Abstract
Major questions remain about the exact role of hormones in cognition. Furthermore, the extent to which early perturbation in steroid function affects human brain development continues to be a wide open area of research. Congenital Adrenal Hyperplasia (CAH), a genetic disorder of steroid dysfunction characterized in part by in utero over-production of testosterone, was used as a natural model for addressing this question. Here, CAH (n=54, mean age = 17.53, 31 female) patients were compared to healthy age- and sex-matched individuals (n=55, mean age = 19.02, 22 female) on a virtual equivalent of the Morris Water Maze task (Morris, 1984), an established measure of sex differences in spatial cognition in rodents. Findings revealed that females with CAH with the most severe form of the disease and expected highest level of in utero exposure to androgens were found to perform similarly to both healthy males and CAH males, whereas strong sex differences were apparent in milder forms of the disorder and in controls. Moreover, advanced bone age, an indicator of long-term childhood exposure to testosterone was correlated with improved performance. The results indicate that individuals exposed to both excess androgens prenatally and prolonged exposure during childhood may manifest long-lasting changes in cognitive function. Such finding suggests a pivotal role of hormonal function on brain development in humans, mirroring results from the animal literature.
Keywords: spatial navigation, hormones, sex differences, steroids, brain development, adolescents
Major questions remain about the influence of sex steroids on the development of core cognitive skills that exhibit strong sexual dimorphism (Voyer et al., 1995; Moffat et al., 1998). For instance, across mammalian species, males commonly outperform females on spatial navigation tasks, in which a hidden platform has to be located using distal cues of the environment (Moffat et al., 1998; Jones and Watson, 2005). Such strong sex differences in spatial cognition, at least in humans, are thought to bear evolutionary underpinnings (Silverman et al., 2000). There is evidence from animal studies that both prenatal and postnatal androgen exposure influences spatial abilities. Litter position in utero affects hormonal milieu: animals that are located between two males are exposed to higher levels of androgen than animals that are located between two females (vom Saal and Bronson 1980). Subsequent studies in rodents reported that such in utero exposure to higher levels of testosterone improved spatial performance of a male-biased litter relative to a female based litter (Galea et al., 1994; Galea et al., 1996). Moreover, postnatal (3-5 days) administration of testosterone to females also improved spatial cognition relative to control females (Roof and Havens, 1992). By contrast, long-term testosterone treatment of young (4.5 months) and middle aged (20 months) rodents led to impairments or no change in these groups on the same task (Goudsmit et al., 1990).
Human studies that examine the impact of steroid contributions to critical periods of development are lacking because it would be unethical to experimentally manipulate hormonal levels. Most human studies that have examined cognitive effects of steroids relied on physiological levels of circulating hormones (e.g. Driscoll et al., 2005). One strategy to examine the cognitive influence of abnormal hormonal levels consists in using natural models of endocrine dysfunction, such as Congenital Adrenal Hyperplasia (CAH). CAH is a genetic disorder characterized by impairment of the enzyme that leads to cortisol biosynthesis. This genetic disorder results in an in utero pattern of adrenal cortisol deficiency and excess androgen production (Merke and Bornstein, 2005). In the severe or classic forms of CAH, girls are born with ambiguous genitalia due to the in utero exposure to excess androgens. Long-term childhood exposure to excess androgen may affect growth and development and manifests itself in advanced bone maturation. Three phenotypic forms of CAH (classic salt-wasting, classic simple virilizing, non-classic) have been described and are determined by the severity of enzyme deficiency (Therrell et al., 1998; Speiser and White, 2003; Merke and Bornstein, 2005). Salt-wasters carry mutations that lead to less than 1 % enzyme activity and are expected to have the most severe endocrine imbalances. Simple-virilizers have mutations associated with weak enzyme activity (<10%), allowing for some mineralocorticoid and cortisol synthesis. Patients with non-classic CAH, a comparatively mild form of the disorder, are characterized by reduced enzyme function to 20%-60% and usually are diagnosed late in childhood or early adulthood. They have normal cortisol production, but at the expense of mildly elevated androgens.
