Abstract
Somatic tinnitus is clinically observed modulation of the pitch and loudness of tinnitus by somatic stimulation. This phenomenon and the association of tinnitus with somatic neural disorders indicate that neural connections between the somatosensory and auditory systems may play a role in tinnitus. Anatomical and physiological evidence supports these observations. The trigeminal and dorsal root ganglia relay afferent somatosensory information from the periphery to secondary sensory neurons in the brainstem, specifically, the spinal trigeminal nucleus and dorsal column nuclei, respectively. Each of these structures has been shown to send excitatory projections to the cochlear nucleus. Mossy fibers from the spinal trigeminal and dorsal column nuclei terminate in the granule cell domain while en passant boutons from the ganglia terminate in the granule cell domain and core region of the cochlear nucleus. Sources of these somatosensory–auditory projections are associated with proprioceptive and cutaneous, but not nociceptive, sensation. Single unit and evoked potential recordings in the dorsal cochlear nucleus indicate that these pathways are physiologically active. Stimulation of the dorsal column and the cervical dorsal root ganglia elicits short- and long-latency inhibition separated by a transient excitatory peak in DCN single units. Similarly, activation of the trigeminal ganglion elicits excitation in some DCN units and inhibition in others. Bimodal integration in the DCN is demonstrated by comparing responses to somatosensory and auditory stimulation alone with responses to paired somatosensory and auditory stimulation. The modulation of firing rate and synchrony in DCN neurons by somatatosensory input is physiological correlate of somatic tinnitus.
Keywords: somatic tinnitus, somatosensory, trigeminal, nonauditory pathways, cochlear nucleus
Introduction: somatic tinnitus
There has long been evidence that connections exist between higher-order neurons subserving different senses (e.g., some neurons serve both the auditory and entorhinal cortices). But only in recent years has it become apparent that these connections exist even in first and second order neurons of the brainstem — and, further, that the connections are functional even in normal animals. How might these connections manifest clinically?
Approximately two-thirds of individuals with tinnitus can modulate the loudness or pitch of their tinnitus by voluntary or external manipulations of the jaw, movements of the eyes, or pressure applied to head and neck regions, including the temporomandibular joint (Rubinstein et al., 1990; Pinchoff et al., 1998). In some cases, the necessary manipulations reported were forceful (e.g., Abel and Levine, 2004), while in others less vigorous manipulations could produce the changes in perceived loudness and or pitch of the tinnitus (Pinchoff et al., 1998). In addition, there is an increased prevalence of somatoform disorders in individuals with tinnitus (Hiller et al., 1997), as well as reports of tinnitus occurring after dental pulpalgia that resolved after endodontic therapy (Wright and Gullickson, 1996). Tinnitus is also associated with upper craniocervical imbalances such as prolapsed intervertebral disks or instability of the craniocervical junction, which can be resolved following stabilization surgery (Montazem, 2000). Similarly, tinnitus occurs more frequently in patients who have craniocervical mandibular disorders such as temporomandibular joint syndrome (Chole and Parker, 1992; Morgan, 1992; Gelb et al., 1997).
These factors have something in common: they all involve stimulation of the somatosensory system, usually associated with the jaw, temporomandibular joint, extra-ocular muscles, and the neck. While most reported changes in tinnitus involve somatosensory regions of the head and neck, changes in tinnitus can even occur with stimulation of the median nerve or upper limbs (Møller et al., 1992; Cacace et al., 1999), all leading to the term “somatic tinnitus.”
These observations imply that there are neural connections between somatosensory centers and auditory centers, at least in individuals with somatic tinnitus. Do these connections also exist in the normal system? How would they function to modulate an existing tinnitus? Could they play a role in generating tinnitus?
This chapter will describe the functional anatomy of these sensory interactions and will demonstrate physiological properties that could account for somatic tinnitus, i.e., the modulation of an existing tinnitus. Some attention will be given to the idea that the generation of tinnitus may be influenced by a functional reorganization of auditory–somatosensory interactions after diminished afferent inputs to either auditory or somatosensory centers, e.g., following noise-induced hearing loss or tooth abscess.
Anatomical substrates of somatic tinnitus
Overview of the somatosensory system
Perception of touch, temperature and pain are all associated with the somatosensory system as is proprioception. They involve neural processes that result in a conscious awareness of a physical stimulus that begin at the periphery of the somatosensory system. Here, specialized sensory receptors transform mechanical stimuli into electrical discharges in the nerve fibers innervating the sensory organ to convey somatic sensory information from various parts of body. Somatic sensation from the trunk, limbs, and neck are mediated by afferent fibers the cell bodies of which are located in the dorsal root ganglia (DRG). Sensation from the head, neck, and face is conveyed by the trigeminal nerve, the cell bodies of which are located in the trigeminal ganglion (TG). The encoded somatic information is then conveyed into the brain via distinct pathways.
Central processes of the DRG ascend in the brain primarily via two pathways: the spinothalamic pathway of the anterolateral system and the dorsal column-medial lemniscal system. Each pathway conveys different sensory modalities. The anterolateral system mediates pain, temperature, and some deep touch, in which nociceptive neurons are mainly located in the superficial layer (lamina I) and the substantia gelatinosa (lamina II) of the dorsal horn. The dorsal column-medial lemniscal system primarily mediates proprioception and discriminative sensation, and consists of two nuclei: nucleus cuneatus, which serves the upper trunk and limbs, and nucleus gracilis, which serves the lower trunk and limbs.
Central processes of the TG terminate in the brainstem trigeminal sensory complex, which comprises three groups of nuclei that mediate different sensory modalities: The principle nucleus receives information about discriminative sensation and light touch; the mesencephalic nucleus receives proprioceptive information from the jaw; and the spinal trigeminal nucleus (Sp5) receives information about pain and temperature as well as gentle pressure and vibrissa deflection (Hayashi et al., 1984; Jacquin et al., 1989). Sp5 also receives proprioceptive inputs from vocal tract/intra oral structures such as the temporomandibular joint and tongue muscles (Romfh et al., 1979; Jacquin et al., 1989; Nazruddin et al., 1989; Takemura et al., 1991; Suemune et al., 1992). The Sp5 can be divided into three groups of nuclei: pars oralis (Sp5O), pars interpolaris (Sp5I), and pars caudalis (Sp5C). The Sp5C contains three layers: (1) the subnucleus magnocellularis; (2) the subnucleus gelatinosus; and (3) the outmost subnucleus marginalis (Darian-Smith et al., 1963; Usunoff et al., 1997). The subnucleus gelatinosus, which is analogous to the lamina II in the spinal cord, primarily receives nociceptive afferents.
Both primary and secondary somatic sensory neurons project to the auditory system. The following section will review the anatomy of these pathways with emphasis on the somatosensory projections to the cochlear nucleus (CN).
Projections from primary somatic sensory neurons to the CN
DRG axons and fibers of the trigeminal nerve project to the ventral CN (VCN) (Pfaller and Arvidsson, 1988; Zhan et al., 2006). The projection cells generally are small in size ranging from 15 to 20 μm in diameter in the DRG (Zhan et al., 2006), and 10 to 45 μm in the TG (Shore et al., 2000). These small projection cells may belong to the category of type B cells, which usually give rise to unmyelinated or lightly myelinated fibers (Zhan et al., 2006).
The CN projection cells in the TG are located in the medial portion (the ophthalmic division) and lateral portion of the ganglion (the mandibular division) (Shore et al., 2000). The terminal field of the TG-to-CN projection is primarily located in the shell regions of the CN but there are also terminals in some magnocellular regions of ventral CN (VCN). The TG-to-CN projection fibers are thin (approximately 1 μm) and typically form en passant boutons, mostly clustering around the medial and lateral edges of VCN. Postsynaptic targets of TG terminals in the VCN include dendrites of granule cells and large cells, and lumina of blood vessels (Shore et al., 2000).
