monolayers cultured at day 7 (Fig. 6A), but almost none in those cultured at day 21 (Fig. 6C). The average labeling index (N=3) was 10.2 % ± 1.1% at day 7, which was significantly reduced to 2.2% ± 0.1% at day 14 and only 0.1% ± 0.03% at day 21 (Fig. 6C, P<0.05). These results confirmed that maturation of intercellular adherent junctions was correlated with a rapid decline of the proliferative potential in HCEC monolayers during the cultivation for 21 days.
Figure 6. Decline of Proliferative Potentical in HCEC Monolayers.
BrdU labeling (red fluorescence) in the nucleus (blue) was performed to assess the proliferative potential of HCEC monolayers cultured at day 7 (A), day 14 (B), and day 21 (C). The labeling index was 10.2% ± 1.1% at day 7, 2.2% ± 0.1% at day 14, while only 0.1% ± 0.03% at day 21 (C, P<0.05). N=3. The bar represents 100 μm.
DISCUSSION
Cadherin-mediated intercellular adherent junctions are present in all solid tissues of many multi-cellular organism [for reviews see40] Therefore, it was not surprising to note such expression in HCECs. Previous studies have shown the cadherins are present at intercellular adherent junctions of in vivo HCECs,36 but the exact type is not resolved because of the use of a non-specific pan-cadherin antibody. Although E-cadherin (type I)37 and N-cadherin (type I)38 transcripts were found later on, the detailed characterization and cytolocalization of other components was not disclosed until our study. We noted that in vivo HCECs expressed mRNAs and proteins of N-cadherin (type I and II for its mRNAs and type I for its proteins), VE-cadherin, and E-cadherin (type I and II for its mRNA and type I for its proteins) and that both N-cadherin (type I) and VE-cadherin were distributed as continuous and discontinuous circular bands, respectively, along the intercellular junction, while E-cadherin (type I) was primarily distributed in the cytoplasm (Fig. 2 and Fig. 3). Although P-cadherin mRNAs were found only in vivo, and N-cadherin (type II) and E-cadherin (type II) were found both in vivo and in vitro by RT-PCR, their proteins were not detected by immunostaining in vivo, collectively suggesting that P-cadherin, N-cadherin (type II) and E-cadherin (type II) might not play a significant role in HCECs. In fact the level of type II N- and E-cadherin mRNAs was substantially lower than that from type I N- and E-cadherin in HCECs in vivo and in vitro according to our unpublished real-time PCR results (data not shown). Such an expression pattern and cytolocalization of cadherins for in vivo HCECs is quite unique and differs from all epithelial cells where the prototypic E-cadherin is distributed in the intercellular junction, from all vascular endothelia where the prototypic VE-cadherin is localized at the intercellular junction while N-cadherin is in the cytoplasm,41 and from neural cells where only N-cadherin is present at the cell junction.42 Further studies of the mechanism by which such a unique cytolocalization pattern is achieved might help unravel how N-cadherin and VE-cadherin work together in endowing HCECs with the crucial function to control the corneal stromal hydration.
Associated with cadherins at the adherent junction are α-catenin, β-catenin, γ-catenin and p120-catenin that collectively help link cadherins to actin filaments [for reviews see43]. Herein, we noted that mRNAs of α-, β- and γ-catenins were expressed by in vivo HCECs (Fig. 2). We did not perform immunostaining to both α- and γ-catenins as the staining pattern has been reported by Petroll et al36 to be located at the intercellular junction by in vivo rabbit corneal endothelia, and that of γ-catenin is weakly expressed at the same location by in vivo HCECs. We noted that β-catenin was clearly localized at the intercellular junction of in vivo HCECs (Fig. 3). For the first time, we noted that both mRNAs and proteins of p120 were also expressed by in vivo HCECs, and distributed in both the intercellular junction and the cytoplasm (Fig. 2 and Fig. 3). Similar to what has been reported by us4 and others,3,36 we also found that both ZO-1 mRNAs and proteins were expressed by in vivo HCECs at intercellular junctions (Fig. 2 and Fig. 3), noticeably localized in the cell junction as continuous circular bands for in vivo HCECs but punctuated bands for in vitro HCECs.44 Because p190, although not a component of adherent junctions, regulates p120,34 our study noted that both mRNAs and proteins of p190 were expressed by in vivo HCEC, and its cytolocalization was close to intercellular junction but also in the cytoplasm (Fig. 2 and Fig. 3). The above expression and cytolocalization pattern by in vivo HCECs from the peripheral cornea was found to be the same as that from the central cornea (data not shown). Similar to what has been reported for other cell types [for reviews see45], such expression patterns of all adherent junction components must play a key role in stabilizing F-actin-based cytoskeletons in vivo HCECs, giving rise to a unique circular pericellular F-actin band reported by earlier studies.36,46
As commonly known, a brief treatment of trypsin-EDTA is sufficient to disrupt intercellular adherent junctions. Herein, we observed that such a treatment, which was reportedly to immediately disperses HCECs into separately scattered cells on plastics47,48 or collagen IV-coated dishes,4 also dramatically downregulated transcript expression of all junction components to nil except for p120 in HCECs cultured only for 2 days (Fig. 2, Lane 2). Such rapid downregulation has been known to follow the physical disruption of cadherin junctions to liberate cadherins and β-catenin into the cytoplasm for ubiquitinmediated proteolysis.49 Under this circumstance, dissimilar to all other catenins, p120 transcript remained stable in the cytoplasm, consistent with a previous report.50 Trypsin/EDTA4 or EDTA alone51 is known to promote proliferation for cultured HCECs. In our hand, we noted that such treated HCECs, although proliferating faster, adopted a fibroblastic morphology, resembling endothelial-mesenchymal transition described by Lee and Kay especially under the stimulation of bFGF.52 Herein, we noted that cells treated with trypsin/EDTA eventually died after only one or two passages or became fibroblastic-like cells even in the absence of bFGF (Fig. 1F). We thus suspect that trypsin-EDTA treatment to disrupt intercellular adherent junctions is at odds with the primary objective of maintaining the normal HCEC phenotype.
