Abstract
An unbiased conditioned place preference paradigm and the microdialysis technique was used to evaluate the effect of (+)-morphine pretreatment on the conditioned place preference produced by (−)-morphine and the increased release of the dopamine produced by μ-opioid ligand endomorphin-1, respectively, in the posterior nucleus accumbens shell of the male CD rat. (−)-Morphine (2.5–10 μ2g) microinjected into the posterior nucleus accumbens shell dose-dependently produced the conditioned place preference. Pretreatment with (+)-morphine (0.1–10 pg) given into the posterior accumbens shell for 45 min dose-dependently attenuated the conditioned place preference produced by (−)-morphine (5 μg) given into the same posterior accumbens shell. However, higher doses of (+)-morphine (0.1 and 1 ng) were less effective in attenuating the (−)-morphine-produced conditioned place preference. Thus, like given systemically, (+)-morphine given into the posterior nucleus accumbens shell also induces an U-shaped dose-response curve for attenuating the (−)-morphine-produced conditioned place preference. Microinjection of μ-opioid agonist endomorphin-1 (1–10 μg) given into the ventral tegmental area dose-dependently increased the release of the extracellular dopamine in the posterior nucleus accumbens shell in the urethane-anesthetized rats. The increased dopamine caused by endomorphin-1 (10 μg) was completed blocked by the (+)-morphine (10 pg) pretreatment given into ventral tegmental area. It is concluded that (+)-morphine attenuates the (−)-morphine-produced conditioned place preference and the μ-opioid receptor-mediated increase of extracellular dopamine in the posterior nucleus accumbens shell of the rat.
Keywords: addiction, mesolimbic system, dopamine, morphine, opioid, rat
1. Introduction
We have previously demonstrated that (+)-morphine at an extremely low dose given systemically attenuates the tail-flick inhibition and the conditioned place preference produced by (−)-morphine given systemically (Wu et al., 2005, 2007a). The antagonistic effects of (+)-morphine against the (−)-morphine-produced tail-flick inhibition and conditioned place preference produced by (−)-morphine appear to be mediated by the activation of the naloxone-sensitive sigma receptors. This view is evidenced by the findings that the attenuation of the tail-flick inhibition and the conditioned place preference produced by (−)-morphine given systemically is reversed by the (+)-naloxone or (−)-naloxone and by the sigma-receptor antagonist BD1047 given systemically (Wu et al., 2007b; Terashvili et al., 2007).
The ventral tegmental area and nucleus accumbens represent two key structures of the mesolimbic dopaminergic system for the reinforcing properties of opioids (Watson et al., 1989; Wise and Rompre, 1989). The dopamine perikarya are located in the ventral tegmental area and nucleus accumbens represents an important terminal field of the fibers arising therein. μ-Opioid agonists such as (−)-morphine or endogenous opioid peptide, endomorphin-1 given into the ventral tegmental area or posterior nucleus accumbens produces the conditioned place preference (Bals-Kubit et al., 1993; Olmstead and Franklin, 1996; Terashvili et al., 2004). Stimulation of μ-opioid receptors by (−)morphine or other μ-opioids in the ventral tegmental area enhances mesolimbic dopaminergic neurotransmission, presumably by inhibition of GABAergic interneuron, thereby disinhibiting mesolimbic dopamine neurons and increasing both somatodendritic and axonal dopamine release (Stinus et al., 1980, 1982; Kalivas and Duffy, 1990; Spanagel et al., 1992; Johnson and North, 1992; Klitenick et al., 1992; Devine et al., 1993). Thus, activation of the μ-opioid receptors by (−)-morphine or other μ-opioids produces conditioned place preference via the increased release of dopamine in the mesolimbic dopaminegic regions.
We have previously demonstrated that the tail-flick inhibition induced by (−)morphine given intrathecally or given into the intracerebral ventral periaquaductal gray is attenuated by (+)-morphine given into the same central sites, indicating that (+)-morphine attenuates the (−)-morphine-produced antinociception at the central injected sites (Wu et al., 2005; Terashvili et al., 2007). Present experiment was then undertaken to determine if (+)-morphine given into the posterior nucleus accumbens attenuated the conditioned place preference produced by (−)-morphine from the posterior nucleus accumbens and to determine if the increased extracellular dopamine in the nucleus accumbens induced by the activation of the μ-opioid receptors by endomorphin-1 can be attenuated by (+)morphine from the ventral tegmental area. We found that pretreatment with (+)-morphine at an extremely low picogram dose attenuated the conditioned place preference produced by (−)-morphine and the increase release of μ-opioid agonist endomorphin-1-induced dopamine release in the posterior nucleus accumbens. Thus, the posterior nucleus accumbens is one of the sites in the central nervous system, which is sensitive to (+)morphine for attenuating the (−)-morphine-produced conditioned place preference in the rat.
2. Materials and Methods
2.1. Animals
Male CD rats (Charles River Laboratories Inc., Wilmington, MA) weighing between 300 to 350 g at the time of surgery were housed in pairs before and after surgery. They were maintained in a room at 22 ± 0.5°C with an alternating 12 h light/dark cycle. Food and water were available ad libitum. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin.