The CN projection cells in the DRG are located at the C2 level. The terminal fields of these projection cells are concentrated along the medial edge of the VCN, dorsal ridge of the AVCN (i.e., subpeduncular corner between the AVCN and the inferior cerebellar peduncle), and lamina of the granule cell domain (GCD) (Pfaller and Arvidsson, 1988; Zhan et al., 2006). The GCD includes the shell region and the fusiform cell layer of the dorsal CN (DCN) that both contain numerous small cells (Weedman and Ryugo, 1996; Zhou and Shore, 2004). Terminal endings of the C2 DRG-to-CN projection fibers have varied size and shapes, and are not readily identified as boutons or mossy fibers (Zhan et al., 2006). Postsynaptic targets of the C2 DRG projection include the primary dendrites of unipolar brush cells, and the distal dendrites of granule cells.
Projections from secondary somatic sensory neurons to the CN
Secondary somatic sensory neurons that project to the CN are located in the Sp5 (Zhou and Shore, 2004; Haenggeli et al., 2005) and dorsal column nuclei (Itoh et al., 1987; Weinberg and Rustioni, 1987; Wright and Ryugo, 1996; Wolff and Kunzle, 1997; Zhou and Shore, 2004).
The Sp5I and the Sp5C contain the majority of projection cells from Sp5 to the CN. In Sp5I, projection cells are located in the dorsomedial and marginal areas (Fig. 1); in Sp5C, projection cells are located in either the subnucleus marginalis or the subnucleus magnocellularis. The subnucleus gelatinosus of Sp5, however, contains very few projection cells (Fig. 1D), suggesting that the nociceptive Sp5 neurons may not directly project to the CN (Zhou and Shore, 2004). The CN projection cells in the Sp5 have varied morphologies: either polygonal somata (ranging from 10 ×12 μm to 25 ×28 μm in diameter), or elongated somata (10 × 30–7 × 40 μm) (Fig. 1), (Wolff and Kunzle, 1997; Zhou and Shore, 2004; Haenggeli et al., 2005). Their projection fibers and terminal endings are either small to medium en passant boutons, or large, irregular terminal swellings that resemble mossy fibers. The small Sp5 terminal endings are scattered across the entire CN, making synaptic contacts with granule cells or large principal cells (Zhou and Shore, 2004). The Sp5 mossy fibers are primarily located in the GCD, making synaptic contacts with granule cells (Zhou and Shore, 2004; Haenggeli et al., 2005).
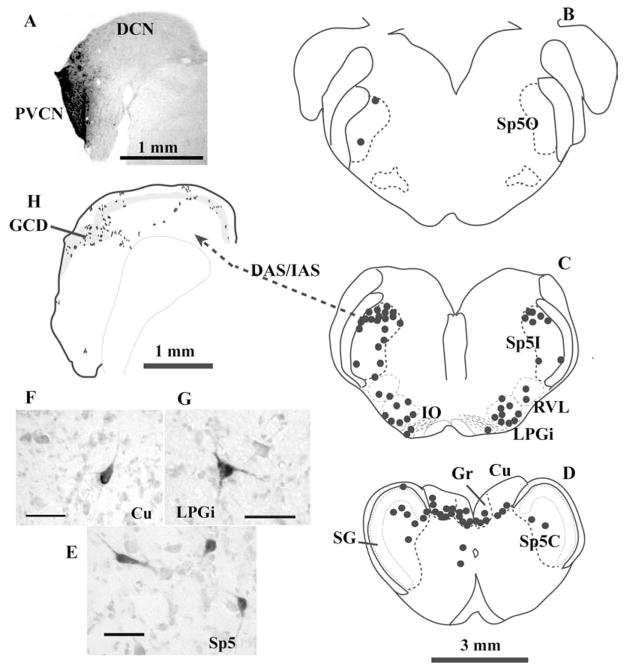
Fig. 1.
Projections from Sp5 to the CN in the guinea pig. (A–G) Retrograde labeling in the brainstem after an injection of biotinylated dextran amine (BDA) into the CN. (A) Photomicrograph of the injection site. The injection site is virtually restricted to the shell region of the PVCN. (B–D): Drawings of 1 mm transverse sections across the medulla. Each dot represents one labeled cell. The labeled neurons are located primarily on the ipsilateral side of Sp5, in the Sp5I and Sp5C. Very few labeled cells, if any, are located in the SG (D). Labeled neurons can also be found in the medular reticular formation (RVL and LPGi, C), inferior olive (IO, C), and dorsal column nuclei (Gr and Cu, D). Projection neurons in Sp5 have either polygonal or elongated somata (E). Projection neurons in dorsal column nuclei and reticular formation are multipolar (F and G). (H) Terminal labeling in the CN after placement of an anterograde tracer into Sp5I. Most Sp5 fibers enter the CN via DAS/IAS and terminate primarily in the GCD (gray area), but also in deep DCN. Each dot represents one to three labeled terminal endings. Scale bars = 25 μm (E–G). (Abbreviations: CN, cochlear nucleus; Cu, cuneate nucleus; DAS, dorsal acoustic striae; DCN, dorsal cochlear nucleus; GCD, granule cell domain; Gr, gracile nucleus; IAS, intermediate acoustic striae; IO, inferior olive; LPGi, lateral paragigantocellular reticular nucleus; PVCN, posteroventral cochlear nucleus; RVL, rostral ventrolateral reticular formation; SG, subnucleus gelatinosus; Sp5, spinal trigeminal nucleus; Sp5C, pars caudalis of Sp5; Sp5I, pars interpolaris of Sp5; Sp5O, pars oralis of Sp5).
The CN projection neurons in the dorsal column nuclei usually have polygonal somata, and aggregate in the ventral edge of the dorsal column nuclei (Fig. 1D). Like the Sp5 projections, the fibers of CN projection neurons from the dorsal column nuclei primarily terminate in the GCD, in the forms of mossy fibers and boutons (Itoh et al., 1987; Weinberg and Rustioni, 1987; Wright and Ryugo, 1996; Wolff and Kunzle, 1997). The postsynaptic targets of these mossy fibers include dendrites of granule cells (Wright and Ryugo, 1996). Secondary neurons in another ascending pathway of the DRG, the anterolateral system that mediates pain and temperature, have not been reported to project to the auditory structures. This again suggests a lack of direct nociceptive projections from the somatosensory system to the auditory system.
Mossy fibers are the predominent terminal endings of projection fibers from the secondary sensory nuclei (i.e., Sp5 and dorsal column nuclei) to the CN, whereas projections from the primary somatic ganglion cells (TG and DRG) terminate in the CN as small, en passant endings. It is currently unknown whether or not these morphological differences in termination may translate into distinct alterations of CN activity. The small terminals of TG projection fibers tend to make contacts with a variety of cells, including principal cells in VCN, and thus may directly affect CN output activity. Mossy fibers are located mostly in the GCD and make contacts with granule cells, whose axons, the parallel fibers, synapse on the apical dendrites of the fusiform cells — the principal output neurons of the DCN. Therefore, the secondary somatic sensory neurons indirectly affect DCN output via the mossy fiber-parallel fiber-fusiform cell pathway (as well as via inhibitory interneurons) (see physiology section “Effects of dorsal root ganglion and dorsal column activation on DCN activity”).
Projections from the somatosensory system to the inferior colliculus
In addition to their projections to the CN, somatic sensory neurons project to other central auditory structures, including inferior colliculus (IC). The IC projection cells are mainly found in the dorsal column nuclei and Sp5 (Zhou and Shore, 2006). Some neurons in the Sp5 and the dorsal column nuclei project to both the CN and the external cortex of IC by way of axon collaterals (Li and Mizuno, 1997). IC projection cells have not been reported in the TG. Projection fibers from these somatic sensory neurons form a laminar pattern of en passant terminal endings from ventromedial to dorsolateral within the ventrolateral regions of ICX, including the ventral border of IC, and the ventromedial edge of IC (or pericentral regions). These regions are collectively termed “the ventrolateral border region of ICX” (ICXV, (Zhou and Shore, 2006). The ICXV is the primary region that receives the convergent projections from CN and somatosensory systems, and thus is likely to be involved in multimodal integration in the IC (Shore, 2005; Jain and Shore, 2006).
