Abstract
Invadopodia are actin-dependent organelles that function in the invasion and remodeling of the extracellular matrix (ECM) by tumor cells. Cortactin, a regulator of the Arp2/3 complex, is of particular importance in invadopodia function. While most of the focus has been on the possible role of cortactin in actin assembly for direct formation of actin-rich invadopodia puncta, our recent data suggest that the primary role of cortactin in invadopodia is to promote protease secretion. In this manuscript, we review our previous work and present new data showing that cortactin is essential for both the localization of key invadopodia matrix metalloproteinases (MMPs) to actin-positive puncta at the cell-ECM interface and for ECM degradation induced by overexpression of MT1-MMP-GFP. Based on these data and results from the literature, we propose potential mechanisms by which cortactin may link vesicular trafficking and dynamic branched actin assembly to regulate protease secretion for invadopodia-associated ECM degradation.
Keywords: Cortactin, Invadopodia, Matrix metalloproteinase, Protease, Membrane trafficking, Vesicle
Introduction
Invasion and metastasis is dependent on the ability of tumor cells to remodel and degrade the extracellular matrix (ECM) (Hoon et al., 2006; Pantel and Brakenhoff, 2004). In vitro, many invasive cancer cell lines have been shown to form specialized structures termed invadopodia for this process. Invadopodia are actin-based protrusions on the basal surface of invading cells that serve as centers in which cellular processes such as branched actin assembly, cell signaling and adhesion, and secretion of proteases spatially converge to promote remodeling of the ECM (Linder, 2007; Weaver, 2006).
Invadopodia were first identified in src-transformed cells and subsequent studies have shown that the formation of invadopodia is dependent on src tyrosine kinase signaling (Chen et al., 1985; Linder, 2007; Weaver, 2006). A number of src substrates, such as cortactin, Tks5/FISH, p130Cas, dynamin2, and N-WASp (Baldassarre et al., 2003; Bowden et al., 1999; Mizutani et al., 2002; Seals et al., 2005; Weaver, 2006; Yamaguchi et al., 2005), also localize to invadopodia and/or the related structures, podosomes (Linder, 2007). These downstream targets presumably function to coordinate the activities of the actin cytoskeleton, focal adhesions, protease activity, and membrane dynamics to the site of invadopodia formation.
Cortactin, an actin assembly protein that functions in both the activation and stabilization phases of branched actin assembly by the Arp2/3 complex (Tehrani et al., 2007; Uruno et al., 2001; Weaver et al., 2001), has been a focus of particular attention in the invadopodia field. Cortactin is present at sites of dynamic actin assembly in cellular protrusions such as lamellipodia and invadopodia (Yamaguchi and Condeelis, 2007); however, the precise role of cortactin in these processes is under much debate. Cortactin overexpression enhances cell migration (Bryce et al., 2005; Hill et al., 2006; Huang et al., 1998; Patel et al., 1998), and cells with cortactin knockdown by siRNA show defects in 2-dimensional migration as well as invasion through Matrigel-coated transwell filters (Bryce et al., 2005; Hill et al., 2006; Rothschild et al., 2006; van Rossum et al., 2006). Cortactin has also been shown to have a major role in the function of invadopodia (Artym et al., 2006; Bowden et al., 1999; Clark et al., 2007) and the related structures, podosomes (Tehrani et al., 2006; Webb et al., 2006, 2007). Bowden et al. (1999) first demonstrated that cortactin is important for invadopodia function, since microinjection with neutralizing antibodies against cortactin blocked degradation of the underlying ECM (Bowden et al., 1999). That study also showed that cortactin is enriched in invadopodia in a complex with paxillin and protein kinase Cµ/protein kinase D. A recent study by Artym et al. (2006) proposed a step-wise model of invadopodia formation. Their live-cell imaging studies, combined with fixed cell analyses, indicate that cortactin and actin are early markers of invadopodia, followed quickly by accumulation of MT1-MMP in the developing invadopodia. Ongoing accumulation of actin, cortactin, and MT1-MMP coincides with matrix degradation (Artym et al., 2006). Using siRNA, Artym et al. (2006) found that downregulation of cortactin decreases the number of actin/cortactin-rich invadopodia puncta along with associated ECM degradation. Based on this work, on studies in podosomes (Tehrani et al., 2006; Webb et al., 2006, 2007), and the important role of cortactin in branched actin assembly (Uruno et al., 2001; Weaver et al., 2001), much of the focus in the field has been on the putative role for cortactin in actin assembly that takes place on site at podosomes and invadopodia.
