Abstract
In axons, organelles move away from (anterograde) and toward (retrograde) the cell body along microtubules. Previous studies have provided compelling evidence that conventional kinesin is a major motor for anterograde fast axonal transport. It is reasonable to expect that cytoplasmic dynein is a fast retrograde motor, but relatively few tests of dynein function have been reported with neurons of intact organisms. In extruded axoplasm, antibody disruption of kinesin or the dynactin complex (a dynein activator) inhibits both retrograde and anterograde transport. We have tested the functions of the cytoplasmic dynein heavy chain (cDhc64C) and the p150Glued (Glued) component of the dynactin complex with the use of genetic techniques in Drosophila. cDhc64C and Glued mutations disrupt fast organelle transport in both directions. The mutant phenotypes, larval posterior paralysis and axonal swellings filled with retrograde and anterograde cargoes, were similar to those caused by kinesin mutations. Why do specific disruptions of unidirectional motor systems cause bidirectional defects? Direct protein interactions of kinesin with dynein heavy chain and p150Glued were not detected. However, strong dominant genetic interactions between kinesin, dynein, and dynactin complex mutations in axonal transport were observed. The genetic interactions between kinesin and either Glued or cDhc64C mutations were stronger than those between Glued and cDhc64C mutations themselves. The shared bidirectional disruption phenotypes and the dominant genetic interactions demonstrate that cytoplasmic dynein, the dynactin complex, and conventional kinesin are interdependent in fast axonal transport.
INTRODUCTION
Neurons depend on fast anterograde axonal transport to move newly synthesized organelles and other macromolecular complexes from the cell body to the axon terminal. Fast retrograde axonal transport is also vital, returning spent organelles and other materials in the endocytic/lysosomal pathway from the terminal to the cell body. Axonal microtubules, 90% of which are oriented with plus ends toward the terminal, provide directional tracks for motor proteins that are attached to the various fast transport cargoes (reviewed by Hirokawa, 1998; Martin et al., 1999). Most kinesin family proteins are anterograde motors, pulling their cargoes specifically toward microtubule plus ends. Other kinesins and cytoplasmic dyneins are retrograde motors, pulling their cargoes toward the minus ends (reviewed by Vale and Fletterick, 1997; Hirokawa, 1998). It is reasonable to suspect that retrograde motors are carried, in some manner, to the terminal by anterograde motors. In return, any anterograde motors that return from the terminal should be transported by retrograde motors. How organelles or other cargoes that carry opposing motors coordinate their activities to accomplish persistent long-range movement in one direction is not known.
Conventional kinesin is a major anterograde motor that has been found in many metazoan cell types. It is abundant in axoplasm, and function disruption studies suggest that it is required for both fast anterograde and fast retrograde axonal transport (Brady et al., 1990; Hurd and Saxton, 1996; Stenoien and Brady, 1997; Gindhart et al., 1998; reviewed by Martin et al., 1999). Mutations of conventional kinesin in Drosophila cause partial posterior paralysis and large axonal swellings filled with organelles normally carried by anterograde and retrograde fast transport (Saxton et al., 1991; Hurd and Saxton, 1996; Gindhart et al., 1998). The composition and distribution of the swellings suggest that a loss of kinesin function increases the frequency of cargo stalling within axons. The stalled kinesin cargoes could cause the equivalent of traffic jams, and hence swellings, by steric hindrance of fast organelle transport (Hurd and Saxton, 1996).
Cytoplasmic dynein is a major fast retrograde motor that is present in all eukaryotic cell types. Drugs or antibodies that inhibit dynein function block fast transport in axoplasmic preparations or dissected axons (e.g., Goldberg, 1982; Ekstrom and Kanje, 1984; Forman et al., 1984; Schnapp and Reese, 1989; Wang et al., 1995), but genetic tests in vivo had not been reported until recently (Bowman et al., 1999). The dynactin complex is a large assembly of proteins that appears to be required for normal cytoplasmic dynein function in a number of motility processes (reviewed by Holleran et al., 1998). Antibodies to p150Glued, a major component of the dynactin complex, inhibit organelle movements in extruded axoplasm (Waterman-Storer et al., 1997). In most of these dynein and dynactin inhibition studies, axoplasmic organelle movements in both directions were inhibited. Although the inhibition of bidirectional transport by functional disruption of all three proteins—kinesin, dynein, and dynactin—can be discounted as nonspecific effects, it could be due to functional linkages between the anterograde and retrograde transport systems.
In Drosophila, the Glued (Gl) gene encodes the homolog of the p150 subunit of the vertebrate dynactin complex (Swaroop et al., 1987; Gill et al., 1991; Holzbaur et al., 1991). Previous work has demonstrated that the dominant Gl1 mutation encodes a truncated polypeptide that acts as a poison product (Swaroop et al., 1985; McGrail et al., 1995). In heterozygotes, the Gl1 mutation produces a rough eye phenotype with a severe disruption in the organization of the retina and in the retinal axonal projections to the optic lobe (Meyerowitz and Kankel, 1978). Certain mutations in cytoplasmic dynein heavy chain (cDhc64C) also produce rough eyes and interact with Gl1 to suppress or enhance the rough eye phenotype (McGrail et al., 1995). Similarly, mutations in Kinesin heavy chain (Khc) produce rough eye phenotypes (Brendza and Saxton, unpublished observations) and exhibit dominant interactions with Gl1 (Hays, unpublished observations). Whether these results reflect the coordinated activity of motors in axonal transport or separate cellular functions is not clear (Fan and Ready, 1997).
To test for interactions between anterograde and retrograde transport systems, and to address questions about dynein and dynactin functions in fast axonal transport in vivo, we conducted genetic and biochemical tests in Drosophila. We analyzed the neuronal phenotypes of mutations in Gl and cDhc64C, which encodes a ubiquitously expressed cytoplasmic dynein heavy chain (Li et al., 1994; Gepner et al., 1996). Our results indicate that dynein and the dynactin complex are critical for fast axonal transport. We also found dominant genetic interactions between the Khc, Kinesin light chain (Klc), cDhc64C, and Gl genes. The genetic interactions show that conventional kinesin, cytoplasmic dynein, and the dynactin complex are interdependent and may physically interact in fast axonal transport.
MATERIALS AND METHODS
Fly Stocks, Culture Conditions, Larval Locomotion Analysis, and Genetic Strategies
All fly stocks were maintained at 22–25°C, and crosses were conducted at 25°C with a 12-h light/dark cycle on a previously described yeast-agar–based fly medium (Hurd and Saxton, 1996). The mutant chromosomes used in this research are listed in Table 1. Recessive lethal chromosomes were maintained over one of three balancer chromosomes carrying the third instar marker, Tubby (Tb) [TM6B, Tb Hu e ca; TM6C, Tb Sb e cu ca; or T(2,3)B3, CyO:TM6B, Tb Hu] (Lindsley and Zimm, 1992; Flybase, 1999). The Tb mutation allowed recognition of test and control genotypes in larval stages, as described by Saxton et al. (1991).
Table 1.
