Abstract
Mucormycosis (zygomycosis) is an acute and often fatal opportunistic fungal infection. Predisposing factors in the development of mucormycosis are nonspecific and include hyperglycemia, hematologic malignancies, neutropenia, pharmacologic immunosuppression, solid organ or bone marrow/stem cell transplantation, burns, trauma, malnutrition, and intravenous drug use. Mucormycosis has also been described in patients with iron and aluminum overload, patients on dialysis, and patients receiving iron chelating therapy. We describe a 75-year-old man with myelodysplastic syndrome and iron overload secondary to multiple red blood cell transfusions who had been treated with deferoxamine chelation therapy. He was admitted to the hospital for atrial fibrillation, developed multiple organ failure, and died. Pulmonary invasive mucormycosis was demonstrated at autopsy. This case further documents an association between invasive mucormycosis, iron overload, and deferoxamine therapy.
CASE REPORT
A 75-year-old man was admitted to the hospital because of recurrent atrial fibrillation with rapid ventricular rate. The patient had a history of myelodysplastic syndrome (refractory anemia with ringed sideroblasts subtype), hypertension, hemochromatosis secondary to repeated transfusions, and coronary artery disease for which he had a four-vessel coronary artery bypass 2 years prior to admission. The patient had no known history of diabetes. He denied use of alcohol, tobacco, or intravenous drugs.
The patient received weekly blood transfusions due to anemia secondary to his myelodysplastic syndrome over the last 2 years. The patient had received a total of 30 transfusions, usually two units of packed red blood cells on each occasion. He frequently developed atrial fibrillation and tachycardia in the days leading up to his transfusions. A cardiac evaluation 2 months prior to presentation showed an ejection fraction of 60% to 65%.
His medications included ramipril 5 mg once daily, metoprolol (extended release) 100 mg once daily, aspirin 81 mg once daily, and deferoxamine (DFO). The patient had begun chelation therapy with DFO 4 weeks prior to admission. He had received continuous intravenous infusions of DFO 2 g over a 10-hour period five times per week for a total of 20 separate infusions.
On admission, the patient was in atrial fibrillation with a heart rate of 150 beats per minute. His blood pressure was 115/67 mm Hg, and his temperature was 98.8°F. He was ill appearing and cachectic but was in no acute distress. A II/VI systolic murmur was heard over the left upper sternal border radiating to the carotids. There was 2+ bilateral lower extremity pitting edema and vascular stasis skin changes. The patient had 4/5 strength in all extremities. Chest x-ray on admission showed small bilateral pleural effusions, bibasilar volume loss secondary to atelectasis (left greater than right), and cardiomegaly without overt failure. His laboratory test results on admission are shown in the Table. Hepatitis A, B, and C serology test results were negative.
Table.
Admission laboratory results
| Test | Result | Reference range |
| Sodium (mEq/L) | 133 | 136–145 |
| Potassium (mEq/L) | 6.1 | 3.6–5.0 |
| Chloride (mEq/L) | 98 | 98–107 |
| Bicarbonate (mEq/L) | 15 | 22–30 |
| Blood urea nitrogen (mg/dL) | 55 | 9–20 |
| Creatinine (mg/dL) | 1.4 | 0.7–1.2 |
| Glucose (mg/dL) | 253 | 75–110 |
| Total bilirubin (mg/dL) | 7.0 | 0.2–1.3 |
| Aspartate aminotransferase (U/L) | 450 | 10–50 |
| Alanine aminotransferase (U/L) | 752 | 10–50 |
| Alkaline phosphatase (U/L) | 108 | 40–129 |
| White blood cell count (K/uL) | 7.2 | 4.5–11.0 |
| Hemoglobin (g/dL) | 14.1 | 13.5–18.0 |
| Hematocrit (%) | 40.1 | 40.0–52.0 |
| Platelet count (K/uL) | 234 | 140–440 |
| Ferritin (ng/mL) | 69,600 | 24–33 |
The patient's atrial fibrillation was treated with an intravenous infusion of diltiazem followed by oral metoprolol therapy. He received one infusion of DFO 2 g intravenously on hospital day 3. During the first 4 days of hospitalization he developed acute renal failure; repeat laboratory tests showed a gradual increase in blood urea nitrogen (110 mg/dL) and serum creatinine (3.5 mg/dL). His liver function test results also increased. An abdominal ultrasound demonstrated passive congestion of the liver, small bilateral pleural effusions, gallbladder sludge, and normal-sized kidneys with slightly increased cortical echogenicity. The patient's acute renal failure was thought to be prerenal (random urine sodium level was 2 mEq/L), and the passive congestion of the liver suggested an element of cardiac failure. A right-sided heart catheterization demonstrated moderate pulmonary hypertension with a pulmonary artery pressure of 53/23 mm Hg, increased right atrial pressure of 18 mm Hg with severe tricuspid regurgitation, and a pulmonary artery wedge pressure of 20 mm Hg. A transthoracic echocardiogram revealed an ejection fraction of 20% to 25% with significant new tricuspid regurgitation since the prior study. In addition, there was a diffuse increase in echogenicity of the cardiac muscle, felt to be likely iron deposition.
