Abstract
Study Design
A bovine intervertebral disc organ culture model was used to study the effect of dynamic compression magnitude on mechanical behavior and measurement of biosynthesis rate, cell viability, and mRNA expression.
Objective
The objective of this study was to examine the effect of loading magnitude on intervertebral disc mechanics and biology in an organ culture model.
Summary of Background Data
The in vivo and cell culture response of intervertebral disc cells to dynamic mechanical loading provides evidence the disc responds in a magnitude dependant manner. However, the ability to link mechanical behavior of the disc with biologic phenomena has been limited. A large animal organ culture system facilitates measurements of tissue mechanics and biologic response parameters on the same sample allowing a broader understanding of disc mechanobiology.
Methods
Bovine caudal intervertebral discs were placed in organ culture for 6 days and assigned to a static control or 1 of 2 dynamic compression loading protocols (0.2–1 MPa or 0.2–2.5 MPa) at 1 Hz for 1 hour for 5 days. Disc structure was assessed with measurements of dynamic modulus, creep, height loss, water content, and proteoglycan loss to the culture medium. Cellular responses were assessed through changes in cell viability, metabolism, and qRT-PCR analyses.
Results
Increasing magnitudes of compression increased disc modulus and creep; however, all mechanical parameters recovered each day. In the anulus, significant increases in gene expression for collagen I and a trend of increasing sulfate incorporation were observed. In the nucleus, increasing gene expression for collagen I and MMP3 was observed between magnitudes and between static controls and the lowest magnitude of loading.
Conclusion
Results support the hypothesis that biologic remodeling precedes damage to the intervertebral disc structure, that compression is a healthy loading condition for the disc, and further support the link between applied loading and biologic remodeling.
Keywords: spine, intervertebral disc, organ culture, bovine, mechanics, dynamic compression loading
Experimental evidence points to a threshold of loading necessary for intervertebral disc (IVD) extracellular matrix maintenance, where too little load (i.e., immobilization) will reduce biosynthesis rates and overloading can cause structural damage and altered biomechanical behaviors.1-3 Dynamic loading is commonly experienced during daily activity, and is particularly important to include when attempting to identify loading patterns that introduce risks to IVD structure, biomechanics, and biosynthesis. Furthermore, a cyclic loading component is necessary to distinguish between immobilization and overloading. In vivo studies demonstrated there is a frequency, magnitude, and duration effect of applied mechanical loading on IVD cells,2,4 further supporting the importance of a better understanding of such loading patterns on the IVD.
The motion segment complex provides 6 degree of freedom mobility, but its structural components are sensitive to damage under distinct loading conditions. Complex loading regimes (e.g., bending and compression), on the spine can result in disc damage and herniation.3,5-8 Compression loading on the spine is known to put the vertebral endplate at risk of fracture, which is then associated with a loss of nucleus pressurization because of damage at the discovertebral junction.6,9 Evidence of biologic remodeling in disc tissue occurs in response to compressive loading magnitudes insufficient to cause vertebral endplate failure,2 raising the possibility that thresholds of structural failure overestimate the levels of loading which are detrimental to intervertebral disc health.
The biologic response of the IVD to dynamic loading has been previously examined in vivo1,2,10-12 and in cell culture studies,4,13,14 whereas the effects of mechanical loading on IVD structure and mechanics have been studied extensively on nonviable tissue in vitro, leaving unanswered questions about the effects of such mechanical changes on living cell populations. Mechanical loading is known to influence the IVD; however, unanswered questions remain regarding the dependence on other signaling pathways existing in vivo (e.g., proinflammatory molecules), and whether the loss of cell-tissue matrix contact in vitro is detrimental to normal mechanical signal transduction. The ability to examine biologic remodeling pathways while also quantifying structural and mechanical changes induced in response to mechanical loading is a critical step towards understanding how the relationship between biomechanical loading and biologic remodeling might contribute toward a progressive degenerative cascade in the IVD.
The use of an organ culture model facilitates investigation into cellular responses to mechanical loading while the disc is largely intact. Organ culture provides complete control over mechanical boundary conditions while allowing for measurement of mechanical properties throughout the culture duration. Chemical boundary conditions can also be controlled, eliminating the effect of other signaling pathways present in vivo, while maintaining viable cells and normal cell-matrix interactions. Currently, however, few studies have investigated the response of the IVD in organ culture to dynamic loading. Developing and testing a large animal organ culture system is important because of its ability to be more directly translated to human IVDs and also because of the ability to evaluate multiple mechanical and biologic dependent variables on the same IVD.
