Abstract
To investigate the involvement of phytohormones during rice microspore/pollen (MS/POL) development, endogenous levels of IAA, gibberellins (GAs), cytokinins (CKs) and abscisic acid (ABA) in the mature anther were analyzed. We also analyzed the global expression profiles of genes related to seven phytohormones, namely auxin, GAs, CKs, brassinosteroids, ethylene, ABA and jasmonic acids, in MS/POL and tapetum (TAP) using a 44K microarray combined with a laser microdissection technique (LM-array analysis). IAA and GA4 accumulated in a much higher amount in the mature anther compared with the other tissues, while CKs and ABA did not. LM-array analysis revealed that sets of genes required for IAA and GA synthesis were coordinately expressed during the later stages of MS/POL development, suggesting that these genes are responsible for the massive accumulation of IAA and GA4 in the mature anther. In contrast, genes for GA signaling were preferentially expressed during the early developmental stages of MS/POL and throughout TAP development, while their expression was down-regulated at the later stages of MS/POL development. In the case of auxin signaling genes, such mirror-imaged expression observed in GA synthesis and signaling genes was not observed. IAA receptor genes were mostly expressed during the late stages of MS/POL development, and various sets of AUX/IAA and ARF genes were expressed during the different stages of MS/POL or TAP development. Such cell type-specific expression profiles of phytohormone biosynthesis and signaling genes demonstrate the validity and importance of analyzing the expression of phytohormone-related genes in individual cell types independently of other cells/tissues.
Keywords: Anther, Auxin, GA, Laser microdissection, Microarray, Rice
Introduction
During anther development, the male sporophyte and the gametophyte tissues undergo unique biological processes, including specific cell divisions, i.e. meiosis and division of the haploid nuclei, cell differentiation of the male gametophyte, cell–cell communication between the tapetum (TAP) and the microspore/pollen (MS/POL), and cell death of TAP cells (Goldberg et al. 1993). The surrounding sporophyte tissues largely support MS/POL development. In particular, TAP cells, which directly associate with the MS/POL, not only provide physical support, but also provide materials and nutrients to the MS/POL.
Although several phytohormones are known to have essential roles during these processes, there is currently no inclusive study of their involvement in anther development. In this study, we analyzed the endogenous level of four kinds of phytohormones, namely indole-3-acetic acid (IAA), gibberellins (GAs), cytokinins (CKs) and abscisic acid (ABA), in the mature rice anther. Data from the 44K microarray combined with the laser microdissection technique (LM-array analysis) of MS/POL and TAP in rice (Suwabe et al. 2008) provided us with an opportunity to analyze further the cell type-specific expresion profiles of the biosynthetic and signaling genes for auxin, GAs, CKs, ABA, brassinosteroids (BRs), ethylene, and jasmonic acids (JAs). These analyses shed light on the overall feature of phytohormone synthesis and signaling during the developmental processes of MS/POL and TAP.
Results
Measurement of endogenous levels of phytohormones in the anther
The endogenous levels of IAA, GAs, CKs, and ABA in the whole anther at the mature tricellular POL stage (TC) were measured (Table 1). At this stage, degradation of TAP has been completed, and consequently POL cells should be the major container of phytohormones in the anther. For comparison, the endogenous levels of the same phytohormones were also measured in the leaf blade and the pistil. A high level of GA4, the 13-non-hydroxylated bioactive GA, but not GA1, the 13-hydroxylated bioactive GA, was detected in the anther, whereas a low level of GA1 but not GA4 was detected in the leaf blade. This is consistent with previous reports of GA1 acting dominantly during the vegetative stages of rice, and GA4 being the predominant bioactive GA in the reproductive organ of rice (Kobayashi et al. 1989, Nakajima et al. 1991, Hasegawa et al. 1995). Results also showed a consistently higher level of 13-non-hydroxylated GAs (GA24 and GA9) compared with their 13-hydroxylated counterparts (GA19 and GA20, respectively), indicating the higher activity in the 13 non-hydroxylation pathway. More importantly, this phenomenon suggests a GA synthesis pathway in the rice anther that proceeds differently relative to that in the other rice organs. Surprisingly, the level of endogenous GA4 in the anther at the TC stage (98.27 ± 6.60 ng g−1 FW) was 140 times higher than that of GA1 in the leaf blade. Such a high level of accumulation of GA has never been detected in other tissues of any plant species (Bensen et al. 1990, Waycott et al. 1990, Smith et al.1992). In the pistil, the level of GA1 was similar to that in the leaf blade, whereas the amount of GA4 was 16 times higher compared with the GA1 level. However, the levels of GA4 and other 13-non-hydroxylated GAs in the pistil were significantly lower than those in the anther. These results indicate that rice predominantly uses GA4 in the floral organs.
Table 1.
Levels of phytohormones in the anther, leaf blade and pistil of rice
| Anther | Leaf blade | Pistil | |
|---|---|---|---|
| GA | |||
| Bioactive GAs | |||
| GA1 | NDa | 2.10 ± 1.01 | 2.13 ± 0.54 |
| GA4 | 295.63 ± 19.87b,c | ND | 34.61 ± 9.82 |
| GA precursors | |||
| GA53 | ND | 33.08 ± 11.16 | 2.25 ± 0.66 |
| GA44 | ND | 3.96 ± 2.64 | 0.58 ± 0.07 |
| GA19 | 2.43 ± 0.37 | 20.65 ± 8.91 | 6.32 ± 0.61 |
| GA20 | 21.65 ± 2.58 | 6.05 ± 1.79 | 0.53 ± 0.11 |
| GA24 | 16.11 ± 2.20 | 17.77 ± 7.07 | 1.46 ± 0.61 |
| GA9 | 88.56 ± 7.65 | ND | 10.62 ± 2.75 |
| Deactivated GA | |||
| GA8 | ND | ND | ND |
| IAA | |||
| Free IAA | 5,346.16 ± 1,230.12 | 83.38 ± 36.67 | 58.90 ± 21.64 |
| IAA conjugates | |||
| IAA-Ala | 142.63 ± 30.53 | ND | ND |
| IAA-Ile | ND | ND | ND |
| IAA-Leu | ND | ND | ND |
| IAA-Asp | 3,231.08 ± 484.79 | ND | ND |
| IAA-Trp | 54.21 ± 8.19 | ND | ND |
| IAA-Phe | ND | ND. | ND |
| CK | |||
| Bioactive CK | |||
| iP | 0.50 ± 0.19 | 21.82 ± 19.13 | 0.12 ± 0.00 |
| tZ | 2.53 ± 0.03 | 1.43 ± 0.63 | 0.31 ± 0.06 |
| Precursors | |||
| iPRMP | 9.87 ± 4.75 | 27.62 ± 21.87 | 4.69 ± 1.02 |
| iPR | 0.20 ± 0.07 | 13.26 ± 12.05 | 0.04 ± 0.01 |
| tZRMP | 0.78 ± 0.05 | 0.27 ± 0.25 | 0.57 ± 0.28 |
| tZR | 1.23 ± 0.32 | 1.16 ± 0.50 | 0.13 ± 0.02 |
| Deactivated CKs | |||
| iP7G | 0.81 ± 0.11 | ND | ND |
| iP9G | 10.73 ± 2.58 | 14.94 ± 9.29 | 0.09 ± 0.00 |
| tZ7G | ND | ND | ND |
| tZOG | 3.03 ± 0.20 | 4.74 ± 3.33 | 0.67 ± 0.00 |
| tZROG | 0.83 ± 0.23 | 0.15 ± 0.11 | 0.19 ± 0.04 |
| tZRMPOG | ND | ND | 0.02 ± 0.00 |
| ABA | 97.06 ± 28.81 | 306.91 ± 141.96 | 49.44 ± 6.06 |
aND, not detected.
bEndogenous phytohormone levels (pmol g−1 FW) in each plant part.
cMeasurements were performed in triplicate and the mean values ± SD are shown.
Similarly, the amount of free IAA in the anther (936.60 ± 215.50 ng g−1 FW) was 64 and 90 times higher than that in the leaf blade and the pistil, respectively. Similar to GA4, such high accumulation of free IAA has never been observed in any other plant organs or tissues including rice (Hall and Medlow 1974, Sweetser and Swartzfager 1978, Schneider et al. 1985, Katayama et al. 1988), indicating that the anther is a unique organ for IAA accumulation. To our knowledge, this is the first report on the direct measurement of IAA in the anther; however, Feng et al. (2006) proposed that Arabidopsis anthers accumulate high levels of auxins at the late developmental stage, through expression analysis of the auxin-inducible marker DR5:GUS in transgenic Arabidopsis. These observations suggest that the accumulation of auxins in the anther at the late developmental stage is a common event in both rice and Arabidopsis.
The levels of isopentenyl adenine-type of CK (iP) and ABA were lower in the anther than the leaf blade, whereas the trans-zeatin-type of CK (tZ) was slightly higher in the anther. Taken together, the results suggest that IAA and GA have important roles during late stages of anther development, whereas the involvement of CK and ABA may not be as important.
Isolation of RNA from MS/POL and TAP cells of rice
To investigate the molecular mechanisms governing the unusually high accumulation of GA and IAA during anther development, the precise site and the timing of GA and IAA synthesis, as well as the subsequent signaling events during anther development were determined using an LM-array analysis. Oryza sativa cv. Nipponbare MS/POL and TAP were individually obtained from cross-sections of anthers at various developmental stages using the LM technique. Anther development was classified into seven stages based on the development of the male gametophyte: meiosis (MEI), tetrad (TET), uninuclear MS (UN), early bicellular POL (EBC), middle bicellular POL (MBC), late bicellular POL (LBC) and the TC (Suwabe et al. 2008). Unless stated otherwise, we combined the three bicellular POL stages (BC). A detailed description of the 44K LM-array analysis of rice MS/POL and TAP has been described by Suwabe et al. (2008) in this special issue.
Analysis of genes related to phytohormones
Genes from the microarray data set that are thought to be involved in biosynthesis and signaling not only of IAA and GA but also of five other phytohormones were initially selected (Supplementary Table S1). For genes whose functions have only been studied in other plant species, the presumed orthologs in rice were used.
To avoid the generation of inaccurate data caused by unreliable basal signals, genes showing low signal intensities (SI < log27) in all of their stages analyzed were excluded from the analysis. The threshold value log27 was determined based on the expression profiles of the IAA/AUX genes and AUXIN RESPONSE FACTOR (ARF) genes as reference (see ‘Expression profile of IAA/AUX and ARF genes’ for a detailed explanation). However, genes with an SI less than the threshold were included if their loss-of-function mutations are known to cause variable anther phenotypes, since this is indicative of their apparent involvement in anther development. Expression profiles of all of the phytohormone-related genes identified on the microarray slide are shown in the heat map data (Figs. 2, 4, 5, and Supplementary figures). Occasionally, different probes corresponding to the same gene can be found on the microarray slide. For these genes, Z-value data from different probes were averaged, and are represented in Figs. 1, 3, 6–10 (figures illustrating the overall expression profile of each phytohormone). Additionally, because the SI is expected to differ depending on the probe used, comparison of expression levels between genes by SI may not, in a strict sense, be completely accurate. Hence, SI was used merely to reflect the relative differences in expression level among the genes when necessary.
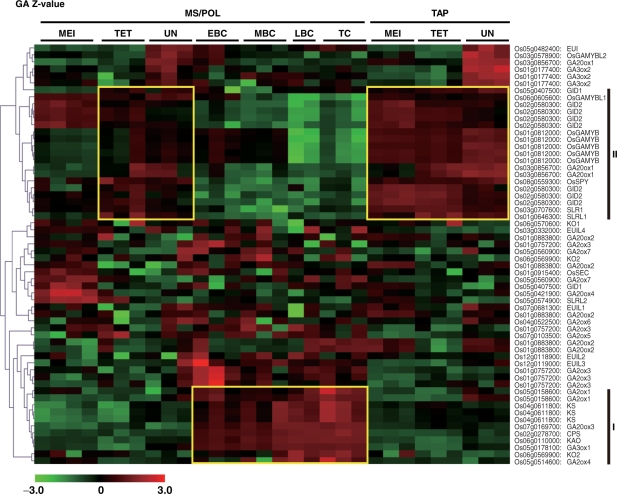
Fig. 2.