2.2. Surgical Procedures
Rats were pretreated with methylatropine bromide (5 mg/kg given intraperitoneally) and anesthetized with pentobarbital sodium (50 mg/kg given intraperitoneally) and were mounted in a stereotaxic apparatus (David Kopf Instrument, Tujinga, CA). The distance of heights between the incision bar and the horizontal lane passing through the interaural line is 3.3 mm (Paxinos and Watson, 1997). A 23-gauge stainless steel guide cannula 12 mm in length was then implanted unilaterally 3 mm down from the surface of the skull and anchored to the skull with three stainless screws and dental cement. Unilateral as opposed to bilateral placement of the cannula was chosen for the reason that bilateral injections are more difficult to perform symmetrically at the intended injection site, and previous reports suggest that unilateral injection is sufficient to produce the conditioned place preference and conditioned place aversion (Bals-Kubik et al., 1993; Suzuki et al., 1997; Zangen et al., 2002). The coordinate for the placement of the cannula was AP 0.7–1.0 mm posterior to bregma and 1.0 mm lateral to the midline for the posterior nucleus accumbens shell (Paxinos and Watson, 1997). After a recovery period of at least 5 days, animals without motor defects were used for the experiments.
2.3. Conditioned place preference
An unbiased conditioned place preference paradigm was used to evaluate the effect of (+)-morphine pretreatment on the conditioned place preference produced by (−)morphine. The place conditioning experiment consisted of pre-conditioning, conditioning and post-conditioning phases. Injections of vehicle or drugs were only done during the conditioning phase. A two-compartment box (60 × 29.2 × 29.2 cm) with a transparent Plexiglas front separated by a gray cylinder platform (10.3 cm in diameter and 12 cm in height) was used. One compartment was white with a textured floor and the other was black with a smooth floor. For pre-conditioning, rats were initially placed on the neutral cylinder gray platform and allowed to step down off of the platform to either the white or black compartment. A sliding wall was then put down on the platform and the rat was free to access either compartment through two openings (9.5 × 12 cm each) on each side of the platform. The amount of time spent in the black or white compartment was manually measured for 15 min. Rats which spent less than 5 min 50 s in either the white or black compartment were considered not to be neutral in preference for either side and were excluded from further study (less than 5 % of rats). This initial preference was measured once in the morning for each rat.
The place conditioning session was carried out on days 2 to 4. The box was divided into two equal-sized compartments by putting down the sliding wall after removing the gray cylinder platform. Conditioning session was conducted twice daily, morning and afternoon, and repeated for 3 days. Rats were placed in either the black or white compartment immediately following the injection of (−)-morphine and left in that compartment for 40 min. Forty min is in agreement with previous studies (Bals-Kubik et al., 1993; Shippenberg and Heidbreder, 1995; Terashvili et al., 2004). One half of rats from each group were confined to either the black or white compartment after injection of (−)-morphine in the morning session of each day, and were confined to the opposite compartment after the injection of vehicle for the afternoon session. The other half of rats were confined to either the black or white compartment after injection with vehicle for the morning session and were confined to the opposite compartment after injection of (−)morphine for the afternoon session of the place conditioning. Animals receiving vehicle in both sessions served as controls. The morning session was carried out at 9–11 AM and the afternoon session was carried out at 3–5 PM of the day.
The post-conditioning session was carried out on day 5 and was exactly the same as the pre-conditioning. The scores for the drug-paired place were then calculated by subtracting the pre-conditioning score from post-conditioning score. A positive score represents conditioned place preference, while a negative score represents conditioned place aversion.
2.4. Histological Identification
At the end of the experiments, rats were injected with 0.5 μl of methylene blue solution (2%) to mark the injection site and were then sacrificed with CO2. The brains were removed, frozen and sectioned sagittally for microscopic identification of the injection sites. The stereotaxic atlas of rats by Paxinos and Watson (1997) was used as a guide for the identification of anatomical injection sites. Only the data obtained from rats, in which the injection sites were accurately identified to be in posterior nucleus accumbens shell were used for further statistical analysis.
2.5. Brain microdialysis and intracerebral injection
The microdialysis system including microdialysis probes, pump, and fraction collector, were purchased from CMA/Microdialysis (Action, MA). Essentially, the microdialysis probes were constructed to a concentric cannula design similar to that described (Hernandez et al., 1986) except that fused silica tubing (75 μm i.d., 150 μm o.d. Polymicrotechnology) were used for the internal cannula. The outer cannula was manufactured from 24-gauge thin-wall stainless tubes. The active part of the probe consisted of a 2.0 mm length of hollow dialysis membrane sealed at the tip with epoxy resin (Maidment et al., 1989). The molecular weight cutoff for the microdialysis probe is 20,000 Dalton.
Rats were injected with 5 mg/kg of methylatropine and anesthetized with urethane (1.2 g/kg given intraperitoneally) and mounted in rat stereotaxic apparatus. A microinjection guide cannula was stereotaxically inserted into the ventral tegmental area site to be used for the drug injection and anchored to the skull with three stainless screws and dental cement. The microdialysis probe, which was connected to a CMA Microdialysis Pump, was stereotaxically inserted into the posterior nucleus accumbens shell. The dialysis tube was perfused with artificial CSF containing 147 mM Na+, 2.25 mM Ca2+, 4 mM K+, and 155.6 mM Cl- (pH 6.0) at a flow rate of 2.5 μl/min. Perfusates were collected every 15 min in Eppendorf tubes containing 5 μl of 0.1 M perchloric acid. Once dopamine in the perfusates was stabilized (about 60 min), two consecutive samples were collected for determination of basal levels of dopamine. Rats were then injected intracerebrally into the ventral tegmental area with (+)-morphine, endomorphin-1, (−)-naloxone or saline and perfusates from microdialysis probe were continually collected every 15 min for 180 min. The body temperature (monitored rectally) were maintained at 37 °C using a heating pad (Homeothermic Blanket Control Unit, Harvard Inc.)