Over the next 2 days the patient developed progressive hypotension, worsening renal failure, and pulmonary congestion. The patient was not felt to be a candidate for dialysis secondary to severe heart failure. The patient remained afebrile throughout the hospital course, and his white blood cell count remained within normal limits.
Due to the development of multiorgan failure, the family requested pain relief and comfort measures only. The patient's condition gradually declined, with progressive uremia and passive pulmonary congestion, and he expired on hospital day 9. A postmortem examination was requested.
The postmortem findings were significant for a 90% hypercellular bone marrow with multilineage dysplasia and 15% blasts, suggesting transformation from refractory anemia with ringed sideroblasts to acute myeloid leukemia, or at least refractory anemia with excess blasts (RAEB-2). Multiple organs had evidence of marked hemosiderin deposition consistent with secondary hemochromatosis (Figures 1 and 2). The left lung contained an abscess with surrounding hemorrhagic infarction. Histologic examination of sections from this area of the lung revealed fungus with broad irregular branching nonseptate hyphae, fungal vascular invasion, vascular thrombosis, and associated hemorrhagic infarction. The fungal morphology was consistent with Zygomycetes (Figures 3 and 4). Histologic examination of other organs did not reveal any further evidence of disseminated zygomycosis.
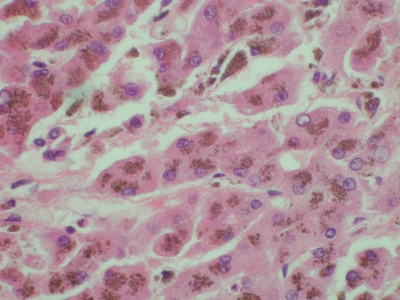
Figure 1.
Liver, high magnification. Hematoxylin and eosin staining demonstrates a large amount of cytoplasmic iron.
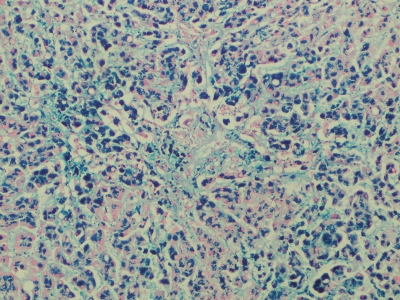
Figure 2.
Liver, medium magnification. Prussian blue stain is strongly positive showing marked iron deposit.
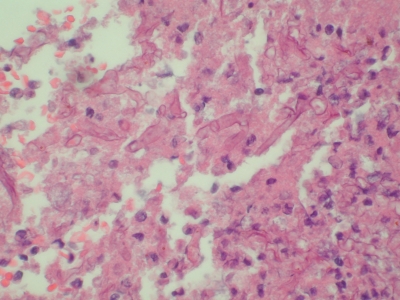
Figure 3.
Left lung, high magnification. Hematoxylin and eosin staining demonstrates fragmented, haphazardly arranged, 10- to 15-μ diameter fungal organisms.
