Abstract
The avian cochlear duct houses both a vestibular and the auditory sensory organ (the lagena macula and basilar papilla, respectively) that each have a distinct structure and function. Comparative mRNA in situ hybridization mappings conducted over the time course of chicken cochlear duct development reveal that Wnt-related gene expression is concomitant with various developmental processes such as regionalization, convergent extension of the cochlear duct, cell fate specification, synaptogenesis, and the establishment of planar cell polarity. Wnts mostly originate from nonsensory tissue domains while the sensory primordia preferentially transcribe Frizzled receptors, suggesting that paracrine Wnt signaling predominates in the cochlear duct. Superimposed over this is the strong expression of two secreted Frizzled-related Wnt inhibitors that tend to show complementary expression patterns. Frzb (SFRP3) is confined to the nonsensory cochlear duct and the lagena macula, whereas SFRP2 is maintained in the basilar papilla along with Fzd10 and Wnt7b. Flanking the basilar papilla are: Wnt7a, Wnt9a, Wnt11, and SFRP2 on the neural side; and Wnt5a, Wnt5b, Wnt7a on the abneural side. The lateral nonsensory cochlear duct continuously expresses Frzb and temporarily expresses Wnt6 and SFRP1. Characteristic for the entire lagena is the expression of Frzb; in the lagena macula are Fzd1, Fzd7, Wnt7b; and in the nonsensory tissues are Wnt4, Wnt5a. Auditory hair cells preferentially express Fzd2 and Fzd9, while the main receptors expressed in vestibular hair cells are Fzd1 and Fzd7, in addition to Fzd2 and Fzd9.
Keywords: Wnts, Frizzled, Wnt-Inhibitors, inner ear, basilar papilla, lagena
Introduction
The inner ear of vertebrates contains several different sensory organs serving hearing and balance. In birds, one organ of each modality is located in close proximity in the cochlear duct. Thus two organs, the auditory basilar papilla (BP) and the vestibular lagena macula (LM), must become differentiated both anatomically and functionally during development. The BP is highly specialized for hearing and shows important spatial gradients (Gleich et al., 2004). Across the width of the organ (the radial dimension) is the axis of maximal sensitivity for hair cell bundle displacement while along the length of the organ (the longitudinal dimension) is the tonotopic axis. Both axes show systematic changes in the mechanosensory hair cells and their innervation patterns (Fischer, 1994). Radially, columnal tall hair cells with relatively small luminal surfaces are situated on the neural (superior) side and give way to short hair cells with broad surfaces located on the abneural (inferior) side. Longitudinally, the lumenal transduction apparatus, the hair cell bundle, varies systematically from a tall, round shape with centrally placed stereocilia for low frequency responses (in the distal apex) to short bundles with a wide rectangular shape for high frequencies (in the proximal base). Auditory hair cell bundles are coupled by a tectorial membrane.
The sensory epithelium of the lagena, situated in the distal end of the cochlear duct, rests on firm tissue. Its tall hair bundles are covered by a membrane containing otoconia similar to other vestibular maculae (Li et al., 2006). The avian LM consists mainly of uniform type II hair cells, except for two central stripes of loosely packed type I hair cells which are restricted to the striola area (Rosenhall, 1970). Stereocilia bundles outside the striola region show an opposing polarity across the striola that reflects anti-parallel directional sensitivity. Although there is still a profound lack of knowledge about the function of the LM, it has been shown that this cochlear vestibular organ has no auditory function (Manley et al., 1991a).
During ontogeny, the cochlear duct emerges from the ventral otocyst and is referred to as pars inferior. The pars inferior includes prosensory domains which give rise to three sensory epithelia: the vestibular saccular and lagenar maculae, as well as the auditory BP. By mid-gestation, the pars inferior splits and the saccule segregates from the cochlear duct. Here we will focus on the cochlear duct while a separate manuscript will present Wnt-related gene expressions during the saccule’s subsequent development together with the other sensory organs of the dorsal inner ear subdivision, the pars superior. By embryonic day 12 (E12, Hamburger & Hamilton stage 38, s38) cochlear differentiation has reached a state where the hair cell types are distinguishable (Cohen and Fermin, 1978) and the inner ear becomes functional. Grier et al. (1967) demonstrated that embryonic chickens respond to sounds as early as E12, when compound action potential (CAP) thresholds are at high levels (Saunders et al., 1973; Rebillard and Rubel, 1981). Vanzulli and Garcia-Austt (1963) recorded microphonic potentials as early as E13. Nonetheless, the hatchling BP is still immature both morphologically and functionally. Synaptogenesis as well as the achievement of final hair cell bundle orientation continues after hatching (Cohen and Fermin, 1978; Cotanche and Corwin, 1991). Between hatching and the first month, the cochlear duct lengthens by 16% (Ryals et al., 1984). Furthermore, intensity functions of primary auditory nerve fibers are not yet mature in post-hatched chickens (Manley et al., 1991b). Moreover, Jones and Jones (2000) found significant developmental changes occurring in the spontaneous activity of both lagenar and auditory neurons of the eighth cranial nerve between E19 and posthatch chickens.
Wnt-related gene expression in the inner ear has been reported previously (Hollyday et al., 1995; Jasoni et al., 1999; Stark et al., 2000; Daudet et al., 2002; Sajan et al., 2007), with functional data now emerging. Examples include the involvement of Wnts in early otic placode specification (Ohyama et al., 2006), Wnts mediating otic dorsalization from the adjacent hindbrain (Riccomagno et al., 2005; Hatch et al., 2007), cell autonomous β-catenin mediated sensory fate specification (Stevens et al., 2003), and Wnt/Frizzled regulation of hair bundle planar polarity and cochlear duct elongation (Dabdoub et al., 2003; Wang et al., 2006; Qian et al., 2007). However, a comprehensive developmental sequence of Wnt-related gene expression in the cochlear duct, presented with topological mapping details, was previously lacking.
Wnt signaling pathways are highly conserved during evolution and they seem to be repeatedly involved in various processes of development (and disease) across the animal kingdom. Wnt ligands elicit various responses via binding to a variety of Frizzled receptors and co-receptors, leading to the activation of distinct intracellular signaling pathways (see e.g. Cadigan and Liu, 2006). Based on biochemical binding preferences and functional data, a classification of Wnt and Frizzled family members has been established. According to their ability to either activate a β-catenin dependent canonical Wnt pathway, or a PCP (planar cell polarity) or Wnt/Calcium pathway, they are classified into canonical and noncanonical signaling factors, respectively. However, lately evidence has accumulated that most Wnts bind to various Frizzleds and that the outcome of signaling is regulated by the receptor context rather than being intrinsic to a particular Wnt or Frizzled (Gordon and Nusse, 2006; Hendrickx and Leyns, 2008). For example, Wnt11, long viewed as a noncanonical Wnt, is clearly also able to signal via β-catenin in a Frizzled-LRP dependent manner (Tao et al., 2005; Zhou et al., 2007). This functional plasticity has also been shown for Frizzleds. For example, Fzd7 is required for Xenopus neural crest induction through canonical Wnt signaling yet later neural crest migration is regulated by Fzd7 together with Wnt11 via a noncanonical pathway (Abu-Elmagd et al., 2006). Furthermore, there can also be crosstalk between Wnt pathways. For example, during convergent extension, Wnt5a inhibits Wnt3a-induced canonical signaling (Schambony and Wedlich, 2007), yet in another context, Wnt5a can activate β-catenin via Fzd4 and LRP co-receptors (Mikels and Nusse, 2006). Additionally, repression of one pathway by another may occur through competition for a common receptor, as proposed by Maye et al. (2004), and via available ligand concentrations. Thus, Wnt expression could have an indirect effect in facilitating or blocking other Wnt signaling pathways and Wnt antagonists, binding either to Wnts, Frizzleds or co-receptors, become important to trigger the expression readout.
Therefore single expression patterns can be obscure as they necessarily neglect the full expression context in which the proteins must function. This motivated us to undertake a comprehensive analysis of spatio-temporal gene expressions of Wnts, Frizzleds and Wnt inhibitors during inner ear development. In this study we analyzed the expression of Wnt-related gene transcripts from outgrowth of the pars inferior (E4) through to an early functional auditory and vestibular organ (E15). The results section describes many diverse expression patterns that coincide with key developmental events in the chicken cochlear duct.
Material and Methods
Tissue preparation
White Leghorn chicken eggs were incubated at 38°C in a humidified incubator up to the desired age. Embryonic stages were discriminated following Hamburger & Hamilton criteria (Hamburger and Hamilton, 1951). All embryonic tissue was handled in RNase-free conditions, fixed in 4% paraformaldehyde (PFA) in PBS (pH 7.4; overnight at 4°C) and washed in phosphate-buffered saline (PBS, pH 7.4). Immersion-fixed s24–25 embryos were embedded intact to provide positive control tissues such as limb buds, kidneys, eyes etc. to confirm probe specificity. Embryos ranging from s26 to s37 were decapitated and the heads were immersion fixed. From s37 onwards, embryos were fixed via an intracardiac perfusion and the heads were postfixed after removing the skin, dissecting the lower jaw and tearing the tympanic membrane to facilitate fixation of the inner ear. These older stage ears were isolated by harvesting the cartilaginous temporal bone and removing ossified tissue by dissection before embedding and sectioning. Tissue was then cryo-protected (in 15% sucrose in PBS) and embedded in TFM (Tissue Freezing Medium, TBS, Triangle Biomedical Sciences). Frozen serial sections of 15μm were collected onto Superfrost Plus slides (Fisher Scientific). Depending on the developmental stage, consecutive sections were placed on a series of 10–20 slides. Every 5th section was probed with the same gene, which allowed for a comparison of 5 genes per specimen, including prosensory markers. Transverse and coronal sections were obtained by cutting angles parallel or perpendicular, respectively, to the longest dorso-ventral axis of the otic anlage. Sections were stored at −80°C until use.
Preparation of probes
Riboprobes for chicken Wnts and Wnt-related genes (Chapman et al., 2004) were made from plasmids provided by the laboratories of G. Schoenwolf and C. Tabin. Sequencing and alignment confirmed the target genes (cf. table 1). Table 1 provides information about 25 Wnt and Wnt-related genes that were studied, the number of experiments for each range of developmental stages and whether expression was detected in the ventral ear. In addition, molecular markers such as Ser1, Lfng (Adam et al., 1998), Msx1, BMP4 (Wu and Oh, 1996), and FGF8 (Sanchez-Calderon et al., 2004) for developing chicken inner ear organs were probed on selected adjacent sections to identify prosensory domains. Antisense riboprobe for each gene was transcribed with either T3, T7, or SP6 RNA polymerase (Roche Applied Science) in the presence of digoxigenin-11-UTP (Roche Applied Science). Digoxigenin-labeled antisense RNA probes were purified with Centri-Sep spin columns (Princeton Separations) and precipitated with LiCl. Resulting probes were tested for RNA integrity by electrophoresis (1% agarose gel).
Table 1.