2.6. Neurochemical analysis for measuring dopamine and its metabolites
The concentrations of dopamine in the dialysis perfusates were determined using a high performance liquid chromatography-electrochemical detection (HPLC-ECD) system. The perfusates were collected and put on ice. Twenty μl of the perfusates will be injected onto the HPLC-ECD. The HPLC system consists of a delivery pump (EP-10, Eicom, Japan), an analytical column (EICOMPAK, MA-5ODS 4.6 f × 150 mm, Eicom) and a guard column (Eicom). The electrochemical detector (EC-100, Eicom) with a graphite electrode (WE-3G, Eicom) were used at voltage setting of +0.70 V vs. an Ag/AgCl reference electrode. The mobile phase were 0.1 M sodium acetate/0.1 M citric acid buffer, pH 3.5, containing 14 % methanol, sodium 1-octanesulfonate (0.74 mM) and EDTA (0.01 mM). The flow rate was set to 0.5 ml/min with a column temperature of 25 °C. The HPLC-ECD system available was extremely sensitive and reliable in measuring biogenic amines and amino acid neurotransmitters. The sensitivity for dopamine was 1–2 pg/μl. The average retention time of dopamine was 14 min.
2.7. Drugs and Drug Administrations
(−)-Morphine sulfate and (+)-morphine base were obtained from National Institute of Drug Abuse (Baltimore, MD). (−)-Morphine sulfate was dissolved in sterile saline solution (0.9% NaCl solution). (+)-Morphine was initially dissolved in10 N hydrochloric acid and then titrated with 1 N sodium hydroxide to pH 6 to 7, which then diluted to the intended dose in 0.9 % saline. The injection volume for each microinjection was 0.5 μl. Injections were made by hand with a 30-gauge injection needle attached to a microsyringe via polyethylene tubing and administered over a 30 s period. The injection needle was left in place for an additional 60 s to ensure complete distribution. The stereotaxic coordinates of the intended injection site were AP 0.7–1.0 mm anterior to bregma, 1.0 mm lateral to the midline and 7.4 mm down from the surface of the skull for posterior nucleus accumbens shell (MacDonald et al., 2003; Bari and Pierce, 2005; Anderson et al., 2006); AP 2.70–2.96 mm anterior to interaural point, 0.5 mm lateral to midline, and 8.4 mm down from the surface of the skull for ventral tegmental area (Paxinos and Watson, 1997).
2.8. Statistical Analysis
Conditioning scores were expressed as means ± S.E.M. The Student paired t-test was used to analyze the differences of the score between pre- and post-conditioning of each group of rats. One-way analysis of variance (ANOVA) followed by Dunnett’s post-test was used to compare the difference between drug treated groups and the vehicle treated group. The results of dopamine release were expressed as percent of basal dopamine release and expressed as means ± S.E.M. The two-way ANOVA followed by Bonferroni post-test were used to test the significance of the difference between groups. In all experiments, P < 0.05 was considered a significant difference. The Prism statistical software was used to perform the statistics (version 4.1; GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Effect of (−)-morphine microinjected into the posterior nucleus accumbens shell on the production of the conditioned place preference
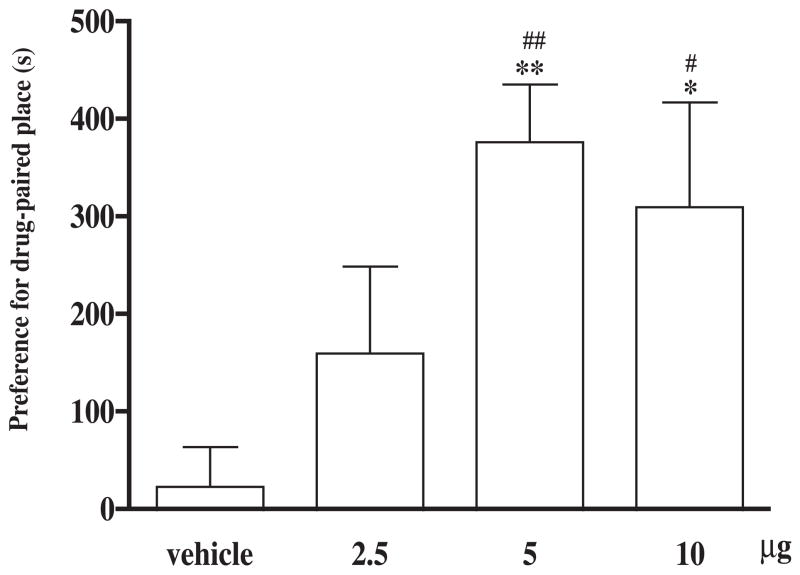
Groups of rats were microinjected with different doses of (−)-morphine or vehicle given into the posterior nucleus accumbens shell for place conditioning repeated for three days. (−)-Morphine at a dose of 2.5 or 5 μg given into the posterior nucleus accumbens shell dose-dependently produced conditioned place preference and at a higher dose of 10 μg, it produced no further increase of conditioned place preference (Fig. 1). Microinjection of the vehicle did not affect the baseline place conditioning response. Five μg of (−)-morphine was then used for place conditioning in the following experiments.
Fig. 1.
(−)-Morphine microinjected into the posterior nucleus accumbens shell produces the conditioned place preference. After completion of the pre-conditioning measurement on the 1st day, groups of rats were place conditioned after microinjection with different doses of (−)-morphine (2.5, 5 or 10 μg) or vehicle given into the posterior nucleus accumbens shell twice a day for three days and the post-conditioning was measured on the 5th day. Each column represents the mean of the conditioned place preference score and the vertical bar represents the S.E.M.; n = 7–13. Paired t test was used to compare production of conditioned place preference of individual dose; for the group of rats microinjected with 2.5, 5 or 10 μg of (−)-morphine or vehicle, t = 1.8, 6.4, 2.9 and 0.04 and df = 7, 9, 6 and 6, respectively, # P < 0.01, ## P < 0.001. One-way ANOVA followed by Dunnett’s post-test was used to test difference between groups, F(3, 34) = 6.12; * P < 0.05, ** P < 0.01.
