Abstract
We have developed a novel multiplex quantitative DNA-based target amplification method suitable for sensitive, specific and quantitative detection on microarray. This new method named NASBA Implemented Microarray Analysis (NAIMA) was applied to GMO detection in food and feed, but its application can be extended to all fields of biology requiring simultaneous detection of low copy number DNA targets. In a first step, the use of tailed primers allows the multiplex synthesis of template DNAs in a primer extension reaction. A second step of the procedure consists of transcription-based amplification using universal primers. The cRNA product is further on directly ligated to fluorescent dyes labelled 3DNA dendrimers allowing signal amplification and hybridized without further purification on an oligonucleotide probe-based microarray for multiplex detection. Two triplex systems have been applied to test maize samples containing several transgenic lines, and NAIMA has shown to be sensitive down to two target copies and to provide quantitative data on the transgenic contents in a range of 0.1–25%. Performances of NAIMA are comparable to singleplex quantitative real-time PCR. In addition, NAIMA amplification is faster since 20 min are sufficient to achieve full amplification.
INTRODUCTION
In 2007, after 12 years of commercialization of genetically modified (GM) crops, the total accumulated land areas sown with GM plants have exceeded 690 millions hectares (1). Consequently, regulations concerning biotech products have been adopted by many countries, and these require traceability of GM products on the market. Additionally, in some countries, compulsory labelling of products containing genetically modified organisms (GMOs) above a certain threshold has been introduced (2).
Currently, methods based on PCR technology are implemented in GMO detection laboratories, which allow for specific identification and quantification of single GM organisms (3,4). Not only the increasing presence of GMOs on the market but also their growing taxonomic (diverse taxon host plants) and biotechnological (diverse genetic constructs) diversity will render the current GMO testing strategies unmanageable both in terms of time and associated testing costs. Consequently, it is necessary to introduce new analytical technologies for high throughput GMO diagnostics. At the moment, the use of microarrays is the method of choice for multiplexing approaches in many fields of research and has already been applied to GMO diagnostics (5–12). However, for the purpose of GMO detection and quantification, direct hybridization of the samples onto microarrays is not possible due to the high sensitivity of GMO target DNA copies needed. Therefore, an amplification step of the DNA targets is applied prior to hybridization on microarrays. PCR technology has thus far, generally, been used for this purpose (5–10,12). However, high-throughput analysis using microarray hybridization may suffer from lack of a true multiplexing solutions for PCR technology due to the number of competitive amplification reactions with different efficiencies (3,13,14). To proceed to the microarray-based analysis, one still needs to use a set-up of multiple PCR reactions; one for each of the PCR assays targeting only a few particular genetic elements (6,8,9,12). As a consequence to such a set-up, the cost and time associated with GMO analyses increase. Furthermore, the accuracy of end-point PCR technology has its limits due to the exponential nature of amplification. This exponential nature limits the quantitative aspects of microarray-based GMO detection systems and therefore, most of these systems are being applied for qualitative analysis. Only one study has reported semiquantitative microarray-based detection (6).
Within the European Integrated Project Co-Extra, several multiplexing amplification methods in combination with microarray hybridization are under investigation (http://www.coextra.eu/library/deliverables.html). We have investigated the potential of NASBA to be used for multiplex target amplification prior to microarray detection in GMO diagnostics to alleviate the lack of multiplexing and quantitative performance associated with end-point PCR technology (15). NASBA is a method that mimics retroviral replication and uses a combination of three enzymes: the avian myeloblastosis virus's reverse transcriptase/DNA polymerase, T7 RNA polymerase and RNase H, along with a T7 promoter-labelled target-specific primer (16). NASBA has been most widely used for clinical diagnosis of human bacterial and viral pathogens, as well as for the detection and quantification of microbes in food and environmental samples (15), using commercial NASBA kits for RNA amplification (bioMérieux SA is the patent holder for NASBA technology). Additionally, it has recently been shown that NASBA is appropriate for human DNA-target amplification (17).
We have developed a novel multiplex quantitative DNA-based target amplification method suitable for use in combination with microarray detection. This new method named NASBA Implemented Microarray Analysis (NAIMA) was applied to the field of GMO detection. This fast and simple integrated method allows sensitive, specific and fully quantitative on-chip GMO detection in a multiplex format. The NAIMA method combined with microarray detection provides a suitable analytical tool for high throughput GMO diagnostics that will be required in the near future.
MATERIALS AND METHODS
Test materials
Mon863 maize flour (9.9% w/w) certified reference material (CRM) was prepared and purchased from the EU Joint Research Centre, IRMM (Institute for Reference Materials and Measurements, Geel, Belgium). Mon810 (100% w/w) maize leaves were obtained from plants grown from seeds (Campero cultivar) kindly provided by CSIC, Barcelona, Spain.
For the specificity assays, CRM RoundupReady® Soya (5% w/w) and CRM EH592-527-1 Potato (100% w/w) were purchased from the EU Joint Research Centre, IRMM (Institute for Reference Materials and Measurements, Geel, Belgium). Feed samples containing RoundupReady® Soya and wild-type maize, feed samples containing wild-type oilseed rape and wild-type maize seeds previously assayed by qPCR for routine diagnostics in our laboratory were also used in these specificity assessment experiments.
For quantitative analyses, samples containing Mon863 and Mon810 transgenic lines were prepared by mixing the Mon810 and Mon863 DNA reference material with DNA from feed samples identified as non-GM in our laboratory, to obtain the appropriate transgenic contents.
DNA purification
Samples were purified using the DNeasy plant mini kit (Qiagen, Valencia, CA) as described by the manufacturer with the incubation time of the sample in the lysis buffer extended to 10 min.
Primer and microarray probes design
Primer design
Oligonucleotide primers used in the NAIMA procedure were designed based on published DNA sequences using the Beacon Designer software (Premier Biosoft International Inc., Palo Alto, CA) and their secondary structure was analysed using the DINAMelt Server (http://frontend.bioinfo.rpi.edu/applications/hybrid/) (18). We have focused the design on the region targeted by singleplex qPCR methods used in routine GMO detection (19–22). Primers designed for the NAIMA procedure are listed in Table 1.
Table 1.