Probe list with numbers of in situ hybridization tests per developmental stage group. Genes (accession number and sequence) for Wnt ligands, Frizzled receptors, and Wnt antagonists analyzed in this study on inner ear tissue from chicken embryos ranging from stage (s)24 to 41 (according to Hamburger and Hamilton, 1951) embryonic day (E)4 to 15.
| s24–s26 | s27–s30 | s31–s36 | s37–s41 | s24–s26 | s27–s30 | s31–s36 | s37–s41 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wnt1 | AY753286 1 – 373 | 2 | 5 | 6 | 1 | Fzd1 | NM_001030337 1148 – (1839) | 3 | 4 | 7 | 5 |
| Wnt2b | NM_204336 687 – 1067 | 1 | 4 | 4 | 1 | Fzd2 | NM_204222 288 – 1430 | 3 | 3 | 8 | 5 |
| Wnt3 | NM_001081696 621 – 1034 | 5 | 8 | 20 | 15 | Fzd4 | NM_204099 818 – 3757 | 3 | 8 | 7 | 5 |
| Wnt3a | NM_204675 697 – 1088 | 3 | 6 | 12 | 8 | Fzd7 | NM_204221 1034 – 3090 | 3 | 6 | 10 | 6 |
| Wnt4 | NM_204783 798 – 1191 | 3 | 5 | 10 | 4 | Fzd8 | XM_418566 728 – 1452 | 4 | 5 | 6 | 3 |
| Wnt5a | NM_204887 994 – 1364 | 6 | 10 | 15 | 11 | Fzd9 | XM_425392 219 – 1746 | 5 | 3 | 16 | 13 |
| Wnt5b | NM_001037269 656 – 1017 | 4 | 4 | 10 | 5 | Fzd10 | NM_204098 1 – 2244 | 5 | 3 | 8 | 6 |
| Wnt6 | NM_001007594 557 – 930 | 7 | 8 | 7 | 3 | ||||||
| Wnt7a | NM_204292 555 – 1244 + 3′UTR | 3 | 1 | 1 | 2 | Dkk1 | XM_421563 478 – 1064 | 4 | 2 | 6 | 4 |
| Wnt7b | NM_001037274 679 – 1067 | 2 | 4 | 13 | 8 | Cer | NM_204823 44 – 877 | 4 | 5 | 6 | 2 |
| Wnt8b | AY753292 440 – 834 | 2 | 4 | 7 | 3 | SFRP1 | NM_204553 250 – 468 | 4 | 6 | 11 | 5 |
| Wnt9a | NM_204981 72 – 1132 | 4 | 9 | 14 | 11 | SFRP2 | NM_204773 1 – 1594 | 6 | 5 | 12 | 4 |
| Wnt11 | NM_204784 1 – 1827 | 4 | 6 | 7 | 4 | Frzb | NM_204772 655 – 1822 | 6 | 6 | 13 | 6 |
Shaded in gray are genes that were not detected in ventral inner ear tissue (Wnt1, Wnt2b, and Cer). Those three probes were not tested beyond s38 (E12).
RNA in situ hybridization and visualization
In situ hybridizations were performed in parallel on sets of 5 slides with sequential sections to test different probes and marker genes on adjacent tissue sections, following a slightly modified protocol provided by M. Hidalgo-Sánchez (Sanchez-Calderon et al., 2005). Briefly, tissue sections were dried at 50°C, postfixed in 4% PFA, washed in PBS, digested for 10 minutes with Proteinase K (Roche Applied Science, 1μg/ml), fixed in 4% PFA, washed in PBS, and acetylated in 0.1M Triethanolamine with 0.25% Acetic Anhydride, before permeabilizing with PBT (PBS with 0.1% TritonX100). All steps, including the RNA hybridization, were performed with slides submerged in slide mailer boxes (Life Science Products). Sections were pre-hybridized in hybridization buffer without probe (2 hours at room temperature), before overnight hybridization at 72°C in formamide hybridization buffer (see Sanchez-Calderon et al., 2005) containing riboprobe at ~1μg/ml concentration. Post-hybridization washes were carried out in 2x SSC and 0.2x SSC first at hybridization temperature and then at room temperature. Anti-Digoxigenin-AP Fab fragments (Roche, 1:3500) were used to detect hybridization and alkaline phosphatase (AP) precipitation was performed with BM Purple AP Substrate (Roche Applied Science) for up to 3 days at varying incubation temperatures from 14° to 37°C. After stopping the precipitation (in 100mM Tris pH 7.5, 1mM EDTA), the slides were either further processed immunhistochemically (see below) or dehydrated and coverslipped with ShurMount mounting medium (TBS, Triangle Biomedical Sciences).
Photomicrographic documentation
Photomicrographs of tissue sections were captured with a SPOT Flex color-mosaic camera (Diagnostic Instruments) mounted on a Nikon E800 microscope. An image database (Apple software Aperture 2.1) was built to store and represent all in situ hybridization results (5,600 photomicrographs with keywords). The figure panels in this study provide exemplary data to support the reported results. Cropping of photomicrographs and a minimal amount of contrast and brightness adjustment was done with Adobe Photoshop CS3.
Neurofilament immunhistochemistry
In addition to comparative in situ hybridizations with marker genes, double labeling with neurofilament-associated monoclonal 3A10 (Developmental Studies Hybridoma Bank, DSHB, University of Iowa, USA) was utilized to mark axons and aid the identification of prosensory epithelia (Adam et al., 1998; Sanchez-Calderon et al., 2004). 3A10 is a neurofilament-associated antigen raised in mouse against chicken ventral spinal cord and labels cells containing a phosphorylated neurofilament associated protein. Previously, Storey et al. (1992) used this antibody as a marker for embryonic chicken nervous tissue and Perez et al. (1999) to reveal sensory neuron fate in the chicken embryo. Following a protocol modified after Sanchez-Calderon et al. (2004), supernatant from hybridoma cells containing anti-3A10 IgG1 was diluted 1:50 with blocking solution. Blocking and antibody incubations were carried out in heat-inactivated 10% Calf Serum, 0.05% TritonX100, 0.05% NaAzide in PBS. Biotinylated goat anti-mouse secondary antibody (IgG H+L; Vector BA-2000; 1:250) was used with extravidin-biotin-horseradish peroxidase complex (Vector Laboratories ABC Standard Kit; 1:200). Peroxidase activity was revealed using diaminobenzidine histochemsitry. Negative controls confirmed that omission of the primary antibody prevented immunostaining.
Results
Ventral otic anlage, the pars inferior at stages 24 to 26 (E4 – E5)
As the otic anlage begins to undergo morphogenesis, the ventral portion consists of the evaginating pars inferior. High mitotic activity characterizes its pseudostratified otic epithelium (Lang et al., 2000). Although not obviously segregated at this stage, the prosensory domains are arranged along the medial wall from proximal to distal in the following order: sacccular macula, BP and LM. The first evidence of neurite ingrowth to the prosensory patches from the adjacent stato-acoustic ganglion begins on E4 with fibers penetrating the basal lamina (Knowlton, 1967). However, in the differentiating auditory receptor the majority of fibers remain below the presumptive sensory epithelium until after E5 (Whitehead and Morest, 1985).
Prosensory
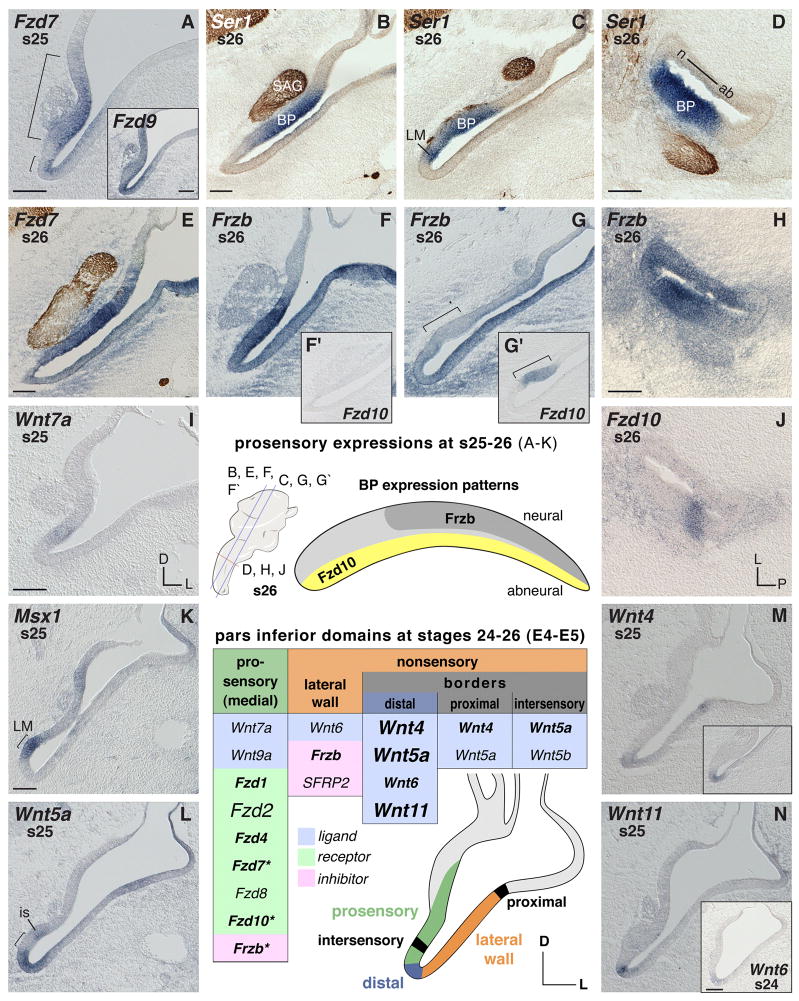
The prosensory medial wall of the pars inferior (Fig. 1A) as revealed by prosensory markers on consecutive serial sections and neurofilament labeling (compare Fig. 1 B–D) is characterized by expressions of Frizzled genes. The details can be summarized as follows: Fzd1 and Fzd7 are most prominent in these domains (Fig. 1A, Fzd7, s25), followed by less intense Fzd4 expression (not shown). By s26, Fzd1 becomes weak in the BP primordium, while Fzd4 and Fzd7 continue to be relatively strong in the developing BP (Fig. 1E, Fzd7). Although also expressed medially, Fzd2 is not as restricted to prosensory domains as the other mentioned receptor genes (not shown). Fzd8 is expressed at relatively low levels in the pars inferior prosensory domains compared to its transcription in the vestibular prosensory patches of the dorsal ear (not shown). Exclusively expressed in the presumptive auditory receptor is Fzd10 (Fig. 1G′), albeit not before s26. At that time, the elongation of the BP primordium is accompanied by complementary expression of particular inhibitor and receptor genes along and across the epithelium (Fig. 1E–H and J). In the neural-basal half of the BP primordium, where prospective hair cells and supporting cells leave the mitotic cycle first, Fzd7 (Fig. 1E) and Frzb (Fig. 1F) are highly expressed, while they are transcribed weakly in the distal BP portion (Fig. 1G, bracket, shows this for Frzb in the same ear as in F). The latter area, where hair cells differentiate last, is where Fzd10 (Fig. 1G′, bracket) is strongest, whereas Fzd10 is absent in the neural-basal papilla (Fig. 1F′). These locally concentrated expression patterns (summarized for Frzb and Fzd10 in the schematic of the auditory epithelium, Fig. 1, center) roughly parallel the later developmental gradient of hair cell births on E5–E7 (Katayama and Corwin, 1989). Genes with different expression levels throughout the BP are marked with asterisks in summary table Fig. 1. The stato-acoustic ganglion shows regional expressions of Fzd1, Fzd8, and Dkk1 by s26 (see Fig. 2I for Fzd8).
Fig. 1. Ventral ear (pars inferior) mRNA expression between s24–26 (E4–5).
