Abstract
Secreted frizzled related proteins (Sfrps) are extracellular attenuators of Wnt signaling that play important roles in both embryogenesis and oncogenesis. Although Sfrps are generally thought to bind and sequester Wnts away from active receptor complexes, very little is known about the specificity of Sfrp family members for various Wnts. In the developing chick neural tube, sfrp-1, 2 and 3 transcripts are expressed in and adjacent to the dorsal neural tube, where Wnt-1 and Wnt-3a are expressed. To better define the possible roles of Sfrp-1, 2 and 3 in the neural tube, we first tested the ability of purified Sfrps to inhibit Wnt-3a induced accumulation of β-catenin in L cells. We find that both Sfrp-1 and Sfrp-2 can inhibit Wnt-3a activity while Sfrp-3 cannot. To determine where Sfrp-1 and Sfrp-2 impinge on the Wnt signaling pathway, we tested the ability of these Sfrps to inhibit Wnt signaling induced by the addition of LiCl, an inhibitor of GSK-3. Sfrp-1 and Sfrp-2 are unable to inhibit the accumulation of β-catenin in LiCl treated cells, suggesting that the ability of Sfrps to inhibit the accumulation of β-catenin is GSK-3 dependent. We have further shown that Sfrp-2 inhibits the ability of ectopic Wnt-3a to stimulate proliferation in the developing chick neural tube. These results provide the framework for understanding how Sfrps function to regulate Wnt-3a activity in developing embryos and in cancer.
Keywords: chick embryo, neural tube, Sfrp-1, Sfrp-2, Sfrp-3, Wnt-3a
Introduction
Wnt-1 and Wnt-3a are functionally redundant Wnt family members that utilize a β-catenin dependent signaling pathway to modulate cell proliferation, survival, specification, and differentiation (Logan and Nusse, 2004). As Wnt-1 and Wnt-3a are extremely potent signals, it comes as no surprise that inappropriate activation of Wnt-1/Wnt-3a signaling has profound consequences on both oncogenesis and embryogenesis. The most notable example of the oncogenic potential of ectopic Wnt expression is the transformation of mouse mammary gland cells as a consequence of MMTV activation of Wnt-1 expression (Nusse et al., 1984). Additional experiments performed in cultured cells further highlight the proliferative/transformative effects of ectopic Wnt-1/Wnt-3a in multiple myeloma cells, mouse mammary cells, and fibroblasts (Brown et al., 1986; Wong et al., 1994; Shimizu et al., 1997; Bafico et al., 1998; Young et al., 1998; Derksen et al., 2004).
The effects of ectopic Wnt-1 and Wnt-3a expression on embryogenesis are equally striking. For instance, injection of fertilized Xenopus eggs with Wnt-1 or Wnt-3a mRNAs leads to the duplication of the embryonic axis (McMahon and Moon, 1989; Wolda et al., 1993). Later in development, ectopic expression of Wnt-1 or Wnt-3a in the mouse/chick neural tube causes dramatic overproliferation (Dickinson et al., 1994; Megason and McMahon, 2002). Application of Wnt-3a to quail neural tube explants also affects neural crest cell lineage specification as it causes the number of melanoblasts to increase at the expense of neuroblasts and glioblasts (Jin et al., 2001). Cumulatively, these data indicate that the restriction of Wnt-1/Wnt-3a activity to appropriate spatiotemporal domains is critical for normal function in both adults and embryos.
Our lab is interested in identifying the molecular mechanisms that control the range of Wnt activity in developing chick embryos. While one obvious mechanism for limiting the range of Wnt activity is to regulate the competence of cells to respond to Wnt signals, the broad expression of frizzled and LRP receptor transcripts suggests that most tissues are competent to respond to Wnts (Stark et al., 2000; Cauthen et al., 2001; Houston and Wylie, 2002; Chapman et al., 2004; Kelly et al., 2004). The identification of the Sfrp family of secreted inhibitors of Wnt proteins has provided insight into one potential mechanism used to regulate Wnt activity in vivo (Hoang et al., 1996; Finch et al., 1997; Leyns et al., 1997; Melkonyan et al., 1997; Rattner et al., 1997; Salic et al., 1997; Wang et al., 1997a). Sfrps contain a cysteine-rich domain (CRD), which possess a high degree of structural and sequence identity with the CRD of Frizzled receptors (Hoang et al., 1996; Finch et al., 1997; Leyns et al., 1997; Melkonyan et al., 1997; Rattner et al., 1997; Salic et al., 1997; Wang et al., 1997a). As the Frizzled CRD is thought to mediate binding to Wnt ligands (He et al., 1997; Wu and Nusse, 2002), the shared sequence homology between the Frizzled and Sfrp CRDs suggests that the binding of Wnt to the Sfrp CRD is responsible for the inhibition of Wnt activity by Sfrps. Consistent with this notion, the CRD of Sfrp has also been shown to be sufficient and necessary for interaction with Wnt proteins (Lin et al., 1997; Bafico et al., 1998; Lescher et al., 1998). However, data showing the existence of Frizzled:Sfrp heterodimeric complexes suggests an alternate mechanism whereby Sfrps inhibit Wnt activity by preventing assembly of Frizzled dimers (Bafico et al., 1999).