Primers and probes used in NAIMA, qPCR and microarrays
| Target | Orientation | Name | Sequence (5′–3′) | References |
|---|---|---|---|---|
| Primers used for NAIMAa | ||||
| Mon810 | Antisense | T7-Mon810a | T7-AATAAAGTGACAGATAGCTGGGCA | this work |
| Sense | SP6-Mon810b | SP6-TGTGCTGATGAAGGTATGTCC | ||
| P35S | Antisense | T7-P35Sa | T7-AAGGGTCTTGCGAAGGATAG | this work |
| Sense | SP6-P35Sb | SP6-TCATTGCGATAAAGGAAAGG | ||
| IVR | Antisense | T7- IVRa | T7-ACAGCCTAGCTAAGAAATGC | this work |
| Sense | SP6-IVRb | SP6-CGTTCGGCCTTCTCGTGCTG | ||
| tNOS | Antisense | T7-tNOSa | T7-GCGATAATTTATCCTAGTTTGC | this work |
| Sense | SP6-tNOSb | SP6-GTCTTGCGATGATTATCATATAATTTCT | ||
| T7-sequence | T7-universalc | AATTCTAATACGACTCACTATAGGGAGATCCAATAG AATCACATCGCTTACAAGGCAAT | this work | |
| SP6-sequence | SP6-universald | CATACGATTTAGGTGACACTATAGAA | ||
| Primers used for TaqMan® qPCR | ||||
| IVR | Antisense | ivr1-TM2-R | AAAGTTTGGAGGCTGCCG | (19) |
| Sense | ivr1-TM1-F | TGGCGGACGACGACTTGT | ||
| Probe | ivr1-pro | VIC-CGAGCAGACCGCCGTGTACTTCTACC-TAMRA | ||
| Mon810 | Antisense | Mon810R1311 | CCTTCATAACCTTCGCCCG | (20) |
| Sense | Mon810F1311 | AATAAAGTGACAGATAGCTGGGCA | ||
| Probe | Mon810pro1311 | FAM-ACGAAGGACTCTAACGTTTAACATCCTTTGCCA-TAMRA | ||
| tNOS | Antisense | tNOSR | CGCTATATTTTGTTTTCTATCGCGT | (21) |
| Sense | tNOSF | GTCTTGCGATGATTATCATATAATTTCTG | ||
| Probe | tNOSpro | FAM-AGATGGGTTTTTATGATTAGAGTCCCGCAA-TAMRA | ||
| P35S | Antisense | TM-35S-R | AAGACGTGGTTGGAACGTCTTC | (22) |
| Sense | TM-35S-F | GCCTCTGCCGACAGTGGT | ||
| Probe | TM-35S-pro | FAM-CAAAGATGGACCCCCACCCACG-TAMRA | ||
| Capture probes present on the microarray | ||||
| Mon810 | cRNA | MON spec | AACATCCTTTGCCATTGCCCAGC | this work |
| ss cDNA | MON spec_B | GCTGGGCAATGGCAAAGGATGTT | ||
| P35S | cRNA | 35S | ATATCTCCACTGACGTAAGGGATGACGC | this work |
| ss cDNA | 35S_B | GTCATCCCTTACGTCAGTGGAGATAT | ||
| IVR | cRNA | IVR | GTGCTGGCGGACGACGACTTGT | this work |
| ss cDNA | IVR_B | ACAAGTCGTCGTCCGCCAGCAC | ||
| tNOS | cRNA | tNOS | GAGATGGGTTTTTATGATTAGAGTCC | this work |
| ss cDNA | tNOS_B | GCGGGACTCTAATCATAAAAACCCATCTC | ||
aAll antisense primers used in NAIMA harbour the T7-cap sequence which is designed so that the T7 segment is bound to the 5′-end in addition to the given sequence.
bAll sense primers used in NAIMA harbour the SP6 sequence which is designed so that the SP6 is bound to the 5′-end in addition to the given sequence.
cThe T7-cap extension primer is composed of the T7-RNA polymerase promoter sequence (5′-end) and an abiotic cap sequence (3′-end, in italic).
dThe SP6-extension primer differs from the SP6 sequence used in sense primers in such a way that it lacks the first four nucleotides (in bold) contained in the latter sequence.
For the multiplex template synthesis reaction, one tailed primer was constructed of a 3′ region specific to the target sequence and of a 5′ region harbouring the sequence promoter for the SP6 RNA polymerase. The second tailed primer was constructed of a 3′ region specific to the target sequence, a 5′ region harbouring the sequence promoter for the T7 RNA polymerase and a central region harbouring an abiotic sequence. The T7 promoter sequence was designed as recommended in literature (23) using an extra purine residue sequence. The abiotic sequence does not show similarities with known biotic sequences. Tailed primers were designed for specific amplification of the maize plant species (invertase, IVR), the CaMV 35S promoter (P35S), the nopaline synthetase terminator (tNOS) and the 5′ maize plant DNA-Mon810 insert junction (MON810).
Criteria for the selection of primers to be used in the first step of the procedure (multiplex template synthesis) were the following: (1) avoiding folding, homodimerization and heterodimerization with other primers in the reaction mix at 55°C; (2) the melting temperature (Tm) of the target-specific regions must be compatible with the annealing temperature at 55°C; (3) possessing the lowest possible difference in Tm between each of the primer pairs; (4) primers were selected so that they could be used for the analysis of degraded genomic DNA as present in highly processed products (each amplicon spans 100–200 bases).
In the second step of NAIMA (universal NASBA), a single pair of universal-primers is used to amplify all templates created during the first step of the procedure. The T7-universal primer is identical to the 5′ sequence of the tailed primer, harbouring the T7 promoter sequence in the first step of NAIMA: it is composed of the T7 RNA polymerase promoter sequence fused with the ‘cap’ abiotic sequence. The SP6-universal primer sequence differs from this SP6 RNA polymerase sequence in such a way that it lacks the first four nucleotides contained in the latter sequence.
Primers were synthesized and HPLC-purified by MWG Biotech AG (Eurofins MWG GmbH, Ebersberg, Germany).
Microarray probe design
The microarrays used in the described experiments were based on the technology developed by Eppendorf Array technologies, S.A. (EAT, Namur, Belgium) (24). The probes are covalently bound by their 5′ ends to glass slides (25). The capture probes were suitably designed for sensitive and specific hybridization of the product to be detected. Capture probes contained 20–30-nt long sequences complementary to their specific target for the specific detection of the maize invertase (NAIMA IVR product), the CaMV 35S promoter (NAIMA P35S product), the nopaline synthetase terminator (NAIMA tNOS product) and the 5′ maize plant DNA–Mon810 insert junction (NAIMA MON810 product) (Table 1). The capture probes were spotted in triplicate. Anti-sense probes corresponding to the reverse-complement sequence of the probes, described above, were also added to the microarray as controls (Table 1). Additional control probes were spotted onto the microarray including (i) positive detection controls consisting of capture probes labelled with Cy3 or Cy5, (ii) negative hybridization controls (non-specific probes) and (iii) negative detection controls (spotting buffer without DNA) (Figure 1). Capture probes were designed to minimize secondary structures, hetero- and self-dimerizations and to have melting temperature values ranging between 77 and 82°C, as described earlier (9).