Drosophila chromosomes used in this research
| Stock | Description | Source |
|---|---|---|
| mwh cDhc64C4–19 jv ca | Amorphic allele, recombinant chromosome | Gepner et al., 1996 |
| cDhc64C6–10 P{FRT w+} | Hypomorphic allele, recombinant chromosome | Gepner et al., 1996 |
| cDhc64C6–6–16h st pp ss es | Hypomorphic allele, recombinant chromosome | J. Gepner and T. Hays |
| Df(3L)10H st e | Deletes cDhc64C (64B10–12;64C5–9) | Garbe et al., 1993; Gepner et al., 1996 |
| Df(3L)GN24 | Deletes cDhc64C (63F04–07;64C13–15) | Bloomington Stock Center (Bloomington, IN) |
| w, P{Dhc+}X | Genomic cDhc64C+ transgene | Gepner et al., 1996 |
| cDhc64Cek1 (synonym = E(Khc)ek1−) | Antimorphic allele, original chromosome | This study |
| ru cDhc64Cek1 sr e & cDhc64Cek1 h th st cu sr e Pr ca | Recombinant chromosomes | This study |
| Gl1 & Gl1 Sb1 | Antimorphic allele, original and recombinant chromosomes | Bloomington Stock Center |
| Df(3L)fz-GF3b | Deletes Glued (70B;70C6) | Bloomington Stock Center |
| Glt.hs | Transgenic, inducible antimorphic allele | Fan and Ready, 1997 |
| pr Khc16 | Null allele | C. Regan, D. Rose, K. Brendza, and W. Saxton |
| b Khc27 lf & c Khc27 Elp | Null allele, parental and recombinant chromosomes | D. Rose, K. Brendza, and W. Saxton |
| P{Khc+, w+} PK15a, PK4c & PK9a | Genomic Khc+ trangene, 3 independent lines | Saxton et al., 1991 |
| Df(3L)8ex94 | Deletes Klc | Gindhart et al., 1998 |
| Df(3L)34ex5 | Deletes Klc and other nearby genes | Gindhart et al., 1998 |
| Df(3L)8ex34 | Deletes Klc and other nearby genes | J. Gindhart |
| P{GEN-KLC+}PC4 | Genomic Klc+ transgene | Gindhart et al., 1998 |
| Df(3R)ry615 | Deletes Arp87C and other genes | Bloomington Stock Center |
| Df(3L)ZN47 | Delete Klp64D and other genes | Bloomington Stock Center |
| Df(3L)vin7 and Df(3L)vin5 | Delete Klp68D and other genes | Bloomington Stock Center |
Scoring of the Posterior-Paralytic Phenotype
Adult flies were mated in vials and transferred every 24 h to prevent overcrowding of progeny. Late third instar larvae were collected, rinsed with water to remove food and debris, and placed on a clean plate of agar-based fly medium. The crawling behavior of each larva was observed for several minutes. Animals were scored as posterior paralytic if at least two posterior segments curved up away from the substrate during the crawling cycle. In most cases, three or four segments were involved in the tail flip, which equates to approximately one-fourth to one-third of body length. Typically, 25–50 larvae of a test class genotype were scored for the posterior-paralytic phenotype from each cross. Each cross was done at least twice.
Induction of Mutant p150Glued
A heat-shock regimen was used to stimulate the production of a truncated, dominant negative p150Glued protein in transgenic flies (Fan and Ready, 1997). Flies homozygous for the transgene (Phs-Glt) were allowed to mate and deposit embryos in vials for 24 h. Vials with progeny were then subjected to 1-h, 36°C heat shocks once a day for 4 d.
Isolation of cDhc64Cek1
A new mutation, E(Khc)ek1, was isolated in an F1 screen for dominant enhancers of a Khc null mutation. Male flies with isogenized second and third chromosomes (w−; +; + or w−; +; e1) were mutagenized with 25 mM ethyl methane sulfonate and mated en masse to virgin pr Khc16/T(2,3)B3, CyO:TM6B, Tb females. Matings were transferred daily to new food until d 4, when the adults were discarded (Saxton et al., 1991). After 5.5 d of development, larvae were harvested by floatation on 3 M NaCl in a separatory funnel (Roberts, 1986). After a distilled water wash, groups of ∼50 larvae were placed on the center of 150-mm hard agar plates that had been dyed dark blue with either food coloring or bromphenol blue. The contrast provided between the white larvae and the dark blue plates allowed easier observation of crawling third instar larvae with the naked eye, magnifying glasses, or stereomicroscopes. Test class larvae (heterozygous for both Khc16 and mutagenized chromosomes) that exhibited the posterior-paralytic phenotype and developed into fertile adults were used to establish permanent lines. Of the 60,000 test class larval genomes screened, 10 E(Khc) loci were identified and designated ek1–ek10. The first dominant enhancer recovered from the screen, E(Khc)ek1, was mapped by meiotic recombination to the left arm of chromosome 3 at 10 ± 4 centimorgans with the use of the flanking mutations roughoid (ru) and hairy (h). Complementation tests with various deletions, cDhc64C mutant alleles, and a cDhc64C wild-type transgene were performed, as described by Gepner et al. (1996), and confirmed that E(Khc)ek1 is a cDhc64C allele. When either of the hypomorphic mutations cDhc64C6–10 or cDhc64C6–6-16 were combined with the small deletion Df(3L)10H or the amorphic allele cDhc64C4–19, lethality occurred during late larval and pupal stages. When cDhc64Cek1 was combined with either of the hypomorphic alleles, the lethal phase was shifted earlier and a greater proportion of deaths occurred during the third larval instar. Thus, in these assays, cDhc64Cek1 is more severe than a null mutation.
Video Imaging and Frame Capturing
Larvae were videotaped with a Dage (Michigan City, IN) 68 Newvicon camera (Hurd and Saxton, 1996). The camera was fitted with three Nikon (Garden City, NY) Vivitar automatic extension tubes (68 mm total lens extension) and a Nikon microNikkor 55-mm lens. Single video frames were digitized for processing in NIH Image 1.62b7 (National Institutes of Health, Bethesda, MD) and Adobe (Mountain View, CA) Photoshop 5.0.
Sample Preparation and Microscopy
Larval dissection, fixation, immunohistochemistry, and confocal microscopy were as described previously (Hurd and Saxton, 1996) with the following exception. Before reactions with the β1 tubulin mAb, formaldehyde-fixed larvae were postfixed in −20°C methanol for 15 min and then washed in PBS with 0.01% Tween 20. The primary antibodies and dilutions used were rabbit polyclonal anti-Drosophila synaptotagmin (1:500) (Littleton et al., 1993), mouse monoclonal anti-Drosophila cysteine string protein (1:250) (Zinsmaier et al., 1990), rabbit polyclonal anti-Drosophila HOOK (1:100) (Kramer and Phistry, 1996), mouse monoclonal anti-β1 tubulin (1:100) (Dettman et al., 1996), rabbit polyclonal anti-Drosophila kinesin heavy chain (1:100) (Saxton et al., 1988), and mouse monoclonal anti-Drosophila dynein heavy chain (1:200) (McGrail and Hays, 1997). The secondary antibodies used were goat polyclonal anti-rabbit conjugated to FITC or goat polyclonal anti-mouse conjugated to TRITC, both at 1:500 (Jackson Immunoresearch Laboratories, West Grove, PA). Sample preparation and thin sectioning for transmission electron microscopy were as described by Hurd and Saxton (1996) except that an accelerating voltage of 80 kV was used for imaging.