In situ hybridization signals (purple) are exemplarily counterstained by labeling of neurofilament (brown) with neurofilament-associated 3A10 antibody in sample images. A–K: Expression in prosensory pars inferior areas. Brackets in A demarcate prosensory regions of the medial pars inferior. A insert: Broad expression of Fzd9 in the ventral otic anlage. B–D: Two independent markers reveal pro-sensory areas: Ser1 (purple), and for neuronal processes, NF-antibody 3A10 (brown). B and C: Two section levels of the same ear through the anterior-basal half and posterior-distal basilar papilla (BP) plus lagenar macula (LM), respectively. E, F, and F′: Anterior section level through the neural-basal half of the BP showing strong expression of Fzd7 and Frzb, while Fzd10, F′, is negative in the neural-basal half of the BP. G and G′: Posterior section level through the abneural pars inferior and the distal BP (bracket) of the same ear, respectively, showing weak Frzb and distinct Fzd10 expression. F and G: Lateral nonsensory pars inferior expresses Frzb. H and J: Cross sections reveal local concentration of Frzb and Fzd10 transcripts in the BP (compare to D; these three are consecutive sections of the same ear). H: Lateral nonsensory pars inferior expresses Frzb. BP shows Frzb expression gradient with highest concentration in the anterior-neural two-thirds. J: Local expression of Fzd10 in the abneural BP. I: Weak expression of Wnt7a in the prosensory pars inferior. K: Msx1 probe used as marker to reveal the LM prosensory patch (bracket). L–N: Wnt expression in border domains. L: Wnt5a transcripts flank the LM (bracket) (compare to K). Abbreviations: ab, abneural; BP, basilar papilla; D dorsal; is, intersensory domain; L, lateral; LM, lagenar macula; n, neural; P, posterior; s, stage; SAG, stato-acoustic ganglion. Each scale bar equals 100μm and applies to consecutive images including inserts, unless otherwise indicated. Center: Orientation and section levels for s26 images are indicated in the cartoon of the otic anlage, lateral view (left); a map of the complementary gene expressions (Frzb and Fzd10) is shown in the schematic outline of the BP (right).
Summary table s24–26 (E4–5): Otic epithelium domains express the listed genes. Table headers refer to otic domains shown in the color-coded schematic. Gene expression over the summarized time span is categorized by different font sizes and font faces to indicate distinct versus weak or temporally-varying expression, as follows: Font face (bold/plain) refers to signal strength: bold indicates obvious (high signal-to-noise) labeling; plain indicates weaker detection or lower signal-to-noise labeling. Relative signal strengths may be probe-dependent and do not necessarily reflect quantitative differences across probes. Font size (small/large) refers to either duration of the expression or local restriction: small means not continuously expressed over the time window covered in this panel description or the gene is expressed only in particular regions of that domain; large refers to broad expression within the domain. Summary table s24–26 (E4–5), does not list Dkk1, Fzd9, Wnt7b that are broadly expressed in the ventral otic anlage and therefore are not specific for a particular domain. Asterisks label genes with local expression or a gradient along and across the BP primordium (see text).
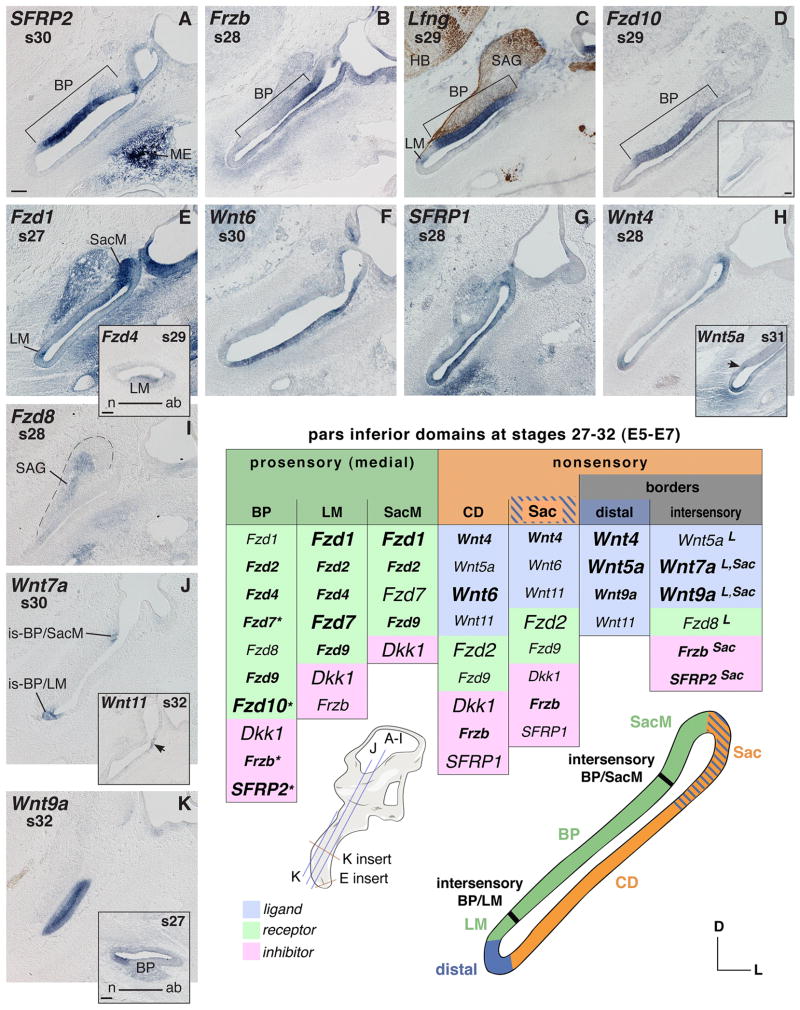
Fig. 2. Pars inferior mRNA expression between s27–32 (E5–7).
A–E Prosensory expression. A, B and D: Basilar papilla (BP) expresses SFRP2 and regionally Frzb and Fzd10. C: Double labeling using Lfng probe as prosensory marker and neurofilament-associated antibody 3A10 reveals innervated prosensory areas. C, D and D insert: Adjacent tissue sections of the same embryo probed for Lfng or Fzd10. D: Robust expression of Fzd10 in the distal and abneural-middle part of the BP. D insert: Fzd10 weakly expressed in the middle-to-neural BP on an adjacent slightly more anterior section level. E and E insert: Frizzled expression in vestibular prosensory domains. E: Maculae of the saccule and the lagena transcribe Fzd1. E insert: Cross section through the distal cochlear duct (CD) shows Fzd4 expression in the lagenar macula (LM). F–H: Wnt and SFRP1 gene transcripts in the nonsensory pars inferior (nonsensory CD and Sac). H insert: Wnt5a expression (1) in the periotic mesenchyme at the distal tip of the CD; (2) the intersensory region between BP and LM (arrow); and (3) the distal nonsensory LM. I: Fzd8 regionally expressed in the SAG (outlined by dashed line). J and K: Border domains expressing Wnt7a and Wnt9a. J insert: Wnt11 positive region (arrow) at the junction of nonsensory Sac and CD. K: Anterior (neural) pars inferior expresses Wnt9a; K insert: cross section through the cochlear duct, showing this neural CD localization of Wnt9a transcripts at a younger stage of development. Abbreviations: ab, abneural; BP, basilar papilla; CD, cochlear duct; D, dorsal; HB, hindbrain; is, intersensory; L, lateral; LM, lagenar macula; ME, middle ear; n, neural; s, stage; Sac, saccule; SacM, saccular macula; SAG, stato-acoustic ganglion. Each scale bar equals 100μm and applies to consecutive images including inserts, unless otherwise indicated. Orientation and section levels are indicated in the 3D-cartoon of the otic anlage, lateral view. Summary table s27–32 (E5–7) (see legend to Fig. 1 for font conventions).
Of the Wnt genes, only Wnt7a and Wnt9a are expressed transiently within prosensory patches of the pars inferior at s25 (Fig. 1I shows this for Wnt7a), although this expression is weak when compared to positive control tissue elsewhere in the embryo (not shown).
Nonsensory
The nonsensory pars inferior expresses mostly Wnts, while Frizzleds are expressed in the prosensory domains (see above). Based on the pattern of gene expression domains that are evident in our samples, we have divided the nonsensory regions into the lateral wall of the ventral otic epithelium and several border domains (described separately below).
Wnt6 is initially concentrated at the distal tip of the pars inferior (at s24) (Fig. 1N insert) before increasing in abundance along the full length of the lateral wall by s25 (for later stage, see Fig. 2F). Frzb and SFRP2 overlap in the nonsensory lateral wall at s24–25 (not shown). However, by s26 Frzb represents the dominant Wnt-inhibitor gene found along the lateral pars inferior (Fig. 1G).
Borders
The most prominent Wnt expression domain in the ventral otic anlage at s24–26 is the distal tip of the pars inferior, which forms a border between the prosensory patch of the LM, revealed by the marker probe Msx1 (cf Wu and Oh, 1996) (Fig. 1K), and the nonsensory lateral wall. Wnt5a (Fig. 1L), Wnt4 (Fig. 1M insert), Wnt11 (Fig. 1N), and Wnt6 (Fig. 1N insert) are each expressed in this distal domain. Another border is established proximally by Wnt4 (Fig. 1M) and Wnt5a, flanking the prosensory patch of the utricular macula at the junction of pars inferior and superior. Finally, on the medial side, Wnt5a (Fig. 1L) and Wnt5b flank the lagenar sensory patch; we have designated this region as “intersensory” border (Fig. 1L, is).
Broad epithelial expression patterns
Several genes are expressed broadly throughout the pars inferior while also showing a more intense signal near the apical (lumenal) surfaces of the otic epithelium at s24–25. These include Dkk1, Fzd9 (Fig. 1A insert), Wnt7b, and Wnt9a. Due to the absence of regionally-restricted expression, these genes are not all listed in the summary table of Fig. 1.
Periotic mesenchyme
With the exception of Fzd1 (not shown), there is no specific Wnt-related gene expression in the mesenchyme adjacent to the pars inferior at s24 and 25. However, from s26 onwards, several genes are found. Fzd1, Fzd8, and the Wnt-inhibitor gene, Frzb, are strongly expressed in the periotic mesenchyme, with the latter being especially prominent lateral and ventral to the pars inferior (Fig. 1F and G). Moderate levels of Fzd7, Fzd9, and Dkk1 transcripts are present in the periotic mesenchyme (Fig. 1E shows Fzd7). In addition, the mesenchyme of the presumptive middle ear anlage has a high accumulation of SFRP2 transcripts (see Fig. 2A).
Pars inferior at stages 27 to 32 (E5 – E7)
By E5 (s27), the developing inner ear has undergone substantial dorsoventral expansion. The lumen of the cochlear duct maintains a broad communication with the lumen of the saccule until the junction begins to constrict at E7.5 (s32) (Cohen and Cotanche, 1992). The BP and the LM do not separate until E6.5 (s30) based on BMP4 as a molecular marker for sensory primordia (Wu and Oh, 1996). However, the distal expression of both Msx1 (s25) and FGF8 (s27) suggest that LM specification takes place earlier, even if segregration of the organs is not yet completed (see Fig. 1K and Sanchez-Calderon et al. 2004). Anatomically, the auditory receptor primordium can first be identified at late E5 to E6 (s28–29) (Cohen and Cotanche, 1992). E5–E6 is when the first hair cell and supporting cell progenitors of the presumptive auditory organ leave the mitotic cycle (Katayama and Corwin, 1989). Cell differentiation, as detected by HCA (Hair Cell Antigen), begins at E5 (s27) in the vestibular saccular macula, and at E6 (s29) in the LM/BP (Bartolami et al., 1991) with just a few cells visible. According to Whitehead and Morest (1985) during E5–E7 (s27–31) there is a phase of massive neurite ingrowth, when most of the ganglion cell fibers invade the sensory primordia.