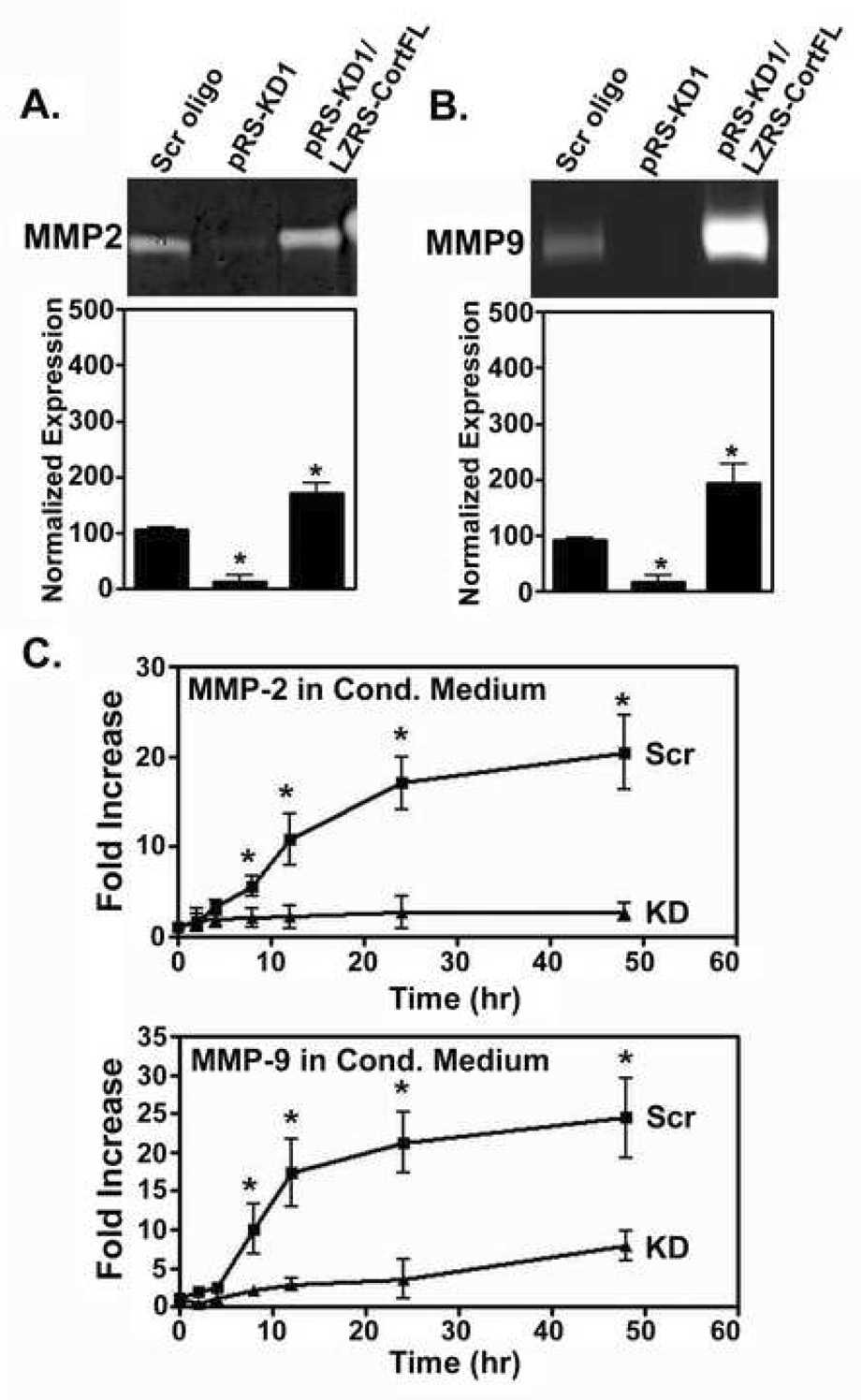
Studies from our laboratory on the role of cortactin in invadopodia also found that knockdown of cortactin leads to a decreased number of actin-based invadopodia puncta per cell when compared with control cells (Fig. 1) (Clark et al., 2007). However, while invadopodia puncta (defined as being positive for β-actin and Arp3, two branched actin invadopodia markers) are still present in cortactin knockdown (cortactin-KD) cells at a level of 40% compared to wild-type cells, there is a striking absence of invadopodia puncta-associated ECM degradation. In addition, in cortactin-overexpressing cells there is a much larger increase in invadopodia-associated ECM degradation than for β-actin/Arp3-positive invadopodia puncta formation (see data for LZRS-CortFL cells in (Clark et al., 2007), and for pRS-KD1/LZRS-CortFL cells in Fig. 1). These findings are surprising since cortactin as an actin assembly protein should affect the earlier actin puncta phase at least equally if not better than the ECM degradation phase. Inhibition of MMPs with either the broad-spectrum inhibitor GM6001 or the more specific TIMP-2 has an effect on invadopodia that is similar to cortactin-KD: fewer invadopodia puncta and abolition of ECM degradation (Clark et al., 2007). These two pieces of evidence led us to suspect that cortactin may have a role in the protease phase of invadopodia function. Western blot analyses of whole-cell lysates from cells with cortactin-KD or cortactin overexpression revealed no change in the overall cellular levels of the key invadopodia metalloproteinases MMP2, MMP9 or MT1-MMP (Clark et al., 2007). However, since these MMPs must reach the outside of the cell to access and degrade ECM, we also quantified the level of these proteins in conditioned medium (MMP2, MMP9) or on the cell surface (MT1-MMP) as a function of cortactin expression. Indeed, secretion of all three MMPs is profoundly affected by cortactin expression (see analyses and zymograms for MMP2 and MMP9 in Fig. 2, analysis of MT1-MMP surface expression in (Clark et al., 2007)), correlating