Neurotransmitters used by somatosensory pathways to the CN
Studies using light and electron microscopy suggest that projections from both primary and secondary somatic sensory neurons to the VCN are excitatory (Wright and Ryugo, 1996; Shore et al., 2000; Zhou and Shore, 2004; Zhan et al., 2006). Mossy fibers in the CN that originate in the cuneate nucleus and Sp5 contain round synaptic vesicles and make asymmetric contacts with postsynaptic targets, features indicative of excitatory synapses (Wright and Ryugo, 1996; Haenggeli et al., 2005). The TG and C2 DRG terminals in the CN have similar ultrastructural characteristics (Shore et al., 2000; Zhan et al., 2006). The MFs from the cuneate nucleus are labeled with an antibody to glutamate, but not to choline acetyltransferase or GABA, thus also suggestive of a glutamatergic excitatory pathway (Wright and Ryugo, 1996).
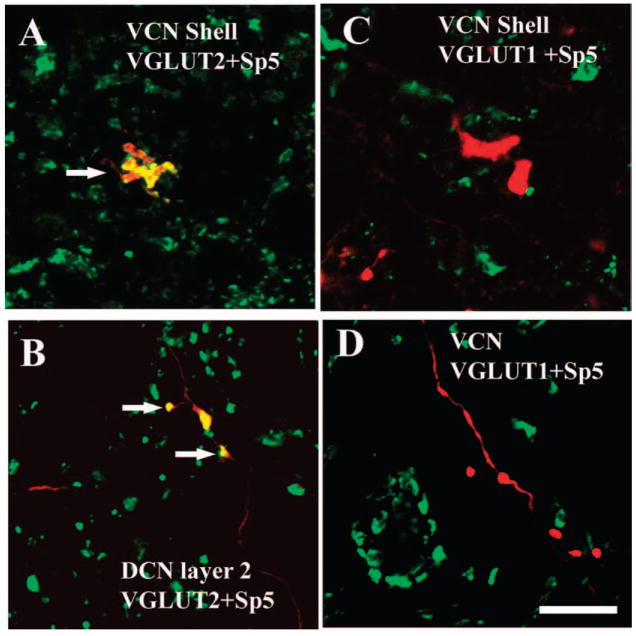
Using double-labeling immunoreactivity techniques, Zhou et al. (2007) demonstrated that the Sp5 projection to CN is glutamatergic, and specifically uses vesicular glutamate transporter 2 (VGLUT2) to mediate glutamate transport at both its MF and small bouton terminal endings. VGLUTs selectively package glutamate into synaptic vesicles and mediate glutamate transport, and are therefore excellent markers of glutamatergic neurons. The expression of VGLUT1 and VGLUT2 is distinct in the CN: the most intense VGLUT1 expression is in the magnocellular regions of the VCN and the molecular layer of the DCN, whereas the most intense expression of VGLUT2 is in the GCD (Zhou et al., 2007). Sp5 terminals colabel only with VGLUT2 in the GCD (Fig. 2) whereas, auditory nerve (AN) endings, including calyceal terminals, colabel only with VGLUT1 in the magnocellular regions of the VCN and deep layer of the DCN (Fig. 3).
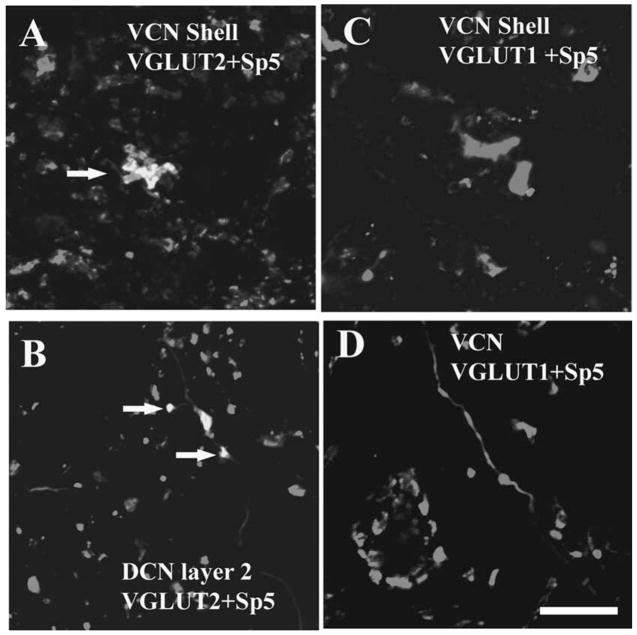
Fig. 2.
High magnification confocal images (63×) showing colocalization of anterogradely labeled Sp5 terminal endings with VGLUT2-ir in the CN. Green, VGLUT-ir. Red, Sp5 labeling. Yellow, double labeled terminals. Figures were obtained from Z projections of stacks of serial 1 μm confocal images. (A) MFs are labeled with BDA from Sp5 and VGLUT2 in the shell region of the VCN (arrow). (B) Small boutons are labeled with BDA from Sp5 and VGLUT2 in the DCN layer 2 (arrows). (C and D) Sp5 terminal endings do not colabel with VGLUT1 in the shell region of the VCN and the core of the VCN. Scale bar = 10 μm in D (applies to A–C). (See Color Plate 10.2 in color plate section.)
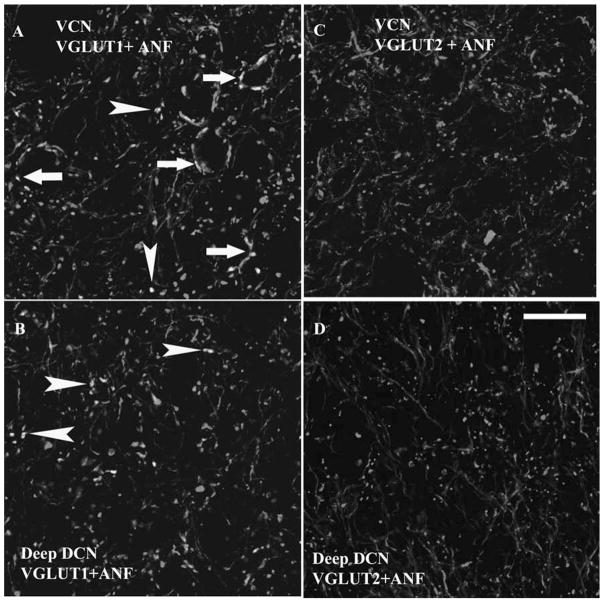
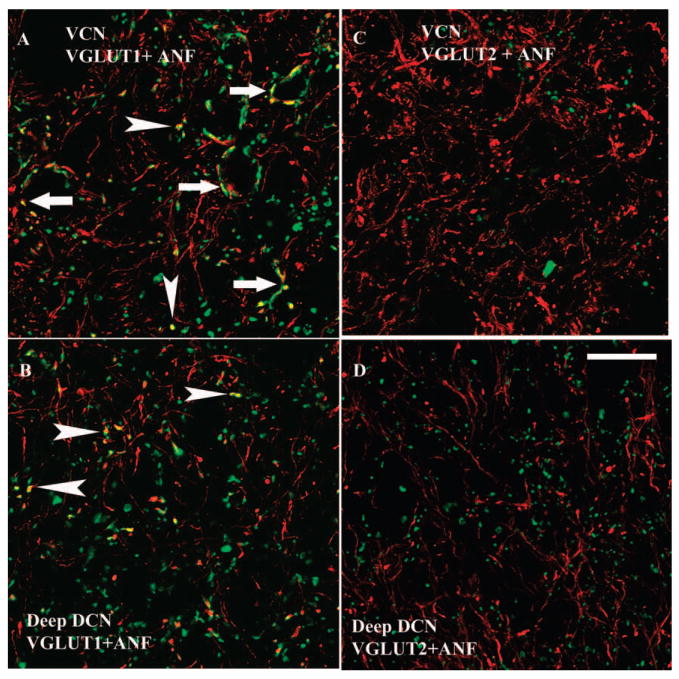
Fig. 3.
High magnification 1 μm confocal images (63×) showing colocalization of AN terminal endings with VGLUT1-ir, but not VGLUT2-ir, in both the VCN and deep layer of the DCN. Green, VGLUT-ir. Red, FG filled AN fibers and endings. Yellow, double-labeled terminals. (A and B) Colocalization of AN terminal endings with VGLUT1-ir in VCN (A) and DCN layer 3 (B). Both endbulb-like AN endings (arrows in A) and small boutons (arrowheads in A and B) colabeled with the VGLUT1. (C and D) VGLUT2 did not label the AN endings in both VCN (C) and DCN layer 3 (D). Scale bar = 20 μm in D (applies to A–C). (See Color Plate 10.3 in color plate section.)