Prosensory
SFRP2 displays the most striking Wnt-related gene expression in the BP, with the highest concentration in the middle and distal regions of this developing epithelium (Fig. 2A). All tested Frizzled receptors are transcribed in the prosensory pars inferior in a differential manner, while none of the Wnt ligand genes are particularly evident there between s27–32. The three presumptive sensory organs have partly overlapping Frizzled expression patterns.
The auditory primordium shows expressions of Fzd4, Fzd7, and the aforementioned unique and regional Fzd10 (Fig. 2D and D insert). Less distinctly is Fzd2, and the BP transcribes weakly Fzd1 and Fzd8 (Fig. 2E and I). From s32, Fzd9 is expressed in the domain of differentiating sensory progenitor cells in the superficial layer of the BP (see Fig. 3D, s34). The localized expressions along and across the developing BP described earlier are maintained until at least s29 (E6). That is, Fzd7 and Frzb are highest in the neural-base (Fig. 2B, Frzb) in a complementary fashion to SFRP2 (Fig. 2A) and Fzd10 (Fig. 2D). Comparison with prosensory marker gene expression (Lfng) (Fig. 2C) reveals further a gradual change from weak Fzd10 transcription in the middle BP to a robust expression in the middle-to-abneural BP (see Fig. 2C, Lfng, and the adjacent section level of the same embryo probed for Fzd10, Fig. 2D insert, compared to Fzd10 in the slightly more posterior middle-to-abneural BP, Fig. 2D). At s30, the gradients of Fzd7 and Frzb are lost and both become confined to only the BP base (not shown). In contrast, the domains of Fzd10 and SFRP2 (Fig. 2A) are maintained. Fzd10 continues to be excluded from the neural BP where the expression of SFRP2 is also weak (Fig. 2A).
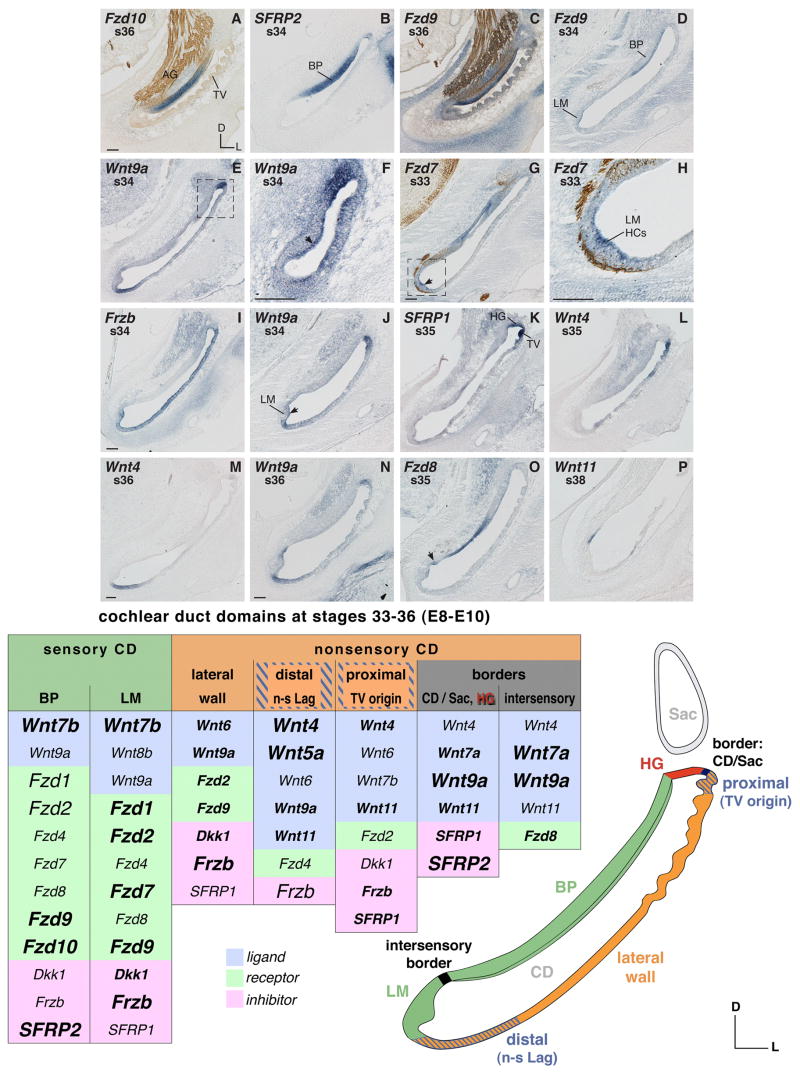
Fig. 3. Cochlear duct mRNA expression between s33–36 (E8–10).
A–D: Frizzled and SFRP2 expression in the basilar papilla (BP). A and B: BP supporting cell domain transcribes Fzd10 and SFRP2. C and D: Hair cells express Fzd9. E: Wnt9a expression (1) in the presumptive homogene cell domain in the proximal CD (frame); (2) in the nonsensory lagena at the distal end of the CD. F: Hair cells (arrow) in the basal BP transcribe Wnt9a at s34 (see frame in E, adjacent section). G–J: LM hair cells transcribe Fzd7, Frzb, and Wnt9a. H: Detail magnification of LM (frame in G). I: Frzb labels the entire nonsensory cochlear duct and the LM. K and L: Transcripts of SFRP1 and Wnt4 in the proximal CD. M–P: Transcripts bordering the LM. O: Fzd8 expressed in the abneural border flanking the long axis of the BP. The arrow marks the intersensory border. Abbreviations: AG, auditory ganglion; BP, basilar papilla; CD, cochlear duct; D, dorsal; HCs, hair cells; HG, homogene cell domain; L, lateral; LM, lagenar macula; n-s Lag, nonsensory lagena; Sac, saccule; s, stage; TV, tegmentum vasculosum. Each scale bar equals 100μm and applies to consecutive images including inserts, unless otherwise indicated. Coun-terstaining of neurofilaments labeled with 3A10 antibody (see A, C, G and H). Summary table s27–32 (E5–7) (see legend to Fig. 1 for explanation and font conventions).
The lagenar primordium can be distiguished by a local increase in the transcription of the prosensory marker, Lfng (LM in Fig. 2C) as well as Fzd1 (Fig. 2E) and Fzd7 (not shown). Although initially broad within the LM, Fzd4 becomes confined to the presumptive hair cell layer at s29 (E6) (Fig. 2E insert). This cellular specificity at s29 and thereafter is also apparent for Fzd2, Fzd9 and the other lagenar-expressed Fzd genes (see e.g. Fig. 3D and G). In addition to the receptors, Frzb shows lagenar transcripts, but rather weakly until after s30 (E6.5) (described later, Fig. 3I).
For the prosensory saccule, a robust expression of Fzd1 (Fig. 2E) is accompanied by locally concentrated Fzd2 and some Fzd7, fading in from the adjacent BP domain (not shown).
The stato-acoustic ganglion expression of Fzd1, Fzd8, and Dkk1 mentioned earlier continues into s27–29 (Fig. 2I shows this for Fzd8).
Broad expression in the pars inferior is displayed by Fzd2, Fzd9 and also Dkk1, albeit they seem to concentrate locally in the forming hair cell layer (for Dkk1 this localization becomes more obvious by s33/34) (not shown). For example, the weak and broad expression of Fzd9 begins to be confined to the apical layer in all three prosensory epithelia at s32 and later (see further in the next stages description).
Nonsensory
Wnt6 and SFRP1 are present along the lateral pars inferior (Fig. 2F, G). An inverse expression pattern is apparent for Wnt4 (Fig. 2H) compared to Wnt6. As a result, although Wnt4 and Wnt6 are broadly overlapping, Wnt6 expression characterizes the nonsensory cochlear duct whereas Wnt4 expression is more characteristic of the forming nonsensory saccule (proximal) and the nonsensory lagena (distal). Particularly at s29 (E6), Wnt5a appears to temporarily extend its otherwise more constricted distal occurrence to an expression along the entire nonsensory pars inferior (not shown). Then, from s31 (E7) onwards, the cochlear Wnt5a expression is again confined to the nonsensory lagena (Fig. 2H insert). Frzb becomes the predominant Wnt antagonist in the entire nonsensory pars inferior from s30 (E6.5) onwards (see later Fig. 3I). Faintly and sporadically, Wnt11 transcripts are detectable in the nonsensory saccule and, particularly at s32, also in the adjacent proximal cochlear duct (Fig. 2J insert). In addition, there is a singular occurrence of weak Wnt7b expression in the nonsensory saccule at s30 (E6.5) (not shown): the transcript is not detectable in this tissue at earlier or later time points. Instead, it is up-regulated in sensory epithelia from s34 (E8) onwards (see description of next developmental stages).
Borders
Based on Wnt-related gene expression compared to molecular markers for prosensory domains, several border domains are apparent in the pars inferior at s27–32. These are: (1) a distal domain which differentiates into the nonsensory lagena; (2) a proximal region where the forming nonsensory saccule is connected to the nonsensory cochlear duct (see under “Sac” in Fig. 2 summary table); two “intersensory” domains bordering the differentiating BP, on one side (3) towards the primordium of the LM (intersensory BP/LM), and on the other side (4) towards the macula of the saccule (intersensory BP/SacM); and finally (5) the anterior flank of the pars inferior bordering the neural BP (not represented in summary table). Expression in these border domains is as follows:
The distal pars inferior expresses Wnt4 (Fig. 2H), Wnt5a (Fig. 2H insert), and faintly Wnt11 (not shown). From s32 (E7.5) onwards, Wnt9a is transcribed in this domain, albeit the expression is relatively weak compared to its occurrence in the intersensory domains (see below).
The nonsensory tissue connection between the saccule and the cochlear duct expresses Wnt11 at s32 (E7.5), a time when the communication between both organ compartments begins to constrict (Fig. 2J insert).
The intersensory BP/LM strongly expresses Wnt7a (Fig. 2J) and Wnt9a (not shown). A faint demarcation is visible also by detection of Wnt5a transcripts in this tissue at s31 (see arrow in Fig. 2H insert). In addition to prosensory markers, differential receptor gene expression patterns demarcate the intersensory BP/LM domain as follows: The expression of Fzd4 (s28) and Fzd7 leaves a gap along the medial wall (see Fig. 1A for Fzd7 at an earlier stage). Fzd8 fills this gap with its “intersensory” expression at s28 (not shown, see a similar pattern later, Fig. 3O), although this receptor gene is rather weakly transcribed in the entire ventral ear in the period of s27–32 (see Fig. 2I).
The intersensory BP/SacM domain, proximal to the auditory organ, resembles the BP/LM domain in that Wnt7a and Wnt9a are both expressed in this region (Fig. 2J shows this for Wnt7a). In addition, this junction transcribes Frzb (Fig. 2B) and SFRP2 (Fig. 2A).
The two intersensory borders are interconnected via an expression domain flanking the auditory epithelium along its entire anterior-neural side, evidenced by a continuous expression of Wnt7a and Wnt9a (Fig. 2K). This domain abuts the cochlear nonsensory expression of Wnt4 and Wnt6 (described above). Cross sections through the cochlear duct probed for Wnt9a reveal this asymmetric neural expression domain adjacent to one side of the developing BP (Fig. 2K insert).