well with the effect on invadopodia-associated ECM degradation (Fig. 1). Interestingly, cortactin is not only essential for secretion of MMP2 and MMP9, but overexpression boosts the levels of secreted proteases (Fig. 2, (Clark et al., 2007)), suggesting that cortactin can actually tune the system up or down. This finding is particularly important in light of the fact that cortactin is frequently overexpressed in cancer as a function of 11q13 amplification (Myllykangas et al., 2007; Ormandy et al., 2003; Rodrigo et al., 2000; Schuuring et al., 1992, 1993).
Fig. 1.
Cortactin regulates invadopodia-mediated ECM degradation more than invadopodia formation. (A) Representative images from the in vitro matrix degradation assay. SCC61 HNSCC cells expressing scrambled oligo siRNA (scrambled oligo) or siRNA targeting human cortactin, either alone (pRS-KD1) or in concert with a mouse wild-type cortactin rescue construct (pRS-KD1/LZRS-CortFL), were cultured for 20 h on crosslinked gelatin overlaid with FITC-fibronectin (FITC-Fn, green in merge), fixed and stained with antibodies against β-actin (blue in merge) and Arp3 (red in merge) tolocalize invadopodia. Pink color in the merged image shows colocalization between Arp3 and actin. Yellow color indicates colocalization between Arp3 and FITC-Fn, turquoise indicates colocalization between actin and FITC-Fn, and white represents colocalization of all three signals. Scale bar = 10 µm. Arrows point to an example invadopodium in each cell. Note the lack of matrix degradation associated with cortactin-KD (pRS-KD1) invadopodia. (B) Quantification of the in vitro matrix degradation assay. Invadopodia were identified by puncta that exhibited double-positive staining for β-actin and Arp3. Data from three independent experiments, 10–12 fields for each cell line per experiment are plotted as mean ± standard error. * p<0.05 compared to scrambled oligo control. Note that pRS-KD1/LZRS-CortFL cells are considered overexpressors, since they express cortactin protein at a level that is 3-fold that of control cells. Modified from (Clark et al., 2007).