The aim of this study was to examine the effects of varying physiologic magnitudes of dynamic compression on intact intervertebral disc structure, biomechanics, cell metabolism, and water content in 3 disc regions. The hypotheses were that low magnitudes of dynamic compression would enhance anabolic remodeling whereas high magnitudes of dynamic compression would demonstrate early signs of disc damage and catabolic remodeling. Specifically, dynamic compression applied to the intervertebral disc structure at low magnitudes of active physiologic loading in a human (0.2–1 MPa, e.g., standing up from a chair15) will promote anabolic remodeling, including increased biosynthesis rates, whereas loading at larger magnitudes of active physiologic loading in a human (0.2–2.5 MPa, e.g., lifting 20 kg with round back15 but less than failure of bovine caudal motion segment16,17) will result in early signs of remodeling including structural damage, loss of cell viability, and catabolic remodeling as measured through biomechanical properties, histology, biochemical measurements, sulfate incorporation, and qRT-PCR.
Methods
Three intervertebral discs, corresponding to caudal levels C2–C3, C3–C4, and C4–C5, were dissected from 12 beef cattle (ages 18–24 months) under sterile conditions within 4 hours of slaughter. Dissection included removal of vertebral endplates from the intervertebral disc using a straight edge razor blade to maintain transport through the endplate route.18 After dissection, discs were rinsed in Tyrode's balanced salt solution (TBSS) containing 0.3 μL/mL penicillin/streptomycin and 0.1 μL/mL fungizone (Invitrogen, Carlsbad, CA).
Discs were assigned to 1 of 3 groups consisting of 2 dynamic loading conditions (low, high) and 1 static control (static). Each group consisted of N = 12 discs, with equal numbers of each disc level assigned to each group as the anatomic level of the disc is known to affect cell metabolism and tissue composition. Discs were placed into a custom built organ culture chamber described previously18,19 and housed in an incubator at 37°C and 5% CO2. Culture media consisting of DMEM (4.5 g/L glucose, 110 mg/L sodium pyruvate, with l-glutamine), supplemented with 100 U/mL of penicillin/streptomycin, 0.1 mg/mL gentamicin, 0.75 mg/L fungizone, 0.02 M HEPES buffer, 50 μg/mL ascorbic acid (Invitrogen, Carlsbad, CA), and 10 mL/L FBS (Atlanta Biologic, Atlanta, GA) was continuously circulated at 1.1 mL/min and replaced every 2 days.
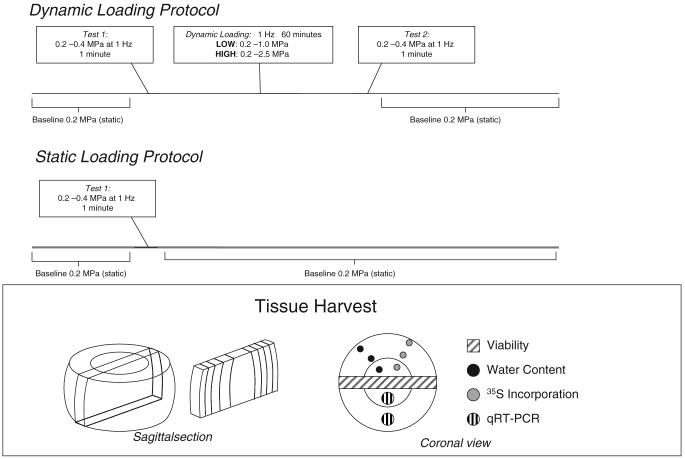
All groups were initially confined with an applied stress of 0.2 MPa for 12 hours.19 Chambers were then individually attached to an incubator-housed loading device for the start of the experimental protocol (Figure 1). First, a 1-minute test (0.2–0.4 MPa, 1Hz) was applied sinusoidally to obtain a preloading dynamic nominal modulus for all groups. Control discs (static group) were removed from the loading device and the 0.2 MPa static load was replaced, and a dynamic load was applied to the 2 test groups (low and high). Dynamic loading consisted of 1 hour of sinusoidal loading at 1 Hz, with amplitudes of 0.2 to 1 MPa for the low load group and 0.2 to 2.5 MPa for the high load group. After the dynamic loading cycle, a repeat of the 1-minute test was performed to obtain a postloading nominal dynamic modulus. After the 3 test cycles, the baseline 0.2 MPa static load was again applied to each chamber and at least 12 hours of recovery was allowed between dynamic load cycles. Each chamber experienced loading once per day, adding to 5 total times during the 6-day culture period.