Immunoprecipitation Experiments
To collect cytosol for immunoprecipitation tests, we used two tissues, fly heads as a source of neural tissue and ovaries because the female germline is rich in microtubule motors and the dynactin complex. Heads or ovaries from either wild-type flies or flies that expressed DHC tagged with three hemagglutinin epitopes (DHC-3HA, which functions as wild type based on mutant rescue experiments [Silvanovich and Hays, unpublished data]) were homogenized in an equal volume of lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.5% Triton X-100, 5 mM EDTA, 3.3 U/ml apyrase) supplemented with protease inhibitors (2 mM PMSF, 10 μg/ml leupeptin, 1 mg/ml pepstatin, 10 mM benzamidine) and centrifuged at 42,000 rpm for 40 min in a Ti50 rotor. The pellet was discarded, and the high-speed supernatant was combined with protein A–agarose beads (Sigma Chemical, St. Louis, MO) to remove proteins that would bind nonspecifically. After brief centrifugation, the preadsorbed high-speed supernatant was transferred to a fresh tube. Typically, 50 μl of this supernatant was used for the immunoprecipitation experiments. Mouse monoclonal anti-Drosophila DHC antibodies (McGrail and Hays, 1997), mouse monoclonal anti-HA (HA.11) antibodies (Babco, Richmond, CA), or p150Glued antibodies (McGrail et al., 1995) were bound to protein A–agarose beads in the presence of 1× Tris-buffered saline (Sambrook et al., 1989) and collected by pelleting. A total of 50 μl of preadsorbed high-speed supernatant was combined with the antibody-bead complexes and 50 μl of NET-gel (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.05% Triton X-100, 0.25% gelatin, 0.02% sodium azide) supplemented with protease inhibitors (2 mM PMSF, 10 μg/ml leupeptin, 1 mg/ml pepstatin, 10 mM benzamidine). The binding reaction was performed at either 4 or 25°C for 4–6 h. The beads were collected by a brief centrifugation and washed four times with buffer 1 (125 mM Tris-Cl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.05% sodium deoxycholate, 0.01% SDS, 0.02% sodium azide), two times with buffer 2 (125 mM Tris-Cl, pH 8.0, 1.0 M NaCl, 5 mM EDTA, 0.02% sodium azide), and once with sterile water. The immunoprecipitates and protein controls were analyzed by standard Laemmli denaturing gel electrophoresis (Gallagher and Smith, 1991) and western blotting with a chemiluminescent detection system (Tropix, Bedford, MA) according to the manufacturer’s instructions.
RESULTS
cDhc64C and p150Glued Are Essential for Axonal Transport in Drosophila
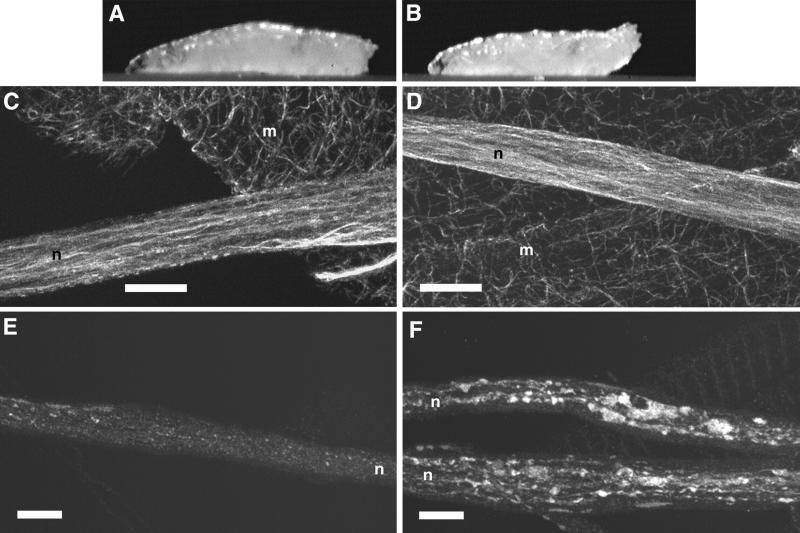
To determine if cytoplasmic dynein functions as a fast axonal transport motor in Drosophila, we studied larvae that carried mutations in cDhc64C (Gepner et al., 1996). Because null or amorphic cDhc64C mutants die too early to observe axonal transport phenotypes, we used hypomorphic alleles that allow development to progress through the larval stages (Gepner et al., 1996). The alleles cDhc64C6–10 and cDhc64C6–6-16, when combined with a deletion of cDhc64C [Df(3L)10H], caused posterior-paralysis phenotypes similar to those caused by conventional kinesin mutations (Figure 1). The penetrance, or percentage of animals that showed the paralytic phenotype, was 100%. Control larvae with the same genotype but carrying a wild-type cDhc64C transgene showed no posterior paralysis, indicating that the effect was specific to the loss of cytoplasmic dynein function.
Figure 1.
Mutations in cDhc64C cause posterior paralysis and defects in fast axonal transport. (A and B) Single video frames of third instar larvae (∼3 mm in length) crawling forward (to the left). (A) The control, heterozygous for a severe cDhc64C mutation (cDhc64Cek1/+), exhibited wild-type posture. (B) The mutant, carrying a moderate mutation over a deletion [cDhc64C6–10/Df(3L)10H], exhibited posterior paralysis whereby the distal-most segments curved upward. (C–F) Laser scanning confocal micrographs of control, P{cDhc64C+}/+; cDhc64C6–10/Df(3L)10H (C and E), and mutant, cDhc64C6–10/Df(3L)10H (D and F), larval segmental nerves stained for tubulin (C and D) and synaptotagmin (E and F). Segmental nerves contain motor and sensory axons that connect the ventral ganglia to the body wall muscles and sensory organs in the abdominal segments. These fields of view each cover about half an abdominal segment. (C and D) Microtubules run longitudinally in the nerves and appeared unaffected by the cDhc64C mutations. A predominantly longitudinal and striated staining pattern was observed in both control and mutant nerves. The meshwork of single microtubules in the background was in muscle cells underlying the nerves. (E) Synaptotagmin staining was distributed in a punctate pattern along the nerve, presumably representing the distribution of anterograde fast transport vesicles. (F) Synaptotagmin in the mutant nerve was much more abundant and concentrated in large accumulations. n, segmental nerve; m, body-wall muscle. Bars, 10 μm.