3.2. Effects of (+)-morphine microinjected into the posterior nucleus accumbens shell on the (−)-morphine-produced conditioned place preference
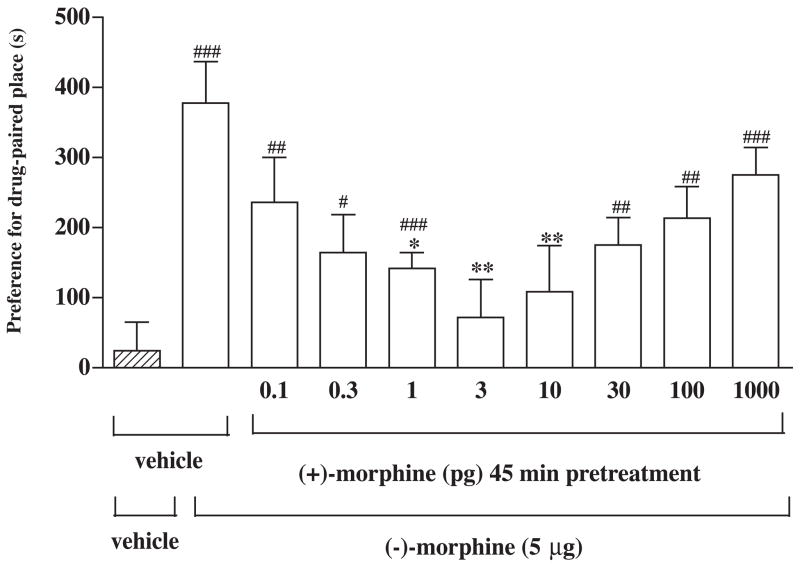
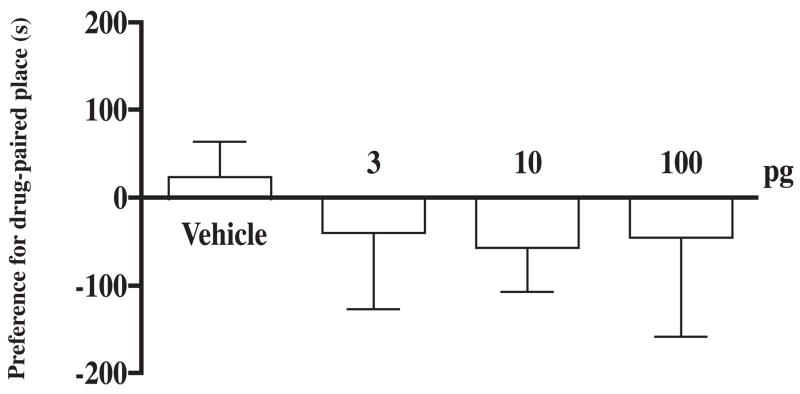
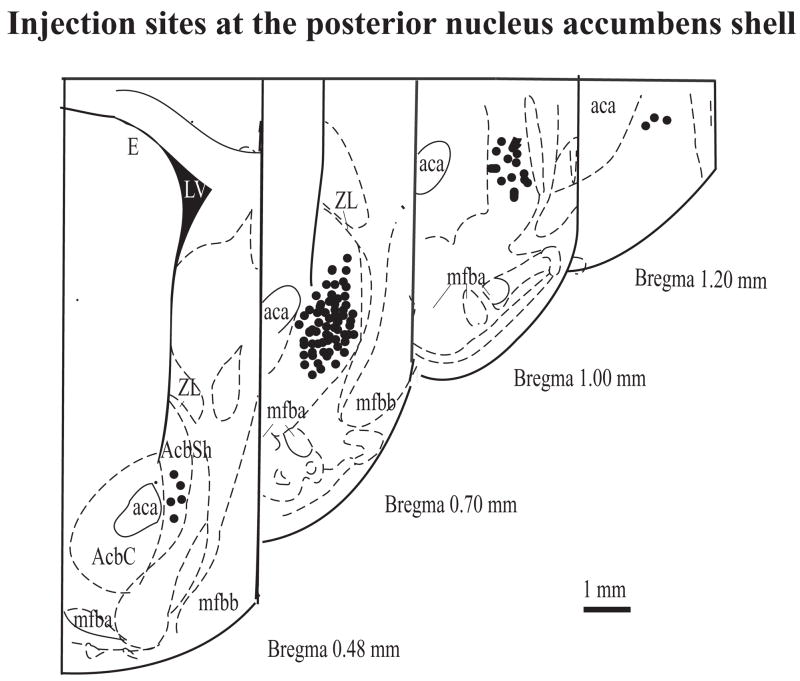
Groups of rats were pretreated in the home cage with different doses (0.1 to 1000 pg) of (+)-morphine or saline vehicle given into the posterior nucleus accumbens shell for 45 min before microinjection of (−)-morphine (5 μg) given into the same site for place conditioning repeated for three days. Pretreatment with (+)-morphine at a dose from 0.1 to 10 pg dose-dependently attenuated the (μ)-morphine-produced conditioned place preference. However, (+)-morphine at a higher dose of 30, 100, and 1000 pg did not attenuate the (+)-morphine-produced conditioned place preference (Fig. 2). Thus, (+)morphine produced a U-shape of the dose-response curve with a maximal inhibition at 3 pg. (+)-Morphine (3 to 100 pg) microinjected into the posterior nucleus accumbens shell given alone did not produce any conditioned place preference in rats (Fig. 3). Histological examination verified that all the injection sites for (+)-morphine and/or (−)morphine intended for the posterior nucleus accumbens shell were within the intended region of the brain site (Fig. 4).