Figure 1.
Test protocol schematic detailing loading protocol (top). Illustration showing tissue harvest protocol (bottom). Note: Schematic diagram not to scale.
Creep during 1 hour of dynamic loading was calculated from displacements that were recorded from the loading device at points corresponding to 0.2 MPa load for the first and last cycles of the 1 hour dynamic loading test, and the initial height at the first cycle was compared between days to compare height lost over the culture duration. Dynamic stiffnesses were calculated using custom written MATLAB code (The MathWorks, Natick, MA) and for ease of comparison across animals, all stiffness measurements were normalized by initial IVD cross-sectional area and presented as a “nominal modulus.”
Structural parameters assessed included changes in intervertebral disc diameter and height. Initial height and diameter measurements were obtained using 3 caliper measurements in each dimension, recorded before the start of the culturing process, and immediately after culture termination. Proteoglycan content released to the culture media was assessed using the DMMB colorimetric assay. Aliquots of culture media were centrifuged at 10,000 rpm for 3 minutes before the application of the DMMB assay. A standard curve was generated using chondroitin-6-sulfate and DMEM, and sample absorbances were read on a microplate reader. Regional tissue water content was calculated after tissue dissection, weighing, and lyophilization to obtain a dry tissue weight.
Cell metabolism was assessed using the 35S incorporation assay. Immediately after culture termination, sections of intervertebral disc tissue from each of the tissue regions (OA, IA, NP) were dissected (Figure 1), weighed, and placed in 2 mL of culture medium without FBS containing 2.5 μCi of 35S (Perkin-Elmer, Boston, MA) and brought to an approximate osmolarity of 400 mOsm by the addition of 1.5% v/v 5 M NaCl and 0.4 M KCl to reduce tissue swelling. Samples were incubated for 6 hours at 37°C and 5% CO2, after which they were removed from the radiolabel medium and digested with proteinase-K (0.5 mL of 1 mg/mL at 57°C). Radioactive media was stored for each tissue sample to allow for later normalization. After digestion, nonincorporated sulfate was removed by exhaustive dialysis against distilled water. Radioactivity of samples was measured using a scintillation counter, and was normalized to incubation media radioactivity and tissue sample dry weight. To minimize potential artifacts because of GAG leaching that may have occurred during the radiolabel incubation step, the sample dry weight was calculated based on measurements of specimen wet weight and water content of paired tissue samples that were taken before the incubation (Figure 1).
Cell viability was examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St Louis, MO) to stain vital cells through the formation of precipitate by active mitochondria, and ethidium homodimer-1 (Invitrogen, Carlsbad, CA) to stain the DNA of nonvital cells with compromised nuclear envelopes. Tissue sections approximately 10 mm by 5 mm were dissected through the disc in the sagittal plane (Figure 1) and placed into a TBSS solution containing 1 mg/mL MTT and 1 μmol/L ethidium homodimer-1. After a 2-hour staining period, samples were removed from the stain solution and placed on a shaker in TBSS for 10 minutes to remove excess dye. The tissue was then frozen in isopentane floated in liquid nitrogen and stored at −80°C until sectioning on a cryotome. Five 10 μm thick sections were taken for each tissue region (OA, IA, and NP) beginning at the tissue surface and every 250 μm thereafter. For each of the 15 resulting slides, representative images were captured at 20× magnification (Zeiss axiocam, Zeiss, Thornwood, NY) first under fluorescent light to capture cells stained with ethidium homodimer-1 (Rhodamine filter: ex/em of 546 nm/617 nm) and then under brightfield light to capture precipitate formed with MTT by vital mitochondria.
QRT-PCR was performed on tissue isolated from the anulus and nucleus regions. The OA and IA were pooled to ensure sufficient tissue quantity for RNA isolation. Expression levels were quantified using SYBR green for aggrecan, versican, collagen types I and II, TIMP-1, MMP -2, -3 and -13, and ADAMTS-4 were normalized to 18S expression levels (to generate ΔCt values). Expression levels for experimental groups (low and high) were normalized to tail-matched static controls (to generate ΔΔCt values).