Glucocorticoid replacement, which reestablishes the negative feedback on the production of testosterone, is initiated upon diagnosis. However, despite early treatment, masculine-like behaviors are common in females with classic CAH. Indeed, studies describe that girls with classic CAH commonly exhibit male-like behavior in play and interests (Berenbaum, 1999), and in adult females with predominantly classic CAH report themselves as being more aggressive relative to reports from unaffected females (Berenbaum and Resnick, 1997; Pasterski et al., 2007). Functional imaging studies have also documented more male-like patterns of activations during processing of emotions in females with classic CAH (Ernst et al., 2007). These studies suggest prenatal androgen imprint on behavior in girls with classic CAH. The findings with regards to spatial abilities in CAH, however, have been mixed (Resnick et al., 1986; Hampson et al., 1998; Malouf et al., 2006). Resnick et al reported large performance differences on spatial tests between females with CAH and unaffected females but no differences in males (Resnick et al., 1986) using mental rotation tests. In addition to Resnick's findings, Hampson (Hampson et al., 1998) reported lower performance for CAH males relative to control males in a spatial relations task. Yet, other studies have failed to detect alterations of spatial abilities in females with CAH (Malouf et al., 2006), but have replicated impaired spatial performance in CAH males relative to controls (Hines et al., 2003). Such inconsistency may reflect the use of suboptimal assays of spatial ability, including rudimentary paper-and-pencil tests of spatial cognition. Recently, more realistic and ecologically valid virtual mazes, well-known tests of hippocampal function, have successfully demonstrated significant differences in spatial performance in neurological (Skelton et al., 2006) and psychiatric (Gould et al., 2007) populations, and are known to elicit robust sex differences (Moffat et al., 1998; Ross et al., 2006). More importantly, previous studies did not differentiate between types of CAH (the study by Malouf et al. is an exception), which could account for the mixed results because the impact of steroids across CAH types may vary considerably. The purpose of the present study is to clarify the contradictory findings of steroid effects on spatial cognition using a state-of-the-art paradigm, and additionally, to examine the contribution of the disease severity to deficits on spatial cognition.
To this goal, we used a computerized virtual water maze task to compare spatial abilities between male and female healthy participants and age- and sex-matched patients with CAH. We hypothesized that, if early steroid exposure critically influences spatial cognition, then females with CAH would perform at an intermediary level between healthy males and healthy females only in the severe forms of the disease (salt wasting and simple virilizing forms). If, however, mild but continued exposure to elevated sex hormones during post-natal life influences spatial cognition, then we also would expect alterations on spatial performance to be present in non-classic CAH patients. The influence of current hormonal status and long-term childhood exposure to excess androgen were also examined.
Methods
Subjects
The CAH patients consisted of three groups: 25 (14 males and 11 females) patients with the salt wasting (SW) form, 13 (7 males and 6 females) patients with the simple virilizing (SV) form, and 16 patients (5 males and 11 females) with the non-classic form. Patients were classified according to clinical and endocrinological evaluation at presentation and all classifications were confirmed by genotype. Additionally, 55 healthy control subjects (22 females and 33 males) participated in the study. Demographic characteristics of the groups are presented in Table 1.
Table 1.
Demographic information about the three CAH subgroups and the control group.
|
salt- wasters (SW) N=25 |
CAH simple virilizers (SV) N=13 |
non- classic N=16 |
Control Group N=55 |
P SW vs. SV vs. NC vs. control |
|
|---|---|---|---|---|---|
| IQ (Mean, StDev) | 99.59 (9.75) |
115.82 (15.82) |
108.73 (12.53) |
113.04 (10.20) |
P<.01 |
| AGE (Years, StDev) | 16.75 (9.26) |
16.73 (7.45) |
19.57 (14.66) |
19.02 (11.88) |
P=.97 |
| SW vs. | |||||
| SV vs. | |||||
| NC | |||||
| Age at diagnosis (Years, StDev) | At birth (0.08) |
2.29 (0.72) |
13.13 (2.88) |
P<.01 | |
| Tanner stage | 2.76 (.38) | 3.00 (.53) | 3.18 (.48) | P=.79 | |
| Height sds | −.44 (.25) | −.15 (.35) | .55 (.31) | P=.06 | |
| Weight sds | .45 (.29) | 1.01 (.41) | 1.19 (.37) | P=.26 | |
| BMI sds | .71 (.26) | 1.18 (.36) | 1.21 (.33) | P=.89 | |
|
History of advanced bone age ( ≥ 2 years )(Number, %) |
9 (36%) | 12 (92.3%) |
6 (37.5%) | P=.03 | |
| High testosterone (Number, %) | 2 (8%) | 4 (30.7%) | 3 (18.7%) | P=.52 | |
|
Progesterone, 17 OH (Median, StDev, Range) |
1460 (5790) 10-27200 |
1340 (6981) 16-22500 |
752 (1938) 16-7050 |
P=.61 | |
BMI = Body Mass Index.