The expression patterns of four Sfrp family members (Sfrp 1-3 and Crescent) have been well characterized in the chick (Duprez et al., 1999; Baranski et al., 2000; Esteve et al., 2000; Ladher et al., 2000; Terry et al., 2000; Jin et al., 2001; Chapman et al., 2002; Frowein et al., 2002; Chapman et al., 2004), with sfrps exhibiting both unique and overlapping areas of expression. In the neural tube, sfrp-1, 2 and 3 are expressed in or adjacent to the Wnt-1/Wnt-3a expression domain in the dorsal neural tube, suggesting that they might serve as regulators of Wnt-1 and/or Wnt-3a activity (Duprez et al., 1999; Baranski et al., 2000; Esteve et al., 2000; Ladher et al., 2000; Terry et al., 2000; Jin et al., 2001). In order to better define the potential roles of Sfrps in the developing neural tube, we sought to rigorously test the ability of Sfrp-1, 2 and 3 to inhibit Wnt-3a activity in vitro and in vivo. We chose to focus our current studies on Wnt-3a due to the availability of soluble (Shibamoto et al., 1998) and purified (Willert et al., 2003) Wnt-3a protein.
Previous studies have shown that Wnt-3a signals via a β-catenin mediated pathway in both L cells and the developing chick neural tube (Shibamoto et al., 1998; Megason and McMahon, 2002; Willert et al., 2003). In this pathway, binding of Wnt-3a to Frizzled/LRP co-receptors results in the inhibition of GSK-3, a serine threonine kinase that phosphorylates β-catenin in unstimulated cells and targets it for degradation (Logan and Nusse, 2004). Upon Wnt-induced inhibition of GSK-3, β-catenin accumulates in the cytosol and translocates to the nucleus where it complexes with TCF/Lef-1 and regulates gene expression. Utilizing purified Sfrps and Wnt-3a in a quantitative assay measuring the accumulation of β-catenin in L cells, we show that Sfrp-1 and 2 are able to attenuate Wnt-3a activity while Sfrp-3 cannot. Sfrp-1 and Sfrp-2 are unable to inhibit the accumulation of β-catenin in LiCl treated cells, suggesting that Sfrps are likely to act upstream of GSK-3. These data are consistent with a mechanism whereby Sfrps inhibit the activation of Frizzled/LRP receptors by Wnt ligands. To extend our inhibition studies in vivo, we co-electroporated Wnt-3a along with either Sfrp-2 or Sfrp-3 into the chick neural tube and compared the resultant phenotype to those obtained by electroporation of Wnt-3a or Sfrps alone. We found that Sfrp-2, but not Sfrp-3, is able to inhibit ectopic Wnt-3a in vivo. Cumulatively, we conclude that Sfrp-1 and 2 are able to inhibit Wnt-3a activity, while Sfrp-3 cannot.
Materials and Methods
We would like to thank Dr. Dave Wilkinson (National Institute for Medical Research, London) and Dr. Angela Nieto (Cajal Institute, Madrid) for providing us with the chick λgt10 oligo-dT primed cDNA library generated from mRNA isolated from Hamburger and Hamilton (HH) stage 12-15 chick embryos (Hamburger and Hamilton, 1951). The full length chick sfrp-2 cDNA (Ladher et al., 2000) was generously provided by Dr. Pip Francis-West (King’s College, London) while L and L-Wnt-3a cells (Shibamoto et al., 1998) were kindly provided by Dr. Shinji Takada (University of Kyoto). We would also like to thank Dr. Paola Bovolenta (Cajal Institute, Madrid) for providing us with MDCK cells.
