Abstract
Amiodarone is a class III antiarrhythmic drug widely used for both ventricular and supraventricular tachyarrhythmias. Due to its high iodine content and structural similarity to thyroxine, abnormalities in thyroid function are common in patients taking amiodarone, especially with long-term use. Both hypo- and hyperthyroidism have been associated with amiodarone, with the former far more common in the United States. We present a patient with medically refractory amiodarone-induced thyrotoxicosis after a 2-year history of amiodarone use, resulting in cardiac arrest and encephalopathy. The patient ultimately required total thyroidectomy for symptomatic control.
CASE REPORT
A 66-year-old man presented to his primary care practitioner after 1 week of worsening shortness of breath associated with cough, orthopnea, palpitations, and malaise. He had a past history of type 2 diabetes mellitus, hypertension, obstructive sleep apnea, cerebrovascular accident, prostate cancer, dyslipidemia, and coronary artery disease. A coronary artery bypass graft he had in September 2005 was complicated by postoperative atrial fibrillation, for which he was started on amiodarone 200 mg tablets—two tablets twice a day for a week and then 200 mg once a day. His most recent echocardiogram in December 2006 showed an estimated ejection fraction of 70%. Besides amiodarone, his daily medications included lisinopril, hydrochlorothiazide, valsartan, clonidine, glyburide, pravastatin, tamsulosin, warfarin, furosemide, amitriptyline, ranitidine, and aspirin. He had no personal or family history of thyroid problems. The patient was an ex-smoker, with a 20 pack-year smoking history.
At his primary care practitioner's office, the patient was found to be in atrial fibrillation with rapid ventricular rate, and he was admitted to a local hospital. His amiodarone was discontinued. However, he remained in atrial fibrillation. Laboratory studies showed a suppressed thyroid-stimulating hormone (TSH) level of 0.008 mlU/L (reference range, 0.35–5.50), with an increased total thyroxine (T4) level of 28.4 mcg/dL (reference range, 4.5–12), total triiodothyronine (T3) level of 5.61 ng/mL (reference range, 0.60–1.81), T3 uptake of 46% (reference range, 24%—39%), and free thyroxine index of 13.1 (reference range, 1.2–4.9). At this point, the patient was transferred to our facility for further care.
On presentation at Baylor University Medical Center, the patient's blood pressure was 130/80 mm Hg, and he had an irregularly irregular pulse at a rate of 95 beats per minute. He was in mild respiratory distress. His thyroid was tender but nonpalpable; he had no thyroid eye disease but did have tremors. His skin was flushed, cold, and clammy. There was no jugular venous distension, and his lungs were clear to auscultation. The precordium was hyperdynamic and reflexes were normal. A 12-lead electrocardiogram demonstrated atrial fibrillation. Laboratory studies at Baylor confirmed hyperthyroidism. Thyroid-stimulating immunoglobulin was undetectable. The patient was started on a diltiazem drip to control his heart rate.
However, the day after admission, he suffered a cardiac arrest with pulseless electrical activity, from which he was successfully resuscitated after about 4 minutes of cardiopulmonary resuscitation. The patient was intubated and was put on mechanical ventilation with sedation. The patient reverted to atrial fibrillation and was put on intravenous heparin for anticoagulation. Diltiazem was stopped, as he needed vasopressor support with norepinephrine and dopamine. Cardiac enzymes, namely, troponin-I and creatine kinase with MB fractionation, were within normal limits. As hyperthyroidism was thought to be the root cause of his clinical presentation, he was started on methimazole 10 mg three times daily, which was then increased to 15 mg three times daily.
The patient was encephalopathic after the cardiac arrest, and it was unclear at that time whether the encephalopathy was due to anoxic brain injury or thyrotoxicosis. He was also having low-grade fevers. Two days after methimazole initiation, vasopressor support could be withdrawn and propranolol was initiated. Subsequently, diltiazem and digoxin were also added to improve rate control.
One week after methimazole initiation, direct current cardioversion was attempted unsuccessfully to convert to sinus rhythm. The patient remained thyrotoxic with continuing fevers and worsening encephalopathy, and hence his methim-azole was changed to propylthiouracil (PTU). A 1000-mg loading dose was given followed by 250 mg every 4 hours. Glucocorticoids, in the form of dexamethasone 2 mg intravenously every 6 hours, were also added. On ultrasonographic imaging, the thyroid gland was enlarged with diffuse heterogeneity, and there was no Doppler evidence of increased vascularity. His cardiac rhythm alternated between atrial fibrillation and atrial flutter. The patient continued to have fevers and remained confused, disoriented, and agitated whenever sedation was withdrawn.