In contrast, we noted a different transcript expression pattern by HCEC aggregates if not submitted to a brief treatment of trypsin/EDTA before cultivation. Specifically, within the cadherin family, expression of N-cadherin (type II) and VE-cadherin transcripts was not downregulated while within the catenin family, expression of β-catenin and p120 transcripts continued at day 2 after cultivation (Fig. 2, Lane 3). Such a difference explained why HCECs continued to maintain a monolayer with close cell-cell contacts by day 7 (Fig. 1) when discontinuous intercellular bands of N-cadherin were maintained while p120 was primarily in the cytoplasm (Fig. 4). Disruption of intercellular junctions without complete suppression of all adherent junction components in this cultivation model was sufficient to trigger active proliferation of HCECs as evidenced a high BrdU labeling index at day 7 (Fig. 6). Such a high proliferative rate during first 7 days of cultivation resembled what has been reported using a brief treatment with EDTA.51 Furthermore, a higher proliferative potential was noted in younger donors (data not shown) similar to what has been noted by others.53
By day 14, there was an increase of transcript expression of all adherent junction components to the level of the in vivo counterpart except for E-cadherin (Fig. 2, Lane 4). Specifically, expression of N-cadherin (type I), α-catenin, γ-catenin, and p190 transcripts were upregulated (Fig. 2, Lane 4) while their cytolocalization pattern of N-cadherin and p120 (Fig. 4) and all other components did not reach to that of in vivo pattern until day 21 (Fig. 5) when the proliferative potential markedly declined (Fig. 6). These results strongly suggested that the proliferative potential of in vitro HCECs was greatly affected by gradual maturation of adherent junctions to an in vivo pattern. Taken together, we speculate that proliferation of HCECs can be promoted while the normal phenotype is maintained by a new strategy to downregulate some, but not all, adherent junction component for achieving ex vivo expansion of HCECs in the future.
Acknowledgments
Supported in part by RO1 EY06819 and RO1 EY015735 (to SCGT) from National Eye Institute, National Institutes of Health National Eye Institute, Bethesda, MD, USA and in part by a research grant from Tissue Tech, Inc., Miami, FL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Proprietary Interests: SCGT but not others has filed a patent on the method of isolation, preservation and expansion of human corneal endothelial cells
Presented in part at the annual meeting of Association of Research in Vision and Ophthalmology in Ft. Lauderdale, FL, April 29, 2008
REFERENCES
- 1.Laing RA, Neubauer L, Oak SS, Kayne HL, Leibowitz HM. Evidence for mitosis in the adult corneal endothelium. Ophthalmology. 1984;91:1129–1134. doi: 10.1016/s0161-6420(84)34176-8. [DOI] [PubMed] [Google Scholar]
- 2.Slettedal JK, Lyberg T, Roger M, Beraki K, Ramstad H, Nicolaissen B. Regeneration with proliferation of the endothelium of cultured human donor corneas with extended postmortem time. Cornea. 2008;27:212–219. doi: 10.1097/ICO.0b013e31815b9723. [DOI] [PubMed] [Google Scholar]
- 3.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48:614–620. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 6.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Mimura T, Yamagami S, Yokoo S, Usui T, Tanaka K, Hattori S, Irie S, Miyata K, Araie M, Amano S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- 8.Mandell KJ, Holley GP, Parkos CA, Edelhauser HF. Antibody blockade of junctional adhesion molecule-A in rabbit corneal endothelial tight junctions produces corneal swelling. Invest Ophthalmol Vis Sci. 2006;47:2408–2416. doi: 10.1167/iovs.05-0745. [DOI] [PubMed] [Google Scholar]
- 9.Mandell KJ, Berglin L, Severson EA, Edelhauser HF, Parkos CA. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Invest Ophthalmol Vis Sci. 2007;48:3928–3936. doi: 10.1167/iovs.06-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu WF, Nelson CM, Pirone DM, Chen CS. E-cadherin engagement stimulates proliferation via Rac1. J Cell Biol. 2006;173:431–441. doi: 10.1083/jcb.200510087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, ls-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- 14.Doherty P, Walsh FS. CAM-FGF Receptor Interactions: A Model for Axonal Growth. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- 15.Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin) J Clin Invest. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–C997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 17.Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandikonda S, Oda D, Niederman R, Sorkin BC. Cadherin-mediated adhesion is required for normal growth regulation of human gingival epithelial cells. Cell Adhes Commun. 1996;4:13–24. doi: 10.3109/15419069609010760. [DOI] [PubMed] [Google Scholar]