The differential colocalization of VGLUT1 and VGLUT2 with AN and Sp5 terminals, respectively, suggests these are different forms of excitatory synaptic transmission that are dependent on the input source. Calyceal AN terminals are necessary for transmission of the acoustic signal with high temporal precision. Thus, VGLUT1 is likely to be involved in fast transport of glutamate in the AN terminals in the CN in order to convey precise temporal information. The transfer of somatic sensory information to the CN, on the other hand, may be slow in nature. MFs in the cerebellum are able to adjust synaptic strength via enhanced neurotransmitter release (Sola et al., 2004). If a similar role in synaptic plasticity is played by MFs in the CN GCD, VGLUT2 may be associated with DCN synaptic plasticity via the Sp5 MF-to-granule cell connections. Plasticity changes in the pathways from Sp5 to CN under certain pathological conditions may then contribute to the enhanced activity of fusiform cells, and thus play a role in the initiation of tinnitus.
Physiological substrates of somatic tinnitus
Physiological correlates of tinnitus in the auditory brainstem
Despite the likely contribution of peripheral factors, most comprehensive theories of tinnitus implicate central processes, especially in the CN. Increased spontaneous rate (SR) and sound-driven activity in DCN principal cells has been observed following noise-induced cochlear damage, and following ossicular disruption (Sumner et al., 2005), and has been proposed as a correlate of behavioral tinnitus in animal models (Kaltenbach, 2000; Kaltenbach et al., 2000, 2002; Brozoski et al., 2002; Chang et al., 2002; Rachel et al., 2002; Zacharek et al., 2002). One mechanism for the increased SR could be a reduction in wideband inhibitory inputs from D-stellate cells in the VCN (Nelken and Young, 1994; Winter and Palmer, 1995) and narrowband inhibitory inputs from vertical cells to the fusiform cells (Nelken and Young, 1994; Zhang and Oertel, 1994; Davis and Voigt, 1997; Rhode, 1999; Salvi et al., 2000), essentially unmasking the excitability of the fusiform cells. Blocking glycine receptors increases sound-driven firing rate in fusiform cells, as indicated by the conversion of nonmonotonic to monotonically rising rate-intensity functions for BF tones (Caspary et al., 1987; Davis and Young, 2000). This could account for the increase in spontaneous and sound-driven responses observed following noise trauma, giving rise to a tonal perception near to the affected frequency (Brozoski et al., 2002).
Another mechanism that could give rise to the percept of a tonal tinnitus is an increased regularity in the firing patterns of affected neurons. In support of this hypothesis, an increase in correlated SRs of simultaneously recorded cells in auditory cortex was reported following quinine administration, as well as a change in the best modulation frequency in response to AM signals (Ochi and Eggermont, 1997). While temporal changes following noise trauma have not been explored in the DCN, one study reported an increased bursting activity of DCN cells following acoustic trauma (Chang et al., 2002).
Thus, in order for somatosensory neurons to alter the intensity and the character of tinnitus, they would have to either alter the spontaneous (i.e., not driven by auditory stimuli) rates, or the synchrony of firing among neurons in the CN, IC, or auditory cortex. Here we will concentrate on the effects of activating somatosensory neurons on neural activity in the CN and IC.
Effects of trigeminal nerve activation on CN activity
Effects on spontaneous rates of CN neurons
Current pulses delivered to the ophthalmic divisions of the TG, at the origins of projecting neurons to the CN (Shore et al., 2000), can excite neurons in the VCN (Shore et al., 2003) and excite or inhibit neurons in the DCN (Shore, 2005; Shore and Zhou, 2006). Figure 4 shows the responses recorded from four channels of a 16-channel recording electrode in the DCN. The onset of the electrical stimulus in the TG is evident as stimulus artifact at 25 ms. Strong inhibitory responses are achieved on three of the four channels. The latencies of these responses are around 12 ms for these recordings but can range from 5 ms to 20 ms, reflecting the multisynaptic pathways that are necessarily stimulated at the origin of the central projections from the trigeminal nerve (Shore, 2005). Figure 5 shows an example of excitatory responses recorded from four different channels on the same mulitchannel electrode in the same animal (see Fig. 6 for electrode configuration). Whether the units are activated or inhibited depends on the location and type of unit rather than on the location of the stimulating electrode. Thus, activity in trigeminal pathways to the CN can alter the spontaneous (i.e., non-sound-driven) activity of neurons in both the VCN and the DCN. Change in the firing rate of CN neurons by incoming trigeminal neurons could be one means by which the loudness of tinnitus is modulated in individuals with somatic tinnitus changing the synchrony between neurons could be a mechanism for altering the pitch of the tinnitus.
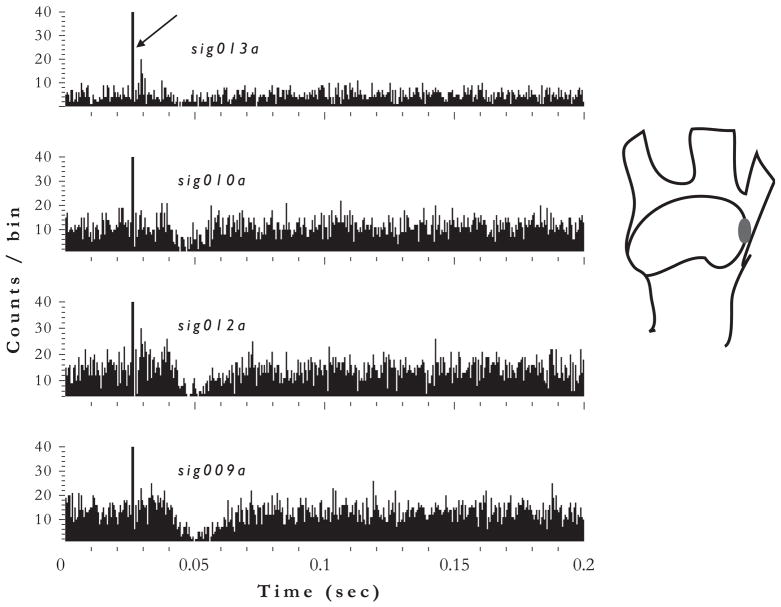
Fig. 4.
Poststimulus time histograms of DCN unit responses to trigeminal ganglion stimulation show inhibition. Responses of four unit clusters from 4 channels (9, 10, 12, 13) of 16-channel electrode are shown. Stimulation was at 80 μA, 100 ms/phase bipolar pulses, 100 presentations. Bin width, 0.5 ms. Arrow — electrical stimulus artifact indicates onset of TG stimulation. Inset at right shows location of the stimulating electrode in the ophthalmic region of the TG.
Fig. 5.
Poststimulus time histograms of DCN unit responses to trigeminal ganglion stimulation show excitation. Responses of four different unit clusters from 4 channels (4, 10, 11, 12) of a 16-channel electrode in the same animal but different penetration as for Fig. 4, are shown. Stimulation was at 80 μA, 100 ms/phase bipolar pulses, 100 presentations. Bin width, 0.5 ms. Arrow — electrical stimulus artifact indicates onset of TG stimulation. Inset at right shows location of the stimulating electrode in the ophthalmic region of the TG.
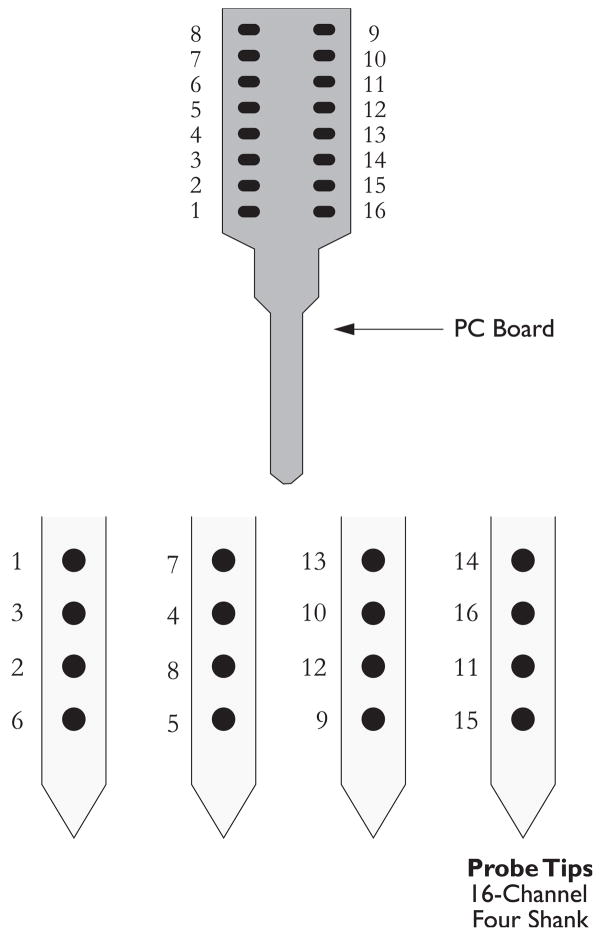
Fig. 6.