To ascertain if the behavioral phenotypes reflected cellular axonal transport defects, mutant and control larval neuromuscular preparations were examined by immunofluorescence microscopy. The distribution of microtubules in segmental nerves was not altered by the dynein mutations (Figure 1). However, the distribution of the endosome protein HOOK (Kramer and Phistry, 1996, 1999), which we presume to be a fast retrograde cargo, and of cDHC itself was abnormal. Large accumulations, similar in appearance to the axonal swellings caused by conventional kinesin mutations, were scattered along the lengths of segmental nerves. The fast anterograde vesicle proteins synaptotagmin and cysteine string protein, and the fast anterograde motor KHC, were also present in the accumulations (Figure 1). These results suggest that cytoplasmic dynein mutations, like conventional kinesin mutations, cause organelle jams, i.e., randomly distributed axon swellings filled with organelles carried by both fast anterograde and retrograde axonal transport.
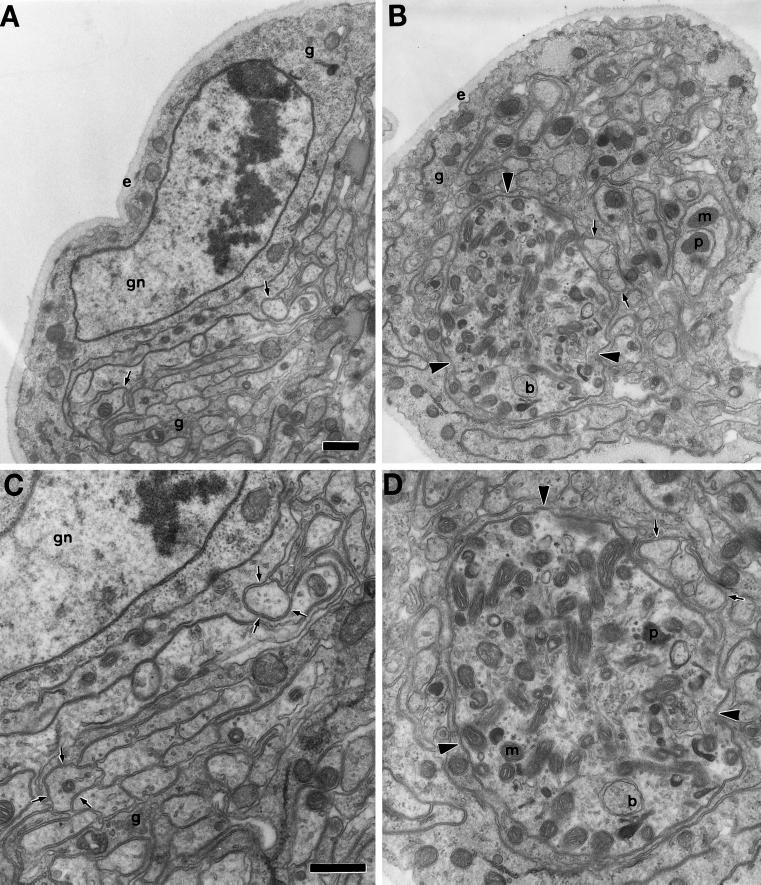
To determine if the abnormal protein accumulations in segmental nerves were indeed organelle jams and, if so, how they compare with those caused by Khc mutations, we examined cDhc64C mutant nerves by transmission electron microscopy. Nerve cross-sections revealed axon swellings filled with retrograde transport organelles, including lysosomal and multivesicular bodies. They also contained mitochondria, many small vesicles, and smooth tubular membranes (Figure 2). The swellings and their contents were not distinguishable from those caused by Khc mutations (Hurd and Saxton, 1996). Thus, cytoplasmic dynein, like conventional kinesin, is required for both retrograde and anterograde fast axonal transport.
Figure 2.
Mutations in cDhc64C cause axonal swellings filled with fast retrograde and anterograde organelles. Transmission electron micrographs of heterozygous control, Df(3L)10H/+ (A and C), and mutant, cDhc64C6–10/Df(3L)10H (B and D), larval segmental nerve cross-sections. (A) One-fourth of a large nerve containing a central cluster of axons (arrows) supported by glial cells (g) and a layer of extracellular matrix (e) is shown. A peripheral glial cell nucleus is also in view (gn). (C) A view of the same section at higher magnification; the predominant axonal organelles were mitochondria and small vesicles. The two axons from A are indicated by triplets of arrows. (B) The overall organization of the mutant nerve and its axons was normal (same magnification as in A). However, some axons were swollen (arrowheads). (D) This higher-magnification view of the largest swollen axon from B (same magnification as in C) contained many prelysosomal bodies (p), multivesicular bodies (b), mitochondria (m), and vesicles. Normal-sized axons are indicated by arrows. Bars, 500 nm.
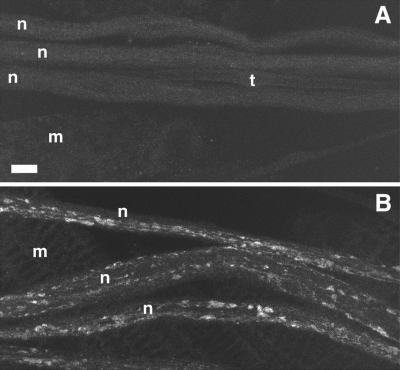
To address the possibility that the dynactin complex is also required for fast axonal transport, we disrupted the function of p150Glued with the use of mutations in Gl. Available Gl alleles, when homozygous, cause lethality too early for assessment of axonal transport phenotypes. To circumvent this effect, we used Phs-Glt, a dominant negative Gl transgene controlled by a heat-shock promoter that expresses a truncated p150Glued protein (Fan and Ready, 1997). C-terminal–truncated forms of p150Glued act in a dominant negative manner, probably by disrupting the association between the dynein motor and the dynactin complex (McGrail et al., 1995). Animals bearing Phs-Glt were exposed to a heat shock once per day during the embryonic and larval stages. They developed mild posterior paralysis by the late third instar (penetrance ∼ 50%) and axonal swellings that contained retrograde and anterograde proteins (penetrance = 100%) (Figure 3). Phs-Glt animals without heat-shock treatments and wild-type animals subjected to heat shocks did not exhibit defects (Figure 3). Thus, disruption of an anterograde motor (kinesin), a retrograde motor (cytoplasmic dynein), or a retrograde activator complex (dynactin) inhibited fast axonal transport in both directions. The bidirectional effects may all be due to steric hindrance effects, as proposed in the “organelle jam” hypothesis (Hurd and Saxton, 1996). Alternatively or additionally, the bidirectional disruptions may be due to important functional interactions between these three components of the fast axonal transport machinery.
Figure 3.
Dominant negative mutation of Glued causes defects in fast axonal transport. Laser scanning confocal micrographs of larval segmental nerves from animals homozygous for Phs-Glt stained for synaptotagmin are shown. (A) A control animal expressing wild-type p150Glued exhibited normal synaptotagmin staining. (B) A test animal expressing a dominant negative form of p150Glued exhibited accumulations of synaptotagmin that denote axonal swellings that contain fast anterograde cargoes. n, segmental nerve; t, tracheal tube; m, muscle. Bar, 10 μm.