A one-way ANOVA was used to evaluate the effect of loading group (static, low, high) on changes in disc mechanics, diameter, height, water content, GAG content in the media, and 35S incorporation. All statistical analyses on qRT-PCR data were performed on the ΔCt values. A one way ANOVA was used to compare loading groups (low and high).8 Fisher PLSD post hoc test was used to detect differences between loading groups with a significance level of P < 0.05 for all ANOVA tests. For qRT-PCR data an additional Student t test with hypothesized mean of zero was used to evaluate statistical differences between static controls (static) and loading groups (low and high), which were normalized to the static controls.
Results
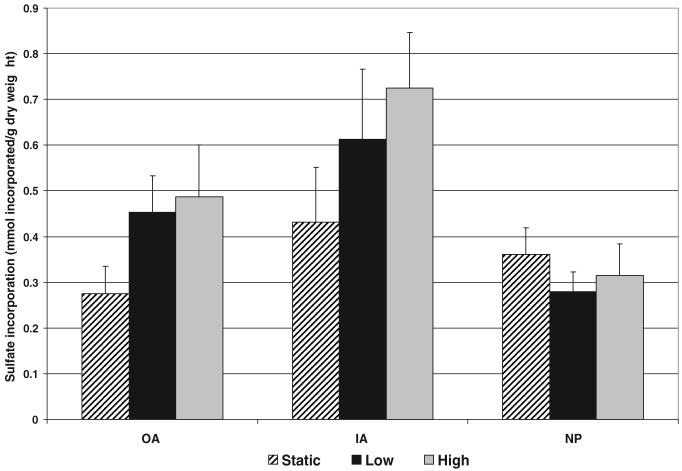
All data are presented as average ± SEM. No significant changes in disc height or diameter were found at the end of the culture period for any of the test groups. The average height loss was 14.8 ± 1.95% and diameter gain was 42.6 ± 1.62%. Proteoglycan loss to the culture media was also not significantly affected by mechanical loading with an average of 0.065 ± 0.004% of the initial disc wet weight. Likewise, no significant differences in regional tissue water content were found between groups, with regional differences in tissue water content maintained. Average tissue water contents were 59.25 ± 0.700% in the OA, 73.59 ± 0.617% in the IA and 79.8 ± 0.562% in the NP. A trend of increasing sulfate incorporation (Figure 2) with increasing load magnitude was observed in the OA and IA; however, no corresponding trend was noted in the NP. Viability was not significantly different between loading groups (Figure 3).
Figure 2.
Average ± SEM sulfate incorporation rates for all testing groups (OA, outer annulus; IA, inner annulus; NP, nucleus pulposus). A trend of increasing sulfate incorporation was seen in the anulus regions, but not in the nucleus.
Figure 3.
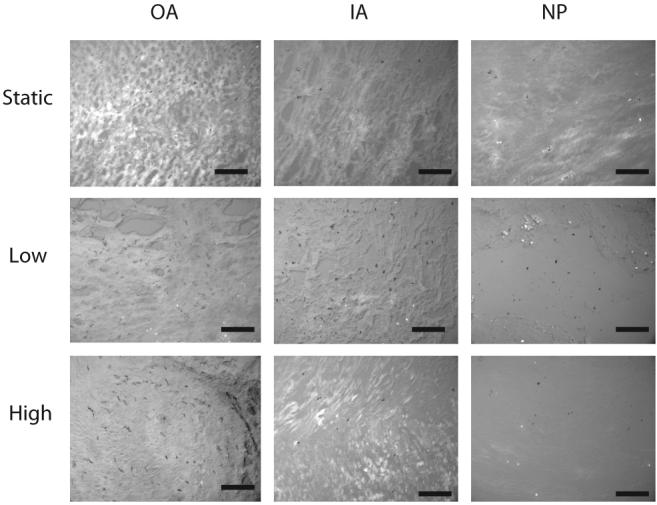
Representative viability images at 20× magnification. Black = live, white = dead. Scale bar in black = 400 μm. Columns represent tissue regions (OA, outer annulus; IA, inner annulus; NP, nucleus pulposus) and rows represent test groups. No changes in cell viability were observed.