- 19.B C, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mech Dev. 1995;53:261–273. doi: 10.1016/0925-4773(95)00442-4. [DOI] [PubMed] [Google Scholar]
- 22.Willert K, Nusse R. Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- 23.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 25.Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and beta-catenin: distinct but closely related. Science. 1992;257:1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- 26.Charpentier E, Lavker RM, Acquista E, Cowin P. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J Cell Biol. 2000;149:503–520. doi: 10.1083/jcb.149.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhurinsky J, Shtutman M, Ben-Ze’ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113(Pt 18):3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- 28.Ireton RC, Davis MA, van HJ, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van RF, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent S, Settleman J. Inhibition of RhoGAP activity is sufficient for the induction of Rho-mediated actin reorganization. Eur J Cell Biol. 1999;78:539–548. doi: 10.1016/S0171-9335(99)80019-3. [DOI] [PubMed] [Google Scholar]
- 32.Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 33.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117:223–231. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- 34.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Hsu JK, Cavanagh HD, Jester JV, Ma L, Petroll WM. Changes in corneal endothelial apical junctional protein organization after corneal cold storage. Cornea. 1999;18:712–720. doi: 10.1097/00003226-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Petroll WM, Hsu JK, Bean J, Cavanagh HD, Jester JV. The spatial organization of apical junctional complex-associated proteins in feline and human corneal endothelium. Curr Eye Res. 1999;18:10–19. doi: 10.1076/ceyr.18.1.10.5392. [DOI] [PubMed] [Google Scholar]
- 37.Gottsch JD, Seitzman GD, Margulies EH, Bowers AL, Michels AJ, Saha S, Jun AS, Stark WJ, Liu SH. Gene expression in donor corneal endothelium. Arch Ophthalmol. 2003;121:252–258. doi: 10.1001/archopht.121.2.252. [DOI] [PubMed] [Google Scholar]
- 38.Joko T, Nanba D, Shiba F, Miyata K, Shiraishi A, Ohashi Y, Higashiyama S. Effects of promyelocytic leukemia zinc finger protein on the proliferation of cultured human corneal endothelial cells. Mol Vis. 2007;13:649–658. [PMC free article] [PubMed] [Google Scholar]
- 39.Petroll WM, Hsu JK, Bean J, Cavanagh HD, Jester JV. The spatial organization of apical junctional complex-associated proteins in feline and human corneal endothelium. Curr Eye Res. 1999;18:10–19. doi: 10.1076/ceyr.18.1.10.5392. [DOI] [PubMed] [Google Scholar]
- 40.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 41.Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cifuentes-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development. 1994;120:1–11. doi: 10.1242/dev.120.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitschke M, Gramm S, Gotze T, Valtink M, Drichel J, Voit B, Engelmann K, Werner C. Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J Biomed Mater Res A. 2007;80:1003–1010. doi: 10.1002/jbm.a.31098. [DOI] [PubMed] [Google Scholar]
- 45.Erez N, Bershadsky A, Geiger B. Signaling from adherens-type junctions. Eur J Cell Biol. 2005;84:235–244. doi: 10.1016/j.ejcb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci. 1995;36:1115–1124. [PubMed] [Google Scholar]
- 47.Engelmann K, Bohnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988;29:1656–1662. [PubMed] [Google Scholar]
- 48.Yokoo S, Yamagami S, Yanagi Y, Uchida S, Mimura T, Usui T, Amano S. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest Ophthalmol Vis Sci. 2005;46:1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- 49.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:2930–2935. [PubMed] [Google Scholar]
- 52.Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279:32325–32332. doi: 10.1074/jbc.M405208200. [DOI] [PubMed] [Google Scholar]
- 53.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45:1743–1751. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]