Fig. 2.
(+)-Morphine pretreatment given into the posterior nucleus accumbens shell attenuates the conditioned place preference produced by (−)-morphine from the posterior nucleus accumbens shell. After completion of the pre-conditioning measurement on the 1st day, groups of rats were pretreated with different doses (0.1 to 1000 pg) of (+)morphine or vehicle for 45 min and were place conditioned after microinjection of (−)morphine (5 μg) or vehicle given into the posterior nucleus accumbens shell twice a day for three days. The post-conditioning was measured on the 5th day. Each column represents the mean of conditioned place preference score and the vertical bar represents the S.E.M.; n = 6–17; Paired t test was used to compare production of the conditioned place preference of individual dose: For the group of rats pretreated with vehicle followed by vehicle or (−)-morphine challenge, t = 0.6 and 6.4 and df = 12 and 9, respectively. For the group of the rats pretreated with different dose of (+)-morphine (0.1, 0.3, 1, 3, 10, 30, 100 or 1000 pg) followed by (−)-morphine challenge, t = 3.7, 2.9, 6.4, 0.8, 0.7, 4.4, 4.2 and 7.2 and df = 7, 5, 8, 6, 12, 5, 12 and 17, respectively. # P < 0.05, ## P < 0.01, ### P < 0.001. One-way ANOVA followed by Dunnett’s post-test was used to test difference between groups, F(8, 91) = 3.02; * P < 0.05, ** P < 0.01.
Fig. 3.
(+)-Morphine microinjected into the posterior nucleus accumbens shell does not have any effect on the conditioned place preference. After completion of the preconditioning measurement on the 1st day, groups of rats were place conditioned after microinjection with different doses of (+)-morphine (3, 10 or 100 pg) or vehicle into the posterior nucleus accumbens shell twice a day for three days. The post-conditioning was measured on the 5th day. Each column represents the mean of the conditioned place preference score and the vertical bar represents the S.E.M.; n = 7–13 rats. Paired t test was used to compare production of conditioned place preference of individual dose: For the group of rats microinjected with vehicle or 3, 10 or 100 pg of (+)-morphine, t = 0.6, 1.7, 1.8 and 1.5 df = 12, 7, 7 and 9, respectively. One-way ANOVA followed by Dunnett’s post-test was used to test difference between groups; F(3, 34) = 0.267.
Fig. 4.
Coronal section of the atlas of Paxinos and Watson (1997) showing the injection sites for drug or vehicle at the posterior nucleus accumbens shell for experiments shown in Fig. 1 through Fig. 3.
3.3. Microinjection of endomorphin-1 into the ventral tegmental area increases the release of dopamine from nucleus accumbens shell and the increased release of dopamine is blocked by (−)-naloxone pretreatment given into the ventral tegmental area in urethane-anesthetized rats
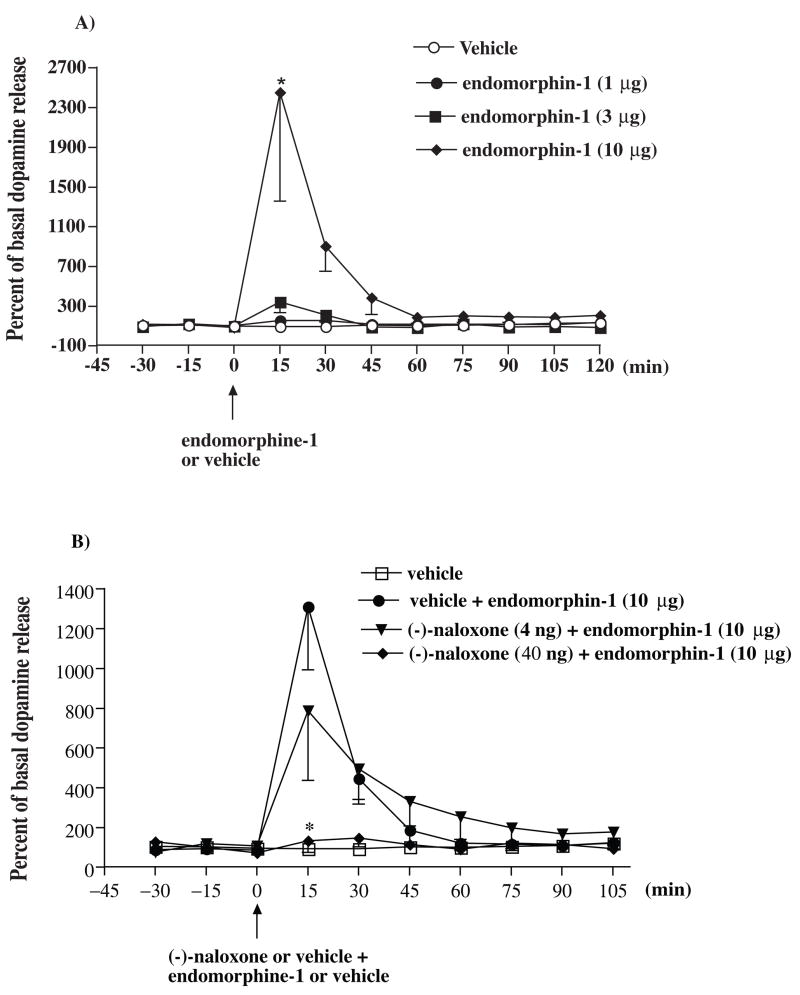
Groups of rats were microinjected with a various dose of endomorphin-1 (1, 3 and 10 μg) or vehicle given into the ventral tegmental area and the release of dopamine from the posterior nucleus accumbens shell were studied. Endomorphin-1 at a dose of 10 μg, but not 1 or 3 μg, caused an increase of the extracellular dopamine in the posterior nucleus accumbens shell 15 min after injection given into the ventral tegmental area. The dopamine level returned to baseline 60 min thereafter (Fig. 5A). To determine if the increased release of dopamine induced by endomorphin-1 is mediated by the stimulation of μ-opioid receptors, the effect of (−)-naloxone on the increased release of dopamine induced by endomorphin-1 was then studied. Co-administration with (−)-naloxone at a dose 40 ng, but not 4 ng, for 45 min attenuated the increase of the extracellular dopamine in the posterior nucleus accumbens shell induced by endomorphin-1 from the ventral tegmental area (Fig. 5B).