Commercially purified preparations of hSfrp-1 and mSfrp-3 were obtained from R&D Systems. Other materials and their respective vendors are as follows: acetone, methanol, bovine serum albumin (BSA), diethanolamine, Hepes, sucrose, fast green, gelatin, MgCl2, NaCl, Tris, KCl, Na2HPO4 and KH2PO4 (Fisher); DMEM, 200mM L-Glutamine, 100x Penicillin/Streptomycin, PBS (Mediatech); Fetal Bovine Serum (FBS) (Hyclone); 5-bromo-4-chloro-3-indolyl-phosphate 4-toluidine (BCIP), 4-nitro blue tetrazolium chloride (NBT), 4-nitrophenol phosphate (PNPP or 4NPP), Triton 100-X, anti-his6 (Roche); mouse anti-β-catenin (BD Biosciences); Goat anti-mouse IgG (H+L) AP, Goat anti-mouse IgG (H+L) Cy5, Goat-anti-Rabbit IgG (H+L) Cy3 (Jackson Immuno Research Laboratories, Inc.); PVDF membrane (VWR); Tween-20, Tyrode’s solution, carboxymethylcellulose sodium salt and n,n-Dimethylformamide (DMF) (Sigma); G418, pcDNA3.1(-)A (Invitrogen); paraformaldehyde (Electron Microscopy Sciences); anti-phosphohistone H3 (ser10) PAbs (Upstate Technologies); Geneporter (Gene Therapy Systems); anti-mouse IgG2b TRITC, anti-mouse IgG1 (Southern Biotech); slowfade light antifade kit (Molecular Probes).
Plasmid constructs
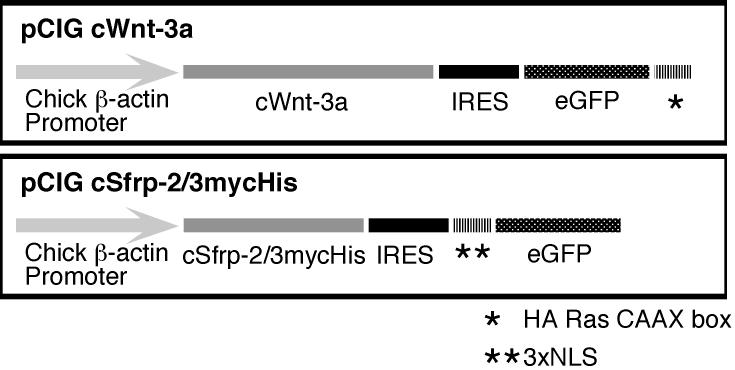
Isolation of the full length chick sfrp-3 cDNA was accomplished by screening a λgt10 cDNA library with digoxigenin labeled cDNA probes generated from a previously isolated partial cDNA encoding chick Sfrp-3 (Baranski et al., 2000). The sequence of the full length cDNA is posted online (Genbank accession number DQ017062). Sequence encoding myc and hexa-his tags was appended onto full length chick sfrp-2 and sfrp-3 by subcloning in frame into the pcDNA3.1(-)A expression vector (Invitrogen). For electroporations, the sequence encoding Sfrp-2-myc/his was subcloned into pCIG. The pCIG electroporation construct uses the chick β-actin promoter to drive the expression of a bicistronic mRNA, which contains the sfrp-2-myc/his coding sequence followed by an internal ribosome entry sequence (IRES) and eGFP with a nuclear localization sequence (NLS). cWnt-3a was subcloned into a variant of pCIG such that expression of this construct results in the production of cWnt-3a and a membrane localized variant of eGFP (GFP was modified at the C-terminus with sequence encoding a Harvey Ras CAAX box motif (Williams, 2003)).
Generation and purification of Sfrp-2 and Sfrp-3 proteins
MDCK cells were grown in DMEM containing 10% fetal bovine serum (FBS), 1X penicillin/streptomycin and 4 mM L-glutamine. Stably transfected MDCK cells were generated by transfecting pCDNA.sfrp-2 and pCDNA.sfrp-3 constructs into MDCK cells using the GenePORTER transfection kit per manufacturer’s instructions. Successfully transfected cells were selected by incubation in 800 μg/ml G418 for 3 weeks, with the medium and G418 being replaced every four days. Individual clones were isolated by limited dilution cloning in 96 well plates. The conditioned media from wells containing single clones were analyzed by SDS-PAGE and Western blot with anti-hexa-his antibodies. Cells in positive wells were then expanded and re-screened via Western blot.