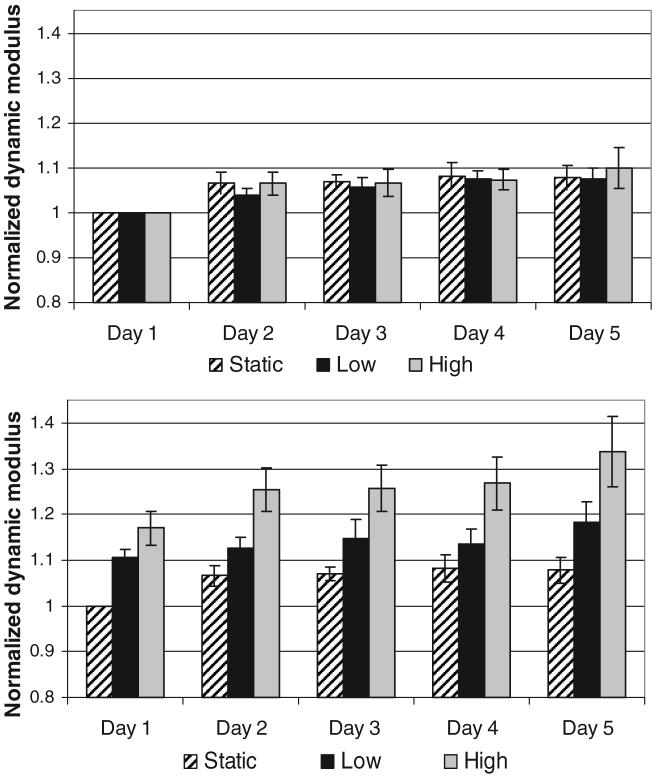
The preloading dynamic modulus was not significantly different between loading groups at any time point (Figure 4). There was a significant increase in the preload modulus between day 1 and day 2 for all groups, but no further significant increases occurred throughout the culture duration for any group. The postload dynamic modulus significantly increased with increasing load magnitude. Likewise, the magnitude of creep observed during the 1 hour of dynamic loading was greater with increasing load magnitudes (Figure 5). No significant difference was noted in the starting disc height between days, indicating a complete recovery of the lost disc height between days.
Figure 4.
Average ± SEM nominal dynamic loading modulus for preload (top) and postload (bottom) tests.
Figure 5.
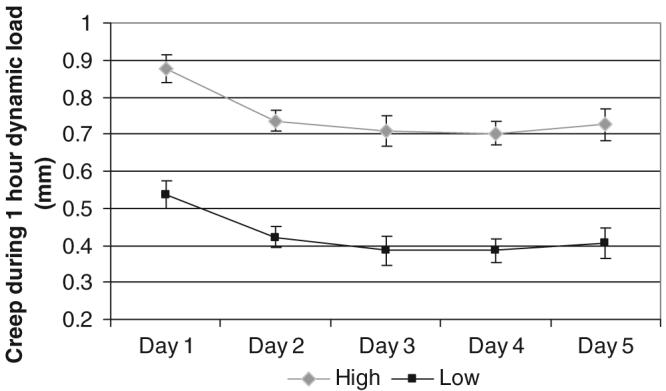
Average ± SEM height loss for the 1 hour dynamic loading protocol. Significantly more height was lost at all time points in the high force dynamic loading groups than in the low force group.
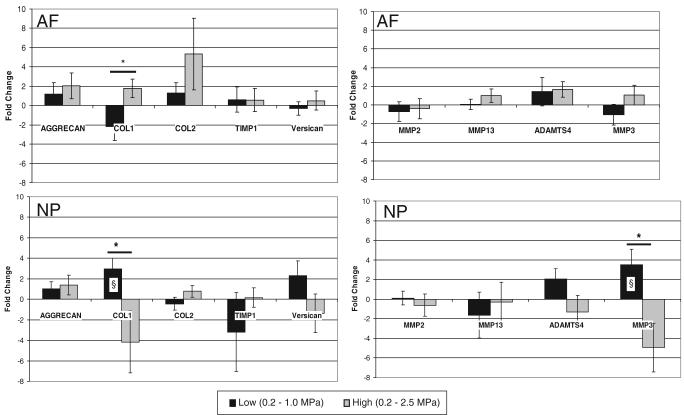
Gene expression in the anulus region was not found to be significantly different with respect to static controls (P > 0.05) (Figure 6). Significant changes in collagen type 1 regulation were found between loading magnitudes, with downregulation observed in the low group and upregulation in the high group (P = 0.48). In the nucleus pulposus, significant upregulation of collagen type 1 (P = 0.002) and MMP3 (P = 0.01) gene expression was observed in the low group relative to static controls. Significant differences in gene expression were also observed between low and high loading groups for collagen 1 (P = 0.018) and MMP3 (P = 0.01).