Configuration of the mulitchannel electrode used to record responses shown in Figs. 3 and 4. The four shank, 16-channel electrodes are positioned on the dorsal aspect of the DCN and lowered into the fusiform cell layer (layer 2) of the DCN. Often responses from multiunit clusters are recorded from each channel, which are then sorted using the Plexon offline sorter, into single units.
Effects of trigeminal stimulation on sound-driven rates of CN neurons
Since tinnitus is manifest as an increase in SR, it may be valid to examine the effects of somatosensory inputs on firing rates driven by low levels of sound, simulating a ‘greater than normal’ firing activity (as in tinnitus).
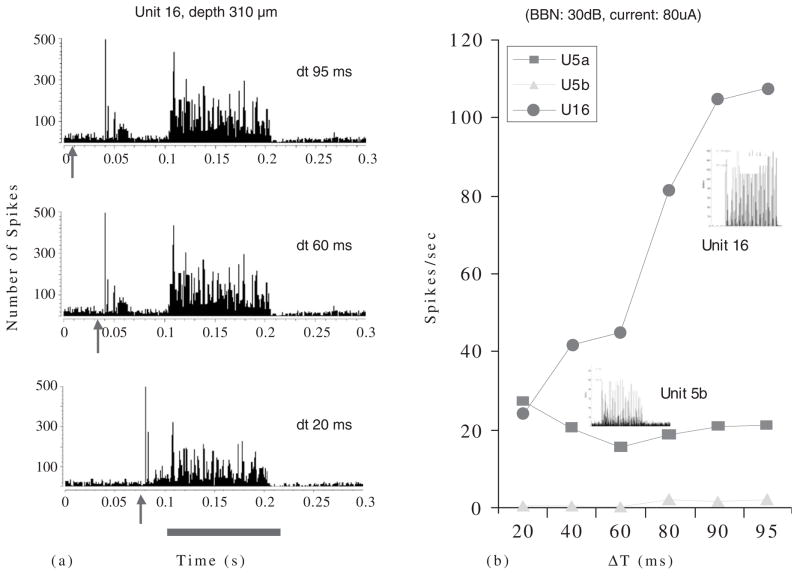
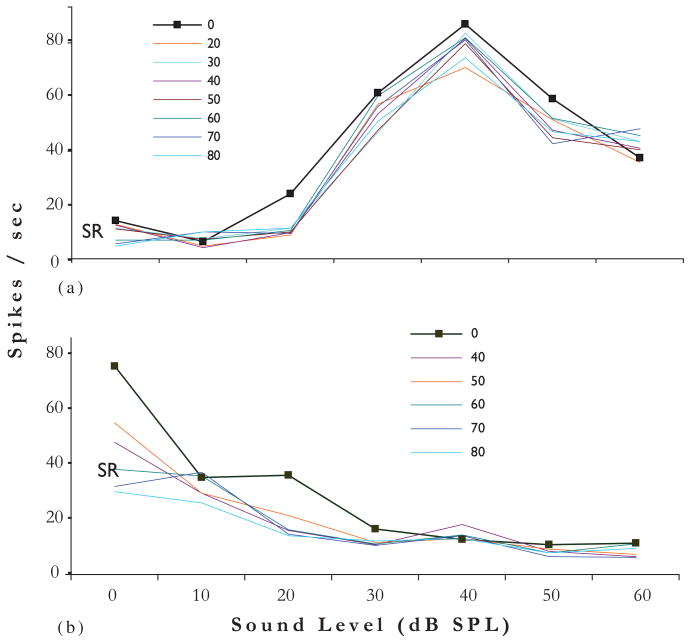
Stimulating the TG has more complicated effects on sound-evoked responses of CN neurons. In some cases, preceding an acoustic stimulus with a current pulse to the TG causes the sound-evoked activity of DCN neurons to decrease (Fig. 7A). The closer in time the two stimuli are, the more pronounced the suppression. Note that the response to the TG stimulation itself (depicted by the arrow) in these examples is excitatory, as is the response to the sound. The combination of the two signals results in a suppression of the response to the sound. This is an example of multisensory integration in which the response to stimulation of both senses results in a nonlinear combination of the responses from each system. Figure 7B shows how the response rate of unit 16 to the sound is most depressed when the trigeminal stimulus is closest to the sound (delta t, dt = 20 ms), and is less depressed as the stimuli are separated in time. Note that the response of unit 16 to a BF tone-burst is “pauser-buildup,” the typical response pattern of pyramidal cells. This figure also shows the responses from two other units (5a and 5b) that are less affected by the trigeminal stimulus. Responses of unit 5b to sound are slightly suppressed at mid dt values. The response type for unit 5b is typical of that shown for cartwheel cells (Parham and Kim, 1995).
Fig. 7.
(A) Poststimulus time histograms of a DCN single unit’s response to TG stimulation combined with acoustic stimulation. The TG pulse precedes the acoustic stimulus (broadband noise at 30 dB SPL) by varying amounts designated by dt. Acoustic stimulus duration is indicated by red bar below histograms. Arrow indicates onset of TG pulse (80 μA, 100 ms/phase bipolar pulses, 100 presentations). Responses to three different dt values are shown. Preceding the acoustic stimulus by TG stimulation results in a diminished response to the acoustic stimulus. The effect is stronger when the two stimuli are closer. Bin width, 0.5 ms. Arrow —electrical stimulus artifact indicates onset of TG stimulation. (B) Quantification of the spike rates achieved in (a) for unit 16. Spike rates for two other units, 5a and 5b, are also shown. Insets: Poststimulus time histograms for responses to BF tonebursts indicate unit types; Unit 16 is a P-Buildup; Unit 5b may be a cartwheel cell.
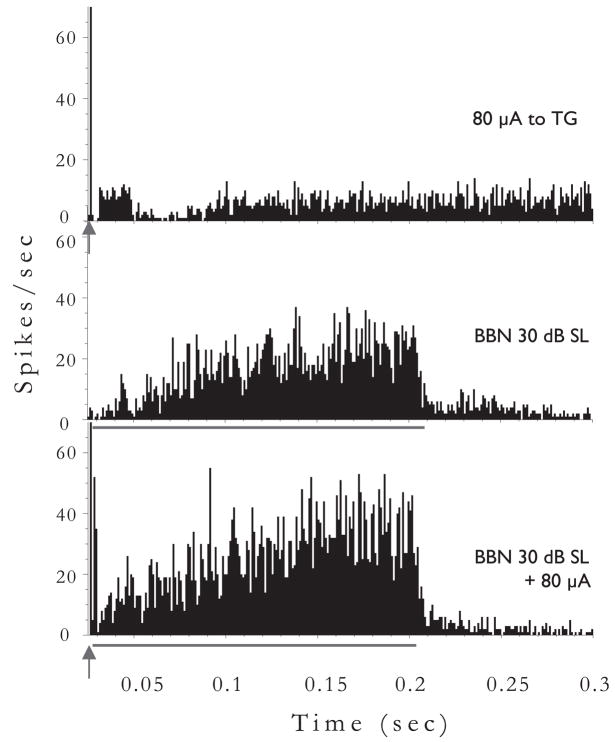
In some neurons, the addition of the TG pulse before the sound can enhance rather than suppress the response to the sound (Fig. 8). Note in this case that the response to the preceding TG pulse is inhibitory, i.e., suppresses SR. In these examples, when the response to TG stimulation is inhibition, the combined response is enhancement and when the response to TG stimulation is excitation, the combined response is suppression.