Conditioned media for Sfrp-2 and Sfrp-3 were collected by growing the transfected MDCK cells until confluence, changing the growth media to DMEM containing 1X penicillin/streptomycin and 4 mM L-glutamine (no FBS) and collecting the media 3 days later. Control and Sfrp-2/3 conditioned media were batch applied to heparin agarose columns. The columns were extensively washed with 100mM Hepes, pH 7.4 containing 0.1 M NaCl until eluted fractions contained no protein as measured by Bradford assay. Bound proteins were eluted by a 0.1M to 4M gradient of NaCl (in 100 mM Hepes, pH 7.4). Fractions with peak protein content were pooled. Pooled proteins were subjected to analysis by SDS-PAGE. Gels were stained with Coomassie Brilliant Blue to verify purity and concentration. Pooled fractions were also subjected to Western Blot analysis with anti-hexa-his antibodies to confirm the identity of the purified proteins.
Preparation of Wnt-3a conditioned media and purified Wnt-3a
L cells and L-Wnt-3a (Shibamoto et al., 1998) cells were grown in a 37°C humidified chamber with 7% CO2 in DMEM supplemented with 10% FBS, 1X penicillin/streptomycin, and 4 mM L-glutamine. Upon reaching 80% confluence, cells were washed once with PBS and transferred into DMEM containing 2% FBS, 1X penicillin/streptomycin and 4 mM L-glutamine. Conditioned media was collected one day past confluence. We refer to the concentration of this media to be 1X. The concentration of active Wnt-3a in the 1X EL-Wnt-3a conditioned medium is estimated to be 5 nM. mWnt-3a was purified as previously described (Willert et al., 2003).
Measurement of the accumulation of β-catenin by ELISA
L cells were grown in a 37°C humidified chamber with 7% CO2 in DMEM supplemented with 10% FBS, 1X penicillin/streptomycin, and 4 mM L-glutamine. Cells were plated on 96 well plates at densities ranging from 10,000 to 360,000 cells/well (100,000 for most experiments) and incubated overnight. Cells were then treated with EL-Wnt-3a or EL conditioned media (at a 0.5X concentration unless otherwise noted), purified Wnt-3a (2.6 nM), 33 mM LiCl or 33 mM NaCl in the presence or absence of purified Sfrp proteins for 0.5 to 8 hrs as indicated. After washing the cells twice with PBS (137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, and 1.8mM KH2PO4 pH 7.4), cells were fixed for 10 min at room temperature with ice cold MeOH:acetone (1:1). The cells were washed again with PBS (twice) and blocked overnight in PBS containing 3% bovine serum albumin (BSA) at 4°C. After two additional washes with PBS, the cells were incubated with anti-β-catenin antibodies diluted 1/500 in PBS containing 3% BSA for 2 hours. The cells were washed again (4 times) with PBS and incubated with anti-mouse IgG conjugated to alkaline phosphatase diluted 1/1000 in PBS containing 3% BSA for 2 hours. After washing the cells four times with PBS and twice with 10mM diethanolamine, pH 9.5 containing 0.5mM MgCl2, cells were incubated with substrate (10mg/ml PNPP in 10mM diethanolamine, pH 9.5 containing 0.5mM MgCl2) for 1 hour. The absorbance was measured on a Bio-Rad Model 550 microplate reader at 405nm with a reference filter at 490nm. Each condition was performed in triplicate. All experiments were repeated two to four times.
Measurement of the accumulation of β-catenin by Western Blot
L cells were grown on a 6 well plate until 70% confluent. Cells were treated with Wnt-3a conditioned medium or control conditioned medium diluted in DMEM and 10% FBS for 3 hours. Cells were lysed in 300 μl of 100mm Hepes (pH 7.4), 100mM NaCl and 1% Triton-X100 and boiled for 5 minutes in SDS-PAGE sample buffer. Samples were electrophoresed on a 10% polyacrylamide gel and electroblotted onto a PVDF membrane. Membranes were blocked for 10 min in blocking buffer (PBS containing 1% non-fat dried milk and 0.1% Tween) and then incubated with anti-β-catenin antibody (1/500 dilution) in blocking buffer for 1 hr. The blots were rinsed 3 times with blocking buffer for a total of 30 minutes and then incubated with anti-mouse IgG AP antibody diluted to 1/500 in blocking buffer for 1 hour. Again the blots were rinsed 3 times with blocking buffer for a total of 30 minutes and briefly incubated with alkaline phosphatase (AP) buffer (100 mM Tris, pH 9.5, 100 mM NaCl, 5mM MgCl2). The blots were then developed in AP buffer containing 165 μg/ml BCIP, and 330 μg/ml NBT.