Broad epithelial expression patterns
Between s27–32 (E5 to E7), several genes are broadly expressed in the ventral ear; these are Dkk1, Fzd2, and Fzd9.
Periotic mesenchyme
The mesenchyme surrounding the cochlear duct shows transcripts in various intensity of all Frizzleds (Fig. 2E, 2I and see e.g. Fig. 3C), with the exceptions of Fzd4 and Fzd10 (cf. Fig. 3A for Fzd10). Also present are transcripts for three Wnt-antagonists, Frzb (Fig. 2B), SFRP1 (Fig. 2G), Dkk1 and one ligand, Wnt5a (Fig. 2H insert). Frzb localizes in condensing mesenchyme around the distal and lateral cochlear duct (Fig. 2B). Other local concentrations are apparent: Wnt5a is strongly expressed in the mesenchyme surrounding the distal tip of the cochlear duct (Fig. 2H insert); transcripts of SFRP1 are found in the periotic mesenchyme of the ventral-lateral half of the pars inferior (Fig. 2G), while Fzd1 shows expression only laterally but not distally (Fig. 2E). All transcripts leave a negative hollow immediately adjacent to the otic epithelium (Fig. 2B, 2G shows that for the inhibitor genes). This becomes particularly striking around s32 (E7–8), when cartilage condensation takes place. More distant from the cochlear duct, the fourth Wnt-antagonist gene, SFRP2, is remarkably strong in the condensing mesenchyme of the forming middle ear (Fig. 2A), as described for younger stages.
Cochlear duct at stages 33 to 36 (E8 – E10)
The ventral ear, described so far as pars inferior, separates into the saccule and the cochlear duct. By E8/9 (s35) the communication between the two compartments constricts and narrows, so that both are finally interconnected solely by the ductus reuniens (Cohen and Cotanche, 1992).
Tissue specification leads to formation of the tegmentum vasculosum, a tissue that is thought to be responsible for the endolymph ionic composition and the endocochlear potential. The first tegmentum-folds form in the proximal cochlear duct at E8–9 (s35) and then subsequently progress throughout its entire length to the nonsensory lagena (Cotanche and Sulik, 1982). Tissue that consists of homogene and clear cells becomes functional at E9 (~ s35) and contributes to the forming tectorial membrane by secretion of the second layer matrix according to Shiel and Cotanche (1990). Finally, the completion of the tectorial membrane coincides with the opening of the perilymphatic spaces (Fermin and Cohen, 1984), which according to our data is evident at s36/37 (E10/E11).
Between E8–10 (s34–36) auditory hair cells begin to mature systematically both across and along the BP. While hair cell production initiates in the middle and neural side (Katayama and Corwin, 1989), biased stereociliary bundle orientation to achieve planar cell polarity starts along the abneural (inferior) BP between E9–11 (Shiel and Cotanche, 1990). Spatially and temporally this coincides with the differentiation of short hair cells in the abneural BP (see below description of cell differentiation).
Sensory
The BP and LM display distinct mRNA expression patterns. A core set of genes, Fzd10 (Fig. 3A), SFRP2 (Fig. 3B), Wnt7b, and Fzd9 (Fig. 3C and D), characterizes the differentiating BP. This ‘core set’ is joined in a broader and less specific manner by all other studied receptor genes, as well as Dkk1. At s34, transcript localization of Wnt9a occurs in the BP where hair cells differentiate (Fig. 3E frame and 3F). Frzb refines its expression to the nonsensory cochlear duct (and the LM) by s35, finally excluding the auditory epithelium. However, at s34, although most of the BP is already negative for Frzb, the neural mid-base still expresses this Wnt-antagonist gene (Fig. 3I). Thus, the region of strongest Frzb expression earlier remains positive the longest.
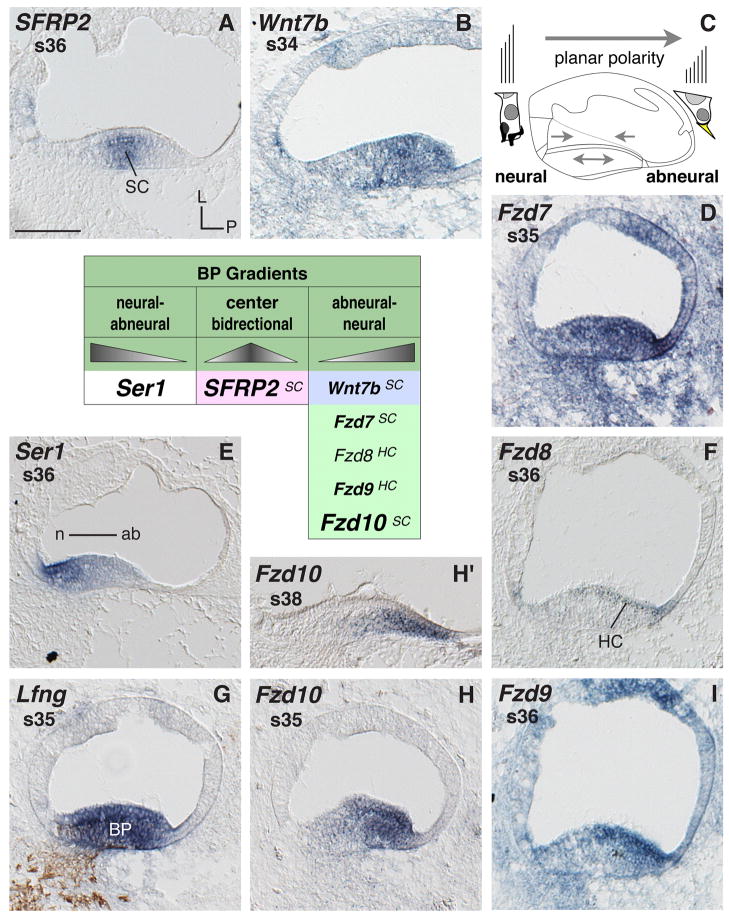
Locally concentrated mRNA expressions are striking across the width of the differentiating auditory epithelium (summarized in the table Fig. 4). SFRP2 shows a central expression, peaking in the middle of the BP (Fig. 4A). The other Wnt-related genes with an asymmetric expression pattern are all concentrated on the abneural side of the BP. These are Wnt7b (Fig. 4B), Fzd7 (Fig. 4D), and Fzd10 (Fig. 4H and H′). Similarly but more specific to hair cells, this is displayed by Fzd8 (Fig. 4F) and Fzd9 (Fig. 4I) at s36 in the BP mid-base. Such local concentration remains for example in the case of Fzd10 at s41 (the latest stage tested) (Fig. 4H′ shows s38). A complementary expression pattern, with the highest expression at the neural side of the BP, is shown by the sensory marker, Ser1, which is a Notch ligand gene (Fig. 3E) (this asymmetry contrasts with the other sensory marker Lfng, Fig. 4G). Thus, the BP expresses several genes in a radially asymmetric fashion that coincides with the onset of planar cell polarity as well as differentiation of hair cell types (see schematic Fig. 4C).
Fig. 4. Basilar papilla mRNA expression.
Gene expression in cross sections through the cochlear duct (CD) and basilar papilla (BP). A, B, D–F and H–I: BP expresses several genes locally concentrated across the neural-to-abneural axis. C: Cartoon cross section of the CD with enlarged neural vs. abneural BP hair cell schematics summarizes the main histological and physiological polarity across the BP (hair cell and bundle shape, innervation, and bundle orientation). Arrows within the CD indicate presumed directional signaling sources based on different gene expression. G: Double-labeling of the BP (Lfng probe) and of neurofilament (antibody 3A10). The table summarizes genes with locally concentrated expressions across the BP. Triangle shapes illustrate the relative signal levels of individual probes. A superscript at the listed gene refers to the main cell type expressing the transcript. Abbreviations: ab, abneural; BP, basilar papilla; HC, hair cells; L, lateral; n neural; P, posterior; s, stage; SC, supporting cells. Scale bar equals 100μm.
In the LM, Wnt7b and Fzd9 (Fig. 3D) overlap with the set of genes described as typical for the auditory organ (excluding Fzd10 and SFRP2, see Fig. 3B). In addition, the LM exhibits some faint Wnt8b expression (not shown). Parallel to this, all tested Frizzled receptor genes are present (with the exception of Fzd10), along with the Wnt-antagonizing genes Frzb (Fig. 3I), Dkk1, and SFRP1. The lagenar transcripts are mostly confined to the differentiating hair cells (Fig. 3D, G, H, I, and J), with the exception of Wnt7b that populates the entire macula (not shown). Wnt9a shows this macular hair cell expression as well (Fig. 3J), although temporally restricted to s34. Of all the expressed genes in LM hair cells, Frzb (Fig. 3I) and Fzd1, -2, and -7 (Fig. 3G and H) are most striking.
Nonsensory
The nonsensory cochlear duct consists of specialized tissue domains surrounding the sensory organs. These are: the lateral wall of the cochlear duct, which subsequently differentiates into the tegmentum vasculosum and the nonsensory lagenar tissue at the distal end; the homogene and clear cells adjacent to the neural (anterior) side of the BP; and the cochlear limbus tissue on the abneural (posterior) side.
The whole nonsensory cochlear duct domain is broadly occupied by transcripts of the Wnt inhibitor gene, Frzb (Fig. 3I). Wnt6, which was the main Wnt ligand gene expressed over the length of the nonsensory cochlear duct during earlier stages, is now widely down-regulated in this domain beyond s34 (E8) (not shown). The same seems to be true for SFRP1: its expression remains strong only in the proximal cochlear duct (Fig. 3K), the initial region of the forming tegmentum vasculosum and the adjacent differentiating homogene cells. The proximal cochlear duct is where partially overlapping and temporally changing gene expression patterns accompany the specification of these two tissue types (see description of expression boundaries and borders below). From s36 (E10), the tegmentum vasculosum shows regionally strong expression of Wnt9a, Fzd2, Fzd9, Frzb and Dkk1 (see e.g. Fig. 5E, Fzd9; and 5F, Wnt9a).
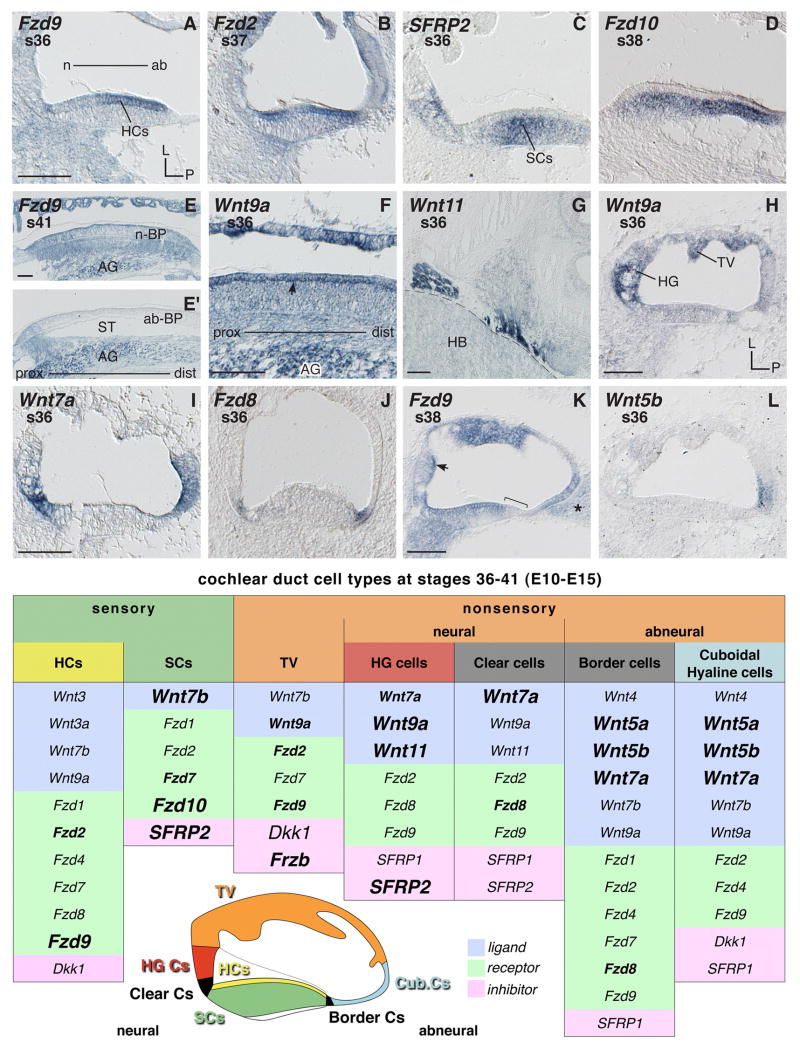
Fig. 5. Cochlear duct mRNA expressions between s36–41 (E10–15).