Immunoprecipitation of Either Cytoplasmic Dynein or the Dynactin Complex Does Not Coprecipitate Conventional Kinesin
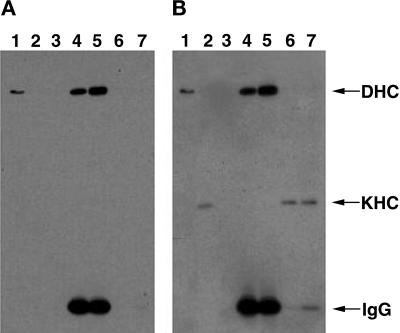
To test for physical interactions of kinesin with either dynein or the dynactin complex, Drosophila cytosol from heads or ovaries was fractionated with either cDHC or p150Glued antibodies conjugated to beads. The conditions used were conducive to coimmunoprecipitation of cytoplasmic dynein intermediate chain with p150Glued (data not shown), as has been described for vertebrate homologues (Vaughan and Vallee, 1995). Western blots of the soluble and immunoprecipitated fractions were probed with anti-KHC antibodies. No coprecipitation of kinesin was detected with either cDHC (Figure 4) or p150Glued (data not shown). Thus, if there are physical interactions between kinesin and dynein or p150Glued, they may be labile or restricted to a small fraction of motors, e.g., those bound to subsets of cargo organelles.
Figure 4.
Immunoprecipitation of cDHC does not coprecipitate KHC. Drosophila cytosol fractions were separated by gel electrophoresis and transferred to a membrane. The membrane was stained first for cDHC (A) and subsequently for KHC (B). Thus, the cDHC bands are present in both panels. The experiment shown here was conducted with ovary cytosol from DHC-3HA–expressing flies immunoprecipitated with anti-HA mAb–agarose bead complexes. However, comparable results were obtained with DHC-3HA head extracts and either ovary or head wild-type extracts with the use of DHC mAbs for the immunoprecipitation. (Lanes 1) Proteins that pelleted from cytosol with taxol-stabilized microtubules in the absence of ATP. cDHC but not KHC was efficiently bound to microtubules under these conditions. (Lanes 2) KHC but not DHC was readily detected in cytosol. (Lanes 3) The pellet from cytosol that was incubated with unconjugated agarose beads. (Lanes 4 and 5) The pellets from cytosol that was incubated with beads conjugated with antibodies to cDHC via the 3HA tag; incubation was at either 4°C (lanes 4) or 25°C (lanes 5). (Lanes 6 and 7) The supernatants of the pellets shown in lanes 4 and 5, respectively. KHC remained in the immunoprecipitation supernatants, and cDHC was detected in the pellets. IgG, immunoglobulin G.
Kinesin, p150Glued, and Cytoplasmic Dynein Mutations Exhibit Genetic Interactions
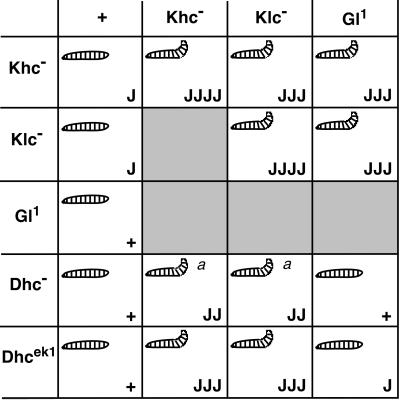
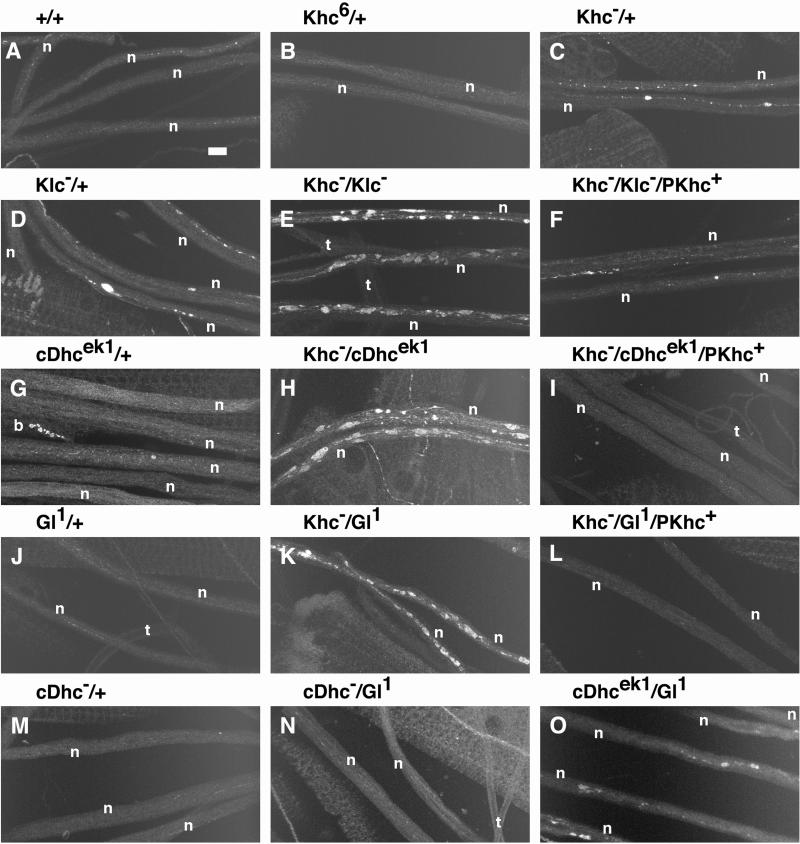
To test for functional interactions between kinesin and dynein/dynactin in axonal transport, we looked for noncomplementation between mutations in Khc, Klc, cDhc64C, and Gl. This sort of dominant genetic enhancer test can reveal functional interactions that are not detected by biochemical fractionation (e.g., Huffaker et al., 1987; Kaiser and Schekman, 1990). As one positive control, we tested for noncomplementation between null mutations in Khc and Klc, whose proteins are known to physically interact. Larvae that were heterozygous for either single mutation showed no posterior paralysis and only a few axonal swellings per nerve. In contrast, larvae that were doubly heterozygous (Khcnull/+; Klcnull/+) developed the posterior-paralytic phenotype (∼50–70% penetrance) and had more axonal swellings (Figures 5–7). This dominant interaction of Khc and Klc was gene specific in that multiple null alleles from different genetic backgrounds interacted and single transgenic copies of either Khc+ or Klc+ suppressed the interaction (Figure 6). In addition, we found that deletions that remove the Klp64D and Klp68D genes fully complemented Khc. Klp64D and Klp68D are expressed in the larval nervous system and encode homologues of the mammalian and sea urchin motor subunits that form the heterotrimeric kinesin II motor (Stewart et al., 1991; Pesavento et al., 1994). Thus, at least one neuronal motor does not show genetic interactions with kinesin.
Figure 5.
Mutations in Khc interact with mutations in Klc, cDhc64C, and Gl to produce the posterior-paralysis phenotype. Single video frames of wandering third instar larvae crawling forward (toward the left) are shown. All of the animals were heterozygous for a Khc null allele (Khc16/+). The animals in B, C, and D were also heterozygous for severe mutations in Klc [Df(3L)34ex5/+], cDhc64C (cDhc64Cek1/+), and Gl (Gl1/+), respectively. The larva in A showed normal locomotion, whereas the double heterozygous larvae all showed a synthetic posterior-paralysis phenotype, as indicated by the upturned tails.