Figure 6.
Gene expression fold change results as average ± SEM for anabolic (aggrecan, collagen types I and II, versican), anticatabolic (TIMP-1) (left column), and catabolic (MMP −2, −3, −13, and ADAMTS-4) (right column) genes for tissue from anulus fibrosus (top row) and nucleus pulposus (bottom row) regions. Significant differences (P < 0.05) between groups are marked with a * whereas significant differences from static controls (using a t test with hypothesized mean of zero) are marked with a § symbol (found in low groups in the NP for collagen type I and MMP3). Note that low and high groups are normalized to tail matched static controls.
Discussion
The effect of dynamic compression on the mechanical and biologic state of the intervertebral disc was examined at 2 loading magnitudes to test the hypotheses that low magnitudes of dynamic compression would increase anabolic remodeling whereas high magnitudes of dynamic compression would demonstrate early signs of disc damage and catabolic remodeling. The use of an organ culture system enabled measurement of mechanical properties throughout the culture duration while maintaining cell viability and metabolism, thus allowing for regional measurements of sulfate incorporation, qRT-PCR, histology, and water content. Overall, the results support the concept that the intervertebral disc tissue structure is tolerant to applied mechanical compression, with no observed permanent damage to the tissue structure. Load magnitude dependent increases in anabolic mRNA expression and sulfate incorporation suggest that dynamic compression increases disc metabolic rate and enhances anabolic remodeling. Significant differences in gene expression were also observed between loading magnitudes in both the anulus fibrosus and nucleus pulposus regions for collagen type I, and in the nucleus pulposus region for MMP3.
The magnitude of applied loading in this study was chosen to reflect expected magnitudes observed in vivo.15 The nominal dynamic modulus and creep magnitude of the disc increased with increasing load magnitude as would be expected due to nonlinear material behaviors associated with tissue compaction at higher loading magnitudes; however, all changes were fully recovered within 12 hours. The recovery times observed in this study are consistent with literature on the topic, with previous studies observing complete recovery after 18 hours.20 Additionally, the removal of the vertebral endplates necessary to maintain cell viability18 has been shown to speed recovery time.21 Although the removal of endplates from the intervertebral disc is anticipated to affect absolute values of local strains in the intervertebral disc, it is not expected to affect relative comparisons between loading magnitudes. It is possible that the magnitude dependent increase in sulfate incorporation in the anulus fibrosus but not the nucleus pulposus is associated with a loss of pressurization in the nucleus due to removal of endplates. The loss of disc height and increase in disc diameter observed is consistent with previous studies on the bovine intervertebral disc in culture,19 and is likely associated with postmortem muscle relaxation resulting in an increase in disc hydration.9 The full recovery of disc properties, combined with no significant changes in disc height, diameter, water content, and GAG loss to the culture media, provide evidence that applied compression loading results in no permanent damage to the intervertebral disc structure even up to magnitudes of 2.5 MPa.
Previous studies examining the biologic response of the intervertebral disc to dynamic loading have been performed in vivo1,2,12 and on cell cultures.4,13,14 Tissue culture and organ culture studies primarily examined the effects of static loading16,18,22 or diurnal loading (applied osmotically23 or through compression19). The current study method links the response of isolated cells to that of the in vivo situation by retaining the in situ cell environment. It is possible that changes in the collagen microstructure may be present, but these did not affect the morphologic structural parameters that were measured in this study, and that are recorded in patients radiologically.24 Although a direct comparison across studies and methodology is difficult due to the use of different species, and varying loading magnitudes, frequencies and modes of application (compression, hydrostatic, tension), it is fairly consistent that collagen type I and MMP3 are affected by applied mechanical stimulation in the intervertebral disc in a region specific manner.2,14
In conclusion, increased cell metabolism as measured on the gene and protein levels was observed at loading magnitudes insufficient to cause observable disc damage, supporting the concept that biologic remodeling precedes intervertebral disc damage. Results also suggest that dynamic compression is a healthy loading condition due to the lack of observable signs of intervertebral disc damage at high stress levels in this study, combined with other studies on risky loading patters on the disc that defined lateral bending and flexion as the loading patterns that place the disc at most risk of injury.8 Furthermore, it is interesting that load magnitude had a progressive increase in expression of many genes in the anulus while collagen-I and MMP3 in the nucleus region were all downregulated after a substantial increase in load magnitude. These observations demonstrate combined mechanical, biologic, and chemical remodeling in response to dynamic compression. Consequently, results motivate the need for further studies on the effects of applied loading on the biologic response of the intervertebral disc under more damaging conditions that might include bending and endplate fracture under compression.