Figure 1.
Microarray used in this study and its specificity. Schematic representation of the probes spotted onto the microarray. negative hyb ctl, capture probe having a non-specific sequence; negative det Ctl, spotting buffer without DNA; det Ctl Cy3, positive detection control consisting of a probe labelled with a Cy3 dye (similar excitation and emission spectrum as the Oyster 550 dye); det Ctl Cy5, positive detection control consisting of probes labelled with a Cy5 dye (similar excitation and emission spectrum as the Oyster 650 dye); P35S, capture probes specific to the Cauliflower mosaic virus-derived promoter NAIMA amplicon; IVR, capture probes specific to the invertase NAIMA amplicon; tNOS, capture probes specific to the nopaline synthase terminator NAIMA amplicon; MON810 spec, capture probes specific to the MON810 event-specific NAIMA amplicon. “B” capture probes are reverse complements of the capture probes that can detect cDNA, secondary products of NAIMA.
Specificity of the sequences
A search using the BLASTN tool (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) has shown no similarity between the non-target sequences used in the primers (T7 promoter, SP6 promoter and abiotic sequences) and any plant genomes or known transgenic sequences introduced in commercial crops. The BLASTN tool was also used to check specificity of the target sequences in the NAIMA primers and of the probes on the microarray in silico.
NAIMA reaction setup
All experiments (specificity, sensitivity, linearity and quantification) were performed in duplicate, and for each of these experiments, measurements at two different dilutions were taken.
First step: multiplex template synthesis
This first step consisted of a multiplexed template synthesis reaction during which several pairs of tailed primers were extended to produce templates bound to the universal regions (Figure 2). For this, we used 5 pmol of each of the SP6- and T7-specific tailed primers (i.e. three pairs of tailed primers for triplex NAIMA), 5× colorless Go Taq® Flexi buffer (Promega, Madison, WI), 1 mM dNTP (Promega, Madison, WI), 1.25 units of Go Taq® Flexi DNA polymerase (Promega, Madison, WI), 2.5 mM MgCl2 (Promega, Madison, WI) and 5 μl of purified DNA in a final reaction volume of 10 μl. Thermal cycling for template synthesis was carried out as follows: 95°C for 2 min 30 s, 55°C for 30 s and 72°C for 7 min 30 s.
Figure 2.
Schematic representation of the NAIMA method. Template synthesis step of NAIMA: after denaturation of the target DNA, tailed primers with a sequence complementary to the target DNA and 5′-end sequences necessary for the multiplex amplification bind to the target DNA and are extended by Taq polymerase (1) to produce the DNA template used in the amplification step. The pair of 5′-end sequences is identical to all the different targets. Multiplex amplification step: the DNA template synthesized in the first step is flanked by a recognition site for DNA dependent T7 RNA polymerase (2). This dsDNA is further transcribed by the T7 RNA polymerase into numerous copies of antisense RNA molecules (3), which are later reverse-transcribed into single-stranded sense DNA (ssDNA) (4 and 5) to form an RNA–DNA duplex. This RNA–DNA duplex is degraded by RNAse H activity (6). The ssDNA is then used as a template by the reverse transcriptase after T7-cap-extension primer annealing (7) to synthesize a second DNA strand (8). This dsDNA can be used as a template for several cycles of amplification. The final product of NAIMA is anti-sense cRNA. Only one pair of primers is needed in this amplification step for all the targets to be amplified.
Second step: universal NASBA amplification
DNA templates from the first step were used directly in the NASBA step (Figure 2). For this, 2.5 μl of template DNA from the first step were added to 5 μl of the NASBA pre-mix including the NucliSens® Basic Kit (bioMérieux, The Netherlands) and the pair of universal primers (T7-universal primer and SP6-universal primer). The optimized protocol was set for final NASBA reaction concentrations of 85 mM Tris–HCl (pH 8.5), 12 mM MgCl2, 70 mM KCl, 15% v/v DMSO, 1 mM for each dNTP, 2 mM for each NTP, 2 μM T7-universal primer and 2 μM SP6-universal primer. This mixture was pre-incubated at 95°C for 15 min and then incubated at 41°C for 2 min 30 s. Afterwards, 2.5 μl of the enzyme mixture (0.08 U/μl RNase H, 32 U/μl T7 RNA polymerase, 6.4 U/μl AMV reverse-transcriptase per reaction) was added. Reactions were incubated at 41°C for 25 min or 60 min in a Gene Amp® PCR system 9700 (PE Applied Biosystems, CA, USA).
Labelling of NAIMA products
The quality and quantity of NAIMA products was assessed with the NanoDrop® ND-1000 UV–Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) according to the manufacturer's instructions. Two microlitres of NAIMA products (1/5 of the NAIMA reaction volume) was ligated directly to 3DNA dendrimers harbouring 15 fluorescent dyes (Oyster-550 dye) per molecule of dendrimer. Ligation was performed with the FlashTag™ RNA Labelling Kit (Genisphere Inc., Hatfield, PA) following recommendations from the manufacturer.
Microarray hybridization
The following steps of hybridization were performed in the dark. The procedure used was derived from the Genisphere (Genisphere Inc., Hatfield, PA) recommended procedure for hybridization of FlashTag™ ligated products on microarrays. Shortly, a hybridization mix was prepared containing the FlashTag™ ligation product, 1× enhanced hybridization buffer (heated to 80°C for 10 min) and 0.8% BSA. The hybridization mix was heated to 65°C for 10 min. Thirty microlitres of the hybridization mix was applied to the microarray, which was covered with a 22 × 25-mm2 glass lifterslip (Erie scientific company, Porthsmouth, NH). The microarrays were then incubated in a GeneMachines® HybChamber™ (Genomic Solutions® Inc., Ann Arbor, MI) for 20 h in the dark at 48°C. Following this incubation, the microarrays were washed for 5 min in 45°C 2× SSC/0.2% SDS, then for 5 min in 2× SSC at room temperature and for 5 min in 0.2× SSC at room temperature. After washing, the microarrays were centrifuged for 2 min at 3,000g and stored in the dark, at room temperature until scanning.
Microarray signal detection and image analysis
The microarrays were scanned using a LS200 scanner (Tecan Trading AG, Switzerland) with the following parameters: laser excitation wavelength λ = 543 nm and filter at λ = 590 nm. Care has been taken that signal measurements have not reached saturation. Image analysis of the scanned microarrays was done using the ArrayPro Analyser® software, version 4.5.1 (Media Cybernetics® Inc., Bethesda, MD). The average local background signal for every probe was calculated. Net signal intensity for each probe with a positive signal was calculated by background subtraction and averaged. A probe signal was considered positive when the net intensity value was higher than the local background value plus one standard deviation.