Figure 7.
Summary of the dominant genetic interactions observed between Khc, Klc, cDhc64C, and Gl. The gene symbols at the top and left represent heterozygous mutant contributions. The internal rectangles contain information on the double heterozygous combinations indicated by the respective row and column. Khc−, Klc−, and Dhc− represent multiple severe alleles and deletions tested for each of these genes. In each box, the upper left-hand symbol indicates the presence or absence of the posterior-paralytic phenotype in third instar larvae. The lower right-hand symbol indicates the abundance of axon swellings typically found with each genotype (with the use of anti-synaptotagmin): +, just a few axon swellings per animal (overlaps wild type, i.e., most nerves have no swellings); J, multiple nerves have a few axon swellings; JJ, all nerves have axon swellings but some parts of nerves are free from swellings; JJJ, all nerves have axon swellings, and there are a few small areas of wild-type staining; JJJJ, large swellings predominate the entire width and length of nerves. These designations are qualitative and represent the typical phenotype from each genotype, although variation generated individuals with more or fewer swellings. a, the tail-flipping phenotype was expressed at low penetrance (10–20% compared with ≥50% for the other genotypes).
Figure 6.
Mutations in Khc interact with mutations in Klc, cDhc64C, and Gl to produce axonal swellings. Laser scanning confocal micrographs of segmental nerves from third instar larvae stained for synaptotagmin are shown. The genotypes are as follows: (A) wild type; (B) Khc6/+ (hypomorphic allele); (C) Khc16/+ (null allele); (D) Df(3L)34ex5/+ (Klc deletion); (E) Khc16/+; Df(3L)34ex5/+; (F) Khc16/+; Df(3L)34ex5, +/+, P{Khc+}; (G) cDhc64Cek1/+; (H) Khc16/+; cDhc64Cek1/+; (I) Khc16/+; cDhc64Cek1, +/+, P{Khc+}; (J) Gl1/+; (K) Khc16/+; Gl1/+; (L) Khc16/+; Gl1, +/+, P{Khc+}; (M) Df(3L)10H/+ (cDhc64C deletion); (N) Df(3L)10H, +/+, Gl1; and (O) cDhc64Cek1, +/+, Gl1. (A, B, G, J, and M) Axonal swellings were rarely seen in wild type, heterozygous hypomorphic Khc mutations, or animals heterozygous for severe cDhc64C or Gl alleles. (C and D) Heterozygotes for severe Khc and Klc mutations did have axon swellings in some regions but showed no swellings in other regions. (E, H, and K) Combination of Klc, cDhc64C, or Gl mutations with Khc in double heterozygotes caused dramatic increases in the number of axon swellings relative to the respective single heterozygous animals (compare C and D with E; compare C and G with H; compare C and J with K). In contrast, combination of the most severe alleles of cDhc64C and Gl led to only a slight increase in axon swellings (compare J and M with N and then compare G and M with O). (F, I, and L) The axon swellings caused by double heterozygous combinations with Khc null mutations were rescued by a single transgenic copy of wild-type Khc. Bar, 10 μm. b, boutons of an underlying neuromuscular junction; n, segmental nerve; t, tracheal tube. All micrographs are at the same magnification.
A functional interaction between the kinesin and dynein pathways in axonal transport was first revealed through genetic interactions with Gl. Gl1/+ larvae exhibited no paralytic phenotypes and very few axonal swellings (Figure 6). However, larvae that were Khcnull/+; Gl1/+ exhibited dramatic posterior paralysis (penetrance ∼ 50–70%) (Figure 5) and had abundant axonal swellings (Figure 6). A deletion of the Gl locus [Df(3L)fz-GF3b] also failed to complement Khcnull/+. Not surprisingly, the interaction phenotypes were milder and less penetrant (∼30%) than those caused by the Gl1 allele, which is antimorphic. We also documented similar specific genetic interactions between mutations in Gl and Klc (Figure 7). Additionally, a deletion that removes a potential homologue of the dynactin complex ARP1 gene (Fyrberg et al., 1994) failed to complement severe Khc mutations. Thus, the functions of conventional kinesin and the dynactin complex are interdependent in fast axonal transport.
A second line of evidence for the interdependence of dynein and kinesin was obtained from a genetic screen for new dominant enhancers of Khc. The first mutation isolated was an allele of cDhc64C that we have named cDhc64Cek1. Larvae that were heterozygous for cDhc64Cek1 showed no posterior paralysis or axonal swellings. However, the posterior-paralysis and axonal-swelling phenotypes of Khcnull/+; cDhc64Cek1/+ larvae were as severe as those generated by the Khc-Klc and Khc-Gl1 interactions described above. The Khc-cDhc64Cek1 interaction was specific; a cDhc64C+ transgene rescued the posterior-paralysis and axonal-swelling phenotypes. Klcnull and cDhc64Cek1 also failed to complement, and the phenotypes generated were very similar to those caused by the double heterozygous Khc-cDhc64Cek1 combination.
To determine if the cDHC-kinesin interactions were specific to the cDhc64Cek1 allele, we tested for genetic interactions between Khc or Klc and other cDhc64C mutations. Both a small deletion of the cDhc64C region, Df(3L)10H, and an amorphic (null-like) allele, cDhc64C4–19, failed to complement Khcnull and Klcnull alleles (Figure 7). Thus, the dominant genetic interactions between kinesin and cDHC are a general property of severe cDhc64C mutations. The interaction phenotypes caused by Df(3L)10H or cDhc64C4–19 were milder than those caused by cDhc64Cek1 (Figures 6 and 7). This was probably due to the fact that cDhc64Cek1 conveyed some genetic properties that were more severe than a null mutation (see MATERIALS AND METHODS). These results confirm that conventional kinesin and cytoplasmic dynein are functionally interdependent in axonal transport.
To complete the investigation of dynein function in axonal transport, we asked if mutations in cDhc64C and Gl failed to complement one another. Strong dominant genetic interactions between mutations in these genes have been shown previously in Drosophila eye development (McGrail et al., 1995), in which dynein and the dynactin complex are thought to participate in mitosis and nuclear positioning (McGrail et al., 1995; Gepner et al., 1996; Fan and Ready, 1997). In our tests of axonal organelle transport, some Gl and cDhc64C mutations did fail to complement, causing increases in axonal swellings. However, the interactions were mild relative to those generated by the kinesin–cytoplasmic dynein or the kinesin-dynactin interactions (Figures 6 and 7). Even when the most severe alleles were combined (Gl1 +/+ cDhc64Cek1), no posterior paralysis was observed. It is striking that the cytoplasmic dynein and dynactin genes, whose products associate and are each individually critical for axonal transport, interact more strongly with conventional kinesin genes than with one another.