Inclusion/Exclusion criteria
Participants were recruited through fliers and newspaper advertisements that invited healthy volunteers to participate in studies of emotion at the National Institute of Mental Health (NIMH). Patients with CAH were enrolled as part of ongoing endocrine studies at the NIH Clinical Center in Bethesda, MD. The study was approved by the IRB of the NIMH and National Institute of Child Health and Human Development. Parents provided written informed consent and children written assent. All participants underwent neurologic, psychiatric and physical examination (Segal et al., 1994; Kaufman et al., 1997). IQ was tested using the WASI (Wechsler, 1999), and IQ scores differed between groups (F(3,94)=8.99, p<.01). However, all had IQ within the normal range. The group differences reflected higher IQ for the simple virilizer (SV )and control groups than for the salt-waster (SW) and non-classic groups (Table1). As expected, patients with the salt-wasting form of CAH were diagnosed earlier than children with the non-classic form of CAH (F(2,56)=22.57, p<.01). Age, height, weight, and BMI did not differ among patient groups, whereas bone age was significantly more advanced in SV patients than in the SW and non-classic groups (Table 1). All SW and SV patients were on glucocorticoid medication while 62.5% of the non-classic CAH patients were medicated. None of the participants was treated with prenatal dexamethasone.
Hormonal Measures and Assays
Hormonal samples were taken at 0800 hrs, prior to medication, and within one or two days of testing. Testosterone and 17-OH-progesterone were measured with high-performance liquid chromatography/tandem mass spectrometry (Mayo Medical Laboratories, Rochester, MN). Age- and sex-appropriate norms were used to determine whether testosterone was elevated (Table 1). Hormones were not measured in healthy control subjects. History of bone age advancement was obtained from medical records and was defined as advanced if it was ever 2 or more years greater than chronological age.
Materials
A virtual equivalent of the Morris Water Maze (NeuroInvestigastions Inc.) was used and presented on a 14 inch monitor linked to a 2.4Ghz Intel Celeron processing computer. The virtual environment consisted of a square room containing a circular pool of water. Peripheral cues to aid orientation consisted of four equally sized abstract rectangular paintings that were distinguishable by their shapes, colours and placements on the walls surrounding the pool. Each painting was placed on a different wall of the room and stretched from the ceiling to the pool wall (see Figure 1). Participants experienced the pool and room from a first-person perspective with the viewing level slightly above the water and navigated around the environment (“swam”) using the ‘up’, ‘left’ and ‘right’ arrow curser keys of the keyboard. They were told not to back up, i.e., use the ‘back’ arrow key. Instead, they were told that if they wanted to turn around they should spin around their left or right axis using the left or right arrow keys.
Figure 1.
Screenshot of a sample trial from the first-person perspective as seen by the participant with the pool filled with water, the arena wall, the wall of the room and a sample distal stimulus (abstract painting). Note that the image was grayscaled for publication purposes and that stimulus presentation is in colour.
Procedure
Each participant completed 18 trials. At the beginning of the experiment, participants were given one practice trial (30 seconds long), in which they were introduced to the environment and were able to practice moving comfortably in this environment. During the practice trial, no platform was present. In the following, visible trial, the platform was introduced to the participants who were asked to simply “swim” towards it. Over the next 16 trials, participants were dropped at four locations of the pool. These 4 locations occurred in a random order. Each time, subjects were required to “swim” directly to the hidden (i.e., invisible) platform. Once the platform was located, a sound occurred and participants remained on the platform for 2 seconds. On each trial, participants were given 60 seconds to find the platform, after which the platform became visible. A written message appeared on the screen indicating the visibility of the platform and encouraged participants to move towards it.