Fig. 8.
Poststimulus time histograms of a different DCN single unit’s response to TG stimulation combined with acoustic stimulation. The TG pulse precedes the acoustic stimulus (broadband noise at 30 dB SL) by 5 ms. Acoustic stimulus duration is 200 ms, indicated by red bar below histograms. Arrow indicates onset of TG pulse (80 μA, 100 ms/phase bipolar pulses, 100 presentations). In this unit, preceding the acoustic stimulus by TG stimulation results in an enhanced response to the acoustic stimulus. Note, the buildup pattern of response indicates that this cell was likely a pyramidal cell.
Effects of dorsal root ganglion and dorsal column activation on DCN activity
Somatic tinnitus includes the modulatory effects of neck and upper limb manipulation on the pitch and loudness of tinnitus. Physiologically, this may correspond to modulation of neural activity in the DCN by activation of the dorsal column nuclei and cervical DRG.
Evoked potentials
Stimulation of cervical nerves can elicit evoked potentials in the DCN. The evoked potentials are largest in response to stimulation of the cervical nerves corresponding to mechanoreception and proprioception in the pinna (C2), neck (C7), and forelimbs (C8) (Kanold and Young, 2001), similar to those evoked by TG stimulation (Shore et al., 2003). In addition, stimulation of the femoral nerve can activate cells in the DCN as evidenced by Fos expression (McKitrick and Calaresu, 1993).
Single unit responses
Activation of dorsal column nuclei with current pulses elicits excitatory and inhibitory single unit responses in the DCN: responses of DCN units have both short- and long-latency inhibitory phases and a transient excitatory phase (Young et al., 1995). The transient excitatory peak can be facilitated by multiple pulses, while the long-latency inhibition can be suppressed by multiple pulses. Single unit responses in the DCN to electrical activation of the DRG are similar to responses to activation of the dorsal column nuclei but with longer latencies (Kanold and Young, 2001). In addition, dorsal column stimulation can alter sound-evoked responses in a similar manner to that described above for trigeminal stimulation (Saade et al., 1989; Kanold et al., 2001), producing bimodal integration.
How could this bimodal integration occur?
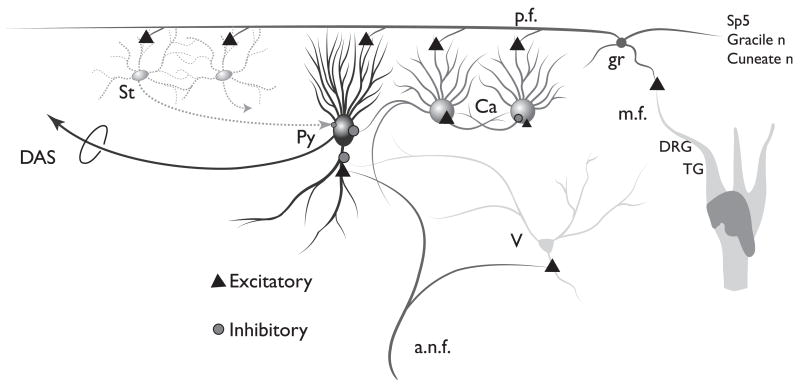
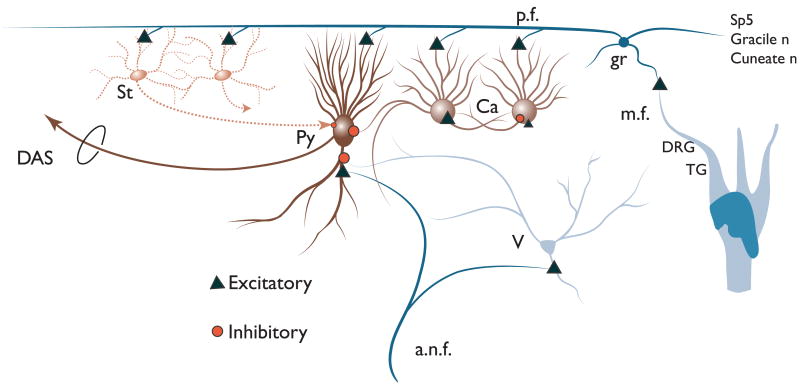
Figure 9 shows a schematic of the DCN circuitry including AN and somatosensory inputs. Somatosensory stimulation activates granule cells (gr), which excite the principal output neurons of the DCN, fusiform (fu), or giant (gi) cells, as well as inhibitory interneurons, the cartwheel (ca) cells (Golding and Oertel, 1997) and stellate cells (st). Cartwheel and stellate cells, in turn, inhibit principal cells (Davis et al., 1996). Sound stimulation (via AN fibers, a.n.f.) strongly excites fusiform cells (Stabler et al., 1996; Young, 1998) and weakly excites cartwheel cells (Parham and Kim, 1995). Suppression of sound-evoked responses of fusiform or giant cells could be achieved by the summation of weak responses from cartwheel cells to sound and stronger cartwheel cell responses to trigeminal input, leading to inhibition of the principal cell response to sound. Similarly, facilitation of responses to sound could occur through summation of granule cell — fusiform/giant cell activation by sound and trigeminal input. Somatosensory stimulation can precede acoustic stimulation by up to 90 ms and still alter the firing rate to acoustic stimulation for the duration of the sound stimulus (Shore, 2005).
Fig. 9.
Schematic of DCN circuitry. Pyramidal cells (Py) in layer II of the DCN receive inputs on their basal dendrites from auditory nerve fibers (a.n.f.) and vertical (v) cells. The apical dendrites of the pyramidal cells receive inputs from the parallel fiber axons (pf) from granule cells (gr) in the VCN, while their cell bodies receive inputs from cartwheel (Ca) and superficial stellate (st) cells. Projections from the trigeminal ganglion (TG), spinal trigeminal nucleus (Sp5), dorsal column nuclei (Gracile and cuneate n), and the dorsal root ganglion (DRG), synapse on granule cells. (See Color Plate 10.9 in color plate section.)
Effects of Sp5 activation on IC activity
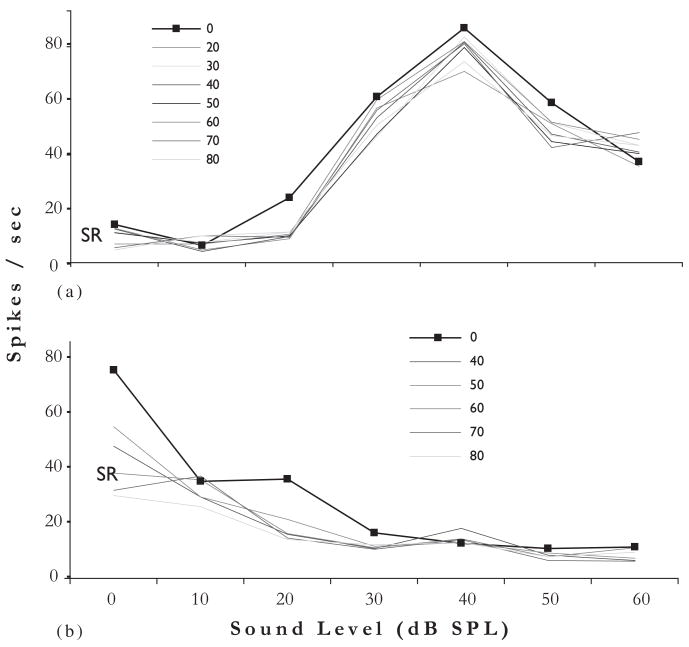
Single unit recordings from ICX reveal that trigeminal stimulation can suppress SRs as well as produce bimodal integration of the type described above in the DCN (Shore, 2005; Jain and Shore, 2006). Units in both locations show mostly suppression, but also show enhancement of acoustically driven responses by trigeminal stimulation. Figure 10 shows two examples of suppression of spontaneous and sound-driven responses in ICX neurons by trigeminal stimulation. In these experiments, the trigeminal stimulation site was in Sp5. The top graph shows a nonmonotonically increasing rate-level response to sound stimulation (broadband noise), decreasing at high levels. The effects of Sp5 stimulation at several current levels are to suppress the SRs as well as the responses to sound especially at low sound levels (20 dB SPL). The bottom graph shows similar effects for a monotonically decreasing rate-level function: Sp5 stimulation further decreases the response to sound around 20 dB SPL. Greater suppression of SR occurs in the lower unit. The importance of this study is its demonstration that the effects on SRs as well as bimodal processing initiated in the DCN are passed on to the next level to be integrated by the extralemniscal system.