Quantitative real-time PCR
Quantitative PCR was used to calibrate DNA copy number analysed in samples, to assess NAIMA amplification and to analyse transgenic contents of DNA samples.
qPCR set-up
The primers and probe for detection of the invertase gene (IVR) were used as described (19). Primers and probes specific for the p35S promoter (P35S) (22) and tNOS terminator (tNOS) (21) were used to target common promoter and terminator sequences as previously described. The event-specific primers and probe for the genetically modified maize MON810 (MON810) were used as previously described (20). Sequences of primers and probes used are listed in Table 1 and their position on the target DNA is indicated on Figure 3. qPCR reactions were performed as previously described (26) using 2 μl of DNA samples or reverse-transcribed NAIMA products. For each sample quantified, dilution controls were included to monitor the possible influence of effectors on qPCR reaction efficiency for each amplicon assayed, as previously recommended (26). Each dilution was assayed in duplicate. Results were analysed using SDS 2.1 software (Applied Biosystems, Foster City, CA) after an automatic adjustment of the baseline and manual adjustment of the fluorescence threshold. After being exported, further data analysis was performed in a basic Excel spreadsheet.
Figure 3.
Description of the GMOs used in this study and approximate position of the regions targeted by NAIMA primers, qPCR primers and probes and microarray probes. P35S, sequence derived from the cauliflower mosaic virus promoter, HSP70, sequence containing the first intron of the 70 kDa heat-shock protein of maize; IVS2, intron from maize alcohol dehydrogenase; CRYA(b), synthetic delta endotoxin gene derived from Bacillus thuringiensis; NPTII, neomycin phosphotransferase II gene from the Escherichia coli transposon Tn5; T-NOS, transcription terminator from the Agrobacter tumefaciens nopaline synthase gene; PR-ACT, 5′ region of the rice actin 1 gene containing the promoter and first intron; OTP, optimized transit peptide sequence; M-EPSPS, a modified form of wild-type 5-enolpyruvyl-3-phosphoshikimate synthase gene from Zea mays; P-GBSS, promoter of the gbss gene involved in starch synthesis from Solanum tuberosum; antigene fragment III, antisense fragment of the gbss gene; GBSS fragment, genomic gbss fragment inverted to sense orientation; CTP, DNA sequences from chloroplast transit peptides from A. thaliana; CP4-EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase gene, isolated from Agrobacterium sp. (strain CP4); *fragment of CP4-EPSPS gene; **rearranged sequence after integration without known homology. Targeted regions are shown by bold lines above the transgenic region.
Determination of target copy number in the samples
The genome copy number of DNA samples was determined by the qPCR TaqMan® invertase assay, using a known copy number of 5% Mon810 certified reference material for the construction of the standard curve. The 5% Mon810 standard reference material was diluted to contain approximately 100, 33, 11, 3.7, 1.2, 0.4 and 0.1 ng of DNA, which corresponds to approximately 18 000, 6100, 2000, 680, 230, 70 and 23 maize genome copies per reaction, respectively, on the basis of maize genome size (1C) (27) and the measured DNA concentration. Genome copy number in the sample prior to amplification was calculated by the interpolation of Ct values generated onto a standard regression curve.
Determination of NAIMA performance
The main final product of NAIMA is cRNA. To estimate the amplification rate of multiplex NASBA with quantitative real-time PCR (qPCR), a reverse transcription was performed on the multiplex NASBA products to get double-stranded cDNA. For this, 50 pmol of SP6-universal primer and 25 μmol of dNTP (Promega, Madison, WI) were added to the 10 μl volume of the NASBA reaction products. The mixture was incubated at 65°C for 5 min and then put immediately on ice for 5 min. Subsequently, 50 pmol of T7-universal primer were added and the mixture was incubated for 2 min at 42°C. Finally, 0.045 U of RNAseH (50 pmol SP6-universal primer) and 60 U of superscript™ II reverse transcriptase were added for a final volume of 13.6 μl and the reaction solution was incubated for 2 h at 42°C followed by 5 min of denaturation at 65°C.
After qPCR, Ct values of the NAIMA products were compared to the Ct values of control samples. The control sample in our experiments was DNA used for NAIMA, which had been diluted in the same proportion as was the amplified DNA in the NAIMA reaction and reverse transcription. The difference in Ct values between the NAIMA products and control indicated the amplification rate of the NAIMA reaction. From this, Ct values of NAIMA products were converted into copy numbers as described above.
To determine the quantitative performance of the NAIMA amplification, the 100% Mon810 and the 9.9% Mon863 standard reference materials were used for preparing the standard curve. Reference materials were diluted as described above (determination of target copy number in the samples), NAIMA-amplified and run on qPCR in duplicate. Target copy number in the sample prior to NAIMA amplification was calculated by the interpolation of Ct values generated onto the standard regression curve.
To determine the transgenic contents of the maize DNA samples, the ratio between the invertase and transgene (P35S, tNOS, Mon8101) copy numbers was calculated and expressed as a percentage. Standard deviations and coefficients of variation were evaluated for replicate measurements. For each series of measurements, the coefficient of variation that would be attributed to the final GMO percent calculation was estimated from the following formula: CV = √(CVinvertase2 + CVtransgene2). Data were analysed by basic spreadsheet software. Measurements with CV values above 50% were excluded from further calculations. For comparison between NAIMA and qPCR performances, the coefficient of variation was estimated using the standard deviation of the GMO content assessed by NAIMA (SDNAIMA) and the GMO content assessed by qPCR considered as the true value (GMOqPCR) as follows: CV = SDNAIMA/GMOqPCR.
RESULTS AND DISCUSSION
We present a new target amplification approach called NAIMA developed to detect and quantify the presence of DNA targets in a multiplex fashion. Moreover, we have shown the possibility of using this new procedure for the quantification of DNA targets on a microarray format. The amplification method is based on a two-step procedure. In the first step, the use of multiple tailed primers allows the synthesis of a template DNA in a single extension reaction. The second step of the procedure consists of a NASBA amplification reaction using a single pair of universal primers to anneal to the universal sequences created by the tailed primers. After amplification, the cRNA are ligated directly with 3DNA dendrimers labelled with fluorescent dyes. The cRNA–dendrimer complexes are then hybridized without further purification onto an oligonucleotide microarray harbouring specific probes complementary to internal segments of the NAIMA products. The procedure was tested for two separate triplex systems.