A, B and H–L: Cross sections through the cochlear duct (CD) and basilar papilla (BP). A and B: Hair cells expressing Frizzleds. C and D: Supporting cells transcribing SFRP2 and Fzd10, respectively. E and F: Longitudinal sections along the BP. E and E′: Neural BP hair cells express Fzd9 (see E); abneural BP hair cells do not transcribe Fzd9 (see E′ middle part where the basilar membrane is situated over the scala tympani). TV cells (see E) as well as afferent cell bodies in the auditory ganglion express Fzd9. F: Expression of Wnt9a (1) in the basolateral region of hair cells (arrow) at s36, (2) TV, and (3) auditory ganglion cell bodies (AG). G: Glia/Schwann cells transcribe Wnt11 at the border to the hindbrain. H–L: Cross sections showing gene expression in different cell types of the CD (compare with schema for details). K: Arrow indicates lumenal surface of HG cells expressing Fzd9; bracket marks abneural BP hair cells negative for Fzd9 transcription; asterisk marks abneural mesenchyme adjacent to the CD. Abbreviations: ab, abneural; ab-BP, abneural BP; AG, auditory ganglion; Cub, cuboidal; Cs, cells; dist, distal; HB, hindbrain; HCs, hair cells; HG, homogene cells; L, lateral; n, neural; n-BP, neural BP; P, posterior; prox, proximal; s, stage; SCs, supporting cells; ST, scala tympani; TV, tegmentum vasculosum. Each scale bar equals 100μm and applies to consecutive images unless otherwise indicated. Summary table s36–41 (E10–15) (see legend to Fig. 1 for font conventions).
Signal in the distal nonsensory cochlear duct is considered to belong to the forming lagena. Wnt4 (Fig. 3M), Wnt5a, and Wnt9a (Fig. 3E and N) show distinct expression in this area, as does Wnt11 after s36 (E10) (not shown). As described earlier, Wnt6 is expressed along the nonsensory cochlear duct overlapping with the nonsensory lagenar domain until s34. Wnt6 differs from Wnt4 and the other nonsensory lagenar expressors in that it fails to reach the lateral edge of the macula. The expression of Frzb, on the other hand, crosses from the nonsensory cochlear duct over into the sensory lagenar domain (Fig. 3I). The only Frizzled receptor that is transcribed sporadically in the nonsensory lagena is Fzd4 (not shown).
Along the posterior (abneural) nonsensory cochlear duct Fzd8 is expressed (Fig. 3O). Shown later, Wnt5a, Wnt5b and Wnt7a transcripts are also abundant in this abneural domain (see Fig. 5I, Wnt7a; and 5L, Wnt5b).
Borders
Expression boundaries and borders in the cochlear duct coincide with: (1) locations of tissue specification; (2) regional compartment separation of the saccule and BP; and (3) the separate fates of cells differentiating in the LM and BP.
Distinctive gene expression patterns in the proximal cochlear duct coincide with the origin of the tegmentum vasculosum and the formation of adjacent homogene cells. Expressions of SFRP1 (Fig. 3K) and Wnt4 (Fig. 3L) are both strong in the proximal tegmentum vasculosum at s35 while they fade out in the distal direction. Yet unlike SFRP1, Wnt4 does not extend into the homogene cell domain, but rather establishes a distinct boundary with it (Fig. 3L). Shortly before the tegmentum vasculosum begins to differentiate, between s32–34, the same abrupt boundary adjacent to but excluding homogene cells can also be seen for Frzb (Fig. 3I), and three Wnts (Wnt6, -7b, -11 not shown). Wnt11 is notable for its spatio-temporal dynamics as follows: in the proximal tegmentum primordium it forms a blunt demarcation with the homogene cell domain but is clearly excluded from it at s32 (E7.5). Between s34 and early s36, Wnt11 becomes restricted to a border domain separating the presumptive homogene cells and the tegmentum vasculosum (see under “border: CD/Sac” in Fig. 3 summary table). Curiously then, from s36 onwards, Wnt11 is expressed specifically in homogene cells, which remain positive for Wnt11 at the latest age tested (s39) (not shown). This means that over time, the expression of Wnt11 shows spatial changes, being first transcribed in presumptive tegmentum tissue preceding its differentiation, then forming a local border, and finally being expressed specifically by the adjacent tissue type, the homogene cells. The other genes listed above, however, continue to exclude the homogene cell domain. In addition, Dkk1, which is expressed in the proximal cochlear duct, becomes downregulated in the homogene cell domain by s37 while continuing its expression in the tegmentum vasculosum (not shown). None of the Frizzled receptors are specifically transcribed in differentiating homogene cells at s33–36.
The tissues of the proximal cochlear duct represent the remaining connection with the saccule. Thus genes expressed specifically in the homogene cell domain (Wnt7a, -9a, -11, SFRP1, and SFRP2) contribute to a boundary not only between the BP and the tegmentum vasculosum but also between the BP and the saccule. Likewise, genes expressed locally at the junction of tegmentum vasculosum and homogene cells (Wnt4 and Wnt11) border the nonsensory saccule as well.
At the intersensory border between BP and LM, Fzd8 transcripts are visible (Fig. 3O). This region also expresses Wnt4, Wnt7a, Wnt9a (Fig. 3N) and Wnt11 (Fig. 3P). Wnt9a, which flanks the LM, extends its expression into the sensory cells of the differentiating LM at s34/35 (E8–9) (Fig. 3J).
Cochlear cell differentiation at stages 36 to 41 (E10 to E15)
E10–12 (s36 to s38) is a phase when the BP undergoes various cell differentiations. Hair cells and supporting cells become distinguishable by E10 (Cohen and Cotanche, 1992). By E11 (s37), the hair cell stereocilia begin to form a staircase by differential growth in length (Cohen and Fermin, 1978) and a cuticular plate forms at the bundle base. Orientation of hair cell bundles with the kinocillium and the row of longest stereocilia at the abneural side is mainly achieved between E10–14 (s36–s40). Yet, in the mid-apical BP a nearly mature bundle orientation is first attained by E18 (s44) and in the distal apex by post hatch day 3 (Cotanche and Corwin, 1991). Particularly the neural apex reorients during the first 30 days after hatching (Cohen and Cotanche, 1992). Thus the main processes of bundle development, rotation and mature orientation take place from E9 to post hatching day 3 in a spatially specific manner along and across the epithelium (Cotanche and Corwin, 1991). Parallel to the establishment of planar cell polarity, hair cells further differentiate and at E11–E12 (s37–s38), cylindrically-shaped tall hair cells, pitcher-shaped short hair cells, and intermediate hair cells, with differential innervation patterns become distinguishable (Cohen and Fermin, 1978). Evidence of synapses appears with the formation of synaptic bars at E11 (according to Cohen and Fermin, 1978).
The nonsensory cochlear duct is now composed of (1) a neural (superior) domain; (2) the tegmentum vasculosum; and (3) an abneural (inferior) domain forming the limbus. Neurally, homogene cells and clear cells are separating the tegmentum vasculosum from the BP. Abneurally, cochlear tissue differentiates into three cell types. These are the cuboidal cells, hyaline cells and, adjacent to the BP, the border cells. In the differentiating tegmentum vasculosum, dark cells become distinguishable from light cells at s37 (Cotanche and Sulik, 1982), perilymphatic spaces open, and the tectorial membrane is completed (Fermin and Cohen, 1984).
Sensory
The first Wnt-related differential gene expression that discriminates auditory hair cells from supporting cells is shown by Fzd9 as early as s32 and distinctly at s34 (E8) when transcripts localize to the prospective hair cell layer of the BP (see Fig. 3D). This coincides with the identification of hair cells and supporting cells histologically (Cohen and Fermin, 1978; Hirokawa, 1978). The broadly expressed Wnt inhibitor gene Dkk1 also shows some mRNA accumulation in the hair cell domain at s34 (not shown). Hair cells preferentially transcribe Fzd9 (Fig. 5A) and, from s37, Fzd2 (Fig. 5B). Separation of hair cell and supporting cell layers is established in the BP by s36 (E10), when supporting cells express SFRP2 (Fig. 5C), Fzd10 (Fig. 5D), Wnt7b, and less distinctly Fzd7 (not shown). Before becoming confined to hair cells, Fzd2 is weakly expressed in supporting cells as well, at least until s36 (not shown). Thus, several genes described before as being characteristic of the BP become restricted later to the supporting cells, with the exception of Fzd9 that restricts to the hair cells. However, in the case of Wnt7b and Fzd7, this restriction is not perfectly exclusive, especially at s38 (E12) and later, when both genes tend to show some weak expression in the hair cells as well (not shown). By s38/39 hair cells transcribe Wnt3 and Wnt3a (not shown), genes that were not expressed in the ventral ear before. The expression of Fzd9 at s36 tends to be stronger in the abneural BP (Fig. 5A, see also Fig. 4I). However later (for example at s38 and s41), when tall hair cells and short hair cells are distinguishable, Fzd9 remains expressed in the domain of the afferently innervated tall hair cells that is in the neural and middle part of the auditory epithelium (Fig. 5E and 5K). Yet abneural hair cells (short hair cells) do not transcribe Fzd9 (Fig. 5E′, and 5K bracket).
At s36 (E10), coinciding with formation of synaptic bars at the base of hair cells, the expression of all genes transcribed in hair cells seems to form a thin line along the hair cell base (Fig. 5F shows this for Wnt9a, see arrow). This local mRNA occurrence is detectable for Wnt9a, Dkk1 and all receptor genes tested (with the exception of Fzd10).
Afferent neuronal cell bodies in the auditory ganglion show a distinct expression of Fzd1, Fzd9 (Fig. 5E) and Dkk1. Ganglion expression to a varied extent is shown by all tested Frizzled receptor genes (with the exception of Fzd10), Frzb and SFRP1. Wnt9a, which is negative for example at s32, is expressed in neuron cell bodies from s34/35 (E8–9) onwards (Fig. 5F). In addition to the auditory ganglion, the expression of Wnt9a is particularly high in other cranial nerve ganglia (not shown). At the border with the hindbrain, Schwann or other glia cells within cranial nerves express three Wnts (Wnt5a, -7b, and -11, Fig. 5G), Fzd7, and Frzb.