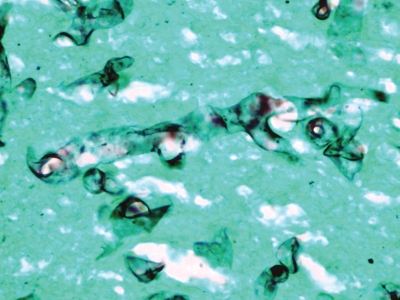
Figure 4.
Left lung, high magnification. Grocott methenamine silver stain reveals broad wavy ribbon-like hyphae, some with 90° branching.
DISCUSSION
Mucormycosis is an acute and often fatal opportunistic fungal infection; it often causes acute angioinvasive infections in immunosuppressed patients, with mortality rates exceeding 60% (1). The most commonly isolated genera include Rhizopus, Mucor, Rhizomucor, and Absidia. Predisposing factors in the development of mucormycosis include diabetes mellitus (with or without ketoacidosis), hematologic malignancies, neutropenia, pharmacologic immunosuppression (e.g., antineoplastic chemotherapy, corticosteroid use, and antirejection therapy), solid organ or bone marrow/stem cell transplantation, burns, trauma, malnutrition, and intravenous drug use (1). Mucormycosis has also been described in patients with iron and aluminum overload, patients on dialysis, and those receiving iron chelating therapy (DFO) (2).
The interrelationship between iron and infectious diseases has been previously described. Iron is essential for the growth and metabolism of microorganisms, including pathogenic microorganisms. Consequently, the human body has a number of mechanisms to bind and sequester iron from potentially dangerous pathogens, including iron-binding proteins such as transferrin and lactoferrin. The hypoferremic response to infection has been known since the early 1930s. After the onset of disseminated infections, serum iron levels become low, due to a rapid shift of iron from plasma into storage areas. Bacteria and fungi counter these defenses by producing siderophores, which are proteins that are synthesized and exported to capture iron, with the resulting iron-siderophore complex endocytosed for use (3).
DFO is a siderophore produced by the fungus Streptomyces pilosus that has been used in clinical practice as a chelating agent for iron or aluminum overload. The binding of DFO with iron or aluminum cations results in a complex (feroxamine in this case) for primary excretion in the urine or, to a lesser extent, in bile. The emergence of DFO as a chelating agent in clinical practice has been paralleled by a rise in reported cases of mucormycosis, the majority due to Rhizopus. The iron-DFO complex is postulated to play a direct role in the pathogenesis of these infections. Feroxamine-mediated iron uptake has been found to stimulate Rhizopus growth in vitro (4, 5) and pathogenicity in vivo (4, 6).
The seminal observation of an association between fulminant and commonly fatal mucormycosis (due to Rhizopus) and either maintenance dialysis or DFO therapy has prompted investigations into the mechanisms of pathogenesis and has led to the creation of an international registry of such cases. The vast majority of the patients registered (78%) were being treated with DFO when the infection developed, and the duration of DFO therapy ranged from 3 weeks to 36 months (7). Results from in vitro and in vivo animal studies suggest that Fe-DFO has a direct effect on the growth of Rhizopus and, to a lesser degree, Aspergillus (8). Furthermore, two independent studies have shown that experimentally induced mucormycosis, either in the guinea pig or in the mouse, is made more fulminant by the simultaneous injection of DFO (4, 6).
Dialysis patients treated with DFO are at increased risk for the development of mucormycosis, whereas DFO-treated patients with normal renal function only rarely develop this infection (7, 9). Pharmacokinetic changes in uremia lead to a 6-fold prolongation of the elimination half time and a 4.9-fold increase in the area under the concentration time curve for Fe-DFO in dialysis patients compared with subjects with normal renal function after a single DFO administration (10). Moreover, the iron-overloaded state itself is associated with higher serum levels of the Fe-DFO complex, sufficient to promote growth of Rhizopus (10). Although the patient described here was not on maintenance dialysis, in his case a uremic milieu in the iron overload may have facilitated mucormycosis in the setting of DFO therapy. Mucormycosis has also been described in patients in other iron-overloaded states such as thalassemia, sideroblastic anemia, and myelodysplasia, where DFO may be used (11–13).