Cluster analysis of expression profiles (presented as log2 Z-values) of rice GA biosynthesis, deactivation and signaling genes in the MS/POL and TAP. Genes were classified into Clusters I and II based on their specific expression patterns. Red and green colors indicate higher and lower expression, respectively. The color scale is shown at the bottom and the RAP-DB accession number and gene product names are shown on the right. EBC, early bicellular POL stage; MBC, middle bicellular POL stage; LBC, late bicellular POL stage. Other details are the same as in Fig. 1.
Fig. 4.
Cluster analysis of expression profiles (presented as log2 Z-values) of rice IAA biosynthesis and auxin signaling genes in the MS/POL and TAP. Genes were classified into Clusters I–IV based on their specific expression patterns. Other details are as in Fig. 1.
Fig. 5.
Cluster analysis of expression profiles of rice IAA/AUX and ARF genes in the MS/POL and TAP. IAA/AUX genes are marked in light blue and ARF genes in dark blue. Genes were classified into Clusters I-1, I-2 and II-1 to II-5 based on their specific expression patterns. Other details are as in Fig. 1.
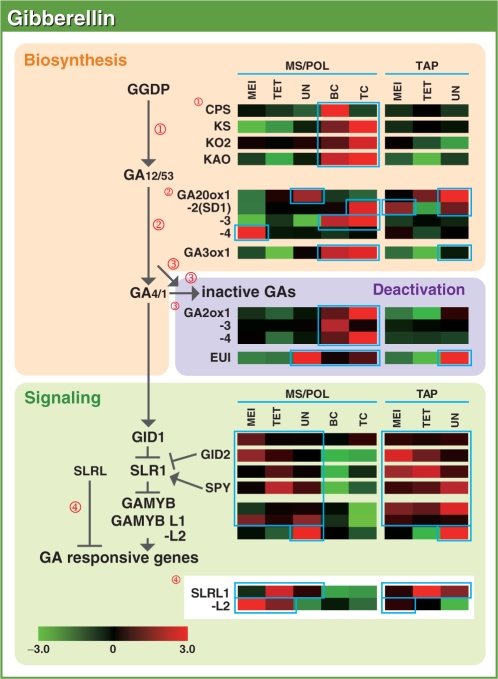
Fig. 1.
Representative gene expression profiles of the GA biosynthesis, deactivation and signaling pathways in the MS/POL and TAP of rice. Red and green colors indicate higher and lower expression, respectively. The color scale (representing the average log2 Z-value normalized by R software) is shown at the bottom. The letters ‘Os’ at the beginning of each gene are omitted for convenience. MS/POL, microspore/pollen; TAP, tapetum; MEI, meiosis, TET, tetrad stage; UN, uninuclear MS stage; BC, bicellular POL stage; TC, tricellular POL stage.
Fig. 3.
Representative gene expression profiles of the IAA biosynthesis and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
Fig. 6.
Representative gene expression profiles of the CK biosynthesis, deactivation and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
Fig. 7.
Representative gene expression profiles of the BR biosynthesis, deactivation and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
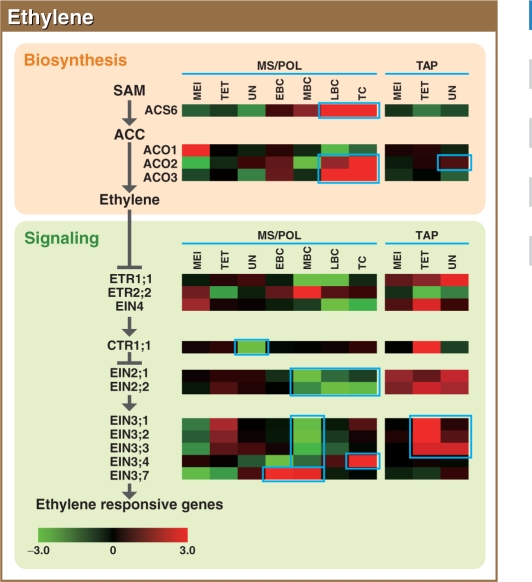
Fig. 8.
Representative gene expression profiles of the ethylene biosynthesis and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
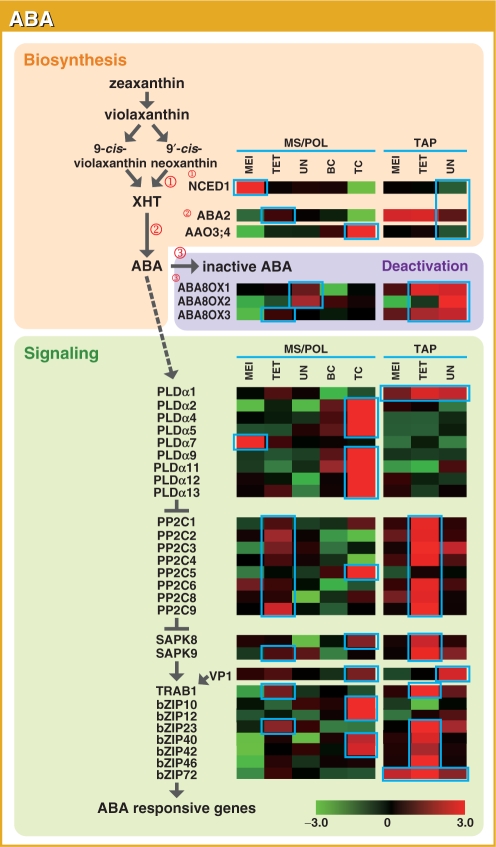
Fig. 9.
Representative gene expression profiles of the ABA biosynthesis, deactivation and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
Fig. 10.
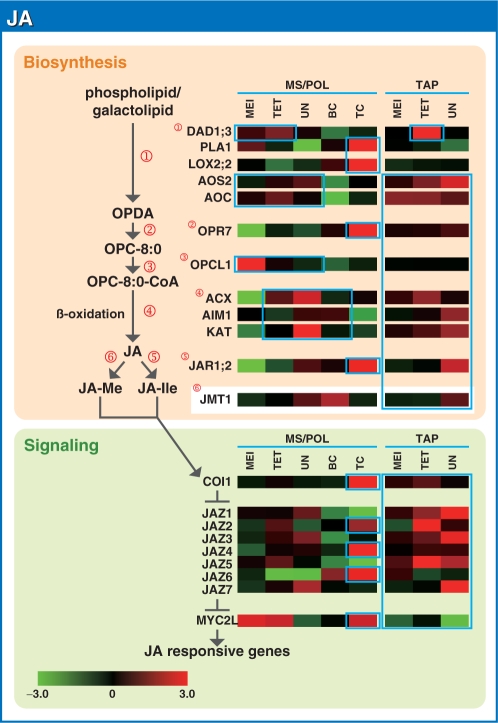
Representative gene expression profiles of the JA biosynthesis and signaling pathways in the MS/POL and TAP of rice. Other details are as in Fig. 1.
The expression profile of GA biosynthesis and signaling genes
Bioactive GAs are synthesized from trans-geranylgeranyl diphosphate (GGDP). In the early steps of GA biosynthesis, GGDP is converted to GA12 by four enzymes, namely ent-copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO) and ent-kaurenoic acid oxidase (KAO). Subsequently, the later steps of GA synthesis, from GA12 or GA53 (a C-13-hydroxylated GA12 derivative), are catalyzed via two parallel pathways (the 13 non-hydroxylation and 13 hydroxylation pathways) through a series of oxidations by GA-20 oxidase (GA20ox) and GA-3 oxidase (GA3ox). GA20ox catalyzes conversion of GA12/GA53 to the immediate bioactive GA precursors GA9/GA20, which are further catalyzed by GA3ox to the bioactive GA4/GA1. The deactivation of GAs is accomplished by GA-2 oxidase (GA2ox). Two additional GA-deactivating enzymes, EUI and GAMT1/GAMT2, have recently been identified (Zhu et al. 2006, Varbanova et al. 2007).
Once bioactive GAs are synthesized, they are perceived by the soluble GA receptor GID1 (Ueguchi et al. 2005). By perceiving GA, GID1 becomes capable of interacting with the DELLA protein (SLR1 in rice), a negative regulator of the GA response (Ikeda et al. 2001). SLR1 within the GA–GID1–SLR1 complex is then degraded through the SCFGID2 complex, resulting in GA responses (Sasaki et al. 2003, Hirano et al. 2008). Apart from these signaling factors, additional components of the GA signaling pathway have also been identified. GAMYB (OsGAMYB in rice) acts downstream of the DELLAs as a positive trans-acting factor (Kaneko et al. 2004, Tsuji et al. 2006), whereas SPINDLY (OsSPY in rice) negatively regulates GA signaling by controlling the function of the DELLA proteins (Jacobsen et al. 1996, Shimada et al. 2006). Furthermore, SLRL1 and SLRL2 function similarly to SLR1 as repressors of GA signaling during the vegetative stages in rice. However, because both lack the domain necessary for GID1 interaction and are not degraded through GA signaling, they function as constitutive GA signaling suppressors (Itoh et al. 2005).
There are only single functional CPS, KS and KAO enzymes in rice. Although there are two functional KO enzymes (KO1 and KO2), KO2 functions dominantly in most tissues of rice, whereas KO1 is assumed to have virtually no role in GA biosynthesis (Itoh et al. 2004, Sakamoto et al. 2004). GA20ox, GA3ox and GA2ox are encoded by four, two and seven genes in rice, respectively (Sakamoto et al. 2004, Lee and Zeevaart 2005, Nakamura et al. 2007). The probes corresponding to the GA2ox2 gene, a gene that was expressed at an undetectable level in all of the tissues and organs analyzed (Sakai et al. 2003), was not arrayed on the slide. For EUI, five homologs were identified (EUI and EUIL1–EUIL4), whereas homologous genes for the GAMT genes were difficult to define clearly in rice and were thus excluded from the analysis. GA signaling factors GID1, SLR1 and GID2 are encoded by single genes. Additionally, there are two genes that are homologous to OsGAMYB (GAMYBL1 and L2) that function similarly to OsGAMYB in the anthers (Tsuji et al. 2006). Two rice homologs of SPINDLY, OsSPY and OsSEC, have also been identified.
The overall pathway of GA synthesis and signaling, along with the expression profiles of genes with SIs higher than the threshold are presented in Fig. 1, whereas clustering of all GA-related genes identified on the microarray slide is shown in Fig. 2. In Fig, 2, two clusters showing clear coordinated expression were observed. The expression of Cluster I genes was observed clearly restricted during the BC and TC stages of the POL. Interestingly, this cluster was composed of the complete set of genes required for the synthesis of bioactive GA (CPS, KS, KO2, KAO, GA20ox3 and GA3ox1) and two genes (GA2ox1 and GA2ox4) that are involved in the deactivation of bioactive GAs. In contrast to GA20ox3, the remaining GA20ox genes showed varying expression patterns in MS/POL (Fig. 1). GA20ox1 was preferentially expressed at the UN stage of MS; GA20ox2, whose loss-of-function mutant causes a dwarf phenotype (sd1; Sasaki et al. 2002), was expressed at the TC stage of POL; and GA20ox4 was expressed at the earliest stage analyzed, the MEI stage. In the case of GA3ox, there is one other GA3ox gene in the rice genome, GA3ox2. Loss-of-function mutation in GA3ox2 causes a severe dwarf phenotype (d18) in rice, indicating that it primarily functions during the vegetative stages (Itoh et al. 2001). In the present study, however, the SI of GA3ox2 was low throughout the stages in both MS/POL and TAP (Supplementary Fig. S1), indicating that the gene is not importantly involved in GA synthesis in anther. GA3ox2 was thus excluded in Fig. 1. Taken together, the results suggest that GA20ox3 and GA3ox1 are the major late step enzymes responsible for the accumulation of GA4 during the TC stage of the anther. Although GA20ox2 was predominantly expressed at the TC stage of the POL (Fig. 1), its SI was lower than that of GA20ox3 (Supplementary Fig. S1), further suggesting that GA20ox3 is the major GA20ox functioning in the mature POL. In summary, the high level accumulation of GA4 observed at later stages of anther may be attributed to the well-coordinated expression of all six GA synthetic genes (Cluster I genes) in the POL.