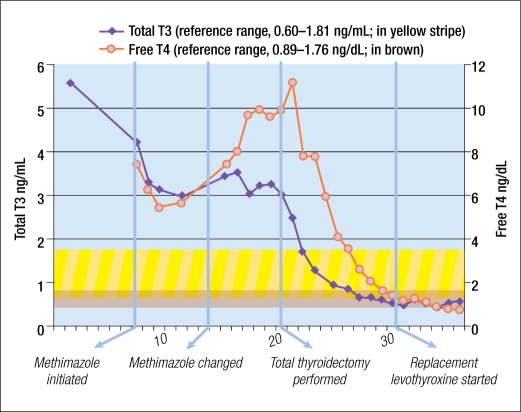
A computed tomographic scan of his head was unremarkable, and workup for his encephalopathy did not reveal any specific cause. Despite maximal medical therapy, the patient's TSH remained below 0.03 mlU/L, and his free T4 and total T3 continued to increase (Figure 1). As a result, surgical intervention was pursued. The patient had a total thyroidectomy under general anesthesia. The surgeon noted that the gland was soft, rubbery, not hypervascular, and relatively easily mobilized.
Figure 1.
Total T3 and free T4 values during hospitalization.
The patient's postoperative course was unremarkable. He was tapered off PTU and glucocorticoids over the next week. His fever resolved and encephalopathy reversed. The patient was clinically and biochemically euthyroid 10 days after the thyroidectomy. Replacement doses of levothyroxine were subsequently started.
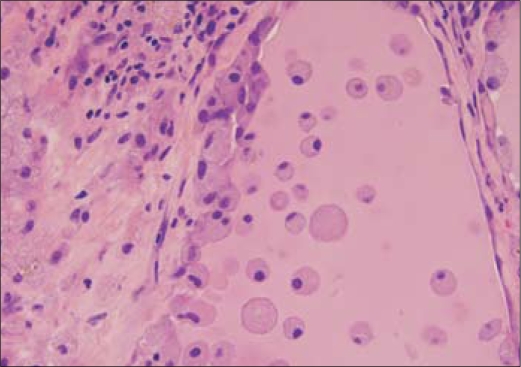
Gross pathological specimen examination demonstrated a vaguely nodular thyroid gland. Microscopically, the epithelium in many of the follicles was degenerated or partially denuded, with vacuolated cytoplasm and pyknotic nuclei. Aggregates of foamy histiocytes were found within the injured follicles, as well as in the adjacent interstitium. These changes are typical for amiodarone-induced thyroid injury (Figure 2).
Figure 2.
High-power view (40⊠) of the thyroid specimen. The follicular epithelium is degenerated and partially denuded. Foamy histiocytes within the follicles represent the characteristic histologic finding of amiodarone-induced thyrotoxicosis.
DISCUSSION
Amiodarone is widely used as an antiarrhythmic agent. It is uniquely rich in iodine; it contains 37.5% iodine by weight, and the usual maintenance dose of 200 to 600 mg translates into 50 to 100 times the daily requirement for iodine (1–3).
Amiodarone is stored in adipose tissue, making its half-life very long—ranging from weeks to months depending on the dose and duration of intake (4). This medication blocks peripheral deiodination of T4 to T3 by inhibiting 5'-deiodinase, resulting in modest increases in serum levels of total T4, free T4, and reverse T3 and a reduction in T3 levels (5). The beta-blocking action of amiodarone complicates the clinical presentation. These changes are nonconsequential in most patients taking amiodarone, as they remain clinically euthyroid.
Amiodarone can cause either hypo- or hyperthyroidism. The relative frequency of amiodarone-induced hypothyroidism (AIH) and amiodarone-induced thyrotoxicosis (AIT) depends on the iodine status of the population. In iodine-deficient areas of Europe, for example, AIT predominates over AIH (2). In the United States, AIH is far more common.