The cause of this patient's death was determined to be myelodysplastic syndrome (refractory cytopenia with multilineage dysplasia in transformation to acute myeloid leukemia) with multiple organ failure. Postmortem examination was instrumental in determining both the extent of iron overload (secondary hemochromatosis) in multiple organs and the diagnosis of invasive pulmonary zygomycosis. Although the pulmonary zygomycosis was not likely the immediate cause of death, it likely contributed to this patient's demise. It is not surprising that postmortem examination was required to diagnose pulmonary zygomycosis in our patient, as autopsy was also required in 61% of the cases listed in the international registry (7). The exact length of time the patient had been treated with DFO prior to the diagnosis was unclear, but he had received two doses during hospitalization just prior to death.
In conclusion, DFO therapy should be considered one of the risk factors for fungal infections, along with diabetes mellitus (particularly with ketoacidosis), liver disease, splenectomy, neutropenia, and steroid or other immunosuppressive therapy. Furthermore, the concept of alternate chelating agents should be explored further. For maintenance dialysis patients, alternate therapy has been facilitated by the transition from aluminum-based to calcium-based phosphate binders. For the nondialysis population with iron overload, a promising development is the newer class of chelator hydroxypyridinone, which seems to be less frequently associated with infectious complications (14).
References
- 1.Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10(Suppl 1):31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 2.Maertens J, Demuynck H, Verbeken EK, Zachée P, Verhoef GE, Vandenberghe P, Boogaerts MA. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24(3):307–312. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 3.Fenves AZ. The interrelationship between iron and infectious diseases. Proc (Bayl Univ Med Cent) 1990;3(2):35–37. [Google Scholar]
- 4.Van Cutsem J, Boelaert JR. Effects of deferoxamine, feroxamine and iron on experimental mucormycosis (zygomycosis) Kidney Int. 1989;36(6):1061–1068. doi: 10.1038/ki.1989.301. [DOI] [PubMed] [Google Scholar]
- 5.Verdonck A, Boelaert JR, Gordts B, et al. Effect of feroxamine on the growth of Rizopus Abstracts of the 91st General Meeting of the American Society of Microbiology, Dallas, Texas, May 5–9, 1991 (abstr F-68).
- 6.Abe F, Inaba H, Katoh T, Hotchi M. Effects of iron and desferrioxamine on Rhizopus infection. Mycopathologia. 1990;110(2):87–91. doi: 10.1007/BF00446996. [DOI] [PubMed] [Google Scholar]
- 7.Boelaert JR, Fenves AZ, Coburn JW. Deferoxamine therapy and mucor-mycosis in dialysis patients: report of an international registry. Am J Kidney Dis. 1991;18(6):660–667. doi: 10.1016/s0272-6386(12)80606-8. [DOI] [PubMed] [Google Scholar]
- 8.Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91(5):1979–1086. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugar AM. Mucormycosis. Clin Infect Dis. 1992;14(Suppl 1):S126–S129. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- 10.Verpooten GA, D'Haese PC, Boelaert JR, Becaus I, Lamberts LV, De Broe ME. Pharmacokinetics of aluminoxamine and ferrioxamine and dose fnding of desferrioxamine in haemodialysis patients. Nephrol Dial Transplant. 1992;7(9):931–938. doi: 10.1093/ndt/7.9.931. [DOI] [PubMed] [Google Scholar]
- 11.Brown AE, Cicogna C, Armstrong D. Mucormycosis in patients with cancer. Infect Med. 1994;11:562–563. [Google Scholar]
- 12.Mucormycosis. Ann Intern Med. 1980;93:93–108. doi: 10.7326/0003-4819-93-1-93. [DOI] [PubMed] [Google Scholar]
- 13.Daly AL, Velazquez LA, Bradley SF, Kauffman CA. Mucormycosis: association with deferoxamine therapy. Am J Med. 1989;87(4):468–471. doi: 10.1016/s0002-9343(89)80836-8. [DOI] [PubMed] [Google Scholar]
- 14.Boelaert JR, Van Cutsem J, de Locht M, Schneider YJ, Crichton RR. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int. 1994;45(3):667–671. doi: 10.1038/ki.1994.89. [DOI] [PubMed] [Google Scholar]