Fig. 5.
Endomorphin-1 given into the ventral tegmental area increases the extracellular dopamine in the posterior nucleus accumbens (A) and the increase of the extracellular dopamine in the posterior nucleus accumbens by endomorphin-1 was blocked by (−)-naloxone given into the ventral tegmental area (B). Groups of rats were microinjected with endomorphin-1 (1, 3 or 10 μg) or vehicle into the ventral tegmental area (A). In other groups of rats, (−)-naloxone (4 or 40 ng) or vehicle was co-microinjected into the ventral tegmental area with endomorphin-1 (10 μg). The perfusates from the microdialysis probe at the nucleus accumbens shell were collected every 15 min from 30 min before microinjection and continuously collected for another120 min (A) or 105 min (B) after microinjection. The perfusates were then analyzed their dopamine level. Each point represents the percent basal dopamine release and the vertical bar represents the S.E.M.; n = 3–9. Two-way ANOVA followed by Bonferroni post-test was used to test the difference between groups; (A) For the groups of the rats injected with different dose of endomorphin-1 versus vehicle Finteraction (24, 189) = 1.573, Ftreatment (3, 189) = 4.44, Ftime (8, 189) = 2.141, * P < 0.005. (B) For the group of rats injected with different dose of (−)-naloxone + endomorphin-1 versus vehicle + endomorphin-1, Finteraction (18, 150) = 3.338, Ftreatment (2, 150) = 7.671, Ftime (9, 150) = 11.17, * P < 0.01; only the data of the dopamine level after the 0 min time point were used for the statistic analysis; the group of rats microinjected with vehicle alone in (B) was not included in the statistic analysis.
3.4 (+)-Morphine pretreatment blocks the increase of the extracellular dopamine in the posterior nucleus accumbens shell induced by endomorphin-1 from the ventral tegmental area
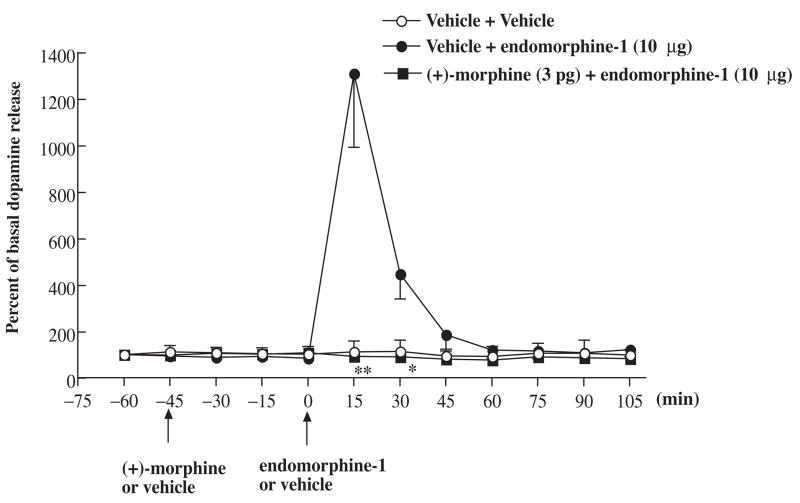
To determine if the increase of the extracellular dopamine produced by the stimulation of μ-opioid receptors from the ventral tegmental area can be blocked by (+)morphine, the effect of (+)-morphine on the increased release of dopamine induced by endomorphin-1 was then studied. Pretreatment with (+)-morphine (3 pg) given into the ventral tegmental area for 45 min completely blocked the increase of the extracellular dopamine in the posterior nucleus accumbens shell produced by endomorphin-1 (Fig. 6). Microinjection of (+)-morphine given into the ventral tegmental area did not affect the basal levels of extracellular dopamine in the posterior nucleus accumbens shell.
Fig. 6.
Effect of (+)-morphine on the extracellular dopamine release at the nucleus accumbens shell induced by endomorphin-1 microinjected into the ventral tegmental area in rats. Groups of rats were microinjected with (+)-morphine (3 pg) or vehicle into the ventral tegmental area 45 min before endomorphin-1 (10 μg) was microinjected into the ventral tegmental area. The perfusates from the microdialysis probe at the nucleus accumbens shell were collected every 15 min from 15 min before first microinjection and continuously collected for another 105 min after last microinjection. The perfusates were then analyzed their dopamine level. Each point represents the percent basal dopamine release and the vertical bar represents the S.E.M.; n = 3–9. Two-way ANOVA followed by Bonferroni post-test was used to test the difference between groups. For the groups of the rats injected with (+)-morphine and endomorphin-1 versus vehicle and endomorphin-1, Finteraction (7, 80) = 11.47, Ftreatment (1, 80) = 26.03, Ftime (7, 80) = 11.58, * P < 0.05, ** P < 0.005; only the data of the dopamine level after the 0 min time point were used for statistic analysis; the vehicle pretreatment followed by vehicle microinjected group was not included in the statistic analysis.