Analysis
Subjects location in the pool, indexed by x and y coordinates, was recorded at 100 ms intervals. The following variables were computed: latency to reach the platform in seconds, the path length covered (relative to the pool diameter) and the subject's heading error (in deg). The heading error is defined as the angle between optimal heading direction and current heading direction. Additionally, the number of failed attempts (latency > 60 seconds) was recorded. In the primary analyses we examined the effect of in utero exposure as reflected in disease type. To assess learning, all 16 experimental trials were binned into four blocks of four, i.e. trials 1-4 (1), trials 5-8 (2), trials 9-12 (3) and trials 13-16 (4). A repeated measures ANOVA for each variable was then performed using a Block (1-4) by Sex (male vs. female) by Group (SW. vs. SV. vs. non-classic vs. control) design. However, because spatial tasks tend to be age sensitive (Voyer et al., 1995), age was used as a covariable of nuisance. History of bone age advancement served as a measure of long-term childhood exposure to sex hormones, and circulating levels of testosterone at time of testing served as a measure of current hormonal status. These measures were used as dichotomous variables, i.e., advanced bone age (≥ 2years from chronological age) and high level of testosterone (yes/no), and were entered as independent factors in multivariate ANOVAs. Given the exploratory nature of these analyses, measures of effect size (Cohen's d) were also computed. Finally, current levels of 17 OH progesterone was employed as an indicator for current control status of the disorder and were correlated with overall performance measures. Due to large interindividual variability, current levels of 17OH progesterone were log-transformed for analysis.
Results
Latency
All participants became faster at finding the platform over the course of the trials as indicated by a significant main effect of Block on latency (F(3,300)=2.76, p<.05). In addition, males were significantly faster in reaching the platform than females (main effect of Sex F(1,100)=12.23, p=.001). Most importantly, a significant Sex by Diagnosis interaction (F(3,100)=3.52, p<.05) indicated that the sex-related differences in latency were modulated by diagnosis. To follow up on this interaction, a univariate ANOVA with Sex as the between subjects factor was performed on latency for each diagnosis group. A significant male advantage relative to females was found in simple virilizer (F(1,10)=6.20, p<.05), non-classic (F(1,13)= 10.39, p<.01), and control (F(1,52)=8.32, p<.01) groups. In contrast, the salt wasting group showed no male advantage on latency (F(1,22)=1.30, p=.27) (Figure 2). There was no significant main effect of Diagnosis or significant interaction of Block by Diagnosis.
Figure 2.
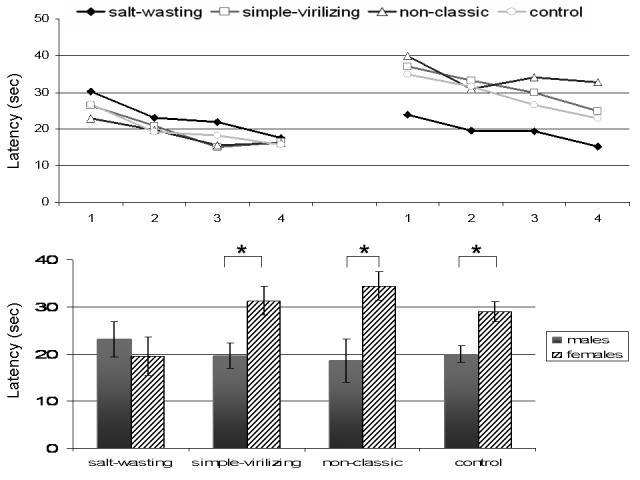
Swim latencies (in seconds) for all groups (SW = filled diamond, SV = open square, non-classic = open triangle, control = open circle) across all four experimental blocks (1= trials 1-4, 2= trials 5-8, 3= trials 9-12, 4= trials 13-16) for males (upper left panel) and females (upper right panel). The collapsed latency across all four blocks is visible in the lower panel for males (grey bars) and females (striped bars). Error bars denote standard error of the mean. An * indicates significance at p<.05.
Path length
As predicted, the distance traveled to the platform significantly decreased over the course of the trials (main effect of Block: F(3,300)=5.13, p<.01) and was apparent in linear (F(1,100)=8.14, p<.01) and cubic trends (F(1,100)=5.39, p<.05). These trial order-related changes are consistent with learning effects. Males, however, traveled shorter distances on average than females (main effect of Sex: F(1,100)=4.30, p<.05), indicative of overall superior performance. There was no significant main effect of Diagnosis and the diagnosis by sex interaction was not significant (F(3,100)=2.17, p=.09).