Fig. 2.
Cortactin expression affects secretion of MMP-2 and MMP-9. (A, B) Representative zymograms from 24-h conditioned medium collected from cortactin-KD (pRS-KD1), scrambled oligo control (Scr oligo), and cortactin-KD/rescue (pRS-KD1/LZRS-CortFL) cells showing secretion of MMP-2 and MMP-9. Note that pRS-KD1/LZRS-CortFL cells are considered overexpressors, since they express cortactin protein at a level that is 3-fold that of control cells (Clark et al., 2007). An equal volume (40 µl) of conditioned medium collected from wells with equal cell number was loaded into each well. The combined densitometric measurements from three separate experiments are shown below the representative zymograms. Data are represented as mean ± standard error. * p<0.05 compared to scrambled oligo control. (C) Time course of 21 MMP-2 and MMP-9 secretion into conditioned medium from scrambled oligo (Scr) and pRS-CortKD1 (KD) cells. Equal numbers of cells (2 × 106) were plated per well for each corresponding time point in 300 µl serum-free medium. At each time, the medium was collected. The zero timepoint is 40 µl serum free medium. Data represented as mean ± standard error (n=3). * p<0.05 compared to control for that timepoint. Modified from (Clark et al., 2007).
To further investigate the role of cortactin in the protease phase of invadopodia function, we performed experiments in which we immunolocalized MMP2, MMP9, and MT1-MMP and β-actin in control and cortactin-KD cells, using confocal microscopy to image only the cell-ECM interface where invadopodia puncta occur.
Materials and methods
Cell culture and antibodies
The HNSCC cell line SCC61 was originally isolated from a tongue squamous cell carcinoma tumor and considered aggressive, as defined by lack of response to radiation therapy and the presence of tumor-positive lymph nodes (Weichselbaum et al., 1986). These cells were maintained in DMEM supplemented with 20% fetal bovine serum and 0.4 µg/ml hydrocortisone. Antibodies against MMP2, MMP9, and MT1-MMP were from Chemicon International (AB809, AB16996, and AB8345, respectively). The anti-Arp3 antibody has been previously described (Yarar et al., 1999). The anti-β-actin antibody was from Sigma (AC-74). The 19-base siRNA sequence to construct pRS-KD1 cells was targeted to nucleotides 552–570 of human cortactin: GCACGAGTCACAGAGAGAT, and the mouse cortactin A isoform was cloned into LZRS-Neo, as previously described (Bryce et al., 2005). All cell lines used were previously described (Clark et al., 2007).