Amiodarone causes overt hypothyroidism in up to 5% of patients, while subclinical hypothyroidism is seen in up to 25% of patients taking the drug (6). The pathogenesis of AIH is related to a failure to escape from the acute Wolff-Chaikoff effect due to defects in thyroid hormonogenesis. The Wolff-Chaikoff effect is an autoregulatory phenomenon of inhibition of thyroid hormone synthesis in the face of a large iodine load. It usually lasts around 10 days, after which it is followed by an “escape phenomenon” in which iodine organification and thyroid peroxidase function are resumed. Failure of this escape mechanism in the presence of a large iodine load such as in amiodarone can result in prolonged hypothyroidism.
AIT is less common than AIH, with a prevalence of about 3% to 5% in amiodarone-treated patients in the United States (2, 6). AIT is divided into two types (Table). Type 1, Graves-like, is due to the Jod-Basedow phenomenon related to the iodine present in amiodarone. The Jod-Basedow phenomenon is an increase in synthesis of T4 and T3 due to exposure to large amounts of iodine, which provides increased substrate available for thyroid hormonogenesis. It is typically seen in patients with preexisting latent/overt Graves disease or multinodular goiter. Type 2, thyroiditis-like, is due to the direct cytotoxic effect of amiodarone on the thyroid gland. The destruction of thyroid follicular epithelial cells results in release of large amounts of thyroid hormones. Type 2 is typically seen in patients with normal thyroid function. The hyperthyroidism lasts weeks to months and is followed by a longer hypothyroid phase with eventual recovery in most patients.
Table.
Comparison between amiodarone-induced thyrotoxicosis type 1 and type 2∗
| Type 1 (Graves-like) | Type 2 (Thyroiditis-like) | |
| Preexisting or latent thyroid disease | Usually present | Absent |
| Prevalence | More common in iodine-deficient areas | More common in iodine-replete areas |
| Duration of amiodarone intake | Usually shorter (<1–2 yrs) | Usually longer (>2 yrs) |
| Pathogenesis | Unregulated hormone synthesis due to excess iodine load (Jod Basedow phenomenon) | Inflammatory destruction of the gland related to direct cytotoxic effect of amiodarone |
| Thyroid examination | Goiter more likely to be present | Normal or tender thyroid gland |
| Thyroid autoantibodies | More likely to be present | Likely absent |
| Radioactive iodine uptake scan (24-hr values) | Increased uptake (>30%) | <1% |
| Interleukin-6 | Normal | High |
| Color-flow Doppler sonography | Increased parenchymal blood flow | Normal or decreased blood flow |
| Treatment | Stop amiodarone; thionamides; perchlorate or lithium | Amiodarone discontinuation may not be required; glucocorticoids |
| Subsequent hypothyroidism | Uncommon | Common |
The widespread practice of thyroid surveillance in patients on amiodarone has allowed AIT to be diagnosed while it is still subclinical. The clinical presentation is consistent with hyperthyroidism due to any other cause with one notable exception. Due to the beta-blocking action of amiodarone, the patient may have less prominent cardiac symptoms. Thyroid function studies would reveal elevated T3 and T4 with a suppressed TSH.
Treatment of AIT depends on the type as well as the severity of thyrotoxicosis on presentation. AIT type 1 is usually treated with thionamides (i.e., methimazole or PTU), since it is an iodine-induced hyperthyroid state. Higher doses may be required, as high levels of iodine in the thyroid gland antagonize the inhibitory action of these drugs on thyroid hormonogenesis (1, 7). Patients need to be closely monitored for potentially life-threatening side effects such as agranulocytosis when using high doses of methimazole or PTU. It is recommended that amiodarone be stopped in an attempt to decrease the iodine load. However, this recommendation is controversial, as amiodarone blocks peripheral conversion of T4 to T3 and has beta-adrenoreceptor-blocking properties—both of which might be beneficial in thyrotoxicosis. Also, the principal metabolite of amiodarone, desethylamiodarone, is a potent T3 receptor antagonist. Worsening of thyrotoxic symptoms and cardiac status have indeed been reported following withdrawal of amiodarone (8). Using other beta-blockers such as propranolol or sotalol might circumvent part of this problem. Potassium perchlorate, which is a competitive inhibitor of thyroidal iodine trapping, may be used as an add-on medication in case of inadequate response (5, 9). Since most patients with type 1 AIT will have underlying Graves disease or toxic multinodular goiter, definitive treatment with radioiodine ablation is recommended (8). The timing of the ablation depends on the severity of hyperthyroidism, the response to antithyroid medications, and the radioactive iodine uptake levels.