Heading error
Heading error while navigating to the platform decreased with time (main effect of Block: F(3,300)=3.78, p=.01) and was evident as a linear trend (F(1,100)=4.97, p<.05), again, consistent with a learning effect. No other effects or interactions reached statistical significance.
Failure to complete
Subjects exhibited marginally fewer failed attempts to reach the platform during later trials of the experiment (main effect of Block: F(3,300)=2.43, p=.06). Overall, males had fewer incomplete trials to find the platform than females (main effect of Sex: F(1,100)=4.89, p=.03). Diagnosis had no influence on failure to complete trials as a main effect or in interaction with Block or Sex.
Effects of long-term childhood exposure and current hormone levels
Patients with a history of an advanced bone age during childhood completed more trials successfully than patients who had experienced normal bone maturation (F(1,43)=5.35, p=.026, d=.70). However, there was no significant interaction between bone age maturation and disease severity (F(4,45)=0.91, p=.46). The measures of latency and path length were also trending in this direction, as reflected in medium strong effect sizes (F(1,43)=3.03, p = .089, d=.47 and F(1,43)=3.08, p=.086, d=.49). This result indicated small beneficial improvements with advanced bone maturation, reflecting long-term childhood exposure to high levels of sex steroids (Figure 4).
Figure 4.
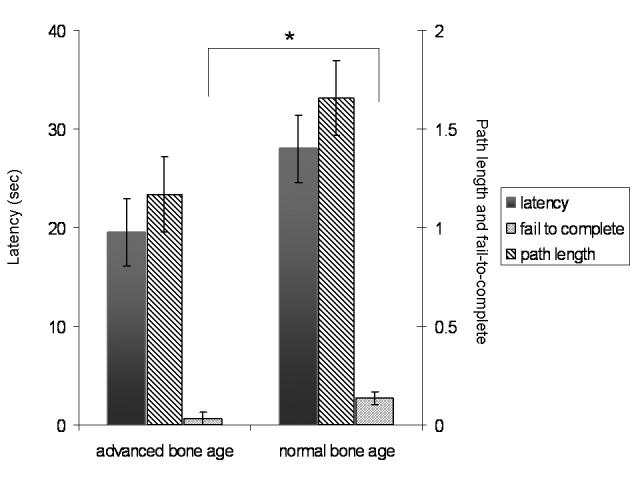
shows the main effect of bone age maturation for latency (left y-axis) and path length and incomplete trials (right y-axis). An * indicates significant at p<.05.
There were no significant effects of current testosterone blood levels. Blood levels of 17-OH- progesterone were negatively correlated at a trend level with latency and path length (r2(50)=−.25, p=.08 and r2(50)=−.26, p=.07), suggesting a possible detrimental effect of poor current hormonal control on performance.
In summary, females with the SW type of CAH performed at similar levels to males on measures of latency in contrast to the other three groups who exhibited strong sex differences. In addition, history of advanced bone age, a marker of prolonged excess sex steroid exposure in childhood, was associated with improved performance.
Discussion
The study examined spatial cognition using an ecologically valid virtual water maze task in patients with androgen excess due to CAH. Two main findings emerged: 1) the severity of the disease, and 2) the history of long-term excess androgen exposure during childhood modulated performance on a spatial task. These findings support the hypothesis of a direct impact of sex steroids on human spatial cognition.
We hypothesized that if prenatal androgen exposure was critical, females with the more severe forms, SW or SV, would perform at intermediate levels between healthy control males and healthy females or females with the mild, non-classic form of CAH. Findings were partially consistent with these predictions and previous data in healthy populations were replicated (Ross et al., 2006; Newhouse et al., 2007). Strikingly, females performed similarly to males only in the most severe CAH form, the SW type, whereas a strong male-advantage on performance was present in all other groups. Both SW and SV are characterized by in utero exposure to androgens leading to ambiguous genitalia in females and the need for early treatment. However, hormonal data suggests that SW exhibit higher androgen levels in utero than SV (New et al., 1983). This is consistent with the notion that only patients with the strongest symptom severity but not patients with milder forms of the disorder (SV and non-classic) show altered performance on spatial cognition.