The expression of the two GA2ox genes, GA2ox1 and 4, which were classified into Cluster I, was also observed predominantly during the BC and TC stages of POL development, with GA2ox1 recording a higher SI than GA2ox4 and the other GA2ox genes (Supplementary Fig. S1). This suggests that GA2ox1 dominantly functions in the anther. EUI also showed predominant expression from the UN to the TC stage of the MS/POL and at the UN stage of the TAP (Fig. 1). The expression of EUI in the anther was expected since EUI preferentially de-epoxidizes the 13-non-hydroxylated form of GAs (Zhu et al. 2006), and because rice uses GA4, rather than GA1, during the reproductive stage (Table 1).
The expression pattern of Cluster II genes showed an almost mirror image of those of Cluster I; preferential expression was observed at the early stages of MS/POL and constitutively in TAP. Cluster II contained all the GA signaling genes that are known to date (GID1, SLR1, GID2, OsSPY and OsGAMYB). This cluster also contained other genes such as OsGAMYBL1, SLRL1 and GA20ox1. Other GA signaling genes such as SLRL2 were preferentially expressed at the MEI and TET stages of the MS, and the MEI stage of the TAP (Fig. 1). GAMYBL2 was expressed preferentially at the UN stage of both MS and TAP.
The expression of early GA synthesis genes at a low level in TAP implies that GA may not be actively synthesized in the TAP. However, high level expression of GA signaling genes in the TAP throughout its development suggests that GA signaling actively functions in TAP cells. In fact, loss-of-function mutants of GA signaling genes show severe defects in TAP degradation (Aya et al. unpublished). Further, GA20ox1 and GA3ox1 genes showed predominant expression in TAP at the UN stage (Fig. 1). These findings indicate that the expression of genes in the later steps of GA biosynthesis and GA signaling is important at least during the later stages of development prior to TAP degradation. There are two possibilities that may explain these incongruous observations. GA12 or GA53, the substrates of GA20ox, could be transported to the TAP from the other parts of the plant, which would allow them to be converted to bioactive GAs in the TAP. Alternatively, although an early GA biosynthetic step may not take place actively in the TAP, some amount of GA12/53 synthesized there could be efficiently catalyzed via the GA20ox1 and GA3ox1 enzymes, leading to the initiation of GA signaling.
The expression profile of IAA biosynthesis and signaling genes
Expression profile of IAA synthesis and receptor genes
IAA is the predominant auxin. It has been proposed to be synthesized through tryptophan (Trp)-dependent and -independent pathways, although the Trp-dependent pathway is likely to be dominant (Zazimalova and Napier, 2003). Trp is synthesized from chorismate via several enzymes in the Trp biosynthetic pathway, including the rate-limiting enzyme anthranilate synthase, which is composed of the two subunits, ASA and ASB (Fig. 3; Morino et al. 2005, Stepanova et al. 2005). Several pathways describing the synthesis of IAA from Trp have been proposed, although not all have been established with certainty. In the most extensively studied pathway, a decarboxylated derivative of Trp, tryptamine, is converted to N-hydroxyl-tryptamine (NHT) by the YUCCA protein (a novel flavin monooxygenase), a step that is assumed to be one of the rate-limiting steps for auxin biosynthesis in Arabidopsis (Zhao et al. 2001). The pathway from NHT to IAA involves the conversion of NHT to indole-3-acetaldoxime (IAOx), followed by the conversion of IAOx to IAA via indole-3-acetonitrile (IAN) or indole-3-acetaldehyde (IAAld; Zhao et al. 2002). In Arabidopsis, an alternative pathway has been suggested to catalyze the conversion of Trp to IAOx. This pathway involves catalysis by the cytochrome P450 proteins CYP79B2/CYP79B3, although rice obviously lacks CYP79B2/CYP79B3 orthologs (Zhao et al. 2002, Yamamoto et al. 2007). Recently, an enzyme from another pathway was identified in Arabidopsis: tryptophan aminotransferase-1 (TAA1), which catalyzes the conversion of Trp to indole-3-pyruvic acid (IPyA; Stepanova et al. 2008, Tao et al. 2008). IPyA is further assumed to be converted to IAAld (Koga et al. 1991). Nitrilase (NIT) and IAAld oxidase 1 (AAO1) are thought to be involved in converting IAN and IAAld to IAA, respectively, but their biological functions have not been well defined (Normanly et al. 1997, Seo et al. 1998). After IAA is synthesized, it is perceived by the F-box-containing auxin receptor, TIR1, or its homologs, AFBs (Dharmasiri et al. 2005).
The genes ASA, ASB, TAA1, AAO1 and NIT are found in rice as two, two, four, three and two homologs, respectively. For YUCCA, eight homologs have been reported in rice, including OsYUCCA1–OsYUCCA7, and COW1 (Woo et al. 2007, Yamamoto et al. 2007), whereas for TIR1/AFB, five homologous genes have been identified. Among these IAA-related genes, OsYUCCA2 and 3 probes were not included in the microarray slide.
The overall pathway of IAA synthesis and signaling, and the expression profiles of the relevant genes with SIs higher than the threshold are presented in Fig. 3, whereas clustering of all IAA-related genes identified on the microarray slide are shown in Fig. 4. Since IAA accumulates to high levels in the mature anther, we expected coordinated expression of IAA synthetic genes at the late stages of POL, similar to the case of GA synthesis genes. During the late stages of MS/POL development, one cluster of genes was preferentially expressed from the BC to TC stages (Cluster I). The preferential expression of these genes paralleled that of the GA synthetic genes (compare Cluster I in Fig. 4 with Cluster I in Fig. 2). This cluster contains OsASB2, OsYUCCA4 and OsTAA1;4, all of which have been reported to be involved in IAA synthesis in Arabidopsis. One IAA receptor gene homolog, OsTIR1;5, was also grouped in the same cluster. Two IAA receptor gene homologs, namely OsTIR1;2 and OsTIR1;3, showed similar expression pattern to Cluster I; they were expressed from one of the three samples of the MBC to the TC stages of POL. Another cluster (Cluster II) was preferentially expressed during the EBC and MBC stages of POL and contains OsASA1, OsASB1 and OsNIT2. Based on the expression profiles and analogy of these profiles to that of the GA synthesis genes, we inferred that the well coordinated expression of OsASB2, OsYUCCA4 and OsTAA1;4 (and possibly including OsASA1, OsASB1 and OsNIT2) may be related to the unusually high level accumulation of IAA in mature anther and thus might have crucial roles in IAA synthesis in mature POL of rice.
Two other clusters, Cluster III and IV, showed preferential expression in TAP and modulated expression at the early stages of MS/POL (Fig. 4). Cluster III included the possible IAA synthesis genes, OsAAO2, OsTAA1;3 and OsNIT1, whereas Cluster IV included OsYUCCA1 and 5 aside from the IAA receptor gene, OsTIR1;1. It is noteworthy that genes expressed in the TAP and at the early stages of MS/POL (Cluster III and IV), and genes actively expressed in the late stages of MS/POL (Cluster I) could be differentiated clearly. This suggests that rice uses different sets of genes within these cell types, although the importance of the synthesis genes classified into Cluster III and IV for IAA synthesis is not yet clear.
Expression profiles of IAA/AUX and ARF genes
Clustering analysis of OsIAA/AUX and ARF genes was also performed. IAA/AUX and ARF are found in multiple copies in plants, and studies from Arabidopsis have shown that specific pair(s) of IAA/AUX and ARF functions depending on the tissue and developmental stage (Kepinski 2007). For example, ARF5/MP, IAA12/BDL and IAA13 show overlapping expression at the provascular tissues during Arabidopsis embryogenesis, where they are assumed to function coordinately to regulate embryo pattern formation (Hamann et al. 2002, Weijers et al. 2005). The identity of the pairs that work together in coordination is determined by both their transcriptional regulation and their protein function (Weijers et al. 2005). Therefore, we expected that cell type-specific expression profiling of IAA/AUX and ARF genes using LM-array analysis is an effective approach to identify initially specific pairs of IAA/AUX and ARF proteins.
In rice, IAA/AUX is composed of 31 members, whereas ARF has 25 members (Wang et al. 2007, Jain et al. 2006). Probes corresponding to OsIAA/AUX28 and 31, and OsARF5, 8, 20 and 22 were not included in the microarray slide (Supplementary Table 1, hereafter OsIAA/AUX genes are designated as OsIAA). We classified the OsIAA and OsARF genes into several clusters (Clusters I-1 to II-5) depending on their expression patterns (Fig. 5). However, some genes (mostly those found in the upper region of the heat map in Fig. 5) exhibited irregular expression patterns that did not cluster within any group. Such genes occasionally showed distinct expression levels even within the identical developmental stages. The raw heat map data for nearly all of the unclassified genes showed low SIs (Supplementary Fig. S3), indicating that low SI genes tend to result in inaccurate data caused by unreliable basal signals.
Analysis of expression patterns of OsIAA/AUX and OsARF genes allowed their rough classification into two clusters (Fig. 5). Cluster I genes were preferentially expressed at the later stages of MS/POL development, whereas Cluster II genes were predominantly expressed in the TAP. Cluster I was further classified into two groups (I-1 and I-2). The I-1 group included OsIAA15 and OsARF14 and 25, and was preferentially expressed from the MBC to the TC stages of POL development. Phylogenetic analysis of Arabidopsis and rice ARFs showed that OsARF25 falls into the same clade as AtARF8, a protein known to be involved in anther dehiscence (Nagpal et al. 2005). The specific expression of OsARF25 at later stages of MS/POL development suggests that OsARF25 is also involved in the dehiscence of anther. The I-2 group included OsIAA18 and 19 and was expressed from the LBC to the TC stage (Fig. 5).
Cluster II genes were further classified into at least five groups, II-1 to II-5. The II-1 group (OsIAA14 and 21, and OsARF16 and 19) was preferentially expressed in TAP at the UN stage and also in MS/POL at the UN to EBC stages. The II-2 group included OsIAA1 and OsARF21, whose expression mainly occurred in the MS at the MEI and TET stages and in the TAP at the UN stage, although OsIAA1 expression was higher in one of three UN samples. The II-3 group (OsIAA30 and OsARF6, 11 and 12) was mainly expressed in the MS at the MEI and TET stages and in the TAP from the MEI to the TET stage, with reduced expression at the UN stage. The II-4 group (OsIAA3, 10, 17 and 24, and OsARF15) was constitutively expressed in TAP, but had low expression in MS/POL, with one of three TET and TC samples of MS/POL expressed at a relatively high level. The last group contained many ARF and IAA/AUX genes (OsIAA5, 6, 7, 13, 16 and 23, and OsARF1, 3, 4, 6, 7, 9, 12, 17, 18, 23 and 24), whose expression mainly occurred in the TAP at all three stages and in the MS at the MEI and TET stages, although expression was lower than in the TAP. Because it is thought that specific pair(s) of IAA/AUXs and ARFs function coordinately in a spatial and temporal manner, IAA/AUX and ARF genes with similar expression patterns may function as pairs.