NAIMA design
Several obstacles needed to be circumvented in the course of NAIMA development. These included the strong preference of NASBA amplification for RNA templates (23), and the low multiplexing capabilities. Also, multiplex amplification, in general, faces the complexity of primer pair combinations and the problem of unspecific background amplification (13). In the context of multiplex PCR, the use of bipartite primers harbouring a universal region on the 5′ end and target-specific sequences on the 3′ end has been shown to improve multiplexing capacities of amplification (6). Also, the addition of primers identical to the universal region of the bipartite primers gave more uniform amplification results of the different targets (6).
With this in mind, we chose to develop a two-step procedure: template synthesis and NASBA amplification. Prior to the amplification procedure, the DNA sample was treated with RNAse A to alleviate the bias caused by RNA amplification in the NASBA step. The first step of NAIMA consists of the synthesis of a DNA template suitable for NASBA amplification, which takes place in the second step. We have designed two types of tailed primers for this first step: bipartite primers and tripartite primers. The bipartite primer is composed of a 5′ region corresponding to the promoter sequence of the SP6 RNA polymerase, and a 3′ region harbouring a target-specific sequence. The SP6 promoter sequence is a non-specific sequence that can be used as universal target in the second step. The tripartite primer is composed of a 5′ region corresponding to the promoter sequence of the T7 RNA polymerase on which NASBA amplification relies, and a 3′ region harbouring a target-specific sequence. The central region of the tripartite primer is composed of an abiotic sequence used to create a non-specific sequence that is used as the universal target in the second step thus enabling reconstruction of functional T7 promoter (step 8 in Figure 2). This sequence was designed to differ from any plant genome or known sequences inserted in GMOs. The resulting product is a short double-stranded DNA product harbouring the target sequence to be amplified, adjoined to the T7 promoter and abiotic sequences on one side, and the SP6-promotor sequence on the other. Such a template has the added advantages of being able to be used as a template by the T7 RNA polymerase, and allowing the use of only one pair of universal primers for all analysed targets during the NASBA amplification. Sequences of all primers designed in this study are available on Table 1.
NAIMA performances
The performance of NAIMA as a DNA target amplification method was tested using two triplex platforms on GMO testing-related targets. One triplex (screening triplex) was designed for the screening of transgenic maize lines authorized in the EU: it targets the endogene maize gene invertase (IVR) and the two main screening elements found in transgenic maize lines authorized in the EU, the cauliflower mosaic virus 35S promoter (P35S) and the terminator of the nopalin synthase (tNOS). The second triplex platform (MON810 triplex) was designed for the detection of the Mon810 transgenic maize lines and targets the IVR endogene and the P35S element, as well as the event-specific 5′-end junction between the plant DNA and the Mon810 transgene (MON810). To comply with GMO testing regulations, the amplification method needs to be specific to the target DNA, sensitive enough to detect low numbers of target copies, allow linear amplification of target sequences over a broad range of target copy numbers (linear range of amplification) and should allow the identification of samples containing GMOs exceeding a set labelling threshold. In this study, we have set the threshold of GMO contents at 0.9%, which is the lowest legal labelling threshold currently in use worldwide (2).
The performance characteristics of NAIMA amplification described in this section were evaluated using qPCR to quantify target copies after amplification.
Kinetics of amplification
We have followed the kinetics of NAIMA amplification in time-course studies using both triplex platforms. After approximately 5 min, NAIMA product accumulation generated a typical sigmoid curve. A semi-logarithmic plot of the increase in the early phase of the reaction (5–20 min) revealed an initial first-order reaction. Following the log-linear phase, the reaction slowed, entering a transitional phase (20–30 min) eventually reaching a plateau (Figure 4). Regarding the performance, NAIMA amplification shows very fast kinetics that are comparable with the fastest qPCR systems currently available, as the duration of a qPCR amplification run varies from 30 min to 2 h (28). NAIMA is also generally a faster amplification method when compared to other multiplex amplification methods combined with microarray detection applied to GMO diagnostics (6–10,12,29). Most of the qPCR methods validated by the CRL for GMO detection include cycles of 75 s, for which a maximum of 7·1010-fold amplification rate (for 36 cycles) is possible in 45 min at 100% reaction efficiency (http://gmo-crl.jrc.it/statusofdoss.htm). The amplification rate of the NAIMA system was determined for both triplex platforms on a series of dilutions of target DNA, and on DNA samples containing different relative amounts of targets. For both triplexes, the amplification rate after 45 min varied from over 105-fold to 108-fold of the starting DNA target copy number, depending on the amplicon. Thus, the maximum amplification rate observed for NAIMA in 45 min is comparable with general qPCR performance.
Figure 4.
Kinetics of NAIMA amplification. Example of the kinetics of invertase (IVR) during NAIMA amplification of the screening triplex platform on Mon863 (10%) reference material. Copy numbers of amplification products were detected by qPCR. Data-points were plotted on a semi-log scale. After a very short period of time, the amplification enters a log-linear reaction. A trendline was drawn for this phase of the amplification. Following this phase, the reaction entered a transitional phase (20–30 min), eventually reaching a plateau. The curve is the average of two independent measurements performed on the same reference material. The log-linear amplification stage of the reaction is shown in the inner graph.
We have analysed the influence of the tailed primer's presence during the first step of NAIMA on the total amplification rate. For this purpose, the universal primers were substituted with water during the second step of NAIMA. In the absence of universal primers, all amplicons were amplified but weak amplification was obtained, ranging from 0.4 to 4.8% of the normal amplification rate. Therefore, the tailed primers only have a minor contribution to the overall NAIMA amplification and hence do not need to be removed by purification or enzymatic digestion prior to the NASBA step. On the other hand, in classical two-step multiplex PCR, the second step multiplex amplification failed if the tailed primers from the first step were not removed (6).
Specificity of amplification
To evaluate the specificity of the system, we performed both NAIMA triplexes using several DNA samples in which at least one of the three targets was absent: RoundupReady® Soya CRM, EH592-527-1 potato CRM, Mon810 maize CRM, a feed sample containing RoundupReady® Soya and wild-type maize, and a feed sample containing wild-type oilseed rape, wild-type maize seed, GA21 maize flour with traces of P35S contamination (Figure 3). In all samples, only the expected targets were amplified by NAIMA and qualitative results were identical to direct qPCR analysis of the same samples. Using this approach, one can consider that our triplexes do not non-specifically amplify transgenic elements inserted in analysed GM lines. Additionally, we have shown that the system does not amplify neither soybean, oilseed rape nor potato genome DNA, the other crops available on the GMO market for food and feed.