AIT type 2 usually responds to glucocorticoids, as it is an inflammatory destruction of the gland. Prednisone at 40 to 60 mg per day is a reasonable dose and often results in improvement in 1 to 2 weeks. Usually, glucocorticoids need to be continued for 2 to 3 months and then tapered slowly to avoid relapse (10). In asymptomatic cases of AIT type 2, withdrawal of amiodarone by itself may suffice (8). Alternative treatment for arrhythmias should be considered. Radioablation is not effective in this type of AIT, as the radioactive iodine uptake is very low (8).
Clinically, “mixed” AIT is common, in which patients seem to respond only to therapy with both thionamides and glucocorticoids. There is also an inherent difficulty in distinguishing the two types of AIT confidently. One approach is to start either glucocorticoids or thionamides and to add the other drug when monotherapy has not worked after a period of several weeks to months (11). Alternatively, patients can be started on both glucocorticoids and thionamides concurrently, recognizing that AIT type 2 would respond within 1 to 2 weeks on glucocorticoids while AIT type 1 usually requires a longer course of methimazole or PTU (1, 11). There have been case reports of using lithium (12), radioactive iodine (13), and plasmapheresis (14, 15) in nonresponders.
Severe AIT requires more urgent intervention. It is recommended that both glucocorticoids and thionamides be started at the same time. Whether amiodarone is continued or stopped depends on the risk of ventricular arrhythmias and efficacy versus contraindications of alternative antiarrhythmic drugs. The decision to stop amiodarone is easier when it is being used to treat non–life-threatening arrhythmias such as paroxysmal atrial fibrillation rather than ventricular tachyarrhythmias; consultation with a cardiologist should be considered. Continuation of amiodarone does not alter the basic approach to the medical management of AIT but does lower the chances of a successful outcome (8).
In the event of worsening thyrotoxicosis in spite of medical management, subtotal or near-total thyroidectomy may be warranted (16–18). There have been over 100 reported cases of total thyroidectomy for AIT (19). Most are performed under general anesthesia with surprisingly low morbidity and mortality (17–19). There are also case reports of thyroidectomy being performed under local anesthesia in patients judged to be too ill for general anesthesia (20, 21). Intraoperatively, the patient is at higher risk of arrhythmias and needs to receive adequate beta-blockade before surgery. Mobilization of the thyroid tissue may result in a “thyroid storm” due to release of stored, preformed thyroid hormone.
CONCLUSION
Amiodarone not uncommonly causes thyroid dysfunction. It is recommended that patients on prolonged amiodarone therapy be screened with TSH levels every 6 months. If those results are abnormal, additional tests for total T3 and free T4 should be conducted to assess for AIH or AIT (8).
Most cases of AIT are amenable to medical therapy with thionamides and/or glucocorticoids. In the minority of patients who do not respond to these measures or are too critically ill to allow for a long enough course of medical management, thyroidectomy is a viable option.
References
- 1.Basaria S, Cooper D. Amiodarone and the thyroid. Am J Med. 2005;118(7):706–714. doi: 10.1016/j.amjmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Martino E, Safran M, Aghini-Lombardi F, Rajatanavin R, Lenziardi M, Fay M, Pacchiarotti A, Aronin N, Macchia E, Haffajee C. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Ann Intern Med. 1984;101(1):28–34. doi: 10.7326/0003-4819-101-1-28. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WM, Trip MD. Amiodarone and thyroid hormone metabolism. Postgrad Med J. 1986;62(732):909–914. doi: 10.1136/pgmj.62.732.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipes DP, Libby PBRO, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia: WB Saunders; 2005. [Google Scholar]