Gene expression of other phytohormone biosynthesis and signaling genes
CK
The initial step in CK synthesis is catalyzed by adenosine phosphate-isopentenyltransferase (IPT), which transfers the prenyl group of dimethylallyl diphosphate (DMAPP) to the N6 position of adenine nucleotides (ATP, ADP or AMP; Fig. 6; Sakakibara 2006). One of the methyl groups on the prenyl side chain is then hydroxylated by CYP735A and, finally, the removal of ribose 5′-monophosphate by LONELY GUY (LOG) produces biologically active CKs (Kurakawa et al. 2007). Bioactive forms of CKs are in turn deactivated by cytokinin oxidase/dehydrogenase (CKX), thereby regulating the amount of bioactive CK (Ashikari et al. 2005, Sakakibara 2006). Sakamoto et al. (2006a) reported that there are eight IPT genes in the rice genome: OsIPT1–OsIPT8. We eliminated OsIPT2 and 4 from the analysis because of their low SIs, whereas OsIPT1, 5, 6 and 8 probes were missing from the microarray slide. We found two homologs of CYP735A (CYP735A3 and A4) in the rice genome, but both were missing from the slide. Although 11 homologs to the CK deactivation gene (OsCKX1–OSCKX11) were found, including Gn1/OsCKX2, which controls the number of grains per panicle (Ashikari et al. 2005), Gn1/OsCKX2 and OsCKX3, 4 and 9 were eliminated because of low SIs, and OsCKX6–OsCKX8 and OsCKX10 probes were missing from the slide.
The expression of the CK synthesis genes OsIPT3, OsIPT7 and LOG occurred predominantly during the early developmental stages and decreased at later stages in MS/POL, whereas the expression of the deactivating genes OsCKX1, 5 and 11 increased starting at the UN stage (Fig. 6). This antiparallel expression pattern between CK synthesis and deactivating genes in MS/POL suggests that CK is actively synthesized in the early stages and then inactivated by CKX at a later stage. In contrast, in TAP cells, OsIPT3 was preferentially expressed at the TET stage, OsIPT7 at the MEI and UN stage, and LOG was moderately expressed. Overall, OsCKXs expression was once again observed at the UN stage, indicating that the deactivation of CK actively occurs in TAP at the UN stage. Although endogenous CK was detected in the anther of the TC stage, CK might accumulate to a higher level during the early stages of anther development.
The perception of CK in plants is mediated by a two-component signaling pathway. The binding of CK to histidine kinase (HK) receptors initiates phospho-relay signaling by transferring the phosphate group to the immediate downstream target histidine phosphotransfer protein (HP) and then to A- or B-type response regulators (A-type RRs or B-type RRs, respectively; Chow and McCourt. 2006, To and Kieber 2008). Once the signal reaches the B-type RR, the transcription of CK-responsive genes including A-type RRs is promoted. In contrast, A-type RRs inhibit this pathway antagonistically by perceiving the phosphate group of the HP (Müller and Sheen 2007, To and Kieber 2008). Based on the observation that ARR1 (an Arabidopsis B-type RR) promotes the expression of ARR6 (A-type RR gene; Sakai et al. 2001), A-type RRs are suggested to function in preventing excess signal from being perceived by the CK-responsive genes. Overexpression of OsRR6, an A-type RR, abolishes shoot regeneration, which also suggests that the A-type RR acts as a negative regulator of CK signaling (Hirose et al. 2007). There are five HK homologs (OsHK1–OsHK4 and OsHKL1/OsCRL4), five HP homologs (OsHP1–OsHP5) and 22 RR homologs in the rice genome (Ito and Kurata 2006, Pareek et al. 2006). In our analysis, OsRR7, 8 and 12 probes were missing from the slide, OsHKL1, OsRR1, 3, 4, 5, 11, 13–16 and 20–22 were eliminated because of low SIs.
The expression of the CK receptor genes OsHK1–OsHK3 occurred predominantly during the early developmental stages and decreased at later stages in MS/POL, whereas OsHK4 was highly expressed not only at the MEI stage, but also at the TC stage. These receptor genes, however, were constitutively expressed in TAP. This expression pattern of the receptor genes is essentially the same as that of the CK synthesis genes in both MS/POL and TAP. The overall expression pattern of OsHP genes was similar to that of OsHK genes in both MS/POL and TAP, but the stage specificity of OsHP expression is less rigorous, and OsHP3–OsHP5 were also expressed during the later stages in MS/POL. In the case of the OsRR genes, the expression pattern of the B-type genes resembled that of HK receptors. This expression pattern of B-type RR genes supports the above idea that CK synthesis and the following signaling occur actively during the early developmental stages and decrease at the later stages in MS/POL. A-type genes had an expression profile similar to that of other CK signaling genes, albeit less specific.
BR
Based on phenotype studies of rice BR-related mutants and the presence of homologs of the BR synthesis genes of Arabidopsis (Hong et al. 2002, Hong et al. 2003, Hong et al. 2005), it has been thought that rice has a BR synthetic pathway similar to that of Arabidopsis. However, rice may not use the most bioactive BR found in other plant species, brassinolide, but rather may use its precursor, castasterone (CS; Nakamura et al. 2006). The early steps of BR synthesis are shared in part with the pathway of sterol synthesis, catalyzed by several enzymes such as SMT1, FACKEL, HYD1, DWF7, DWF5 and DIM (Fig. 7; Clouse 2002). The importance of this pathway for BR synthesis in rice has been revealed by a loss-of-function mutant of rice DIM homologous gene, which shows a BR-deficient phenotype (Hong et al. 2005). The later steps that specifically function in BR synthesis are catalyzed by several enzymes including DET2, DWARF4/CYP90B1, D11/CYP724B1, D2/CYP90D2, CPD/CYP90A and DWARF/CYP85A1, finally producing a bioactive BR (Hong et al. 2002, Hong et al. 2003, Tanabe et al. 2005, Ohnishi et al. 2006b, Sakamoto et al. 2006b). Bioactive BR is, in turn, inactivated by another member of the cytochrome P450 group called BAS1 (Neff et al. 1999, Ohnishi et al. 2006a). In the rice genome, there are two homologous genes for each of FACKEL, DET2 and CPD/CYP90A, and three for BAS1, whereas other enzymes are each encoded by a single gene. OsDET2;1, OsDWARF4/CYP90B2, D2/CYP90D2, OsCPD2/CYP90A4, OsBAS1L1 and OsBAS1L3 were eliminated from the analysis because of low SIs, although the loss-of-function mutant of D2/CYP90D2 shows semi-dwarfism with typical BR-related phenotypes at the vegetative stage (Hong et al. 2003). This mutant produces normal POL, which are inherited normally by the next generation, indicating that D2/CYP90D2 is not significantly involved in anther development.
In MS/POL, some of the genes for the early biosynthetic enzymes were preferentially expressed during the UN to TC stages without clear synchronization (Fig. 7). OsSMT1, OsFACKEL1and 2, and OsDWF7 were highly expressed at the UN stage, and OsHYD1 and OsDWF5 at the TC stage. OsDIM was preferentially expressed at the TET and UN stages. In contrast, genes involved in the BR-specific pathway were expressed discretely. OsDET2;2 was predominantly expressed at the TC stage, D11/CYP724B1 at the UN stage, OsCPD1/CYP90A3 at MEI, and OsDWARF/CYP85A1 at the TET stage. The differential expression patterns of these BR synthesis genes raise the possibility that BR synthesis may not actively occur in MS/POL. In contrast, all of the genes were constitutively expressed in TAP. The gene for the inactivating enzyme (OsBAS1L2) showed preferential expression during the early stages of MS/POL development, but was constitutively expressed in TAP.
The perception of BR by the cell surface receptor BRI1 leads to the activation of BZR1/BES1, which are transcription factors that directly regulate the expression of BR-responsive genes. During this activation process, BAK1 and BSU1 act positively, whereas BKI1 and BIN2 negatively regulate BR signaling (Fig. 7; Belkhadir et al. 2006, Li and Jin 2007). The rice genome contains one homolog each for BKI1 and BZR/BES1, whereas there are four, two, two and two homologs for BRI1, BAK1, BSU1 (OsBSLs) and BIN2, respectively. The loss-of-function mutant of a rice BRI1 homolog, osbri1/d61, shows a typical BR-insensitive phenotype (Yamamuro et al. 2000). Recent studies of OsBZR1/BES1 (Bai et al. 2007) and OsGSK1 (OsBIN2; Koh et al. 2007) further support the idea that rice has a signaling pathway similar to that reported for Arabidopsis. OsBRL1–OsBRL3, OsBKI1 and OsBIN2;4/OsSKetha were eliminated from the analysis because of low SI levels.
Our microarray profiling demonstrated that most of the BR signaling genes were preferentially expressed during the early stages of MS/POL development and constitutively in TAP, except for OsBIN2/OsGSK1, which demonstrated an expression pattern with preferential expression at later stages of MS/POL development. Because the SI of OsBIN2/OsGSK1 was high throughout the stages, OsBIN2/OsGSK1 might be constitutively expressed during MS/POL development (Supplementary Fig. S7). OsBAK1;2 also showed a different expression pattern, with predominant expression at the TC stage of POL, although it was also preferentially expressed at the early stages of MS/POL development and at all stages of TAP. Based on these observations, it appears that BR synthesis, signaling and deactivation occur actively and constitutively in TAP. Although the expression is ambiguous in MS/POL, the preferential expression of most of the BR signaling and deactivation genes in the early stages of MS/POL suggests some sort of biological relevance.
Ethylene
Two protein families are required for ethylene synthesis (Fig. 8). The first step, S-adenosylmethionine (SAM) to 1-aminocyclopropane-1-carboxylic acid (ACC), is catalyzed by ACC synthase (ACS), and ACC to ethylene is catalyzed by ACC oxidase (ACO; Hamilton et al. 1990). The rice genome contains six homologs for ACS and seven homologs for ACO. OsACS3 and 4 probes were missing from the microarray slide, and OsACS1, 2 and 5, and OsACO4–OsACO7 were eliminated because of low SIs. With regard to the ethylene-related genes, the BC stage was further divided into three distinct stages because gene expression changed dramatically during this stage. During MS/POL development, OsACS6, and OsACO2 and 3 were predominantly expressed from the LBC to TC stages, indicating that ethylene synthesis actively occurs during the later stages of POL development. Interestingly, the expression of OsACO2 and 3 occurred moderately at the EBC stage, was decreased at the MBC stage and rapidly increased from MBC to LBC in a similar manner to the ACS6 expression. In TAP, the expression of these genes was not clearly synchronized, but the SIs of OsACS6 and OsACO2 were strong (Supplementary Fig. S9). Thus, ethylene synthesis may also be active in TAP cells.
In the absence of ethylene, ethylene receptors activate CONSTITUTIVE TRIPLE RESPONSE-1 (CTR1), which is a negative regulator of ethylene signaling (Kieber et al. 1993). Upon ethylene perception, ethylene receptors and subsequently CTR1 are inactivated, which relieves the suppressive state of ETHYLENE INSENSITIVE2 (EIN2). EIN2 activates the downstream transcription factor EIN3, resulting in the expression of ethylene-responsive genes. The rice genome contains five homologs for ethylene receptors, two each for CTR1 and EIN2, and seven for EIN3. OsETR1;2, OsETR2;1, OsCTR1;2, and OsEIN3;5 and 6 were eliminated from the analysis because of their low SIs.
Overall, the expression of the ethylene receptors occurred in both MS/POL and TAP at all times (Fig. 8). According to the raw microarray data, the SIs of OsCTR1;1 and OsEIN2;1 were relatively high throughout all stages analyzed (see Supplementary Fig. S9), although the Z-value data indicated reduced expression in the UN stage of MS for OsCTR1;1 and the MBC to TC stages of POL for the EIN2 homologs. The overall expression of the EIN3 homologs was also observed in MS/POL and TAP at all times. In particular, high expression of OsEIN3;1– OsEIN3;3 was observed in TAP at the TET and UN stages, OsEIN3;4 at the TC stage of POL, and OsEIN3;7 in the EBC and MBC stages of POL. Similar to the OsACO genes, some of the EIN3 homologs showed apparently reduced expression at the MBC stage. Except for the sudden decrease in expression for some genes during the MBC stage, these results indicate that ethylene synthesis and signaling occur in the MS/POL at the late stages and in TAP throughout anther development.