4. Discussion
4.1. (−)-Morphine, but not (+)-morphine, given into the mesolimbic nucleus accumbens produces the conditioned place preference
Unlike naturally occurring (−)-morphine, which produces analgesia and other μ-opioid receptor mediated pharmacological effects, the synthetic (+)-morphine does not have any affinity and efficacy for μ-opioid receptors and therefore does not produce analgesia and other effects mediated by the stimulation of μ-opioid receptors (Jacquet et al., 1977). We have previously demonstrated that only (−)-morphine, but not (+)morphine, given systemically produces the conditioned place preference in rats (Wu et al, 2007a). The finding is consistent with previous findings by Mucha and Herz (1986) that (+)-morphine (4 mg/kg) given systemically does not produce any conditioned place preference or conditioned place aversion. (−)-Morphine at the same dose, on the other hand, produces the conditioned place preference. Thus, the conditioned place preference produced by morphine is stereospecific; it is only produced by the opioid receptor active isomers, such as levorotatory (−)-morphine, but not dextrorotatory (+)-morphine.
The ventral tegmental area and nucleus accumbens represent two key structures of the mesolimbic dopaminergic system in the CNS for the reinforcing properties of opiates (Watson et al., 1989; Wise and Rompre, 1989). The microinjection technique was then used to deliver (−)-morphine or (+)-morphine into the posterior nucleus accumbens shell and the conditioned place preference produced by (−)-morphine or (+)-morphine given into the same posterior nucleus accumbens shell was then measured. We found in the present study that only (−)-morphine, but not (+)-morphine delivered into nucleus accumbens shell produced the conditioned place preference (Fig. 1 and 3). The conditioned place preference produced by (−)-morphine is blocked by co-administration with μ-opioid receptor antagonist naltrexone, indicating that the effect is mediated by the stimulation of μ-opioid receptors (Olmstead and Burns, 2005).
4.2. (+)-Morphine at an extremely low picogram dose given into the mesolimbic posterior nucleus accumbens shell attenuates the (−)-morphine-produced conditioned place preference
We found in the present study that (+)-morphine at an extremely low picogram dose attenuated the conditioned place preference produced by (−)-morphine. Paradoxically, a higher dose of (+)-morphine was ineffective in attenuating (−)-morphine-produced conditioned place preference (Fig 2.). Thus, (+)-morphine produced a U-shape dose-response curve with a maximal attenuation at a dose of 3 pg. Similarly, (+)morphine given systemically also produces a U-shaped dose-response curve in attenuating the (−)-morphine-produced conditioned place preference (Wu et al., 2007a).
The U-shaped dose-response curve is also known as hormesis (Calabrese and Baldwin, 2003). A well-documented example of a U-shaped dose-response relationship concerns the actions of corticosteroid hormones in the CA1 area of the hippocampus (Diamond et al., 1992; Joels and de Kloet, 1994; Joels, 2006), a brain region that is important for learning and memory formation. The neurosteroids have been proposed to be the endogenous ligand for sigma-1 receptors in the central nervous system (Maurice, 2004; Maurice et al., 2001; Monnet and Maurice, 2006). In a modified passive-avoidance learning task in mice, pretraining or posttraining administration of neurosteroids, pregnenolone sulfate or dehydroepiandrosterone sulfate enhances memory retention of passive-avoidance training. In both treatments, an inverted U-shaped dose-response curve is obtained covering 2- to 5-fold dose range in a manner typical for memory-enhancing substance. The neurosteroid-induced facilitation of memory retention may involve central sigma receptors, because the effect of neurosteroids is completely antagonized by sigma receptor antagonist haloperidol (Reddy and Kulkarni, 1998). It is postulated that (+)-morphine may mimic the effect of endogenous neurosteroids to stimulate the sigma receptors for producing the anti-addictive effect (see details in the next paragraph below). The U-shaped dose-response curve for (+)-morphine to attenuate the (−)-morphine conditioned place preference is consistent with this hypothesis.
It is hypothesized that (+)-morphine attenuates the (−)-morphine-produced conditioned place preference via the activation of the naloxone-sensitive sigma receptor. The hypothesis is supported by our previous findings that the attenuation of the (−)morphine-produced conditioned place preference induced by (+)-morphine was reversed by the pretreatment with the sigma receptor antagonist BD1047 (Wu et al., 2007a). We have previously demonstrated that (+)-morphine pretreated systemically or given into periaqueductal gray attenuates the antinociception produced by (−)-morphine given systemically. The attenuation of the (−)-morphine-produced antinociception is blocked or reversed by the sigma receptor antagonist BD1047 and by the (+)-naloxone or (−)-naloxone pretreatment, indicating that the anti-morphine effects of (+)-morphine are mediated by the activation of the naloxone-sensitive sigma receptor (Wu et al., 2007b; Terashvili, et al., 2007).