In vitro matrix degradation assay
The matrix degradation assay was performed essentially as described (Chen et al., 1994). Briefly, fibronectin (BD Biosciences) was labeled with FITC (Sigma) or with Texas Red succinimidyl ester (Molecular Probes) by dialysis in borate buffer (0.17 M borate, 0.075 M NaCl, pH 9.3) or 0.1 M sodium bicarbonate buffer, pH 9.0, respectively. The buffer was changed to phosphate-buffered saline (PBS), and the sample was dialyzed extensively over 3–4 days, followed by dialysis against 50% glycerol in PBS and storage at −20°C. To coat MatTek dishes, 2.5% gelatin/2.5% sucrose in PBS was heated to 37 °C and added to the dish, followed by crosslinking with 0.5% glutaraldehyde in PBS. After ultracentrifugation for 15 min to remove aggregates, a 50-µg/ml solution of labeled fibronectin was prepared in PBS and incubated with the crosslinked gelatin in Mat-Tek dishes in the dark for 1 h. The dish was sterilized with 70% ethanol, washed with DMEM, and equilibrated with invadopodia medium (DMEM supplemented with 20% FetalClone III (Hyclone) and 10% Nu-Serum (Invitrogen)) for 30 minutes prior to the addition of cells. For invadopodia assays, 5 × 104 cells were suspended in 2 ml invadopodia medium and added to the plate for 20 h. For the experiment shown in Figure 4, 5 × 105 cells were transfected in 100-mm tissue culture plates with 4 µg MT1-MMP-GFP DNA in 4 ml medium or mock transfected using Lipofectamine (25 µl (Invitrogen)). Following 4 h incubation in the Lipofectamine/DNA mixture, the medium was changed and the cells were further incubated for 10 h before trypsinization and replating on Texas Red-labeled fibronectin/crosslinked gelatin substrates for the in vitro matrix degradation assay. The cells were fixed in 3% paraformaldehyde in PIPES buffer (127 mM NaCl, 5 mM KCl, 1.1 mM NaH2PO4, 0.4 mM KH2PO4, 2 mM MgCl2, 5.5 mM glucose, 1 mM EGTA, and 20 mM PIPES), permeabilized with 0.4% Triton X-100 in PBS (for the experiments shown in Fig. 1, Fig 4) or fixed and permeabilized with ice-cold methanol (for the experiments shown in Fig. 3) and blocked with 3% bovine serum albumin in PBS + 0.1% Tween-20 and incubated with appropriate primary and secondary antibodies or fluorescent phalloidin.
Fig. 4.
Overexpression of MT1-MMP does not rescue matrix degradation in cortactin-deficient cells. (A) Representative images from the in vitro matrix degradation assay. Control (Scrambled oligo) or cortactin-knockdown cells (pRS-KD1) were transfected with 4 µg MT1-MMP-GFP DNA (green in merge) using Lipofectamine or mock transfected. Cells were cultured for 20 h on gelatin overlaid with Texas Red-fibronectin (red in merge), fixed and stained with Phalloidin-633 (blue in merge) to stain actin filaments and localize invadopodia. Note the high level of ECM degradation in MT1-MMP-transfected controls, but not in transfected KD cells. In some MT1-MMP-transfected control cells (“Scrambled oligo, MT1-MMP-GFP”, upper image series) the ECM degradation clearly localizes to invadopodia actin puncta, whereas in the most aggressively degrading cells (“Scrambled oligo, MT1-MMP-GFP”, lower image series) there is too much degraded ECM by the 20 h timepoint to identify focal sites. Scale bar = 10 µm. (B) Quantification from two independent experiments, 10–12 fields for each cell line per experiment. Data are shown as mean ± standard error. ** p<0.01 compared to mock transfected control.
Fig. 3.
Cortactin is required to target MMPs to actin puncta. Cells were cultured for eighteen hours on cross-linked gelatin overlaid with unlabeled fibronectin. Samples were then fixed with ice-cold methanol, blocked with 3% bovine serum albumin, and stained with an antibody against β-actin (Sigma) as a marker of invadopodia (green in merge) and either MMP-2-, MMP-9-, or MT1-MMP-specific antibodies (Chemicon) (red in merge). Images were obtained by confocal microscopy, scanning at the basal surface of the cell. Notice that colocalization between actin puncta and MMPs occurs only when cortactin is expressed (see enlargements of white boxes below the appropriate images). Scale bar = 10 µm
Microscopy
Fluorescent images were captured using a Nikon Eclipse TE2000-E wide-field fluorescence microscope equipped with a 40x Plan Fluor 1.3NA objective or a Zeiss LSM510 confocal microscope with a 40x Plan-Neofluar 1.3 NA objective, pinhole = 1 airy unit. For quantitative image analysis, at least 10 randomly chosen fields were imaged per trial. Image analysis was performed using Metamorph software. Invadopodia were manually counted and graphed as either number of invadopodia/cell or the percent cells with invadopodia. For the analyses shown in Figure 1, invadopodia were defined as actin puncta that were also positive for Arp3. Cell area was determined by tracing the cell footprint of the β-actin channel and using the region tools in Metamorph to calculate area. Degradation area was determined by performing an inclusive threshold of the FITC or Texas Red channels, as appropriate, to include the dark, degraded areas of labeled fibronectin, then region tools were used to calculate the threshold area. Data were collected in an Excel spreadsheet and used to calculate the degradation area per cell area.