Sensitivity of amplification
The sensitivity of the triplex platforms to the template concentration was assayed using a dilution series of Mon863 (9.9%, w/w) and Mon810 (100% w/w) DNA. For the screening triplex, IVR was detectable in the range between 5400 and 6 copies, while the P35S amplicon was detectable from 530 to 4 copies. For tNOS, a signal was obtained from 265 to 2 copies. With the MON810 triplex platform, IVR was detectable in the range of 5500 to 7 copies of the endogene. The P35S amplicon was detectable from 2750 to 3 copies, while a signal for the Mon810 event-specific amplicon was detectable from 2750 to 7 copies. These results show that the sensitivity of the newly developed method is comparable to qPCR method, where best assays allow detection of one to ten molecules (4,30).
Linearity of amplification
We investigated the influence of target copy number on the linearity of the NAIMA amplification to assess the linear range of amplification. For this, both triplex platforms were assayed using a series of Mon863 (9.9%, w/w) and Mon810 (100% w/w) DNA samples. Using Mon863 (9.9% w/w) DNA samples, a linear response to copy number was obtained with the IVR amplicon from 5380 copies to 11 copies (with a squared regression coefficient of 0.9145). For the P35S amplicon, amplification was quantitative from 530 to 4 copies (with a squared regression coefficient of 0.8980). With the tNOS amplicon, linear amplification was obtained from 265 to 4 copies (with a squared regression coefficient of 0.9336) (Figure 5). Using Mon810 (100% w/w) DNA samples, linear amplification of the IVR amplicon was obtained from 5500 to 11 copies (the squared regression coefficient being 0.8621). For the P35S amplicon, amplification was linear between 2750 and 21 copies (with a squared regression coefficient of 0.9077), while for the Mon810 amplicon this ranged from 2750 to 21 copies (the squared regression coefficient of which was 0.9798). The linearity of amplification was sufficient to allow broad range of quantitative analysis on the samples.
Figure 5.
Linearity of NAIMA amplification. Example of the amplification using the screening triplex. A dilution series of template DNA was amplified with NAIMA. Copy number of template DNA was estimated by qPCR before NAIMA amplification (control DNA, plotted with circles) and compared to the copy number of the same sample amplified by NAIMA (NAIMA products, plotted with triangles) estimated by qPCR. As for the plots from the non-amplified control DNA, plots obtained from NAIMA-amplified DNA are linear showing the linearity of the NAIMA amplification method. Linearity of amplification was assessed for all three amplicon IVR (A), P35S (B) and tNOS (C).
Quantification trueness
Both triplex platforms were evaluated for trueness of quantification based on a series of maize samples containing different ratios of P35S, Mon810 and tNOS targets copies in maize DNA from Mon810 and Mon863 GM maize background. Quantification results were compared on the same samples with those of singleplex qPCR analysis that is a golden standard in GMO detection (Table 2). The amount of GMO present was calculated as a ratio of transgene and endogene DNA copy numbers.
Table 2.
Trueness of GMO quantification: comparison between NAIMA and qPCR
| Triplex platform | 35S/IVR NAIMA | 35S/IVR qPCR | Cv% between NAIMA and qPCR | Mon810/IVR NAIMA | Mon810/IVR qPCR | Cv% between NAIMA and qPCR |
|---|---|---|---|---|---|---|
| MON810 (IVR × 35S × Mon810) | 20.0 ± 8.1 | 25.7 ± 8.0 | 15.5 | 11.5 ± 5.0 | 8.7 ± 1.0 | 23.1 |
| 8.4 ± 6.0 | 11.5 ± 3.2 | 19.2 | 5.0 ± 2.1 | 5.0 ± 0.4 | 0.2 | |
| 7.4 ± 2.9 | 5.1 ± 0.6 | 32.6 | 2.6 ± 0.8 | 4.1 ± 0.4 | 25.2 | |
| 1.5 ± 0.7 | 3.9 ± 1.1 | 44.1 | 1.4 ± 0.6 | 1.2 ± 0.5 | 16.0 | |
| 0.9 ± 0.6 | 2.6 ± 0.8 | 45.7 | 0.3 ± 0.2 | 0.7 ± 0.3 | 37.4 | |
| 0.3 ± 0.2 | 0.6 ± 0.2 | 35.6 | 0.14 ± 0.11 | 0.13 ± 0.04 | 1.5 |
| Triplex platform | 35S/IVR NAIMA | 35S/IVR qPCR | Cv% between NAIMA and qPCR | tNOS/IVR NAIMA | tNOS/IVR qPCR | Cv% between NAIMA and qPCR |
|---|---|---|---|---|---|---|
| screening (IVR × 35S × tNOS)s | 17.1 ± 5.9 | 13.4 ± 4.3 | 20.0 | 10.9 ± 5.9 | 9.2 ± 3.4 | 12.9 |
| 12.6 ± 4.7 | 10.1 ± 1.7 | 17.7 | 9.6 ± 2.9 | 9.9 ± 1.0 | 2.3 | |
| 4.0 ± 1.8 | 6.2 ± 1.9 | 24.4 | 3.5 ± 0.6 | 3.4 ± 0.8 | 3.7 | |
| 0.9 ± 0.5 | 1.0 ± 0.4 | 6.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 2.4 | |
| 0.5 ± 0.2 | 0.4 ± 0.1 | 21.0 | 0.13 ± 0.13 | 0.3 ± 0.1 | 33.0 | |
| ND | 0.04* | ND | 0.00* | 0.02* | ND |
Both triplex platforms were evaluated for trueness of quantification on a series of maize samples containing different ratio of P35S, Mon810 and tNOS target copies in maize DNA from Mon810 and Mon863 GM maize background. Results of quantification were compared with those of singleplex qPCR analysis on the same samples.
Results are expressed as percentage of the transgenic element copy number (35S, tNOS or Mon810) in haploid genome (estimated by the IVR copy number).
*one of the amplifications has failed.
ND, not determined.
For samples with GM contents between 0.1 and 25%, analyses results were in agreement with qPCR quantification with a correlation of variation (CV) between results of both methods ranging from 2.3 to 33.0% for the screening triplex, and from 0.2 to 45.7% for the Mon810 triplex (Table 2). Also, comparable standard deviation values of calculated results were observed for qPCR and NAIMA quantification (Table 2). Thus, one can consider that NAIMA behaves similarly to singleplex qPCR for these samples in quantitative analyses.
NAIMA as a target amplification method for on-chip GMO detection
NAIMA product is a single-stranded RNA allowing direct hybridization to probes on microarrays without a prior denaturation step. To additionally increase sensitivity of the system, we have included signal amplification, as fluorescent tagged dendrimers were used for labelling. The capture probes present on the microarray allowed specific recognition of the maize plant species (IVR gene), the CaMV 35S promoter (35S), the nos terminator (tNOS) and the event-specific Mon810 sequence (5′ junction between the maize host plant DNA and the transgenic Mon810 construct).