ABA
In higher plants, ABA is synthesized from a β-carotene compound, zeaxanthin, which is epoxidized to all-trans-violaxanthin by zeaxanthin epoxidase (ZEP; Fig. 9; Marin et al. 1996). Subsequent catalysis by neoxanthin synthase (NSY; ABA4) and 9-cis-epoxycarotenoid dioxygenase (NCED) produces xanthoxin (XHT) via two pathways where NCED acts as the rate-limiting enzyme of ABA synthesis during water deficiency (Schwartz et al. 1997). XHT is finally converted to ABA through a short-chain dehydrogenase/reductase (SDR) and aldehyde oxidase, which are encoded by ABA2 and AAO3 in Arabidopsis, respectively (Seo et al. 2000, Cheng et al. 2002, Gonzalez-Guzman et al. 2002). AAO3 requires a molybdenum cofactor (MoCo) for its catalytic activity, and mutations in the Arabidopsis MoCo biosynthesis gene ABA3 lead to ABA deficiency (Xiong et al. 2001). Major deactivation of ABA is catalyzed by CYP707A, a subfamily of cytochrome P450 monooxygenase (ABA8OX), a reaction converting ABA to phaseic acid (Kushiro et al. 2004). Although ABA biosynthesis in rice has not been studied in depth, the loss-of-function mutant of the rice ZEP homolog (OsZEP1) shows an ABA-deficient phenotype (Agrawal et al. 2001). There are three, six and three paralogs of genes to NCED (OsNCED1–OsNCED3), AAO3 (OsAAO3;1–OsAAO3;6) and ABA8OX (OsABA8OX1–OsABA8OX3) in rice, respectively, whereas ZEP and ABA2–ABA4 are encoded by single genes. OsZEP1, OsABA4, OsNCED2 and 3, OsAAO3;1–OsAAO3;3 and OsABA3 were eliminated from the analysis because of low SIs. OsAAO3;5 and 6 probes were absent from the microarray slide.
Our microarray profiling could not clearly define whether ABA was actively synthesized in the anther, because the analyzed ABA biosynthetic genes did not show synchronized expression (Fig. 9). In MS/POL, OsNCED1 was preferentially expressed at the MEI stage, OsABA2 at the TET stage and OsAAO3;4 at the TC stage. In TAP, the expression of all of the biosynthetic genes analyzed decreased at the UN stage. Genes that encode the deactivation enzymes, OsABA8OX1 and 2, were preferentially expressed at the UN stage of MS, and OsABA8OX3 at the TET stage of MS; in TAP, they were preferentially expressed during the TET and UN stages. The antiparallel expression of ABA biosynthesis and deactivating genes during the UN stage of TAP suggests that the amount of ABA might be reduced during this stage.
Various components of the ABA signaling pathway have been identified in Arabidopsis; however, the complete map of ABA signaling has yet to be elucidated. Phospholipase D alpha (PLDα), SAPK, VP1 and TRAB-related bZIP proteins positively regulate ABA signaling, whereas phosphatase 2C (PP2C) proteins are negative regulators of ABA signaling (Gosti et al. 1999, Yoshida et al. 2006, Nijhawan et al. 2008). PLDα, PP2C, SAPK and TRAB-related bZIP protein are encoded by 13, 9, 10 and 14 genes, respectively, whereas VP1 is encoded by a single gene. OsbZIP29 and 77 probes were missing from the microarray slide, and OsPLDα3, 6, 8 and 10, OsPP2C7, SAPK10 and OsbZIP9, 24, 62 and 69 were eliminated because of low SIs. Because SAPK8–SAPK10 are the only SAPK genes known to be activated by ABA, SAPK1–SAPK7 were not analyzed. The overall expression pattern of signaling factors was similar within each gene family, and most of the OsPLDα genes, OsbZIP10 and 12 were preferentially expressed during the TC of POL development. Most of the OsPP2C genes, SAPK9, TRAB1 and OsbZIP23 were expressed in the TET of both MS and TAP. However, some genes showed different expression patterns. OsVP1 showed high expression during the TC stage of POL and the UN stage of TAP, and SAPK8, OsbZIP40 and 42 during the TC stage of POL and the TET stage of TAP. OsPLDα1 and OsbZIP72 were strongly expressed in TAP at all stages, OsPLDα7 during MEI, OsPP2C5 at the TC of POL, and OsbZIP46 at the TET stage of TAP. ABA signaling may preferentially function in anthers during the TET and TC stages of MS/POL and the TET stage of TAP.
JA
JA biosynthesis has been elucidated by studies of Arabidopsis. The α-linolenic acid, which is released from chloroplast membrane lipids by specific lipases, including DAD1 and PLA1 (Ishiguro et al. 2001, Wasternack 2007, Yang et al. 2007), is transformed to 12-oxo-phytodienoic acid (OPDA) by a series of enzymatic reactions involving chloroplast-localized lipoxygenase (LOX), allene oxide synthase (AOS) and allene oxide cyclase (AOC; Fig. 10; Bell et al. 1995, Feussner et al. 1995, Maucher et al. 2000, Ziegler et al. 2000, Froehlich et al. 2001, Stenzel et al. 2003). OPDA is then transported to the peroxisome and converted to JA by OPDA reductase (OPR), an acyl-activating enzyme (OPCL1), three cycles of β-oxidation by acyl-CoA oxidase (ACX), a multifunctional protein (MFP; AIM1) and 3-keto-acyl-CoA thiolase (KAT; Stintzi and Browse 2000, Cruz Castillo et al. 2004, Li et al. 2005, Koo et al. 2006, Wasternack 2007). JA is further modified by specific enzymes such as JAR1 (Staswick et al. 2002, Staswick and Tiryaki. 2004) and JMT (Seo et al. 2001) to produce JA-isoleucine (JA-Ile) and methyl-JA (JA-Me), respectively. In yeast cells, JA-Ile promotes the interaction between the F-box protein CORONATINE-INSENSITIVE1 (COI1) and jasmonate ZIM-domain (JAZ) proteins (Xie et al. 1998, Chini et al. 2007, Thines et al. 2007), suggesting that JA-Ile may be the true biologically active compound (see below).
The rice genome contains five homologs for DAD1 and PLAI (OsDAD1;1–OsDAD1;4 and OsPLA1), five homologs for LOX2 (OsLOX2;1– OsLOX2;5) and two homologs for AOS (OsAOS1 and 2). There are six homologs for JMT (OsJMT1–OsJMT6) and three homologs for JAR1 (OsJAR1;1–OsJAR1;3). The other homologous genes involved in JA synthesis were found as the single genes OsAOC, OsOPR7, OsOPCL1, OsACX, OsAIM1 and OsKAT. OsDAD1;2 and 4, OsLOX2;1 and 3–5, OsAOS1, OsJAR1;3 and OsJMT2–OsJMT5 were eliminated from the analysis because of low SIs, whereas OsDAD1;1, OsJMT6 and OsJAR1;1 probes were missing from the microarray slide.
The microarray results suggest that the coordinated expression of the genes required for JA-Ile synthesis is not obvious in MS/POL, although it may occur actively in TAP at all stages. Of the DAD1 and PLA1 family, OsDAD1;3 was expressed in MS/POL at MEI and the TET stage and in TAP at the TET stage, whereas OsPLA1 was predominantly expressed during the TC stage of POL development. OsLOX2;2 and OsOPR7, which catalyze the conversion of linolenic acid to 3-oxo-2-(2′-pentenyl)-cyclopentane-1-octanoic acid CoA (OPC-8:0-CoA), were highly expressed during the TC stage of POL development, whereas OsAOS2, OsAOC and OsOPCL1 were primarily expressed in the early stages of MS/POL development. The SI of the OsLOX2;2 gene, however, was much lower than those of the OsAOS2, OsAOC and OsOPCL1 genes, suggesting that the reaction catalyzed by OsLOX2;2 might be the rate-limiting step in this metabolic pathway (see Supplementary Fig. S13). The genes involved in β-oxidation, i.e. OsACX, OsAIM1 and OsKAT, were mainly expressed in MS/POL at the TET to BC stages, and constitutively in TAP. Their SIs were higher than those of the DAD1, PLA1 and LOX2 genes (see Supplementary Fig. S13). The SI of OsJAR1;2 was high in both MS/POL and TAP, with the predominant expression occurring in MS/POL at the TC stage and in TAP at the UN stage. The SI of another JA modification gene, OsJMT1, was much lower than that of OsJAR1;2 (see Supplementary Fig. S13). These results suggest that JA synthesis may occur in all three stages in TAP, while it may preferentially occur in MS/POL at the TC stage since OsLOX2;2 is predominantly expressed at this stage.
In the JA signaling pathway, COI1 may function as a JA receptor and interact with the JAZ transcriptional repressors in the presence of JA-Ile, leading to the disruption of JAZ proteins through the 26S proteasome pathway (Thines et al. 2007, Katsir et al. 2008). As a result of JAZ repressor disruption, the MYC2 transcription factor is able to regulate the transcription of JA-responsive genes (Boter et al. 2004, Lorenzo et al. 2004, Chini et al. 2007). In the rice genome, there is one homolog each for COI1 (OsCOI1) and MYC2 (OsMYC2L), and nine for JAZ (OsJAZ1–OsJAZ9). The OsJAZ8 probe was missing from the microarray slide and OsJAZ9 was eliminated from the analysis because of low SIs.
The expression of OsCOI1 occurred predominantly at the TC stage in MS/POL development. Preferential expression during the TC stage of POL was also observed for OsMYC2L, indicating that JA signaling is active in MS/POL at the TC stage, consistent with the hypothesis that JA is actively synthesized during the TC stage of POL development. The active expression of JA signaling genes was also observed in TAP at all stages, although OsMYC2L was not actively expressed as evaluated using the Z-value data. The raw data, however, show high SI of OsMYC2L in TAP (Supplementary Fig. S13), suggesting that JA signaling is also active in TAP at all stages. In Arabidopsis, JA-responsive genes are regulated by several transcription factors, including AtMYC2 (an ortholog of OsMYC2L), ethylene response factor 1, WRKY70 and ORA family proteins (reviewed in Dombrecht et al. 2007, Wasternack. 2007). The reduced expression of OsMYC2L in TAP may alternatively indicate that the expression of JA-responsive genes is enhanced by transcription factors other than OsMYC2L in TAP.
Discussion
GA
Role of GA biosynthesis during the later stages of MS/POL development
Chhun et al. (2007) reported that GA biosynthesis mutations in rice are not transmitted as Mendelian traits to the next generation in self-pollinated F1 heterozygous plants. Namely, the penetration of the GA biosynthesis mutants (oscps, osks, osko2 and oskao) in the F2 generation is significantly lower than the expected frequency of 1 : 3. Further investigation showed that the reduced penetration is caused by defects in POL germination and POL tube elongation of the mutant POL in the F1 plants. However, although GA signaling is also necessary for proper POL germination and its tube elongation, the penetration of GA signaling mutants such as gid1, gid2, slr1 and gamyb was normal. Based on these observations, Chhun et al. (2007) proposed that GA signaling proteins expressed prior to meiosis are inherited (carried over) to the haploid POL and are necessary for POL germination and its tube elongation, whereas GA biosynthetic enzymes are not inherited or are inherited only at low levels and consequently need to be expressed after meiosis depending on the genome of the MS. In the present study, the observation of a high level of GA4 accumulation in the mature anthers (Table 1) supports the idea that GA synthetic enzymes carried over to POL are not sufficient to synthesize such a large amount of GA4.