Gelatin zymography
The gelatin zymography protocol was previously described (Brabek et al., 2004; Clark et al., 2007).
Results
Our published analysis of MMP secretion consisted of bulk assays, in which we determined whether MMP2 and MMP9 secretion into conditioned media (Fig. 2) or MT1-MMP cell surface levels were affected by cortactin expression (Clark et al., 2007). However, the ECM-degrading activity of cells is focused at invadopodia, as is evident by the typical fluorescent ECM degradation assay (Fig. 1). To determine whether cortactin affects the focal localization of MMPs to invadopodia puncta, we performed immunostaining for β-actin as an invadopodia marker and either MMP2, MMP9, or MT1-MMP on HNSCC cells plated on crosslinked gelatin/fibronectin substrate. Confocal microscopy analysis of these samples shows strong focal colocalization of each of these MMPs with β-actin puncta at the basal surface of control cells (Fig. 3). However, while the β-actin puncta are present in cortactin-KD cells, there is a striking absence of MMPs from those structures (Fig. 3). Because the matrix in these experiments was unlabeled, we cannot definitively identify the MMP/β-actin puncta in control cells as ECM-degrading invadopodia. Nonetheless, the loss of MMP localization to cortactin-KD puncta, coordinate with the previously described defects in ECM degradation at Arp3/β-actin-positive puncta as well as in MMP secretion in cortactin-KD cells (Fig. 1, Fig 2, (Clark et al., 2007)), is highly suggestive that cortactin is important for the focal localization of MMPs to invadopodia puncta. These data, along with our previous finding that there is no change in the diameter of actin and Arp2/3 complex-positive invadopodia puncta in cortactin-KD cells (Clark et al., 2007), supports the idea that the primary role for cortactin in invadopodia is not on-site actin assembly, but rather to promote the secretion of proteases.
We also determined whether the defect in ECM degradation in cortactin-KD cells can be rescued by overexpression of MT1-MMP, a key invadopodia protease (Artym et al., 2006). Since cortactin-KD cells still express actin-/Arp2/3-positive invadopodia puncta at a level that is ~40% of control cells (Fig. 1), we reasoned that there should be sufficient foci to connect with and organize secreted proteases for ECM degradation. However, whereas overexpression of GFP-MT1-MMP in control cells leads to a ~4-fold increase in ECM degradation, cortactin-KD cells remain unable to degrade ECM (Fig. 4). These data are consistent with the previously identified block in secretion in cortactin-KD cells (Fig. 2, (Clark et al., 2007)), such that even overexpression of proteases cannot rescue the ECM degradation defect. However, it is possible that cortactin might also affect activation of MT1-MMP before it reaches the cell surface. In future studies, it will be useful to confirm a change in surface expression of MT1-MMP-GFP with cortactin-KD, as we have previously seen with endogenous MT1-MMP (Clark et al., 2007), as well as investigate whether cortactin affects protease activation inside cells.