Nonsensory – tegmentum vasculosum
The differentiating tegmentum vasculosum, which was initially positive (s35) for Wnt4 (see Fig. 3L), SFRP1 (see Fig. 3K) and locally restricted Wnt11, becomes mostly negative for these genes by s36 (not shown). At this time, the cells express Wnt9a (Fig. 5F, H) and, at least in the proximal region, also Wnt7b (not shown). Fzd2, Fzd9 (Fig. 5E), Frzb, Dkk1 and Fzd7 (the latter from s38 onwards) are all present in a locally strong and patchy manner that seems characteristic of the tegmentum.
Nonsensory – neural side
Homogene and clear cells in the neural cochlear duct are characterized by Wnt9a (Fig. 5H), Wnt11 (the latter from s36–37, not shown) and SFRP2 (Fig. 5C). Wnt7a, which mainly concentrates its neural-edge expression to the clear cells (located between the homogene cells and the BP), shows some extension of its mRNA distribution into the homogene cell domain (Fig. 5I). This overlaps with Wnt9a and Wnt11, which are mainly expressed throughout the homogene cells but show in some instances their highest expression in the lower half of that domain which corresponds to the clear cells. At s36, a restricted expression of Fzd8, and less distinctly expression of Fzd2 and -9, demarcate the clear cell domain in some regions of the cochlear duct. For example, Fzd8 shows a distinct occurrence in the clear cells (and abneural border cells) but only in the basal portion of the papilla (Fig. 5J). Later (s38), the expression of those receptor genes transcribed by homogene and clear cells localizes to the lumenal surface of cells in this tissue (the arrow in Fig. 5K indicates this for Fzd9). In addition, the expression of SFRP1 in the homogene cell domain, described for s35 (see above) remains (latest test s38) but is often also reduced to the subcellular region facing the lumen and the adjacent hair cells.
Nonsensory – abneural side
Most prominent in abneural tissue domains are Wnt7a (Fig. 5I), Wnt5a and -5b (Fig. 5L). Their expression labels the complex of cuboidal, hyaline and border cells. Also prominent is Fzd8 yet its expression is restricted to the border cells (Fig. 5J) and may extend into the BP (cf. Fig. 4F), but not hyaline and cuboidal cells. From s38 onwards, abneural cells also express Wnt4, -7b, -9a, Fzd2, -4, -9 (Fig. 5K shows this for Fzd9), Dkk1, and SFRP1, although in some cases these expressions are comparatively weak. In addition, Fzd7 and Fzd1 expression cross from the abneural edge of the BP into the border cell domain (not shown).
Otic Mesenchyme (abneural)
Located adjacent to hyaline and cuboidal cells, the abneural periotic mesenchyme is the last remaining mesenchyme in the opening perilymphatic space. This tissue shows transcripts of Fzd9 (Fig. 5K, asterisk) and Frzb abundantly and also expresses Fzd2, Fzd7, and Dkk1 (not shown).
Discussion
Wnt-related gene expression in chicken, mouse and rat inner ears
Our data expand and refine the list of known Frizzled transcripts in the ventral inner ear beyond the otocyst stage. Altogether we find evidence for the expression of 7 Frizzled genes during cochlear duct development: Fzd1, -2, -4, -7, -8, -9 and -10. Fzd2 and Fzd9 are broadly expressed in both prosensory and nonsensory domains (until they become confined to hair cells and later to specific tissue types as for example the tegmentum vasculosum). Fzd1, -5, -6, -7, and -10 were previously reported to be transcribed in chicken otic tissue (Lewis and Davies, 2002; Stevens et al., 2003). We show the expression patterns of four additional Frizzleds, including Fzd2 and Fzd8 that were not tested previously and Fzd4 and Fzd9 that were presumed to be negative. The exclusive transcription of Fzd10 in the BP reported earlier can now be refined to an earlier time point, as we detected it at s26 (E4–5), whereas Stevens et al. (2003) did not find Fzd10 at E4, E5 or E6 but instead described its onset as E7 (s31).
Our analysis reveals the expression of 11 Wnts in the developing ventral inner ear: Wnt3, -3a, -4, -5a, -5b, -6, -7a, -7b,-8b (faint), -9a, and -11. The vast majority of the Wnt transcripts are present in nonsensory domains, with very few (Wnt7b and to a limited extent Wnt7a, Wnt9a) in the sensory primordia of the BP and LM. We also demonstrate strong expression of the Wnt inhibitors, SFRP2 and Frzb, broad expression of Dkk1, and variable, mostly weak expression of SFRP1. The Wnt inhibitors segregate into subdomains of the ventral ear, possibly to modulate the activity of specific Wnts or Frizzleds at particular times and places.
The chicken expression patterns described here mostly complement previous data from mammals in which Wnts and Fzds were comprehensively studied by transcript profiling. Sajan et al. (2007) found temporally-regulated expression of Wnt7a, Wnt4 and Wnt5a in the mouse cochlea using microarrays. A similar profiling approach for the neonatal mouse cochlea supports expression of three to five of the 14 Wnt genes present on GeneChips: Wnt4, -5a, -7b were robust, while Wnt5b and -10a were marginal (Chen and Corey, 2002). PCR amplification of the postnatal rat cochlea shows transcripts of Wnt2b, -4, -5a, -5b, and -7a in addition to Fzd1, -2, -3, -4, -6, and -9 (Daudet et al., 2002). Our data confirm that 8 of these 11 genes are also expressed in the chicken cochlear duct at mid-gestation; we did not sample Fzd3 or Fzd6 and found Wnt2b only in the dorsal ear (data to be reported in a separate manuscript). In addition, we report mRNA expression of Wnt9a in the chicken inner ear.
Previous in situ hybridization studies of Wnt-related expression in the otocyst indicated that ligands classically associated with β-catenin-dependent canonical Wnt signaling are mostly confined to the dorsal half. Examples include Wnt2b, Wnt3a, and Wnt6 (Hollyday et al., 1995; Jasoni et al., 1999; Lillevali et al., 2006). On the other hand, cells that receive canonical Wnt signaling can be traced from their dorsal otocyst origin into the ventral ear in mice (Riccomagno et al., 2005); the purpose of this intriguing migration is still unknown. Wnt7a was reported in differentiating pillar cells of the perinatal mouse cochlea (Dabdoub et al., 2003). In the chicken cochlear duct, Wnt7a is restricted to the nonsensory flanks of the auditory epithelium, whereas Wnt7b’s presence in the abneural half of the BP may be a closer match to the pattern of Wnt7a in the mouse cochlea. In both mouse and chicken, Wnt5b localizes to nonsensory tissue bordering the auditory epithelium, albeit on both sides in mouse (Daudet et al., 2002) and only abneurally in chicken. Further comparison matches Wnt4 immunoreactivity in the outer spiral sulcus of the mouse (Daudet et al., 2002) to our results of Wnt4 transcription by abneural border and cuboidal cells at later stages of development (from s38, E12).
Functional data pertaining to the role of Wnt-related genes in cochlear development is mostly confined to the mouse and is still rather limited in scope. Fzd4-mutant mice show hair cell loss and progressive deafness (Wang et al., 2001; Xu et al., 2004). A pair of Frizzleds, Fzd3 and Fzd6, is involved in hair cell bundle orientation as indicated by double mutants with disrupted bundle polarity (Wang et al., 2006). In another study of PCP, Qian et al. (2007) demonstrated that Wnt5a antagonizes Frzb in regulating hair cell bundle orientation as well as convergent extension of the cochlea.
Wnt signaling and sensory specification
Stevens et al. (2003) report that the forced expression of canonical Wnt/β-catenin signaling, including Wnt3a misexpression, can induce ectopic sensory patches with LM phenotype in the chicken BP and elsewhere. This observation implies that Wnt/β-catenin signaling is normally kept under tight spatiotemporal regulation, but it also raises the possibility that an endogenous Wnt ligand specifies LM fate. Although Wnt3a is not expressed at the appropriate time and place to serve this function, Wnt4, Wnt6, Wnt5a and Wnt11 are. All four are present in the distal cochlear duct adjacent to where the LM arises. Wnt4 is known for canonical signaling in other systems, for example during joint formation (Guo et al., 2004), while Wnt6 signals through the β-catenin-dependent pathway in dermomyotomes (Linker et al., 2005). Wnt5a and Wnt11 are preferentially noncanonical Wnt cues but can also mediate canonical Wnt signaling (Tao et al., 2005; Mikels and Nusse, 2006).
Wnt inhibitors may constrain Wnt-mediated sensory fate specification
If the LM is specified, at least in part, through canonical Wnt signals, how does the nearby BP primordium normally avoid this influence? We note the strong and persistent expression of transcripts for the Wnt inhibitor, SFRP2, in the BP primordium but not in the LM primordium. SFRP2 inhibits β-catenin stabilization (Yin et al., 2006) and reduces the activity of Wnt1, Wnt3a and Wnt4 (Lee et al., 2000; Wawrzak et al., 2007). We wish to speculate that the strong presence of SFRP2 in the BP primordium actively blocks canonical Wnts that may be diffusing from adjacent tissues, or through the cochlear duct lumen, thus preventing the BP domain from initiating an intracellular β-catenin cascade that would otherwise induce the LM vestibular fate. Our hypothesis is supported by the finding that the few Wnts expressed in the BP (Wnt7b or Wnt9a) that are known to have the ability to signal through β-catenin (Person et al., 2005; Wang et al., 2005), are not transcribed in sensory domains before s34 (E8).
The presumptive homogene cell domain on the neural side of the BP may also need to block canonical Wnt signaling to prevent it from taking on a sensory fate. When challenged with activated β-catenin transgene before s23, these cells are able to convert to a sensory fate (Stevens et al., 2003). The homogene cell domain continuously expresses both Wnt9a and SFRP2. The SFRP2 inhibitor may be important to commit the homogene cell domain to a nonsensory fate by preventing it from activating a β-catenin response during a critical period at early stages. At s35 (~E9), SFRP2 is joined by SFRP1 in the homogene cell region. By s36, homogene and clear cells also express Wnt11. Interestingly, Wnt11 is reported to be effective in blocking canonical Wnt signaling (Maye et al., 2004).
Possible autocrine Wnt activity in non-sensory domains
We do not rule out potential roles for Wnt signaling in other parts of the ventral ear, particularly once cell specification and differentiation are underway. In particular, several Wnt genes are ideally placed to play an autocrine role in non-sensory domains, such as Wnt9a in the tegmentum vasculosum. Other potential sites of autocrine signaling include border cells, cuboidal cells, hyaline cells, clear cells, homogene cells and intersensory border regions. On the other hand, nearly all of these emerging cell types also express one or more Wnt inhibitors, which are generally associated with a block of canonical Wnt signaling. Thus, it would appear that either there is tight spatiotemporal regulation of canonical Wnt signaling or there is predominantly non-canonical Wnt signaling in these non-sensory territories.