Our microarray data allowed us to make a detailed presumption about how the inheritance of GA-related proteins is determined. Genes that encode the enzymes that catalyze the initial steps of GA biosynthesis (CPS, KS, KO2 and KAO) were expressed at very low levels in MS during MEI. In contrast, GA signaling genes (GID1, SLR1, GID2, OsGAMYB and OsSPY) were predominantly expressed during the early stages of microsporogenesis. These findings strongly support the hypothesis that the inheritance of GA-related proteins in POL is determined by whether or not the corresponding genes can be expressed prior to MEI. Except in aleurone cells, the non-overlapping expression patterns between GA synthesis and signaling genes are an unusual phenomenon during the entire life cycle of rice and are also unusual for many tissues and organs of rice where synchronized expression of GA biosynthesis and signaling are commonly observed (Kaneko et al. 2003). The high level of GA4 accumulation in the anther at the TC stage, and high expression of GA biosynthetic genes at the later stages of MS/POL development without the synchronized expression of GA signaling genes, is suggestive that GA4 synthesized during these stages might not be used immediately, but kept as a storage GA until POL germination and POL tube elongation.
Different roles of GA20 and GA3 oxidases in different rice organs
Our LM-array profile indicated that GA20ox3 and GA3ox1 are dominantly involved in bioactive GA synthesis in the POL during the BC and TC stages where GA4 accumulates to an extraordinarily high level (Table 1, and Figs. 1, 2). It is noteworthy that rice uses different sets of GA20 and GA3 oxidases for the production of bioactive GA at the vegetative stage: GA20ox2 (SD1) and GA3ox2 (D18; Itoh et al. 2001, Sasaki et al. 2002). Such differential usage of GA20 and GA3 oxidases may be related to the difference in the dominant bioactive GA between the vegetative and anther tissues, i.e. the rice vegetative tissues dominantly use GA1, whereas the rice anther uses GA4 (Table 1). A differential expression pattern of GA3ox genes has also been observed in Arabidopsis, which contains four GA3ox genes (AtGA3ox1–AtGA3ox4) (Mitchum et al. 2006). The expression of AtGA3ox1 was observed throughout plant development, whereas AtGA3ox2 was expressed mainly during seed germination and vegetative growth, and AtGA3ox3 and AtGA3ox4 were predominantly expressed in the flowers and silique. In stamens, AtGA3ox1 was expressed in the anther filament, whereas other oxidases were expressed in the anthers and in the MS/POL (Mitchum et al. 2006, Hu et al. 2008). Although it has not been investigated, an interesting possibility is that GA20ox3 and GA3ox1 in MS/POL have higher affinities for the 13-non-hydroxylated GAs such as GA12 and GA9, whereas GA20ox2 (SD1) and GA3ox2 (D18) prefer 13-hydroxylated GAs such as GA53 and GA20.
Auxin
Our LM-array analysis demonstrates that the expression of OsASB2, OsYUCCA4 and OsTAA1;4 is synchronously induced at the later stages of POL development (Figs. 3, 4). At present, it is not yet clear how IAA is synthesized in rice, although lines of evidence pointing to the importance of some YUCCA genes in IAA synthesis in the vegetative organs of rice have been reported (Woo et al. 2007, Yamamoto et al. 2007). The well coordinated expression of the Cluster I genes suggests their involvement in the high level accumulation of IAA in the mature POL, thus implicating that reactions catalyzed by YUCCA and TAA1 enzymes (each catalyzing different IAA synthetic pathway) are important steps in IAA synthesis of rice anthers. Cluster III and IV, including OsTAA1;3 and OsYUCCA1 and 5, whose expression pattern is almost a mirror-image of Cluster I, suggest that different sets of IAA synthesis genes are used in a cell type-specific manner similar to GA20ox and GA3ox in the case of GA synthesis. Similar to biosynthetic genes, the expression of OsTIR1 varied depending on the developmental stage. This is consistent with the differential expression pattern of TIR1 and AFB1–AFB3 in Arabidopsis anthers (Cecchetti et al. 2008).
Several studies have suggested the importance of auxin during anther development. In the floral organs of Arabidopsis, for example, auxin predominantly accumulates in the MS/POL and the TAP, with the highest levels of accumulation occurring during mitosis (Feng et al. 2006). When the bioactive auxin level decreased in the anther through the overproduction of IAAL (an enzyme that converts free IAA to IAA-lysine conjugate), the development of the POL following the mitotic division is inhibited, resulting in a reduced number of mature POL (Feng et al. 2006). Furthermore, treatment of Torenia fournieri with IAA stimulates POL tube growth, causing tubes to be slender and straighter, possibly by disorganization of cellulose microfibrils in the tube (Wu et al. 2008). Additionally, loss of the function of all four auxin receptors in Arabidopsis results in the precocious maturation of the POL, anther dehiscence and reduced filament elongation (Cecchetti et al. 2008), indicating that auxin signaling is also required during anther development. Our data are consistent with these observations. Significantly high accumulation of free IAA in mature anthers and the preferential expression of IAA synthetic genes and TIR1 homologs at the later stages of MS/POL development, support the idea that IAA synthesized in the POL is crucial for POL maturation, and the subsequent POL germination and tube elongation.
From the expression pattern of IAA/AUX and ARF genes, we propose possible pairs of IAA/AUX and ARF proteins in the anther to be those that occurred in the same group (i.e. I-1 to II-5). Despite their importance, the combinations of IAA/AUXs and ARFs that function during each plant developmental stage have only been partly identified even in Arabidopsis due to functional redundancies among these genes (Okushima et al. 2005, Overvoorde et al. 2005). Expression profiling using laser microdissected cells, which is expected to show very specific expression patterns of IAA/AUX and ARF genes, may be an efficient way to identify specific pairs of IAA/AUXs and ARFs initially.
Gene expression of other phytohormone biosynthesis and signaling genes
CK
There are currently only a few reports concerning the role of CK in the anther. When CKX was specifically expressed in transgenic maize under the control of anther-specific and POL-specific promoters, both resulted in male sterility (Huang et al. 2003), suggesting that CK is required during male organ development in both the sporophytic and gametophytic tissues. Our results support this hypothesis; endogenous CKs were detected in the mature anther to some extent, and CK synthesis and perception genes were also actively expressed in both developing MS/POL and TAP. Our results further suggest that CK synthesis preferentially occurs during the early stage of MS/POL and that active CK is deactivated by CKX in the later stages of MS/POL development. The production of CK during MEI to the UN stage might be related to meiosis and mitosis of MS/POL, but the deactivation of CK at the later stage in POL is an unexpected but interesting phenomenon that apparently differs from the rapid increase in production of GA and IAA. Thus, it will be interesting to investigate the biological significance of this unique deactivation of CK in the later stages of POL development.
BR
BR was initially identified in POL of rape (Brassica napus); several BRs were later isolated from POLs of various plant species (Grove et al. 1979, Mandava et al. 1988). Further, biological functions of BR in POL were confirmed by the promotion of POL tube elongation in Prunus avium in vitro (Hewitt et al. 1985). The Arabidopsis BR-deficient and -insensitive mutants cpd and bri1, respectively, fail to produce viableseed because of male-sterile POLs (Szekeres et al. 1996, Li and Chory 1997). These observations suggest that BR is accumulated in POLs and functions in POL tube elongation. However, we found no clear trend in the expression of BR synthesis genes, especially in the BR-specific pathway during the POL developmental stages, which is in clear contrast to the synchronized expression of GA and IAA synthesis genes during the later stages of POL development. This leads us to suspect that there is active BR production in developing rice MS/POL. Supporting this speculation, all of the rice BR-deficient mutants, including osdwarf, d11, d2 and osdim, are inherited normally from the F1 plants to the F2 generation, in contrast to the GA synthesis mutants (Hong et al. 2002, Hong et al. 2003, Hong et al. 2005, Tanabe et al. 2005). This indicates that gametophytic BR synthesis is not necessary for POL fertility. Further studies should be conducted to clarify the importance of BR for rice POL development.
In contrast to MS/POL cells, genes for BR synthesis and signaling were highly expressed in TAP cells. Ariizumi et al. (2008) found that BR is important for exine layer formation in Arabidopsis POL. The det2 mutant has a defect in the initial step of exine formation, although this defect is restored at later stages. The major constituent of exine, sporopollenin, is supplied from TAP cells in both Arabidopsis and rice, so it is possible that in rice, BR functions in POL wall layer formation in TAP through the regulation of sporopollenin synthesis or transport.
Ethylene
There are currently only a few descriptions of the role of ethylene in anther development, with the exception of its role in anther dehiscence for the release of POL (Davies 2004). The introduction of a constitutively active form of the melon ethylene receptor into tobacco under the control of an anther-specific promoter caused a reduced number of POL grains and a delay in the programmed cell death (PCD) of TAP cells (Takada et al. 2006). Because ethylene is a key regulator of PCD (Drew and Morgan 2000, Rubinstein 2000, Young 2000, Gunawardena and Arunika 2008), these observations suggest that ethylene is involved in the PCD of TAP cells. The preferential expression of ethylene signaling genes in TAP, especially at the TET and UN stages, supports this idea, although clear induction of ACS expression was not observed in TAP cells at this stage. It is possible that ethylene is produced in other cells of the anther and that the ethylene level in locules increases, or, alternatively, the ACS expression is sufficient for ethylene production in TAP under the increased expression of OsACO2.
ABA
Although active ABA synthesis and signaling in the anther was not clearly demonstrated, the reduced expression of ABA synthetic genes and the elevated expression of genes encoding the ABA-deactivating enzyme at the late developmental stages of TAP suggest that the level of ABA might be reduced at these stages. Since ABA is known to suppress PCD of aleurone cells (Fath et al. 2000), induction of its deactivation enzymes at the late stages of TAP development may have a significant role in promoting PCD in TAP cells. Additionally, our microarray analysis revealed an interesting expression pattern for OsVP1. The transcription factor OsVP1 is required for the activation of ABA-regulated genes during embryo maturation, and its expression is not normally detectable during the vegetative stages (Nakagawa et al. 1996). We observed a significant level of OsVP1 expression at the TC stage of the POL and at the UN stage of the TAP, suggesting that OsVP1 may have functions in these cells.
JA
The microarray results imply that JA synthesis and signaling are active in TAP at all stages, suggesting that JA is important for TAP development. In MS/POL, although we could not clearly demonstrate the synthesis of JA from the Z-value heat map, observation of SI data suggested that JA might be preferentially synthesized at the TC stage. Ishiguro et al. (2001) and Sanders et al. (2000) reported that the specific expression of AtDAD1 and AtOPR3 in Arabidopsis occurs in the anther filament such that JA synthesis preferentially occurs in anther filaments but not in MS/POL or TAP. They also hypothesized that JA synthesized in the filaments regulates water transport in the stamens to induce anther dehiscence and desiccation of the locule, resulting in POL maturation. However, biologically active JA derivatives were identified in POL of Pinus mugo and Petunia hybrida, and in anthers of Camellia species (Yamane et al. 1982, Knöfel et al. 1995, Miersch et al. 1998). Further, these JA derivatives inhibited POL germination and its tube elongation to maintain POL dormancy (Yamane et al. 1982, Knöfel et al. 1995). These observations strongly suggest that high levels of JA localized in the anther and MS/POL may function in a cell-autonomous manner. Inconsistent observations between Arabidopsis and these other plant species led us to speculate that the JA/COI1 signaling pathway may function differently in distinct developmental processes depending on the plant species, as previously discussed by Li et al. (2004).