Additional analyses revealed that improved performance was also related to a history of advanced bone age maturation, an index of childhood exposure to excess sex steroids (Bayley and Pinneau, 1952). Although it is possible that disease severity and bone age overlap to some extent and are confounded, the absence of a significant interaction of these two variables suggests that it is unlikely in the present case. This is further supported by the fact that only 36% of the SW group but ∼90% of the SV group were characterized by advanced bone age and that all groups showed similar current hormonal levels. Future studies will need to investigate the possible interplay of these factors. However, these data suggest two points of vulnerability for impact of androgens on cognition: an in utero effect and an effect related to prolonged androgen exposure during childhood. One possible explanation for the mixed findings reported in previous CAH studies of spatial cognition (Resnick et al., 1986; Hampson et al., 1998; Hines et al., 2003) could be related to the failure to separate patients as a function of disease severity. Another possibility might be the use of less sensitive tests of spatial cognition, including paper-and-pencil tests.
Previous research on the impact of sex steroids on spatial cognition during development has been predominantly investigated in rodents. Studies have shown that androgens critically influence spatial performance when rodents were exposed in utero (Galea et al., 1994; Galea et al., 1996) or at young age (i.e., postnatal) (Roof and Havens, 1992) but had no effect in aged rats (Goudsmit et al., 1990). Likewise, early (postnatal day 4) exposure to dexamethasone has been shown to impair performance on the water maze task (DeKosky et al., 1982). However, when rodents were exposed to dexamethasone in utero but reared by a healthy mother as opposed to a rearing mother treated with dexamethasone, young rodents exhibited adequate learning (Brabham et al., 2000). These findings suggest a distinct window of vulnerability and a lasting impact of steroid hormones on spatial cognition. The current study is consistent with this literature and suggests similar windows of vulnerability in humans.
A limitation to the interpretation of findings based on the CAH model is the potential contribution of corticosteroid dysfunction on performance, given the CAH phenotype of combined androgen excess and cortisol deficiency (Merke and Bornstein, 2005). Based on current knowledge, androgen effects rather than corticosteroid effects appear to be the most logical interpretation, because of the well recognized sex differences on spatial navigation, and of the dearth of known association between corticosteroids and spatial cognition in humans. Furthermore, this interpretation is consistent with previous work that has shown associations between spatial cognition and circulating testosterone but not cortisol (Driscoll et al., 2005). Further studies however are needed to replicate the present findings and clarify the role of corticosteroids on spatial cognition. One other potential confound is the level of computer gaming experience that might differ between individuals or gender. Although, unfortunately, we do not have any measure of computer gaming experience of the subjects, previous studies in similar (Newhouse et al., 2007) and older (Moffat et al., 1998, Mueller et al, submitted) age groups have not found any influence of prior computer experience on performance of the virtual water maze task.
These preliminary findings highlight a potentially crucial involvement of androgens in shaping cognitive function in early stages of development. Based on the relatively well known neurocircuitry underlying spatial navigation, i.e., hippocampus and parietal cortex, the next steps could involve the examination of regional brain activity in response to performance on the water maze task, comparing patients with varied degrees of early androgen perturbations. Moreover, future research will need to clarify on which specific domains of higher cognition steroid hormone changes in the brain operate and to what extent.
Figure 3.