4.3. (+)-morphine attenuates the increase of the extracellular dopamine in the nucleus accumbens shell produced by μ-opioid agonist endomorphin-1 from the ventral tegmental area
We found in the present study that endomorphin-1 given into the ventral tegmental area caused the increase of the extracellular dopamine in the posterior nucleus accumbens shell. The increase of the extracellular dopamine in the posterior nucleus accumbens shell by the endomorphin-1 is blocked by (−)-naloxone pretreatment, indicating that the effect is mediated by the μ-opioid receptor activation (Fig 5). Thus, stimulation of μ-opioid receptors by (−)-morphine or other μ-opioids in the ventral tegmental area enhances mesolimbic dopaminergic neurotransmission, presumably by inhibition of GABAergic interneurons, thereby disinhibiting mesolimbic dopaminergic neurons and increasing both somatodendritic and axonal dopamine release (Stinus et al., 1982; Kalivas and Duffy, 1990; Spanagel et al., 1992; Johnson and North, 1992; Klitenick et al., 1992; Devine et al., 1993). An increase in the extracellular dopamine in the nucleus accumbens has been reported by systemic (−)-morphine and by intracerebroventricular injection of the μ-receptor agonist, D-Ala2-N-MePhe4-Gly-ol5]enkephalin (Stinus et al., 1980; Spanagel et al., 1990). We found in the present study that the increased release of dopamine in the posterior nucleus accumbens induced by the μ-opioid receptor agonist endomorphin-1 was blocked by the (+)-morphine given into the ventral tegmental area (Fig. 6). Thus, the behavioral response to (+)-morphine in attenuating the (−)-morphine-produced conditioned place preference is correlated with the biochemical finding that (+)-morphine blocks the increase of extracellular dopamine in the posterior nucleus accumbens elicited by the specific μ-opioid receptor agonist endomorphin-1 from the ventral tegmental area. The finding supports the hypothesis that (+)-morphine, which activates naloxone-sensitive sigma receptors, inhibits the (−)morphine-produced conditioned place preference by attenuating the increase of extracellular dopamine in the mesolimbic ventral tegmental area-nucleus accumbens system.
The results of the present study indicate that (+)-morphine can be used for the treatment of opiate addiction. The therapeutic window of the dose of (+)-morphine for the anti-addiction therapy should be in the picogram dose range. Higher doses of (+)morphine are not effective in providing the therapeutic effect.
Acknowledgments
This study was supported by Grant DA12588 from the National Institute on Drug Abuse, National Institute of Health (PI: L.F.T.), Medical College of Wisconsin Research Affairs Committee (PI: H-E.W.) and in part by the Intramural Research Program of the NIH/HIEHS (PI: J-S Hong).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Stanley BG, Hoebel BG. A small removable microdialysis probe. Life Sci. 1986;39:2629–2637. doi: 10.1016/0024-3205(86)90119-0. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecific and nonstereospecific effects of (+)- and (−)-morphine: evidence for a new class of receptors. Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implication for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurones by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effects of acute and daily neurotensin and enkephalin treatment on extracellular dopamine in the nucleus accumbens. J Neurosci. 1990;10:2940–2949. doi: 10.1523/JNEUROSCI.10-09-02940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Peptides that regulate food intake, effect of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans EJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neurosci. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptor, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37(Suppl):S171–182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the s1 receptor function: behavioral consequences and therapeutic opportunities. Brainm Res Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active) steroids: molecular, physiological, and behavioral aspects. J Pharmacol Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Preference conditioning produced by opioid active and inactive isomers of levorphanol and morphine in the rat. Life Sci. 1986;38:241–249. doi: 10.1016/0024-3205(86)90309-7. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH. Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychoparmacology. 2005;12:1–6. doi: 10.1007/s00213-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. Differential effects of ventral striatal lesions on the conditioned place preference induced by morphine or amphetamine. Neuroscience. 1996;3:701–708. doi: 10.1016/0306-4522(95)00486-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learnin task in mice. Brain Res. 1998;791:108–116. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. The delta-opioid receptor antagonist naltrindole prevents sensitization to the conditioned rewarding effects of cocaine. Eur J Pharmacol. 1995;280:55–61. doi: 10.1016/0014-2999(95)00185-n. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathways. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in-vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Stinus L, Herman JP, Moal ML. GABAergic mechanisms within the ventral tegmental area: Involvement of dopaminergic (A 10) and non-dopaminergic neurons. Psychopharmacology. 1982;77:186–192. doi: 10.1007/BF00431946. [DOI] [PubMed] [Google Scholar]
- Stinus L, Koob GF, Ling N, Bloom FE, Moal ML. Locomotor activation induced by infusion of endorphin into the ventral tegmental area: evidence for opiate-dopamine interactions. Proc Natl Acad Sci USA. 1980;77:2323–2327. doi: 10.1073/pnas.77.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H. Involvement of dopamine-dependent and -independent mechanisms in the rewarding effects mediated by delta opioid receptor subtypes in mice. Brain Res. 1997;744:327–334. doi: 10.1016/S0006-8993(96)01119-5. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Hung K, Clithero AD, Schwasinger ET, Tseng LF. Differential conditioned place preference responses to endomorphin-1 and endomorphin-2 microinjected into the posterior nucleus accumbens shell and ventral tegmental area in the rat. J Pharmacol Exp Ther. 2004;309:816–824. doi: 10.1124/jpet.103.059287. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Moore RM, Harder DR, Tseng LF. (+)-Morphine and (−)-morphine stereoselectively attenuate the (−)-morphine-produced tail-flick inhibition via the naloxone-sensitive sigma receptor in the ventral periaqueductal gray of the rat. Eur J Pharmacol. 2007;571:1–7. doi: 10.1016/j.ejphar.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Trujillo KA, Herman JP, Akil H. In: Molecular and Cellular Aspects of the Drug Addiction. Goldstein A, editor. Springer; New York: 1989. pp. 29–91. [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- Wu HE, Schwasinger ET, Terashivili M, Tseng LF. dextro-Morphine attenuates the morphine-produced conditioned place preference via the sigma1receptor activation in the rat. Eur J Pharmacol. 2007a;562:221–226. doi: 10.1016/j.ejphar.2007.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharmacol. 2007b;571:145–151. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: Anterior-posterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22:7225–7233. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]