Detection on microarray was tested with products of both NAIMA triplexes. The screening triplex was tested on Mon863 CRM material (9.9% w/w) and the Mon810 triplex was tested on Mon810 material (100% w/w). For each of the expected capture probes, a statistically significant positive signal was generated, while no signal was observed for other capture probes showing high specificity of the system (Figure 6).
Figure 6.
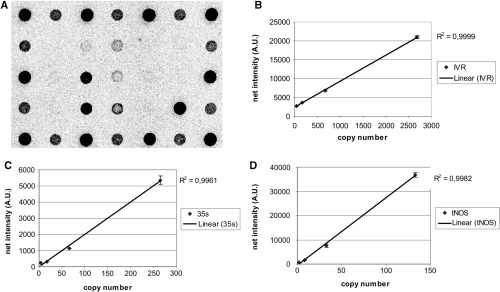
NAIMA in combination with detection on microarray. (A) An example of a microarray scanning image after the hybridization of the screening triplex NAIMA product performed on Mon863 (10%) reference material. Only the expected capture probes (P35S, IVR, tNOS) emitted a signal. A low signal was also observed for the cDNA NAIMA secondary products (P35S_B, IVR_B and tNOS_B). A Cy3 positive detection control yielded a signal as expected, but Cy5 also yielded a low signal (2% of Cy3 signal) due to fluorescence leakage of the dye spectrum. (B) Linearity of the fluorescent signals for IVR (B), P35S (C) and tNOS (D) capture probes using dilution series of template DNA. Each graph is the results of three measurements.
Linearity and sensitivity of the detection method were tested using a dilution series of CRM Mon863 (9.9% w/w) DNA. The detection on microarrays was proportional to the starting copy number of the target (before NAIMA) with coefficients of linear correlation ranging from 0.9961 to 0.9999 (Figure 6). The detection system appeared to be very sensitive, since as few as 2 copies of tNOS could be amplified and detected (4 copies for P35S and 42 copies for IVR, this being the lowest concentration tested for invertase). This corresponds to 60 pg of starting maize genomic DNA (27). Considering the maize genome size, this means that the NAIMA-microarray platform should reliably identify the presence of transgenic events at 0.1% level.
In comparison to other array-based GMO detection methods, the method presented in this study shows comparable performances in terms of specificity and relative sensitivity; most other methods being able to detect as low as 0.1% GM contents (6,9,29,31), and some being slightly less sensitive (8,10). The absolute sensitivity of the NAIMA amplification method combined with microarray detection (∼60 pg) is also favourable when compared to the above-cited methods, since several methods require starting amounts of DNA from 10 to 500 ng (8,10,31), two methods requiring 60 pg (29) and 300 pg (6). The microarray detection of NAIMA-amplified GM targets overpowers other mentioned methods by being the only method enabling quantitative detection on microarrays reported so far. Only the MQDA method combined with macroarray detection can also result in semi-quantitative data on analysed samples (6).
CONCLUSION
The aim of our work was to develop a system allowing the detection and quantification of GMO presence in a multiplex fashion. Thus, we have adapted the NASBA amplification strategy for multiplex DNA amplification and used it in combination with microarray technology. The NAIMA method was shown to be equivalent in sensitivity and specificity to the qPCR method, allowing similar qualitative and quantitative performance for GMO diagnostics. Linearity of the NAIMA method shows quantification of GMO DNA targets in the range of 0.1–25%. This allows reliable detection and quantification of GM content in samples (food, feed and seeds) according to the requirements of most countries’ regulations where thresholds are established for the labelling of GMOs. This method is a proof of concept for developing high multiplexing amplification and detection systems. This approach is a proof of concept, and the results show great potential for the NAIMA method to be quantitative also at a higher multiplexing level, since the first step template synthesis primers do not contribute or interfere with the overall amplification. Along with the described performance characteristics, the advantages of the NAIMA method lie also in its applicability, the short time of amplification and its modularity suitable with the future needs in GMO testing. NAIMA has been successfully combined with microarray-based detection, the most advanced currently existing method for multiplex target detection. The use of dendrimers in signal amplification additionally increases sensitivity of the method without trade-off in linearity. The NAIMA approach combined with microarray hybridization, as described here, is a general approach for specific and sensitive multiplex DNA target identification and quantification and could similarly be applied to other domains where diagnostics rely on DNA-based sequence detection such as clinical diagnosis of human bacterial pathogens, as well as for the detection of microbes in food and environmental samples.
FUNDING
European Commission through the Integrated Project Co-Extra, under the 6th Framework Programme, priority 5, food quality and safety (contract no. 007158); Slovenian Research Agency (contract no. P4-0165). Funding for open access charge: Project Co-Extra.
ACKNOWLEDGEMENTS
The authors thank Tina Dolinšek for her critical review of the manuscript and her technical assistance.