DISCUSSION
To determine if dynein and the dynactin complex have important functions in fast axonal transport and to investigate possible interactions between anterograde and retrograde transport systems in vivo, we conducted genetic and biochemical tests in Drosophila. The neuronal phenotypes of mutations in cDhc64C and Gl indicate that dynein and the dynactin complex do indeed have important functions in axonal transport. The organelle-filled axonal-swelling and posterior-paralysis phenotypes observed are similar to those caused by conventional kinesin mutations. Both retrograde and anterograde fast transport cargoes accumulate in the swellings, despite the fact that dynein is a retrograde motor. The same bidirectional axonal transport phenotypes are generated by pair-wise heterozygous combinations of mutations in Khc, Klc, cDhc64C, and Gl. These dominant genetic interactions and the shared single-mutant phenotypes indicate that the kinesin and dynein motor systems are interdependent during fast axonal transport.
It is curious that disruption of either the kinesin or the dynein motor system inhibits both anterograde and retrograde fast axonal transport. We consider three nonexclusive models to explain the bidirectional effects. The first model is based on evidence that known microtubule motors are unidirectional and that >90% of microtubules in axons have uniform polarity, i.e., plus ends toward the terminal (Burton and Paige, 1981; Heidemann et al., 1981; Baas et al., 1988; Topp et al., 1994). Therefore, most minus end–directed motors such as dynein, which are synthesized in the cell body, are probably carried into the axon as fast anterograde cargoes before they are used to drive retrograde transport. If kinesin transports dynein down the axon, then kinesin mutations should reduce the concentration of dynein in the axon. Thus, retrograde as well as anterograde transport would suffer. The weakness in this model is that it does not explain why cDhc64C and Gl mutations disrupt anterograde transport. Because kinesin is presumably degraded at the terminal (Hirokawa et al., 1991), kinesin levels should not be affected directly by changes in retrograde transport. In a second model proposed by Hurd and Saxton (1996), the stalling of an organelle as a result of faulty kinesin activity hinders the passage of other anterograde and retrograde motor-cargo complexes, encouraging them to detach from their microtubule tracks at that same point. Detached cargoes then accumulate and generate axonal swellings. The bidirectional transport inhibition caused by cytoplasmic dynein or dynactin complex mutations could also be attributed to this sort of steric hindrance.
In a third model, the activities of conventional kinesin, cytoplasmic dynein, and the dynactin complex are linked through physical contacts. Thus, in neurons without dynein, kinesin does not function normally and vice versa. This model is interesting because such a linkage could ensure proper coordination of opposing motor activities and provide an even distribution of motors along the axon. To date, there is no biochemical evidence for a direct dynein-kinesin interaction. However, this does not eliminate the possibility of such interactions. For example, although it is generally accepted that the dynactin complex and cytoplasmic dynein have important physical contacts in vivo, strong evidence of such contacts remained elusive for a number of years. Now, a demonstration of binding of p150Glued to a dynein intermediate chain, combined with shared phenotypes and genetic interactions between dynactin complex and dynein mutations, provide a compelling argument for substoichiometric but important direct interactions (Muhua et al., 1994; Plamann et al., 1994; Karki and Holzbaur, 1995; McGrail et al., 1995; Vaughan and Vallee, 1995; Bruno et al., 1996; Tinsley et al., 1996). There have been several observations that are consistent with an association between dynein, the dynactin complex, and kinesin in fast axonal transport. Kinesin and dynein localize to fast anterograde organelles (Hirokawa et al., 1990, 1991). In extruded axoplasm and intact axons, individual organelles can move in both directions on microtubules (e.g., Allen et al., 1985; Schnapp et al., 1985; Overly et al., 1996). Some purified plus end–directed vesicles bind both plus end– and minus end–directed motors (Muresan et al., 1996). Inhibition of kinesin in extruded squid axoplasm with specific mAbs impairs both anterograde and retrograde vesicle motility (Brady et al., 1990; Stenoien and Brady, 1997). Likewise, p150Glued antibodies that disrupt the interaction of dynein and the dynactin complex inhibit vesicle motility in both directions (Waterman-Storer et al., 1997). Organelle motility on peripheral single microtubules and interior groups of microtubules was affected similarly in those antibody disruption studies. Thus, it seems unlikely that simple steric hindrance between anterograde and retrograde organelle movement is the explanation for bidirectional inhibition (Brady et al., 1990; Stenoien and Brady, 1997; Waterman-Storer et al., 1997). Our finding that single and double heterozygous cDhc64C and Gl mutations exhibit less severe phenotypes than single or double heterozygous combinations that include kinesin mutations might be explained by a greater flux of anterograde cargoes versus retrograde cargoes. However, in light of the previous studies discussed above, our current genetic observations support the idea of a physical linkage between anterograde and retrograde fast transport motors. This linkage may be indirect, such as both motors binding the same cargo but at distinct sites. Or the linkage could be direct in the form of physical or regulatory contacts between kinesin, dynein, and the dynactin complex at the same or closely placed cargo-binding sites.
To determine the validity and relative influences of the three models for anterograde-retrograde linkage discussed above, more experimentation will be needed. Direct measurements of organelle motility in wild-type and mutant Drosophila provide an excellent approach to such questions and the more general issues of motor regulation and coordination. Analysis of single-lipid-droplet motility in wild-type and mutant embryos suggests that both dynein and a plus end–directed kinesin are active and that the dynactin complex is important for coordinating their activities (Welte et al., 1998; Gross, Welte, Block, and Wieschaus, personal communication). We are developing tests of single-organelle motility in wild-type and mutant Drosophila axons that should prove very informative. Continued biochemical and genetic investigations of motors and their associations should identify new regulatory and cargo-linkage proteins. The integration of results obtained from these differing approaches will lead to a definitive understanding of the relationship between kinesin, dynein, and the dynactin complex in fast axonal transport.
ACKNOWLEDGMENTS
The research described here was initiated as a result of the discovery of dominant genetic interactions between Khc and Gl in eye development, and we thank Hays laboratory members John Robinson, Susan Ludmann, and Min-Gang Li for this key contribution. Theresa Werner, Aaron Pilling, Laura Scheibel, Mike Spence, Vicki Lawless, Phil Spence, Chris Ficklin, and Lori Cooper all contributed to the E(Khc) screening effort. Daryl Hurd and Bob Brendza provided expert microscopy tutelage. We thank Steve Gross, Michael Welte, Krishanu Ray, Aaron Bowman, and Larry Goldstein for insightful discussions. We thank members of the Saxton, Hays, and Raff laboratories for advice, help, and discussions. Improvements to the manuscript were contributed by two anonymous reviewers. Postdoctoral fellowships were awarded by the Walther Cancer Institute (M.A.M.), the American Heart Association Indiana Affiliate (M.A.M.), and the National Institutes of Health (M.A.M. and J.G.G.), with additional funding for J.G.G. from the Howard Hughes Medical Institute via L.S.B. Goldstein. Other support was provided by the National Institutes of Health (grants GM46295 to W.M.S. and GM44757 to T.S.H.). W.M.S. and T.S.H. are Established Investigators of the American Heart Association (with funds contributed in part by the American Heart Association Indiana and Minnesota Affiliates, respectively).