Wnt signaling sources across the auditory epithelium
A perusal of Wnts previously associated with PCP in other systems reveals several transcripts that unilaterally border the hair cell domains in the BP, including Wnt5a on the abneural edge. Wnt5a has been implicated in hair bundle polarity in the mouse (Qian et al., 2007). Other non-canonical spatial asymmetries include an abneural source of Wnt5b and a neural source of Wnt11. Also notable is the expression of Wnt7b in a graded fashion across the BP sensory epithelium from s34 (E8). Wnt7b signals canonically as mentioned before (Wang et al., 2005) but also non-canonically (Rosso et al., 2005; Tu et al., 2007). Furthermore, four Frizzled genes show graded expression across the same axis, from high abneurally to low neurally, with Fzd10 being the most robust and prolonged, and Fzd9 as well as Fzd8 appearing at s36. Fzd10 usually operates in the context of canonical signaling (e.g. Wang et al., 2005). If these Frizzleds are activated by the asymmetrically expressed Wnts, our prediction is that this arrangement would steepen radial Wnt activity gradients originating from the abneural side, while flattening activity gradients from the neural side. A radial gradient of PCP Wnt signaling could contribute to the establishment of bundle polarity (towards the abneural edge). Other spatially graded phenotypes evident in the radial dimension, such as hair cell type (tall hair cells vs. short hair cells) or synaptic terminal size, number and type (afferents vs. efferents), may utilize either canonical or non-canonical signaling pathways. We note that Wnt expression asymmetries precede both hair bundle polarization (on E9) and the differentiation of tall and short hair cells (on E12).
Superimposed over the radial gradients is the coincident expression of SFRP2, with its peak in the center of the BP, which confounds the interpretation. If the purpose of SFRP2 at this location is to repress canonical signals, such as strong Wnt9a arising from the neural side or Wnt7a from both sides, then it may simply be permissive for non-canonical signaling across the BP. Alternatively, if any of the bordering Wnts are acting canonically (including possibly Wnt5a), then SFRP2 may sharpen their functional gradients. Or, it may confine the reach of the bordering Wnt ligands to the cells located on only one half of the BP.
Hair cells on the neural half of the BP will differentiate into hair cells receiving mostly afferent innervation (tall hair cells), while hair cells located abneurally will differentiate into hair cells receiving predominantly or only efferent innervation (short hair cells). The presence of neural/abneural asymmetric distributions of both BP innervation and expression of some Wnt-related transcripts highlights a need for determining Fzd protein expression within afferent and efferent growth cones. Wnt3, Wnt4, Wnt5a, Wnt7b and SFRP1 have all been implicated in axon guidance in other systems, where they can function as either attractants or repellants (Endo and Rubin, 2007).
Whether or how these potential Wnt gradients across the sensory BP may interact with radial expression gradients of two other important ligands, Serrate1 (Figure 4E) or BMP4 (Cole et al., 2000 Fig. 1D) remains unexplored.
In summary, our data indicate that there are many potential roles for Wnt signaling during development of the ventral ear. These data will provide a baseline for interpreting both gain-of-function and loss-of-function analyses, and will facilitate comparative studies for unraveling the role of these genes in cochlear development in other vertebrates, including mammals.
Acknowledgments
Riboprobes were kindly provided by Susan Chapman, Gary Schoenwolf, Cliff Tabin, Doris Wu and Julian Lewis. Matías Hidalgo-Sánchez generously shared detailed protocols. We thank Deborah Biesemeier and Katie Holmes for technical assistance. This work was supported by the NIDCD002756 (D.M.F.).
References
- Abu-Elmagd M, Garcia-Morales C, Wheeler GN. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev Biol. 2006;298(1):285–298. doi: 10.1016/j.ydbio.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Bartolami S, Goodyear R, Richardson G. Appearance and distribution of the 275 kD hair-cell antigen during development of the avian inner ear. J Comp Neurol. 1991;314(4):777–788. doi: 10.1002/cne.903140410. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229(3):668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Corey DP. An inner ear gene expression database. JARO. 2002;3:140–148. doi: 10.1007/s101620020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM, Cotanche DA. Development of the sensory receptors and their innervation in the chick cochlea. In: Romand R, editor. Development of Auditory and Vestibular Systems. 2. Vol. 4. Amsterdam: Elsevier; 1992. pp. 101–138. [Google Scholar]
- Cohen GM, Fermin CD. The development of hair cells in the embryonic chick’s basilar papilla. Acta Otolaryngol. 1978;86:342–358. doi: 10.3109/00016487809107513. [DOI] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424(3):509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cotanche D, Sulik K. Scanning electron microscopy of the developing chick tegmentum vasculosum. Scan Electron Microsc Pt. 1982;3:1283–1294. [PubMed] [Google Scholar]
- Cotanche DA, Corwin JT. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear Res. 1991;52(2):379–402. doi: 10.1016/0378-5955(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130(11):2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ripoll C, Molès JP, Rebillard G. Expression of members of Wnt and Frizzled gene families in the postnatal rat cochlea. Brain Res Mol Brain Res. 2002;105:1–2. 98–107. doi: 10.1016/s0169-328x(02)00397-2. [DOI] [PubMed] [Google Scholar]
- Endo Y, Rubin JS. Wnt signaling and neurite outgrowth: Insights and questions. Cancer Sci. 2007;98(9):1311–1317. doi: 10.1111/j.1349-7006.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin CD, Cohen GM. Developmental gradients in the embryonic chick’s basilar papilla. Acta Otolaryngol. 1984;97:39–51. doi: 10.3109/00016488409130963. [DOI] [PubMed] [Google Scholar]
- Fischer FP. General pattern and morphological specializations of the avian cochlea. Scanning Microsc. 1994;8(2):351–363. [PubMed] [Google Scholar]
- Gleich O, Fischer FP, Köppl C, Manley GA. Hearing organ evolution and specialization: Archosaurs. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. New York: Springer-Verlag; 2004. pp. 224–255. [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Grier JB, Counter SA, Shearer WM. Prenatal auditory imprinting in chickens. Science. 1967;155(770):1692–1693. doi: 10.1126/science.155.3770.1692. [DOI] [PubMed] [Google Scholar]
- Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci USA. 2004;101(25):9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–91. [PubMed] [Google Scholar]
- Hatch EP, Noyes CA, Wang X, Wright TJ, Mansour SL. Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development. 2007;134(20):3615–3625. doi: 10.1242/dev.006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50(4):229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. The ultrastructure of the basilar papilla of the chick. J Comp Neurol. 1978;181(2):361–374. doi: 10.1002/cne.901810208. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52(1):9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn. 1999;215(3):215–224. doi: 10.1002/(SICI)1097-0177(199907)215:3<215::AID-AJA4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Jones T, Jones S. Spontaneous activity in the statoacoustic ganglion of the chicken embryo. J Neurophysiol. 2000;83(3):1452–1468. doi: 10.1152/jn.2000.83.3.1452. [DOI] [PubMed] [Google Scholar]
- Katayama A, Corwin JT. Cell production in the chicken cochlea. J Comp Neurol. 1989;281(1):129–135. doi: 10.1002/cne.902810110. [DOI] [PubMed] [Google Scholar]
- Knowlton VY. Correlation of the development of membranous and bony labyrinths, acoustic ganglia, nerves, and brain centers in the chick embryo. J Morph. 1967;121:179–208. [Google Scholar]
- Lang H, Bever MM, Fekete DM. Cell proliferation and cell death in the developing chick inner ear: spatial and temporal patterns. J Comp Neurol. 2000;417(2):205–220. doi: 10.1002/(sici)1096-9861(20000207)417:2<205::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127(1):109–118. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Lewis J, Davies A. Planar cell polarity in the inner ear: How do hair cells acquire their oriented structure? J Neurobiol. 2002;53(2):190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- Li CX, Gong M, Huang YN, Tang ZQ, Chen L. Morphometry of otoliths in chicken macula lagena. Neurosci Lett. 2006;404:1–2. 83–86. doi: 10.1016/j.neulet.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Haugas M, Matilainen T, Pussinen C, Karis A, Salminen M. Gata3 is required for early morphogenesis and Fgf10 expression during otic development. Mech Dev. 2006;123(6):415–429. doi: 10.1016/j.mod.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Linker C, Lesbros C, Gros J, Burrus LW, Rawls A, Marcelle C. beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development. 2005;132(17):3895–3905. doi: 10.1242/dev.01961. [DOI] [PubMed] [Google Scholar]
- Manley GA, Haeseler C, Brix J. Innervation patterns and spontaneous activity of afferent fibres to the lagenar macula and apical basilar papilla of the chick’s cochlea. Hear Res. 1991a;1–2(56):211–226. doi: 10.1016/0378-5955(91)90172-6. [DOI] [PubMed] [Google Scholar]
- Manley GA, Kaiser A, Brix J, Gleich O. Activity patterns of primary auditory-nerve fibres in chickens: development of fundamental properties. Hear Res. 1991b;57(1):1–15. doi: 10.1016/0378-5955(91)90068-k. [DOI] [PubMed] [Google Scholar]
- Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279(23):24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133(5):865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Perez S, Rebelo S, Anderson D. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126(8):1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol. 2005;278(1):35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306(1):121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebillard G, Rubel EW. Electrophysiological study of the maturation of auditory responses from the inner ear of the chick. Brain Res. 1981;229(1):15–23. doi: 10.1016/0006-8993(81)90741-1. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19(13):1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U. Some morphological principles of the vestibular maculae in birds. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1970;197(2):154–182. doi: 10.1007/BF00306164. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Creech HB, Rubel EW. Postnatal changes in the size of the avian cochlear duct. Acta Otolaryngol. 1984;98:93–97. doi: 10.3109/00016488409107539. [DOI] [PubMed] [Google Scholar]
- Sajan SA, Warchol ME, Lovett M. Toward a systems biology of mouse inner ear organogenesis: gene expression pathways, patterns and network analysis. Genetics. 2007;177(1):631–653. doi: 10.1534/genetics.107.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martín-Partido G, Hidalgo-Sánchez M. Otx2, Gbx2, and Fgf8 expression patterns in the chick developing inner ear and their possible roles in otic specification and early innervation. Gene Expr Patterns. 2004;4(6):659–669. doi: 10.1016/j.modgep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martín-Partido G, Hidalgo-Sánchez M. Pax2 expression patterns in the developing chick inner ear. Gene Expr Patterns. 2005;5(6):763–773. doi: 10.1016/j.modgep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12(5):779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Shiel MJ, Cotanche DA. SEM analysis of the developing tectorial membrane in the chick cochlea. Hear Res. 1990;47:147–158. doi: 10.1016/0378-5955(90)90172-l. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93:1–2. 195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol. 2003;261(1):149–164. doi: 10.1016/s0012-1606(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Storey K, Crossley J, De Robertis E, Norris W, Stern C. Neural induction and regionalisation in the chick embryo. Development. 1992;114(3):729–741. doi: 10.1242/dev.114.3.729. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120(6):857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt Signaling through G Protein-Linked PKCdelta Activation Promotes Bone Formation. Dev Cell. 2007;12(1):113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzulli A, Garcia-Austt E. Development of cochlear microphonic potentials in the chick embryo. Acta Neurol Latin Am. 1963;9:19–23. [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26(8):2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21(13):4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25(12):5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzak D, Métioui M, Willems E, Hendrickx M, de Genst E, Leyns L. Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun. 2007;357(4):1119–1123. doi: 10.1016/j.bbrc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The development of innervation patterns in the avian cochlea. Neurosci. 1985;14:255–276. doi: 10.1016/0306-4522(85)90177-0. [DOI] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16(20):6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu O, Wang YS, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yin YJ, Katz V, Salah Z, Maoz M, Cohen I, Uziely B, Turm H, Grisaru-Granovsky S, Suzuki H, Bar-Shavit R. Mammary gland tissue targeted overexpression of human protease-activated receptor 1 reveals a novel link to beta-catenin stabilization. Cancer Res. 2006;66(10):5224–5233. doi: 10.1158/0008-5472.CAN-05-4234. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39(10):1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]