Fig. 10.
Rate-level functions for two different units in ICx (A and B) in response to broadband noise stimuli (100 ms, 5 ms risefall times) with and without Sp5 stimulation. Trigeminal stimulus was at the onset of the acoustic stimulus. Response to Sp5 stimulation alone was minimal, but Sp5 stimulation reduced the responses of units in A and B to sound stimulation. The effects were more pronounced at low sound levels. (Adapted with permission from Jain and Shore, 2006.) (See Color Plate 10.10 in color plate section.)
How do responses of DCN and IC neurons to combined sensory stimulation relate to somatic tinnitus?
The suppression or enhancement of sound-evoked activity in DCN and ICX could be analogous to the suppression or enhancement of tinnitus experienced by some individuals with tinnitus. Maneuvers that alter the tinnitus such as clenching of the jaw or lateral gaze would be analogous to electrical stimulation of the TG or Sp5, as described above, that alters the neurons’ spontaneous activity or responses to sound. The underlying mechanisms for these alterations could involve long-term depression (LTD)/potentiation (LTP). Such long-term plasticity of synapses occurs between parallel fibers and their targets, the fusiform, giant, and cartwheel cells. When postsynaptic depolarization is preceded by stimulation of parallel fibers in slice experiments, LTP or LTD is produced in fusiform and cartwheel cells (Fujino and Oertel, 2003; Tzounopoulos et al., 2004). Parallel fiber inputs to fusiform cells are strengthened (LTP) when paired with subsequent postsynaptic firing, whereas in cartwheel cells they are weakened (LTD) (Tzounopoulos et al., 2004). The induction of LTP in fusiform cells and LTD in cartwheel cells has the net effect of increasing the firing rates of fusiform cells because cartwheel cells provide feed-forward inhibition to fusiform cells. Reversing the order of pre- and postsynaptic activation produces LTD in fusiform cells, but does not change LTD to LTP in cartwheel cells, resulting in a net depression of firing rate in fusiform cells.
Another mechanism that could contribute to these effects is activation of GABAB receptors in the DCN that regulate dendritic excitability and excitatory inputs (Caspary et al., 1987). GABAB receptors in CN are indeed strategically placed to modulate glutamatergic neurotransmission between the granule cells and their targets, the fusiform, cartwheel, and stellate cells in the superficial layers. The sources of GABAergic inputs to these regions could be vertical cells or superficial stellate cells, which cocontain GABA as well as glycine (Mugnaini, 1985; Altschuler et al., 1991; Davis and Young, 2000). These mechanisms would be reflected also in neurons in the ICX that receive direct inputs from DCN neurons. Additional processes within the IC may enhance the effects already evident at the level of the DCN.
Plate 10.2.
High magnification confocal images (63 ×) showing colocalization of anterogradely labeled Sp5 terminal endings with VGLUT2-ir in the CN. Green, VGLUT-ir. Red, Sp5 labeling. Yellow, double labeled terminals. Figures were obtained from Z projections of stacks of serial 1 μm confocal images. (A) MFs are labeled with BDA from Sp5 and VGLUT2 in the shell region of the VCN (arrow). (B) Small boutons are labeled with BDA from Sp5 and VGLUT2 in the DCN layer 2 (arrows). (C and D) Sp5 terminal endings do not colabel with VGLUT1 in the shell region of the VCN and the core of the VCN. Scale bar = 10 μm in D (applies to A–C). (For B/W version, see page 112 in the volume.)
Plate 10.3.
High magnification 1 μm confocal images (63 ×) showing colocalization of AN terminal endings with VGLUT1-ir, but not VGLUT2-ir, in both the VCN and deep layer of the DCN. Green, VGLUT-ir. Red, FG filled AN fibers and endings. Yellow, double-labeled terminals. (A and B) Colocalization of AN terminal endings with VGLUT1-ir in VCN (A) and DCN layer 3 (B). Both endbulb-like AN endings (arrows in A) and small boutons (arrowheads in A and B) colabeled with the VGLUT1. (C and D) VGLUT2 did not label the AN endings in both VCN (C) and DCN layer 3 (D). Scale bar = 20 μm in D (applies to A–C). (For B/W version, see page 113 in the volume.)
Plate 10.9.
Schematic of DCN circuitry. Pyramidal cells (Py) in layer II of the DCN receive inputs on their basal dendrites from auditory nerve fibers (a.n.f.) and vertical (v) cells. The apical dendrites of the pyramidal cells receive inputs from the parallel fiber axons (pf) from granule cells (gr) in the VCN, while their cell bodies receive inputs from cartwheel (Ca) and superficial stellate (st) cells. Projections from the trigeminal ganglion (TG), spinal trigeminal nucleus (Sp5), dorsal column nuclei (Gracile and cuneate n), and the dorsal root ganglion (DRG), synapse on granule cells. (For B/W version, see page 119 in the volume.)
Plate 10.10.
Rate-level functions for two different units in ICx (A and B) in response to broadband noise stimuli (100 ms, 5 ms risefall times) with and without Sp5 stimulation. Trigeminal stimulus was at the onset of the acoustic stimulus. Response to Sp5 stimulation alone was minimal, but Sp5 stimulation reduced the responses of units in A and B to sound stimulation. The effects were more pronounced at low sound levels. (Adapted with permission from Jain and Shore, 2006.) (For B/W version, see page 120 in the volume.)
Acknowledgments
This work supported by grants from the National Institutes of Health (R01 DC 004825; P30 DC 05188), Tinnitus Research Consortium, Tinnitus Research Initiative and American Tinnitus Association. We thank Sana Syed and Naveen Nannapaneni for technical assistance and Ben Yates for graphic design and technical writing assistance.