REFERENCES
- Allen RD, Weiss DG, Hayden JH, Brown DT, Fujiwake H, Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985;100:1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P, Deitch J, Black M, Banker G. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffrey JM, Goldstein LS, King SM. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J Cell Biol. 1999;146:165–180. [PMC free article] [PubMed] [Google Scholar]
- Brady S, Pfister K, Bloom G. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno KS, Tinsley JH, Minke PF, Plamann M. Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa. Proc Natl Acad Sci USA. 1996;93:4775–4780. doi: 10.1073/pnas.93.10.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P, Paige J. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci USA. 1981;78:3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman RW, Turner FR, Raff EC. Genetic analysis of the Drosophila beta3-tubulin gene demonstrates that the microtubule cytoskeleton in the cells of the visceral mesoderm is required for morphogenesis of the midgut endoderm. Dev Biol. 1996;177:117–135. doi: 10.1006/dbio.1996.0150. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Kanje M. Inhibition of fast axonal transport by erythro-9-[3-(2-hydroxynonyl)]adenine. J Neurochem. 1984;43:1342–1345. doi: 10.1111/j.1471-4159.1984.tb05392.x. [DOI] [PubMed] [Google Scholar]
- Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- Flybase. A genome database. 1999. http://flybase.bio.indiana.edu/ http://flybase.bio.indiana.edu/. . [Google Scholar]
- Forman DS, Brown KJ, Promersberger MW, Adelman MR. Nucleotide specificity for reactivation of organelle movements in permeabilized axons. Cell Motil. 1984;4:121–128. doi: 10.1002/cm.970040205. [DOI] [PubMed] [Google Scholar]
- Fyrberg C, Ryan L, Kenton M, Fyrberg E. Genes encoding actin-related proteins of Drosophila melanogaster. J Mol Biol. 1994;241:498–503. doi: 10.1006/jmbi.1994.1526. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Smith J. Electrophoretic separation of proteins. In: Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current Protocols in Molecular Biology. Boston, MA: Current Protocols; 1991. pp. 10.2.1–10.2.18. [Google Scholar]
- Garbe J, Yang E, Fristrom J. IMP-L2: an essential secreted immunoglobulin family member implicated in neural and ectodermal development in Drosophila. Development. 1993;119:1237–1250. doi: 10.1242/dev.119.4.1237. [DOI] [PubMed] [Google Scholar]
- Gepner J, Li M, Ludmann S, Kortas C, Boylan K, Iyadurai SJ, McGrail M, Hays TS. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JJ, Desai C, Beushausen S, Zinn K, Goldstein L. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DJ. Microinjection into an identified axon to study the mechanism of fast axonal transport. Proc Natl Acad Sci USA. 1982;79:4818–4822. doi: 10.1073/pnas.79.15.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S, Landers J, Hamborg M. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister K, Bloom G, Brady S. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran EA, Karki S, Holzbaur EL. The role of the dynactin complex in intracellular motility. Int Rev Cytol. 1998;182:69–109. doi: 10.1016/s0074-7696(08)62168-3. [DOI] [PubMed] [Google Scholar]
- Holzbaur EL, Hammarback JA, Paschal BM, Kravit NG, Pfister KK, Vallee RB. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Huffaker TC, Hoyt MA, Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Hurd D, Saxton W. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Kramer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133:1205–1215. doi: 10.1083/jcb.133.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H, Phistry M. Genetic analysis of hook, a gene required for endocytic trafficking in Drosophila. Genetics. 1999;151:675–684. doi: 10.1093/genetics/151.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, McGrail M, Serr M, Hays TS. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J Cell Biol. 1994;126:1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D, Zimm G. The Genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Martin, M.A., Hurd, D.D., and Saxton, W.M. (1999). Kinesins in the nervous system. Cell. Mol. Life Sci. (in press). [DOI] [PMC free article] [PubMed]
- McGrail M, Gepner J, Silvanovich A, Ludmann S, Serr M, Hays TS. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM, Kankel DR. A genetic analysis of visual system development in Drosophila melanogaster. Dev Biol. 1978;62:112–142. doi: 10.1016/0012-1606(78)90096-9. [DOI] [PubMed] [Google Scholar]
- Muhua L, Karpova TS, Cooper JA. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994;78:669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Muresan V, Godek C, Reese T, Schnapp B. Plus-end motors override minus-end motors during transport of squid axon vesicles on microtubules. J Cell Biol. 1996;135:383–397. doi: 10.1083/jcb.135.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overly C, Rieff H, Hollenbeck P. Organelle motility and metabolism in axons versus dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109:971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- Pesavento P, Stewart R, Goldstein L. Characterization of the KLP68D kinesin-like protein in Drosophila: possible roles in axonal transport. J Cell Biol. 1994;127:1041–1048. doi: 10.1083/jcb.127.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M, Minke PF, Tinsley JH, Bruno KS. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994;127:139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. Basic Drosophila care and techniques. In: Roberts D, editor. Drosophila: A Practical Approach. Oxford, UK: IRL Press; 1986. pp. 1–38. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Saxton W, Hicks J, Goldstein L, Raff E. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64:1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- Saxton W, Porter ME, Cohn SA, Scholey JM, Raff EC, McIntosh JR. Drosophila kinesin: characterization of microtubule motility and ATPase. Proc Natl Acad Sci USA. 1988;85:1109–1113. doi: 10.1073/pnas.85.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B, Reese T. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp BJ, Vale RD, Sheetz MP, Reese TS. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985;40:455–462. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- Stenoien D, Brady S. Immunochemical analysis of kinesin light chain function. Mol Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Pesavento P, Woerpel D, Goldstein L. Identification and partial characterization of six members of the kinesin superfamily in Drosophila. Proc Natl Acad Sci USA. 1991;88:8470–8474. doi: 10.1073/pnas.88.19.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Paco-Larson ML, Garen A. Molecular genetics of a transposon-induced dominant mutation in the Drosophila locus Glued. Proc Natl Acad Sci USA. 1985;82:1751–1755. doi: 10.1073/pnas.82.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Swaroop M, Garen A. Sequence analysis of the complete cDNA and encoded polypeptide for the Glued gene of Drosophila melanogaster. Proc Natl Acad Sci USA. 1987;84:6501–6505. doi: 10.1073/pnas.84.18.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JH, Minke PF, Bruno KS, Plamann M. p150Glued, the largest subunit of the dynactin complex, is nonessential in Neurospora but required for nuclear distribution. Mol Biol Cell. 1996;7:731–742. doi: 10.1091/mbc.7.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp KS, Meade LB, LaVail JH. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R, Fletterick R. The design plan of kinesin motors. Annu Rev Cell Biol. 1997;13:745–777. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Asai D, Robinson K. Retrograde but not anterograde bead movement in intact axons requires dynein. J Neurobiol. 1995;27:216–226. doi: 10.1002/neu.480270208. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C, Karki S, Kuznetsov S, Tabb J, Weiss D, Langford G, Holzbaur E. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc Natl Acad Sci USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–557. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- Zinsmaier KE, Hofbauer A, Heimbeck G, Pflugfelder GO, Buchner S, Buchner E. A cysteine-string protein is expressed in retina and brain of. Drosophila. J Neurogenet. 1990;7:15–29. doi: 10.3109/01677069009084150. [DOI] [PubMed] [Google Scholar]