Key Points.
The biologic and mechanical responses of the cultured intervertebral disc to applied dynamic compression were examined.
No permanent compromise in disc mechanical properties was observed throughout the culture period.
Biosynthesis rates and gene expression responses were affected by disc region and dynamic compression magnitude, particularly for collagen type 1 and MMP3.
Dynamic compression magnitude increased biosynthesis rates and did not result in structural disruption, suggesting it is a healthy loading condition for the disc.
Results suggest biologic changes occur before structural damage to the intervertebral disc.
Acknowledgments
The authors gratefully acknowledge Dr. Karin Wurtz for primer sequences and Arthur Michalek for assistance with the creation of computer code for mechanical parameter analysis.
Federal and Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. Supported by NIH grant R01 AR051146.
References
- 1.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–37. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 2.Maclean JJ, Lee CR, Alini M, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29:2724–32. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasra M, Merryman WD, Loveless KN, et al. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24:1967–73. doi: 10.1002/jor.20253. [DOI] [PubMed] [Google Scholar]
- 5.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165–75. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams MA, Freeman BJ, Morrison HP, et al. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–36. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Farfan HF, Cossette JW, Robertson GH, et al. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg Am. 1970;52:468–97. [PubMed] [Google Scholar]
- 8.Costi JJ, Stokes IA, Gardner-Morse M, et al. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: Motions that place disc tissue at risk of injury. J Biomech. 2007;40:2457–66. doi: 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfirrmann CW, Resnick D. Schmorl nodes of the thoracic and lumbar Spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology. 2001;219:368–74. doi: 10.1148/radiology.219.2.r01ma21368. [DOI] [PubMed] [Google Scholar]
- 10.MacLean JJ, Lee CR, Grad S, et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–81. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 11.MacLean JJ, Lee CR, Alini M, et al. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–7. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Ching CT, Chow DH, Yao FY, et al. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech (Bristol, Avon) 2003;18:182–9. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 13.Kasra M, Goel V, Martin J, et al. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21:597–603. doi: 10.1016/S0736-0266(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 14.Wuertz K, Urban JP, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. doi: 10.1002/jor.20436. In press. [DOI] [PubMed] [Google Scholar]
- 15.Wilke HJ, Neef P, Caimi M, et al. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–62. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara H, McNally DS, Urban JP, et al. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80:839–46. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- 17.Ochia RS, Tencer AF, Ching RP. Effect of loading rate on endplate and vertebral body strength in human lumbar vertebrae. J Biomech. 2003;36:1875–81. doi: 10.1016/s0021-9290(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 18.Lee CR, Iatridis JC, Poveda L, et al. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine. 2006;31:515–22. doi: 10.1097/01.brs.0000201302.59050.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korecki CL, MacLean JJ, Iatridis JC. Characterization of an in vitro intervertebral disc organ culture system. Eur Spine J. 2007;16:1029–37. doi: 10.1007/s00586-007-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannessen W, Vresilovic EJ, Wright AC, et al. Intervertebral disc mechanics are restored following cyclic loading and unloaded recovery. Ann Biomed Eng. 2004;32:70–6. doi: 10.1023/b:abme.0000007792.19071.8c. [DOI] [PubMed] [Google Scholar]
- 21.MacLean JJ, Owen JP, Iatridis JC. Role of endplates in contributing to compression behaviors of motion segments and intervertebral discs. J Biomech. 2007;40:55–63. doi: 10.1016/j.jbiomech.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handa T, Ishihara H, Ohshima H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–66. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 24.Pezowicz CA, Schechtman H, Robertson PA, et al. Mechanisms of anular failure resulting from excessive intradiscal pressure: a microstructural-micromechanical investigation. Spine. 2006;31:2891–903. doi: 10.1097/01.brs.0000248412.82700.8b. [DOI] [PubMed] [Google Scholar]