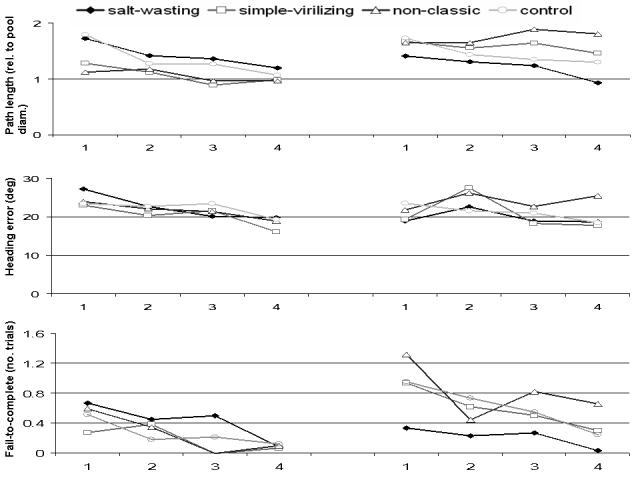
Path length (relative to pool diameter), heading error (in deg) and average number of incomplete trials over the experimental blocks (upper, middle and lower panel, respectively) for males (left side graphs) and females (right side graphs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J. Pediatr. 1952;40:423–441. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Horm. Behav. 1999;35:102–110. doi: 10.1006/hbeh.1998.1503. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22:505–515. doi: 10.1016/s0306-4530(97)00049-8. [DOI] [PubMed] [Google Scholar]
- Brabham T, Phelka A, Zimmer C, Nash A, Lopez JF, Vazquez DM. Effects of prenatal dexamethasone on spatial learning and response to stress is influenced by maternal factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1899–1909. doi: 10.1152/ajpregu.2000.279.5.R1899. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Nonneman AJ, Scheff SW. Morphologic and behavioral effects of perinatal glucocorticoid administration. Physiol. Behav. 1982;29:895–900. doi: 10.1016/0031-9384(82)90340-7. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm. Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Ernst M, Maheu FS, Schroth E, Hardin J, Golan LG, Cameron J, Allen R, Holzer S, Nelson E, Pine DS, Merke DP. Amygdala function in adolescents with congenital adrenal hyperplasia: a model for the study of early steroid abnormalities. Neuropsychologia. 2007;45:2104–2113. doi: 10.1016/j.neuropsychologia.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J. Exp. Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ossenkopp KP, Kavaliers M. Performance (re-acquisition) of a water-maze task by adult meadow voles: effects of age of initial task acquisition and in utero environment (litter sex-ratio) Behav. Brain Res. 1994;63:177–185. doi: 10.1016/0166-4328(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Van de Poll NE, Swaab DF. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in young and middle-aged animals. Behav. Neural. Biol. 1990;53:6–20. doi: 10.1016/0163-1047(90)90729-p. [DOI] [PubMed] [Google Scholar]
- Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, Burgess N, Manji HK, Zarate CA., Jr. Performance on a virtual reality spatial memory navigation task in depressed patients. Am. J. Psychiatry. 2007;164:516–519. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet JF, Altman D. Spatial reasoning in children with congenital adrenal hyperplasia. Developmental Neuropsychology. 1998;14:299–320. [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, Wisniewski AB. Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm. Res. 2006;65:142–150. doi: 10.1159/000091793. [DOI] [PubMed] [Google Scholar]
- Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” Maze: Sex Differences and Correlation With Psychometric Measures of Spatial Ability in Humans. Evol. Hum. Behav. 1998;19:73–87. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- New MI, Lorenzen F, Lerner AJ, Kohn B, Oberfield SE, Pollack MS, Dupont B, Stoner E, Levy DJ, Pang S, Levine LS. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J. Clin. Endocrinol. Metab. 1983;57:320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav. Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Hindmarsh P, Geffner M, Brook C, Brain C, Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH) Horm. Behav. 2007;52:368–374. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman II, Bouchard TJ. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Dev. Psychol. 1986;22:191–198. [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Ross SP, Skelton RW, Mueller SC. Gender differences in spatial navigation in virtual space: implications when using virtual environments in instruction and assessment. Virtual Reality. 2006;10:175–184. [Google Scholar]
- Segal DL, Hersen M, Van Hasselt VB. Reliability of the Structured Clinical Interview for DSM-III-R: an evaluative review. Compr. Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Silverman II, Choi J, Mackewn A, Fisher M, Moro J, Olshansky E. Evolved mechanisms underlying wayfinding: further studies on the hunter-gatherer theory of spatial sex differences. Evol. Hum. Behav. 2000;21:201–213. doi: 10.1016/s1090-5138(00)00036-2. [DOI] [PubMed] [Google Scholar]
- Skelton RW, Ross SP, Nerad L, Livingstone SA. Human spatial navigation deficits after traumatic brain injury shown in the arena maze, a virtual Morris water maze. Brain Inj. 2006;20:189–203. doi: 10.1080/02699050500456410. [DOI] [PubMed] [Google Scholar]
- Speiser PW, White PC. Congenital adrenal hyperplasia. N. Engl. J. Med. 2003;349:776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- Therrell BL, Jr., Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol. Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, Tx: 1999. [Google Scholar]