REFERENCES
- 1.James C. The International Service for the Acquisition of Agri-Biotech Applications (ISAAA) Ithaca, NY: 2007. Global Status of Commercialized Biotech/GM Crops 2007. The International Service for the Acquisition of Agri-Biotech Applications (ISAAA). ISAAA Brief No 37. [Google Scholar]
- 2.Gruere GP, Rao SR. A review of international labeling policies of genetically modified food to evaluate India's proposed rule. AgBioForum. 2007;10:51–64. [Google Scholar]
- 3.Hernandez M, Rodriguez-Lazaro D, Ferrando A. Current methodology for detection, identification and quantification of genetically modified organisms. Curr. Anal. Chem. 2005;1:203–221. [Google Scholar]
- 4.Holst-Jensen A, Ronning SB, Lovseth A, Berdal KG. PCR technology for screening and quantification of genetically modified organisms (GMOs) Anal. Bioanal. Chem. 2003;375:985–993. doi: 10.1007/s00216-003-1767-7. [DOI] [PubMed] [Google Scholar]
- 5.Birch L, Archard CL, Parkes HC, McDowell DG. Evaluation of LabChipTM technology for GMO analysis in food. Food Control. 2001;12:535–540. [Google Scholar]
- 6.Rudi K, Rud I, Holck A. A novel multiplex quantitative DNA array based PCR (MQDA-PCR) for quantification of transgenic maize in food and feed. Nucl. Acids Res. 2003;31:e62. doi: 10.1093/nar/gng061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bordoni R, Germini A, Mezzelani A, Marchelli R, De Bellis G. A microarray platform for parallel detection of five transgenic events in foods: a combined polymerase chain reaction-ligation detection reaction-universal array method. J. Agric. Food Chem. 2005;53:912–918. doi: 10.1021/jf0486949. [DOI] [PubMed] [Google Scholar]
- 8.Germini A, Rossi S, Zanetti A, Corradini R, Fogher C, Marchelli R. Development of a peptide nucleic acid array platform for the detection of genetically modified organisms in food. J. Agric. Food Chem. 2005;53:3958–3962. doi: 10.1021/jf050016e. [DOI] [PubMed] [Google Scholar]
- 9.Leimanis S, Hernandez M, Fernandez S, Boyer F, Burns M, Bruderer S, Glouden T, Harris N, Kaeppeli O, Philipp P, et al. A microarray-based detection system for genetically modified (GM) food ingredients. Plant Mol. Biol. 2006;61:123–139. doi: 10.1007/s11103-005-6173-4. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Miao H, Wu H, Huang W, Tang R, Qiu M, Wen J, Zhu S, Li Y. Screening genetically modified organisms using multiplex-PCR coupled with oligonucleotide microarray. Biosens. Bioelectron. 2006;22:71–77. doi: 10.1016/j.bios.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Tengs T, Kristoffersen AB, Berdal KG, Thorstensen T, Butenko MA, Nesvold H, Holst-Jensen A. Microarray-based method for detection of unknown genetic modifications. BMC Biotechnol. 2007;7:91. doi: 10.1186/1472-6750-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Zhu S, Miao H, Huang W, Qiu M, Huang Y, Fu X, Li Y. Event-specific detection of seven genetically modified soybean and maizes using multiplex-PCR coupled with oligonucleotide microarray. J. Agric. Food Chem. 2007;55:5575–5579. doi: 10.1021/jf070433m. [DOI] [PubMed] [Google Scholar]
- 13.Markoulatos P, Siafakas N, Moncany M. Multiplex polymerase chain reaction: a practical approach. J. Clin. Lab. Anal. 2002;16:47–51. doi: 10.1002/jcla.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipp M, Shillito R, Giroux R, Spiegelhalter F, Charlton S, Pinero D, Song P. Polymerase chain reaction technology as analytical tool in agricultural biotechnology. J. AOAC Int. 2005;88:136–155. [PubMed] [Google Scholar]
- 15.Morisset D, Stebih D, Cankar K, Zel J, Gruden K. Alternative DNA amplification methods to PCR and their application in GMO detection: a review. Eur. Food Res. Technol. 2008 doi:10.1007/s00217-008-0850-x. [Google Scholar]
- 16.Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans EC, Tebas P, Ruiter JP, Wanders RJ, de Ronde A, de Baar MP. Real-time nucleic acid sequence-based amplification assay to quantify changes in mitochondrial DNA concentrations in cell cultures and blood cells from HIV-infected patients receiving antiviral therapy. Clin. Chem. 2006;52:979–987. doi: 10.1373/clinchem.2005.062901. [DOI] [PubMed] [Google Scholar]
- 18.Markham NR, Zuker M. DINAMelt web server for nucleic acid melting prediction. Nucl. Acids Res. 2005;33:W577–W581. doi: 10.1093/nar/gki591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodmann PD, Ilg EC, Berthoud H, Herrmann A. Real-time quantitative polymerase chain reaction methods for four genetically modified maize varieties and maize DNA content in food. J. AOAC Int. 2002;85:646–653. [PubMed] [Google Scholar]
- 20.Holck A, Vaitilingom M, Didierjean L, Rudi K. 5′-Nuclease PCR for quantitative event-specific detection of the genetically modified Mon810 MaisGard maize. Eur. Food Res. Technol. 2002;214:449–454. [Google Scholar]
- 21.Kuribara H, Shindo Y, Matsuoka T, Takubo K, Futo S, Aoki N, Hirao T, Akiyama H, Goda Y, Toyoda M, et al. Novel reference molecules for quantitation of genetically modified maize and soybean. J. AOAC Int. 2002;85:1077–1089. [PubMed] [Google Scholar]
- 22.Pauli U, Schouwey B, Hubner P, Brodmann P, Eugster A. Quantitative detection of genetically modified soybean and maize: method evaluation in a Swiss ring trial. Mitt. Geb. Lebensmittelunters Hyg. 2001;92:145–158. [Google Scholar]
- 23.Deiman B, van Aarle P, Sillekens P. Characteristics and applications of nucleic acid sequence-based amplification (NASBA) Mol. Biotechnol. 2002;20:163–179. doi: 10.1385/MB:20:2:163. [DOI] [PubMed] [Google Scholar]
- 24.Hamels S, Gala JL, Dufour S, Vannuffel P, Zammatteo N, Remacle J. Consensus PCR and microarray for diagnosis of the genus Staphylococcus, species, and methicillin resistance. Biotechniques. 2001;31:1364–1372. doi: 10.2144/01316md04. [DOI] [PubMed] [Google Scholar]
- 25.Zammatteo N, Jeanmart L, Hamels S, Courtois S, Louette P, Hevesi L, Remacle J. Comparison between different strategies of covalent attachment of DNA to glass surfaces to build DNA microarrays. Anal. Biochem. 2000;280:143–150. doi: 10.1006/abio.2000.4515. [DOI] [PubMed] [Google Scholar]
- 26.Cankar K, Stebih D, Dreo T, Zel J, Gruden K. Critical points of DNA quantification by real-time PCR—effects of DNA extraction method and sample matrix on quantification of genetically modified organisms. BMC Biotechnol. 2006;6:37. doi: 10.1186/1472-6750-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett MD, Smith JB. Nuclear DNA amounts in angiosperms. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1991;334:309–345. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- 28.Csako G. Present and future of rapid and/or high-throughput methods for nucleic acid testing. Clin. Chim. Acta. 2006;363:6–31. doi: 10.1016/j.cccn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Bordoni R, Mezzelani A, Consolandi C, Frosini A, Rizzi E, Castiglioni B, Salati C, Marmiroli N, Marchelli R, Bernardi LR, et al. Detection and quantitation of genetically modified maize (Bt-176 transgenic maize) by applying ligation detection reaction and universal array technology. J. Agric. Food Chem. 2004;52:1049–1054. doi: 10.1021/jf034871e. [DOI] [PubMed] [Google Scholar]
- 30.Gruden K. Gene expression analysis by DNA microarrays and other methods—advantages and disadvantages. In: Freitag J, editor. Plant Genomics and Bioinformatics Expression Micro Arrays and Beyond—A Course Book. National Institute of Biology: Ljubljana, Slovenia; 2006. pp. 36–45. [Google Scholar]
- 31.Peano C, Bordoni R, Gulli M, Mezzelani A, Samson MC, Bellis GD, Marmiroli N. Multiplex polymerase chain reaction and ligation detection reaction/universal array technology for the traceability of genetically modified organisms in foods. Anal. Biochem. 2005;346:90–100. doi: 10.1016/j.ab.2005.08.004. [DOI] [PubMed] [Google Scholar]