- 5.Martino E, Bartalena L, Bogazzi F, Baverman LE. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22(2):240–254. doi: 10.1210/edrv.22.2.0427. [DOI] [PubMed] [Google Scholar]
- 6.Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM, SAFE-T investigators Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation. Am J Med. 2007;120(10):880–885. doi: 10.1016/j.amjmed.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas G, Cabral JM, Leslie CA. Amiodarone-induced thyrotoxicosis: diagnostic and therapeutic strategies. Cleve Clin J Med. 2003;70(7):624–631. doi: 10.3949/ccjm.70.7.624. [DOI] [PubMed] [Google Scholar]
- 8.Newman CM, Price A, Davies DW, Gray TA, Weetman AP. Amiodarone and the thyroid: a practical guide to the management of thyroid dysfunction induced by amiodarone therapy. Heart. 1998;79(2):121–127. doi: 10.1136/hrt.79.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;50(1):89–105. [PubMed] [Google Scholar]
- 10.Bartalena L, Brogioni S, Grasso L, Bogazzi F, Burelli A, Martino E. Treatment of amiodarone-induced thyrotoxicosis, a difficult challenge: results of a prospective study. J Clin Endocrinol Metab. 1996;81(8):2930–2933. doi: 10.1210/jcem.81.8.8768854. [DOI] [PubMed] [Google Scholar]
- 11.Franklyn JA, Gammage MD. Treatment of amiodarone-associated thyrotoxicosis. Nat Clin Pract Endocrinol Metab. 2007;3(9):662–666. doi: 10.1038/ncpendmet0592. [DOI] [PubMed] [Google Scholar]
- 12.Dickstein G, Shechner C, Adawi F, Kaplan J, Baron E, Ish-Shalom S. Lithium treatment in amiodarone-induced thyrotoxicosis. Am J Med. 1997;102(5):454–458. doi: 10.1016/S0002-9343(97)00047-8. [DOI] [PubMed] [Google Scholar]
- 13.Martino E, Bartalena L, Mariotti S, Aghini-Lombardi F, Ceccarelli C, Lippi F, Piga M, Loviselli A, Braverman L, Safran M. Radioactive iodine thyroid uptake in patients with amiodarone-iodine-induced thyroid dysfunction. Acta Endocrinol. 1988;119(2):167–173. doi: 10.1530/acta.0.1190167. [DOI] [PubMed] [Google Scholar]
- 14.Erbil Y, Tihan D, Azezli A, Salmaslioglu A, Ozluk Y, Buyukoren A, Ozar-magan S. Severe hyperthyroidism requiring therapeutic plasmapheresis in a patient with hydatidiform mole. Gynecol Endocrinol. 2006;22(7):402–404. doi: 10.1080/09513590600842372. [DOI] [PubMed] [Google Scholar]
- 15.Aghini-Lombardi F, Mariotti S, Fosilla PV, Grasso L, Pinchera A, Braverman LE, Martino E. Treatment of amiodarone iodine-induced thyrotoxicosis with plasm apheresis and methimazole. J Endocrinol Invest. 1993;16(10):823–826. doi: 10.1007/BF03348934. [DOI] [PubMed] [Google Scholar]
- 16.Hamoir E, Meurisse M, Defechereux T, Joris J, Vivario J, Hennen G. Surgical management of amiodarone-associated thyrotoxicosis: too risky or too effective? World J Surg. 1998;22(6):537–543. doi: 10.1007/pl00024613. [DOI] [PubMed] [Google Scholar]
- 17.Farwell AP, Abend SL, Huang SK, Patwardhan NA, Braverman LE. Thyroidectomy for amiodarone-induced thyrotoxicosis. JAMA. 1990;263(11):1526–1528. [PubMed] [Google Scholar]
- 18.Batori M, Nardi M, Chatelou E, Straniero A, Makrypodi M, Ruggieri M. Total thyroidectomy in amiodarone-induced thyrotoxicosis. Preoperative, intraoperative and postoperative considerations. Eur Rev Med Pharmacol Surg. 2006;10(4):187–190. [PubMed] [Google Scholar]
- 19.Gough J, Gough IF. Total thyroidectomy for amiodarone-associated thyrotoxicosis in patients with severe cardiac disease. World J Surg. 2006;30(11):1957–1961. doi: 10.1007/s00268-005-0673-x. [DOI] [PubMed] [Google Scholar]
- 20.Williams M, Lo Gerfo P. Thyroidectomy using local anesthesia in critically ill patients with amiodarone-induced thyrotoxicosis: a review and description of the technique. Thyroid. 2002;12(6):23–525. doi: 10.1089/105072502760143926. [DOI] [PubMed] [Google Scholar]
- 21.Mehra A, Widerhorn J, Lopresti J, Rahimtoola SH, Amiodarone-induced hyperthyroidism: thyroidectomy under local anesthesia Am Heart J. 1991;122(4 Pt 1):1160–1161. doi: 10.1016/0002-8703(91)90488-4. [DOI] [PubMed] [Google Scholar]