Discussion
The discovery that cortactin affects secretion of invadopodia-associated proteases raises the question of where cortactin acts within the cell to regulate secretion (Fig. 5). Our recent finding that cortactin is essential not only for the secretion of key invadopodia proteases but also for a non-invadopodia protein, apolipoprotein A (Clark et al., 2007), is consistent with a general role for cortactin in secretion. Indeed, a study from the McNiven laboratory previously implicated cortactin in trafficking from the trans-Golgi network (TGN) to the plasma membrane (Cao et al., 2005). In that study, expression of dominant-negative cortactin reagents led to accumulation of the model trafficking protein VSV-G in the TGN, possibly due to mislocalization of the cortactin SH3-domain binding partner dynamin-2 away from the TGN (Cao et al., 2005). Trafficking of the mannose-6-phosphate receptor from the TGN to lysosomes was also affected (Cao et al., 2005). In addition to the TGN, cortactin is strongly localized to invadopodia (Bowden et al., 1999), suggesting the possibility that cortactin could act as a linker protein in invadopodia to mediate vesicle capture or fusion on site (Fig. 5). This putative mechanism is consistent with the finding that cortactin overexpression boosts secretion of proteases and invadopodia-associated ECM degradation above the level of control cells (Fig. 1, Fig 2, (Clark et al., 2007)). Live-cell imaging studies using vesicle markers would be useful to test these possibilities.
Fig. 5.
Proposed model for the role of cortactin in invadopodia function. Based on our data, we propose a model in which the primary role of cortactin in invadopodia is to regulate the secretion of proteases. At a subcellular level, cortactin (indicated by the eight-pointed star) could act at many steps, including promoting fission of vesicles from the trans-Golgi network, the assembly of actin comet tails for the propulsion of vesicles toward the plasma membrane (PM), the capture of vesicles at the PM and/or promoting vesicle fusion at the PM. In all of these processes, cortactin likely functions through promotion of branched actin assembly, just as has been proposed for the actin assembly that takes place in invadopodia puncta. Cortactin may also function to bridge branched actin networks to vesicular trafficking proteins, such as dynamin2.
At the molecular level, cortactin is ideally suited to link branched actin assembly and vesicular trafficking. This could occur through a number of potential mechanisms: actin nucleation, actin branch stabilization, and/or a link to membrane trafficking proteins such as the dynamin2 pinchase at assembled branched actin networks (Lanzetti, 2007; Uruno et al., 2001; Weaver et al., 2001). The actin cytoskeleton is being increasingly implicated in membrane trafficking, with putative mechanisms including vesicle fission, fusion, capture and/or movement (Lanzetti, 2007). Cortactin as a central regulator of branched actin assembly and linker to dynamin2 is likely to play an important role in most or all of these processes. Indeed, in endocytosis, cortactin and dynamin are both important and may function together in vesicle fission and/or movement away from the membrane (Cao et al., 2003; Lanzetti, 2007; Merrifield et al., 2005; Weed and Parsons, 2001; Zhu et al., 2005). Vesicle fusion is another potential step that cortactin might affect in invadopodia-associated protease secretion (Fig. 5). Although this mechanism has not been shown for cortactin, the cortactin-binding partner and branched actin assembly molecule N-WASp was shown to promote exocytosis of docked vesicles in neuroendocrine cells, suggesting that branched actin assembly can act at almost every stage of vesicle trafficking (Gasman et al., 2004).
Invadopodia function is a complex process, requiring the coordination of many cellular functions, including signal transduction, actin assembly, and secretion of proteases. Actin puncta formation is thought to be an initial step; however once the puncta are formed, molecules that are necessary for invasion such as proteases need to be delivered to the extracellular space to allow for ECM degradation and presumably invadopodia protrusion and maturation. Therefore, vesicular trafficking of invasive components from the Golgi apparatus and other intracellular membrane compartments to the plasma membrane is an often overlooked, but extremely important process in overall invadopodia function. Possibly to aid in this process, the Golgi apparatus has been demonstrated to reorient toward and come in close proximity to invadopodia, allowing the concentrated delivery of proteases and other components to the extracellular space (Baldassarre et al., 2003).
While most of the focus for cortactin has been on its role in the formation of the initial actin precursor structures in invadopodia, we propose that a critical function for cortactin in this process is to promote the secretion of proteases for ECM degradation and invasion (Fig. 5). In our studies, the effect of MMP inhibitors and cortactin-KD on invadopodia puncta formation and ECM degradation are indistinguishable (Clark et al., 2007). The known cortactin-binding partners and our recent studies provide a link between processes that until recently have been viewed as spatially and temporally distinct: that of cell motility and invasion, and vesicular trafficking. In light of this newly-identified role for cortactin in regulating the targeting and/or secretion of components necessary for invasion, we propose that future studies on the role of cortactin and other branched actin assembly molecules in invadopodia should consider this important component of invadopodia formation and/or maturation: protease secretion.