Involvement of phytohormones during anther development
Our comprehensive transcriptome analyses of genes related to seven phytohormones suggest important functional roles for each phytohormone at each specific stage of MS/POL and TAP development. We schematically present the assumed changes in the concentration of each hormone in MS/POL in Fig. 11. IAA, GA and ethylene synthesis genes showed preferential expression during later stages of POL development in MS/POL (Fig. 1–4,and8; see also Fig. 11), and, consistently, significant high level accumulation of free IAA and GA4 was actually observed in the anther of the TC stages. In contrast, the reduced expression of GA signaling genes in the later stages implies that GA synthesized during the BC and TC stages is not used immediately, but is stored for later POL germination and POL tube elongation events. In fact, GA produced at the late stages of POL development is necessary for POL germination and its tube elongation in rice (Chhun et al. 2007). On the other hand, CK synthesis genes had a unique expression pattern among the analyzed phytohormones, with active expression limited to the early stages of MS development. The predominant expression of deactivation genes (CKX genes) at the later stages of MS development further suggests that CKs are deactivated after the UN stage. In contrast to IAA and GA, non-coordinate expression of genes encoding the BR synthesis enzymes suggests that BR may not be actively synthesized in MS/POL. For ABA and JA, we also could not define clear patterns of synthesis from the Z-value heat map, whether they were synthesized or not. However, although the SIs may not accurately reflect the absolute expression of the genes, the raw heat map data suggest that ABA biosynthetic genes are also relatively well expressed during anther development and, consistent with our hypothesis, ABA was detected in the TC stages of anthers. Similarly, expression of JA synthesis was suggested from the raw heat map data. Other types of analysis are necessary to confirm our observations.
Fig. 11.
Schematic representation of changes in phytohormone levels in the MS/POL during development, as predicted from the microarray data. vn; vegetative nucleus, gc; generative cell.
In TAP, although the relative activities of the different phytohormones varied, synthesis and signaling appeared to occur for all of the phytohormones analyzed, indicating that they actively function in TAP. These findings are in contrast to those for MS/POL. The expression of ethylene and JA synthesis genes in TAP and preferential expression of ABA deactivation genes at late stages of TAP are consistent with previous reports of the function of ethylene and JAs in the PCD of TAP cells and of ABA inhibiting the PCD of aleurone cells (Fath et al. 2000).
Clear differences in the expression profiles of MS/POL and TAP demonstrate the powerfulness of LM technology when combined with global transcriptome analysis. This strategy should be applicable to other tissues, plant developmental stages and sets of genes, thereby providing better accuracy and deeper insight into the dynamics of plant development.
Materials and Methods
Plant materials
Rice (O. sativa L. ssp. japonica cv. Nipponbare) plants were grown in a greenhouse under normal conditions. Samples were collected from various stages of developing anthers. The developmental stages were confirmed by microscopy. The BC stage was further classified into EBC (early BC), MBC (middle BC) and LBC (late BC) stages by observing the expression profiles of the genes during the BC stage; samples that showed relatively similar expression to the UN stage were designated as EBC, similar expression to the TC stage as LBC and the remainder as MBC (Suwabe et al. 2008). Whole anther, the leaf blade and the pistil of the TC stage were used for phytohormone quantification.
Quantification of phytohormones
About 100 mg (fresh weight) of each plant part was subjected to analysis. Quantification of phytohormones was performed as described (Nakagawa et al. 2005, Hirano et al. 2007, Naito et al. 2007) using a liquid chromatography–mass chromatography system (UPLC/Quattro Premier XE; Waters) with an ODS column (AQUITY-UPLC BEH-C18, 1.7 μm, 2.1 × 100 mm, Waters).
Laser microdissection, isolation and linear amplification of mRNA, and microarray analyses
Sections of rice anther in each developmental stage were laser microdissected using the PixCell II LCM system (Arcturus, Mountain View, CA, USA) according to the manufacturer's instructions and the methods of Nakazono et al. (2003). Total RNA was quantified using a Quant-iT™ RiboGreen RNA reagent and assay kit (Invitrogen). We used a rice genome 44K oligo microarray containing approximately 42,000 oligonucleotides synthesized based on the nucleotide sequence and full-length cDNA data of the Rice Annotation Project (RAP; http://rapdb.dna.affrc.go.jp/). Fluorescent probe labeling, hybridization and scanning were performed according to the manufacturers’ instructions. Microarray data were analyzed statistically for variance stabilization (VSN) and mean − SD (Z) scaling, using R software (http://www.r-project.org/). Cluster analysis was performed using the Cluster and TreeView software (Eisen et al. 1998). Please refer to the methods of Suwabe et al. (2008) in this special issue for descriptions of experimental procedures.
Identification of phytohormone-related genes in rice
The identification of rice phytohormone biosynthesis and signaling genes whose functions have only been studied in other plant species was conducted through BLAST searches (Altschul et al. 1997, http://rapdb.dna.affrc.go.jp/tools/blast) using the genes of other plant species as queries. Genes that were obtained from the BLAST search and query sequence were aligned using ClustalW version 1.81 (http://align.genome.jp/), followed by manual alignment. A neighbor-joining (NJ) tree (Saitou and Nei 1987) was obtained (http://align.genome.jp/sit-bin/clustalw); sequences that had the closest phylogeny to the query sequences were assumed to be orthologs of rice genes (Supplementary Table S1).
Funding
The Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) Grants-in-Aid for Special Research on Priority Areas (No. 18075006).
Supplementary Material
Acknowledgments
The authors thank Dr. Hidehiro Fukaki of Kobe University, and Dr. Tomoaki Sakamoto, Dr. Motoyuki Ashikari, Dr. Tsukaho Hattori and Dr. Sumie Ishiguro of Nagoya University for their valuable suggestions and critical reading of the manuscript.
Glossary
Abbreviations:
- BC
bicellular POL stage
- BR
brassinosteroid
- CK
cytokinin
- GA
gibberellin
- JA
jasmonic acid
- MEI
meiosis
- MS
microspore
- PCD
programmed cell death
- POL
pollen
- SI
signal intensity
- TAP
tapetum
- TC
tricellular POL stage
- TET
tetrad stage
- UN
uninuclear MS stage
References
- Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H. Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel OsTATC gene. Plant Physiol. 2001;125:1248–1257. doi: 10.1104/pp.125.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T, Kawanabe T, Hatakeyama K, Sato S, Kato T, Tabata S, et al. Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant Cell Physiol. 2008;49:58–67. doi: 10.1093/pcp/pcm167. [DOI] [PubMed] [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, et al. Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc. Natl Acad. Sci. USA. 2007;104:13839–13844. doi: 10.1073/pnas.0706386104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl Acad. Sci. USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen RJ, Beall FD, Mullet JE, Morgan PW. Detection of endogenous gibberellins and their relationship to hypocotyl elongation in soybean seedlings. Plant Physiol. 1990;94:77–84. doi: 10.1104/pp.94.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boter M, Ruíz-Rivero O, Abdeen A, Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004;18:1577–1591. doi: 10.1101/gad.297704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell. 2008;20:1760–1774. doi: 10.1105/tpc.107.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W-H, Endo A, Zhou L, Penney J, Chen H-C, Arroyo A, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhun T, Aya K, Asano K, Yamamoto E, Morinaka Y, Watanabe M, et al. Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell. 2007;19:3876–3888. doi: 10.1105/tpc.107.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Chow B, McCourt P. Plant hormone receptors: perception is everything. Genes Dev. 2006;20:1998–2008. doi: 10.1101/gad.1432806. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Arabidopsis mutants reveal multiple roles for sterols in plant development. Plant Cell. 2002;14:1995–2000. doi: 10.1105/tpc.140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Castillo M, Martínez C, Buchala A, Métraux JP, León J. Gene-specific involvement of beta-oxidation in wound-activated responses in Arabidopsis. Plant Physiol. 2004;135:85–94. doi: 10.1104/pp.104.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Davies PJ. Plant Hormones: Biosythesis, Signal Transduction, Action!. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2004. [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Bostein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R. Programmed cell death in cereal aleurone. Plant Mol. Biol. 2000;44:255–266. doi: 10.1023/a:1026584207243. [DOI] [PubMed] [Google Scholar]
- Feng XL, Ni WM, Elge S, Mueller-Roeber B, Xu ZH, Xue HW. Auxin flow in anther filaments is critical for pollen grain development through regulating pollen mitosis. Plant Mol. Biol. 2006;61:215–226. doi: 10.1007/s11103-006-0005-z. [DOI] [PubMed] [Google Scholar]
- Feussner I, Hause B, Vörös K, Parthier B, Wasternack C. Jasmonate-induced lipoxygenase forms are localized in chloroplast of barley leaves (Hordeum vulgare cv. Salome). Plant J. 1995;7:949–957. [Google Scholar]
- Froehlich JE, Itoh A, Howe GA. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001;125:306–317. doi: 10.1104/pp.125.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM. Anther development: basic principles and practical applications. Plant Cell. 1993;5:1217–1229. doi: 10.1105/tpc.5.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, et al. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell. 2002;14:1833–1846. doi: 10.1105/tpc.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell. 1999;11:1897–1910. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. [Google Scholar]
- Gunawardena AH. Programmed cell death and tissue remodelling in plants. J. Exp. Bot. 2008;59:445–451. doi: 10.1093/jxb/erm189. [DOI] [PubMed] [Google Scholar]
- Hall SM, Medlow GC. Identification of IAA in phloem and root pressure saps of Ricinus communis L. by mass spectrometry. Planta. 1974;119:257–261. doi: 10.1007/BF00429049. [DOI] [PubMed] [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002;16:1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Nakajima M, Takeda K, Yamaguchi I, Murofushi N. Endogenous levels of gibberellins in normal and male sterile anthers of rice (Oryza sativa cv. Nihonmasari). Biosci. Biotech. Biochem. 1995;59:1716–1720. [Google Scholar]
- Hewitt FR, Hough T, O’Neill P, Saase JM, Williams EG, Rowan KS. Effect of brassinolide and other growth regulators on germination and growth of pollen tubes of Prunus avium using a multiple hanging-drop assay. Aust. J. Plant Physiol. 1985;12:201–211. [Google Scholar]
- Hirano K, Nakajima M, Asano K, Nishiyama T, Sakakibara H, et al. The GID1 mediated GA perception mechanism is conserved in the lycophyte Selaginella moellendorffii but not in the bryophyte Physcomitrella patens. Plant Cell. 2007;19:3058–3079. doi: 10.1105/tpc.107.051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Ueguchi-Tanaka M, Matsuoka M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008;13:192–199. doi: 10.1016/j.tplants.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hirose N, Makita N, Kojima M, Kamada-Nobusada T, Sakakibara H. Over-expression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007;48:523–539. doi: 10.1093/pcp/pcm022. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, et al. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell. 2005;17:2243–2254. doi: 10.1105/tpc.105.030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, et al. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 2002;32:495–508. doi: 10.1046/j.1365-313x.2002.01438.x. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, et al. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell. 2003;15:2900–2910. doi: 10.1105/tpc.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, et al. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell. 2008;20:320–336. doi: 10.1105/tpc.107.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, et al. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003;131:1270–1282. doi: 10.1104/pp.102.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, et al. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Shimada A, Ueguchi-Tanaka M, Kamiya N, Hasegawa Y, Ashikari M, et al. Overexpression of a GRAS protein lacking the DELLA domain confers altered gibberellin responses in rice. Plant J. 2005;44:669–679. doi: 10.1111/j.1365-313X.2005.02562.x. [DOI] [PubMed] [Google Scholar]
- Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, et al. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 2004;54:533–547. doi: 10.1023/B:PLAN.0000038261.21060.47. [DOI] [PubMed] [Google Scholar]
- Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M. Cloning and functional analysis of two gibberellin 3 beta-hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl Acad. Sci. USA. 2001;98:8909–8914. doi: 10.1073/pnas.141239398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Kurata N. Identification and characterization of cytokinin-signaling gene families in rice. Gene. 2006;382:57–65. doi: 10.1016/j.gene.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc. Natl Acad. Sci. USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct. Integr. Genomics. 2006;6:47–59. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Inukai Y, Ueguchi-Tanaka M, Itoh H, Izawa T, Kobayashi Y, et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell. 2004;16:33–44. doi: 10.1105/tpc.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, et al. Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J. 2003;35:104–115. doi: 10.1046/j.1365-313x.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- Katayama M, Thiruvikraman SV, Marumo S. Localization of 4-chloroindole-3-acetic acid in seeds of Pisum sativum and its absence from all other organs. Plant Cell Physiol. 1988;29:889–891. [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl Acad. Sci. USA. 2008;105:7100–7105. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S. The anatomy of auxin perception. Bioessays. 2007;29:953–956. doi: 10.1002/bies.20657. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Knöfel HD, Sembdner G. Jasmonates from pine pollen. Phytochemistry. 1995;38:569–571. [Google Scholar]
- Kobayashi M, Sakurai A, Saka H, Takahashi N. Quantitative analysis of endogenous gibberellins in normal and dwarf cultivars of rice. Plant Cell Physiol. 1989;30:963–969. [Google Scholar]
- Koga J, Adachi T, Hidaka H. Molecular cloning of the gene for indolepyruvate decarboxylase from Enterobacter cloacae. Mol. Gen. Genet. 1991;226:10–16. doi: 10.1007/BF00273581. [DOI] [PubMed] [Google Scholar]
- Koh S, Lee SC, Kim MK, Koh JH, Lee S, An G, et al. T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 2007;65:453–466. doi: 10.1007/s11103-007-9213-4. [DOI] [PubMed] [Google Scholar]
- Koo AJ, Chung HS, Kobayashi Y, Howe GA. Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem. 2006;281:33511–33520. doi: 10.1074/jbc.M607854200. [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Zeevaart JA. Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol. 2005;138:243–254. doi: 10.1104/pp.104.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, Lee GI, Jayanty S, Sageman C, et al. Role of beta-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell. 2005;17:971–986. doi: 10.1105/tpc.104.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plant Sci. 2007;12:37–41. doi: 10.1016/j.tplants.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava NB. Plant growth-promoting brassinosteroids. Annu. Rev. Plant Phsiol. Plant Mol. Biol. 1988;39:23–52. [Google Scholar]
- Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, et al. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996;15:2331–2342. [PMC free article] [PubMed] [Google Scholar]
- Maucher H, Hause B, Feussner I, Ziegler J, Wasternack C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): tissue specific regulation in seedling development. Plant J. 2000;21:199–213. doi: 10.1046/j.1365-313x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- Miersch O, Knofel HD, Schmidt J, Kramell R, Parthier B. A jasmonic acid conjugate, N-[(–)-jasmonoyl]-tyramine, from Petunia pollen. Phytochemistry. 1998;47:327–329. [Google Scholar]
- Mitchum MG, Yamaguchi S, Hanada A, Kuwahara-Yoshioka Y, Kato T, Tabata S, et al. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J. 2006;45:804–818. doi: 10.1111/j.1365-313X.2005.02642.x. [DOI] [PubMed] [Google Scholar]
- Morino K, Matsuda F, Miyazawa H, Sukegawa A, Miyagawa H, Wakasa K. Metabolic profiling of tryptophan-overproducing rice calli that express a feedback-insensitive alpha subunit of anthranilate synthase. Plant Cell Physiol. 2005;46:514–521. doi: 10.1093/pcp/pci051. [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J. Advances in cytokinin signaling. Science. 2007;318:68–69. doi: 10.1126/science.1145461. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development. 2005;132:4107–4118. doi: 10.1242/dev.01955. [DOI] [PubMed] [Google Scholar]
- Naito T, Yamashino T, Kiba T, Koizumi N, Kojima M, Sakakibara H, et al. A link between cytokinin and ASL9 (ASYMMETRIC LEAVES 2 LIKE 9) that belongs to the AS2/LOB (LATERAL ORGAN BOUNDARIES) family genes in Arabidopsis thaliana. Biosci. Biotech. Biochem. 2007;71:1269–1278. doi: 10.1271/bbb.60681. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Jiang CJ, Sakakibara H, Kojima M, Honda I, Ajisaka H, et al. Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. Plant J. 2005;41:512–523. doi: 10.1111/j.1365-313X.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ohkura E, Ohmiya K, Hattori T. The seed-specific transcription factor VP1 (OSVP1) is expressed in rice suspension-cultured cells. Plant Cell Physiol. 1996;37:355–362. doi: 10.1093/oxfordjournals.pcp.a028953. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamaguchi I, Kizawa S, Murofushi N, Takahashi N. Semi-quantification of GAX and GA4 in male-sterile anthers of rice by radioimmunoassay. Plant Cell Physiol. 1991;32:511–513. [Google Scholar]
- Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, et al. The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 2006;140:580–590. doi: 10.1104/pp.105.072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Hakata M, Amano K, Miyao A, Toki N, et al. A genome-wide gain-of function analysis of rice genes using the FOX-hunting system. Plant Mol. Biol. 2007;65:357–371. doi: 10.1007/s11103-007-9243-y. [DOI] [PubMed] [Google Scholar]
- Nakazono M, Qui F, Brsuk LA, Schnable PS. Laser-capture microdissection, a tool for the global analysis of gene expression in specific plant cell type: identification of genes expressed differentially in epidermal cell or vascular tissues of maize. Plant Cell. 2003;15:583–596. doi: 10.1105/tpc.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, et al. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc. Natl Acad. Sci. USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan A, Jain M, Tyagi AK, Khurana JP. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008;146:333–350. doi: 10.1104/pp.107.112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Grisafi P, Fink GR, Bartel B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell. 1997;9:1781–1790. doi: 10.1105/tpc.9.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Nomura T, Watanabe B, Ohta D, Yokota T, Miyagawa M, et al. Tomato cytochrome P450 CYP734A7 functions in brassinosteroid catabolism. Phytochemistry. 2006a;67:1895–1906. doi: 10.1016/j.phytochem.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos SC, et al. C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell. 2006b;18:3275–3288. doi: 10.1105/tpc.106.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell. 2005;17:3282–3300. doi: 10.1105/tpc.105.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL. Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol. 2006;142:380–397. doi: 10.1104/pp.106.086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Regulation of cell death in flower petals. Plant Mol. Biol. 2000;44:303–318. doi: 10.1023/a:1026540524990. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sakai H, Honma T, Aoyama T, Sato S, Kato T, Tabata S, et al. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science. 2001;294:1519–1521. doi: 10.1126/science.1065201. [DOI] [PubMed] [Google Scholar]
- Sakai M, Sakamoto T, Saito T, Matsuoka M, Tanaka H, Kobayashi M. Expression of novel rice gibberellin 2-oxidase gene is under homeostatic regulation by biologically active gibberellins. J. Plant Res. 2003;116:161–164. doi: 10.1007/s10265-003-0080-z. [DOI] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu. Rev. Plant. Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–1653. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006b;24:105–109. doi: 10.1038/nbt1173. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, et al. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006a;142:54–62. doi: 10.1104/pp.106.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, et al. The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;12:1041–1061. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature. 2002;416:701–702. doi: 10.1038/416701a. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299:1896–1898. doi: 10.1126/science.1081077. [DOI] [PubMed] [Google Scholar]
- Schneider EA, Kazakoff CW, Wightman F. Gas chromatography–mass spectrometry evidence for several endogenous auxins in pea seedling organs. Planta. 1985;165:232–241. doi: 10.1007/BF00395046. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, et al. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate-regulated plant responses. Proc. Natl Acad. Sci. USA. 2001;98:4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Akaba S, Oritani T, Delarue M, Bellini C, Caboche M, et al. Higher activity of an aldehyde oxidase in the auxin-overproducing superroot1 mutant of Arabidopsis thaliana. Plant Physiol. 1998;116:687–693. doi: 10.1104/pp.116.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, et al. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl Acad. Sci. USA. 2000;97:12908–12913. doi: 10.1073/pnas.220426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Ueguchi-Tanaka M, Sakamoto T, Fujioka S, Takatsuto S, Yoshida S, et al. The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J. 2006;48:390–402. doi: 10.1111/j.1365-313X.2006.02875.x. [DOI] [PubMed] [Google Scholar]
- Smith VA, Knatt CJ, Gaskin P, Reid JB. The distribution of gibberellins in vegetative tissues of Pisum sativum L.: I. Biological and biochemical consequences of the le mutation. Plant Physiol. 1992;99:368–371. doi: 10.1104/pp.99.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol. Biol. 2003;51:895–911. doi: 10.1023/a:1023049319723. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis, Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl Acad. Sci. USA. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe K, Suzuki G, Takahashi H, Shiono K, Endo M, et al. Separated transcriptomes of male gametophyte and tapetum in rice: validity of a laser microdissection (LM) microarray. Plant Cell Physiol. 2008;49:1407–1416. doi: 10.1093/pcp/pcn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser PB, Swartzfager DG. Indole-3-acetic acid levels of plant tissue as determined by a new high performance liquid chromatographic method. Plant Physiol. 1978;61:254–258. doi: 10.1104/pp.61.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takada K, Ishimaru K, Kamada H, Ezura H. Anther-specific expression of mutated melon ethylene receptor gene Cm-ERS1/H70A affected tapetum degeneration and pollen grain production in transgenic tobacco plants. Plant Cell Rep. 2006;25:936–941. doi: 10.1007/s00299-006-0147-0. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005;17:776–790. doi: 10.1105/tpc.104.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF (COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- To JP, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Aya K, Ueguchi-Tanaka M, Shimada Y, Nakazono M, et al. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 2006;47:427–444. doi: 10.1111/j.1365-313X.2006.02795.x. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Varbanova M, Yamaguchi S, Yang Y, McKelvey K, Hanada A, et al. Methylation of gibberellins by Arabidopsis GAMT1 and GAMT2. Plant Cell. 2007;19:32–45. doi: 10.1105/tpc.106.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pei K, Fu Y, Sun Z, Li S, Liu H, et al. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene. 2007;394:13–24. doi: 10.1016/j.gene.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waycott W, Smith VA, Gaskin P, Macmillan J, Taiz L. The endogenous gibberellins of dwarf mutants of lettuce. Plant Physiol. 1991;95:1169–1173. doi: 10.1104/pp.95.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, et al. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005;24:1874–1885. doi: 10.1038/sj.emboj.7600659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YM, Park HJ, Su’udi M, Yang JI, Park JJ, Back K, et al. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol. Biol. 2007;65:125–136. doi: 10.1007/s11103-007-9203-6. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Lin Y, Zhang XL, Pang DW, Zhao J. IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J Exp Bot. 2008;59:2529–2543. doi: 10.1093/jxb/ern119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell. 2001;13:2063–2083. doi: 10.1105/TPC.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007;143:1362–1371. doi: 10.1104/pp.106.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, et al. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell. 2000;12:1591–1606. doi: 10.1105/tpc.12.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Abe H, Takahashi N. Jasmonic acid and methyl jasmonate in pollens and anthers of three Camellia species. Plant Cell Physiol. 1982;23:1125–1127. [Google Scholar]
- Yang W, Devaiah SP, Pan X, Isaac G, Welti R, Wang X. AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. J. Biol. Chem. 2007;282:18116–18128. doi: 10.1074/jbc.M700405200. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, et al. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006;140:115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Gallie DR. Programmed cell death during endosperm development. Plant Mol. Biol. 2000;44:283–301. doi: 10.1023/a:1026588408152. [DOI] [PubMed] [Google Scholar]
- Zazimalova E, Napier RM. Points of regulation for auxin action. Plant Cell Rep. 2003;21:625–634. doi: 10.1007/s00299-002-0562-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, et al. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, et al. ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell. 2006;18:442–456. doi: 10.1105/tpc.105.038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J, Stenzel I, Hause B, Maucher H, Hamberg M, Grimm R, et al. Molecular cloning of allene oxide cyclase. The enzyme establishing the stereochemistry of octadecanoids and jasmonates. J. Biol. Chem. 2000;275:19132–19138. doi: 10.1074/jbc.M002133200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.