Abbreviations
- CN
cochlear nucleus
- DCN
dorsal cochlear nucleus
- DRG
dorsal root ganglion
- GCD
granule cell domain
- IC
inferior colliculus
- ICX
external cortex of IC
- MF
mossy fiber
- PSTH
poststimulus time histogram
- Sp5
spinal trigeminal nucleus
- Sp5C
caudal spinal trigeminal nucleus
- Sp5I
interpolaris spinal trigeminal nucleus
- SR
spontaneous rate
- TG
trigeminal ganglion
- VCN
ventral cochlear nucleus
- VGLUT
vessicular glutamate transporter
References
- Abel MD, Levine RA. Muscle contractions and auditory perception in tinnitus patients and nonclinical subjects. Cranio. 2004;22(3):181–191. doi: 10.1179/crn.2004.024. [DOI] [PubMed] [Google Scholar]
- Altschuler R, Juiz J, Shore S, Bledsoe S, Helfert R, Wenthold RJ. In: Inhibitory amino acid synapses and pathways in the ventral cochlear nucleus. Merchan MA, Juiz JM, Godfrey DA, Mugnaini E, editors. Plenum Press; New York: 1991. [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AT, Cousins JP, Parnes SM, Semenoff D, Holmes T, McFarland DJ, Davenport C, Stegbauer K, Lovely TJ. Cutaneous-evoked tinnitus. I Phenomenology, psychophysics and functional imaging. Audiol Neurootol. 1999;4(5):247–257. doi: 10.1159/000013848. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kossl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417(2):273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res. 2002;164(1–2):59–68. doi: 10.1016/s0378-5955(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Chole RA, Parker WS. Tinnitus and vertigo in patients with temporomandibular disorder. Arch Otolaryngol Head Neck Surg. 1992;118(8):817–821. doi: 10.1001/archotol.1992.01880080039010. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Phillips G, Ryan RD. Functional organization in the trigeminal main sensory and rostral spinal nuclei of the cat. J Physiol. 1963;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol. 1996;76(5):3012–3024. doi: 10.1152/jn.1996.76.5.3012. [DOI] [PubMed] [Google Scholar]
- Davis KA, Voigt HF. Evidence of stimulus-dependent correlated activity in the dorsal cochlear nucleus of decerebrate gerbils. J Neurophysiol. 1997;78(1):229–247. doi: 10.1152/jn.1997.78.1.229. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83(2):926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci USA. 2003;100(1):265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb H, Gelb ML, Wagner ML. The relationship of tinnitus to craniocervical mandibular disorders. Cranio. 1997;15(2):136–143. doi: 10.1080/08869634.1997.11746004. [DOI] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78(1):248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484(2):191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sumino R, Sessle BJ. Functional organization of trigeminal subnucleus interpolaris: nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol. 1984;51(5):890–905. doi: 10.1152/jn.1984.51.5.890. [DOI] [PubMed] [Google Scholar]
- Hiller W, Janca A, Burke KC. Association between tinnitus and somatoform disorders. J Psychosom Res. 1997;43(6):613–624. doi: 10.1016/s0022-3999(97)00188-8. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. Direct projections from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res. 1987;400:145–150. doi: 10.1016/0006-8993(87)90662-7. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Barcia M, Rhoades RW. Structure–function relationships in rat brainstem subnucleus interpolaris: IV Projection neurons. J Comp Neurol. 1989;282(1):45–62. doi: 10.1002/cne.902820105. [DOI] [PubMed] [Google Scholar]
- Jain R, Shore S. External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett. 2006;395(1):71–75. doi: 10.1016/j.neulet.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. J Am Acad Audiol. 2000;11(3):125–137. [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88(2):699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147(1–2):282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci. 2001;21(19):7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Young ED, Manis PB. In: Auditory–somatosensory interactions in the dorsal cochlear nucleus. Breebaart DJ, Houtsma AJM, Kholrausch A, Prijs VF, Schoonhoven R, editors. Shaker Publishing BV; The Netherlands: 2001. pp. 215–220. [Google Scholar]
- Li H, Mizuno N. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: a fluorescent retrograde double-labeling study in the rat. Neurosci Res. 1997;29(2):135–142. doi: 10.1016/s0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- McKitrick DJ, Calaresu FR. Expression of Fos in rat central nervous system elicited by afferent stimulation of the femoral nerve. Brain Res. 1993;632(1–2):127–135. doi: 10.1016/0006-8993(93)91147-k. [DOI] [PubMed] [Google Scholar]
- Møller AR, Møller MB, Yokota M. Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope. 1992;102(10):1165–1171. doi: 10.1288/00005537-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Montazem A. Secondary tinnitus as a symptom of instability of the upper cervical spine: operative management. Int Tinnitus J. 2000;6(2):130–133. [PubMed] [Google Scholar]
- Morgan DH. Tinnitus of TMJ origin. J Craniomandib Pract. 1992;10(2):124–129. doi: 10.1080/08869634.1992.11677900. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. GABA neurons in the superficial layers of the rat dorsal cochlear nucleus: light and electron microscopic immunocytochemistry. J Comp Neurol. 1985;235(1):61–81. doi: 10.1002/cne.902350106. [DOI] [PubMed] [Google Scholar]
- Nazruddin Suemune S, Shirana Y, Yamauchi K, Shigenaga Y. The cells of origin of the hypoglossal afferent nerves and central projections in the cat. Brain Res. 1989;490(2):219–235. doi: 10.1016/0006-8993(89)90240-0. [DOI] [PubMed] [Google Scholar]
- Nelken I, Young ED. Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus type IV units to narrowband and wideband stimuli. J Neurophysiol. 1994;71(6):2446–2462. doi: 10.1152/jn.1994.71.6.2446. [DOI] [PubMed] [Google Scholar]
- Ochi K, Eggermont JJ. Effects of quinine on neural activity in cat primary auditory cortex. Hear Res. 1997;105(1–2):105–118. doi: 10.1016/s0378-5955(96)00201-8. [DOI] [PubMed] [Google Scholar]
- Parham K, Kim DO. Spontaneous and sound-evoked discharge characteristics of complex-spiking neurons in the dorsal cochlear nucleus of the unanesthetized decerebrate cat. J Neurophysiol. 1995;73(2):550–561. doi: 10.1152/jn.1995.73.2.550. [DOI] [PubMed] [Google Scholar]
- Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol. 1988;268(1):91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19(6):785–789. [PubMed] [Google Scholar]
- Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164(1–2):206–214. doi: 10.1016/s0378-5955(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Vertical cell responses to sound in cat dorsal cochlear nucleus. J Neurophysiol. 1999;82(2):1019–1032. doi: 10.1152/jn.1999.82.2.1019. [DOI] [PubMed] [Google Scholar]
- Romfh JH, Capra NF, Gatipon GB. Trigeminal nerve and temporomandibular joint of the cat: a horseradish peroxidase study. Exp Neurol. 1979;65(1):99–106. doi: 10.1016/0014-4886(79)90251-6. [DOI] [PubMed] [Google Scholar]
- Rubinstein B, Axelsson A, Carlsson GE. Prevalence of signs and symptoms of craniomandibular disorders in tinnitus patients. J Craniomandib Disord. 1990;4(3):186–192. [PubMed] [Google Scholar]
- Saade NE, Frangieh AS, Atweh SF, Jabbur SJ. Dorsal column input to cochlear neurons in decerebrate-decerebellate cats. Brain Res. 1989;486(2):399–402. doi: 10.1016/0006-8993(89)90532-5. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147(1–2):261. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Shore S, El-Kashlan HK, Lu J. Effects of trigeminal ganglion stimulation on unit activity of ventral cochlear nucleus neurons. Neuroscience. 2003;119(4):1085–1101. doi: 10.1016/s0306-4522(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci. 2005;21(12):3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol. 2000;419(3):271–285. doi: 10.1002/(sici)1096-9861(20000410)419:3<271::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hear Res. 2006;216–217:90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sola E, Prestori F, Rossi P, Taglietti V, D’Angelo E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J Physiol. 2004;557(Pt 3):843–861. doi: 10.1113/jphysiol.2003.060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SE, Palmer AR, Winter IM. Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. J Neurophysiol. 1996;76(3):1667–1688. doi: 10.1152/jn.1996.76.3.1667. [DOI] [PubMed] [Google Scholar]
- Suemune S, Nishimori T, Hosoi M, Suzuki Y, Tsuru H, Kawata T, Yamauchi K, Maeda N. Trigeminal nerve endings of lingual mucosa and musculature of the rat. Brain Res. 1992;586(1):162–165. doi: 10.1016/0006-8993(92)91389-v. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol. 2005;94(6):4234–4243. doi: 10.1152/jn.00401.2005. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Shigenaga Y. Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol. 1991;111(3):324–331. doi: 10.1016/0014-4886(91)90099-x. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7(7):719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Marani E, Schoen JH. The trigeminal system in man. Adv Anat Embryol Cell Biol. 1997;136(I–X):1–126. doi: 10.1007/978-3-642-60779-0. [DOI] [PubMed] [Google Scholar]
- Weedman D, Ryugo D. Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol. 1996;371(2):311–324. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Rustioni A. A cuneocochlear pathway in the rat. Neuroscience. 1987;20(1):209–219. doi: 10.1016/0306-4522(87)90013-3. [DOI] [PubMed] [Google Scholar]
- Winter IM, Palmer AR. Level dependence of cochlear nucleus onset unit responses and facilitation by second tones or broadband noise. J Neurophysiol. 1995;73(1):141–159. doi: 10.1152/jn.1995.73.1.141. [DOI] [PubMed] [Google Scholar]
- Wolff A, Kunzle H. Cortical and medullary somatosensory projections to the cochlear nuclear complex in the hedgehog tenrec. Neurosci Lett. 1997;221(2–3):125–128. doi: 10.1016/s0304-3940(96)13305-x. [DOI] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365(1):159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wright EF, Gullickson DC. Dental pulpalgia contributing to bilateral preauricular pain and tinnitus. J Orofac Pain. 1996;10(2):166–168. [PubMed] [Google Scholar]
- Young E. Cochlear nucleus. In: Shepherd G, editor. The Synaptic Organization of the Brain. Oxford University Press; Oxford: 1998. pp. 121–158. [Google Scholar]
- Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol. 1995;73(2):743–765. doi: 10.1152/jn.1995.73.2.743. [DOI] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res. 2002;172(1–2):137–143. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]
- Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006;496(3):335–348. doi: 10.1002/cne.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J Neurophysiol. 1994;71(3):914–930. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500(4):777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78(6):901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495(1):100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]