Studies over the past fifteen years since the discovery of cortactin have linked this unique protein to a diverse array of cellular processes including cell motility, invasion, synaptogenesis, endocytosis, exocytosis, intercellular adhesion assembly, and host-pathogen interactions (Cosen-Binker and Kapus, 2006; Weed and Parsons, 2001). Clearly one connection with cortactin and all of these processes is the dependence on F-actin dynamics to drive their activity. However, possibly the more general common denominator in all of these processes is the need to couple dynamic membrane trafficking and signaling with the cytoskeleton. Future studies of cortactin should address this possibility as well as determine the role of the many cortactin-interacting proteins in this coupling.
Acknowledgements
The MT1-MMP-EGFP cDNA was a kind gift of Dr. Sarah Netzel-Arnett. Thanks to Dr. Susette Mueller for many fruitful discussions. This work was supported by NIH grants R01GM075126 and R21DE018244 to A.M. Weaver.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell. 2003;14:1074–1084. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Brabek J, Constancio SS, Shin NY, Pozzi A, Weaver AM, Hanks SK. CAS promotes invasiveness of Src-transformed cells. Oncogene. 2004;23:7406–7415. doi: 10.1038/sj.onc.1207965. [DOI] [PubMed] [Google Scholar]
- Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, Stamnes M, McNiven MA. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat. Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- Chen WT, Chen JM, Parsons SJ, Parsons JT. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–158. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- Chen WT, Yeh Y, Nakahara H. An in vitro cell invasion assay: Determination of cell surface proteolytic activity that degrades extracellular matrix. J. Tissue Culture Methods. 1994;16:177–181. [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell. 2004;15:520–531. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, Ouhtit A, Johnston PG, Harkin DP, McCormick D, Waugh DJ. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- Hoon DS, Kitago M, Kim J, Mori T, Piris A, Szyfelbein K, Mihm MC, Jr, Nathanson SD, Padera TP, Chambers AF, Vantyghem SA, MacDonald IC, Shivers SC, Alsarraj M, Reintgen DS, Passlick B, Sienel W, Pantel K. Molecular mechanisms of metastasis. Cancer Metastasis Rev. 2006;25:203–220. doi: 10.1007/s10555-006-8500-x. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr. Opin. Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- Myllykangas S, Bohling T, Knuutila S. Specificity, selection and significance of gene amplifications in cancer. Semin. Cancer Biol. 2007;17:42–55. doi: 10.1016/j.semcancer.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat. Rev. Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- Rodrigo JP, Garcia LA, Ramos S, Lazo PS, Suarez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2000;6:3177–3182. [PubMed] [Google Scholar]
- Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, Chen L, Varella-Garcia M, Sacks PG, Frederick B, Raben D, Weed SA. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell. Biol. 1993;13:2891–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Tehrani S, Faccio R, Chandrasekar I, Ross FP, Cooper JA. Cortactin has an essential and specific role in osteoclast actin assembly. Mol. Biol. Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. USA. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- van Rossum AG, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp. Cell Res. 2006;312:1658–1670. doi: 10.1016/j.yexcr.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: roles of the protein interaction domains. Exp. Cell Res. 2006;312:760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Webb BA, Jia L, Eves R, Mak AS. Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur. J. Cell Biol. 2007;86:189–206. doi: 10.1016/j.ejcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- Weichselbaum RR, Dahlberg W, Beckett M, Karrison T, Miller D, Clark J, Ervin TJ. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head-and neck-cancer patients. Proc. Natl. Acad. Sci. USA. 1986;83:2684–2688. doi: 10.1073/pnas.83.8.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarar D, To W, Abo A, Welch MD. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 1999;9:555–558. doi: 10.1016/s0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J. Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]