Chick Neural Tube Electroporations
India Ink diluted in Tyrode’s solution was injected underneath the embryo to enhance visualization of embryos in windowed eggs. After making a small tear in the vitelline membrane at the site of injection, embryos were overlaid with 1 ml of Tyrode’s solution containing 1X penicillin/streptomycin. HH stage 12-14 chick embryos were injected in the neural tube at the level of segmental plate with a solution containing 1-2 mg/ml DNA, 333 μg/ml fast green dye and 4 μg/ml high viscosity carboxymethyl cellulose. Homemade electrodes constructed with 100 μm (diameter) platinum wires that were spaced 4 mm apart were used for the electroporations. Embryos were electroporated with four 50 msec pulses at 30V with a BTX square wave generator. The embryos were incubated for 48 hours, harvested and fixed in PBS containing 4% paraformaldehyde for 1 to 2 hours at 4°C. Embryos were washed twice with PBS, incubated in PBS containing 5% sucrose and 0.01% NaN3 for at least 3 hours, and then incubated in PBS containing 15% sucrose and 0.01% NaN3 overnight. Embryos were then equilibrated in PBS containing 15% sucrose and 7.5% gelatin at 40°C for 1-4 hours, oriented at room temperature and then frozen in liquid nitrogen. Serial sections (25-30 μm) were cut on a Leica cryostat.
Immunostaining of tissue sections
Tissue sections on slides were de-gelatinized by washing once in PBS at 50°C for 10 minutes and then twice in PBS at room temperature. The slides were then blocked for 1 hour in blocking buffer (3% sheep serum in PBS with 0.1%Tween). Slides were incubated in primary antibody diluted in blocking buffer overnight at 4°C and then washed two to four times in PBS plus 0.1%Tween. The slides were blocked for 30 minutes and incubated in secondary antibody diluted in blocking buffer for 2 hours at room temperature. They were then washed four times in PBS with 0.1% Tween, post-fixed in PBS containing 4% paraformaldehyde and mounted in Slowfade.
Confocal Microscopy
For confocal microscopy, specimens were excited using the 488 nm line of an argon ion laser for visualization of GFP, the 543.1 nm line of a green helium-neon laser for visualization of Cy3, and the 647 nm line of the red helium-neon laser for visualization of Cy5. Fluorescence emission was captured on a Nikon E600 Physioscope attached to the Nikon PCM2000 confocal laser scanning unit. Images were collected with a 20x objective. The EZ-C1 software (Nikon) controlled the confocal microscope for zeta-axis image acquisitions. Adobe Photoshop version 7.0 was used for final image data processing.
Statistics
Data were analyzed using a two-tailed unpaired student’s t-test.
Results
Development of a quantitative assay to measure the accumulation of β-catenin in L cells
In vertebrates, Wnt ligands signal through multiple pathways, including a β-catenin dependent pathway and several β-catenin independent pathways (Ishitani et al., 1999; Kuhl et al., 2000; Kuhl et al., 2001; Ishitani et al., 2003; Topol et al., 2003; Westfall et al., 2003; Logan and Nusse, 2004; Smit et al., 2004). In some tissues and cell lines, there is evidence that the β-catenin dependent and independent pathways antagonize each other via crosstalk between the two pathways (Ishitani et al., 1999; Kuhl et al., 2001; Ishitani et al., 2003; Topol et al., 2003; Westfall et al., 2003). For proper interpretation of our studies, it was imperative that we exclusively measure the primary effects of Sfrps on Wnt-3a signaling via the β-catenin mediated pathway and not secondary effects of Sfrps on endogenous Wnt ligands that signal via β-catenin independent pathways. Thus, we chose to assess Wnt-3a activity in L cells by measuring the accumulation of cytosolic and nuclear β-catenin rather than by measuring the activation of Tcf/Lef reporters (such as TopFlash), which could be influenced by multiple pathways.
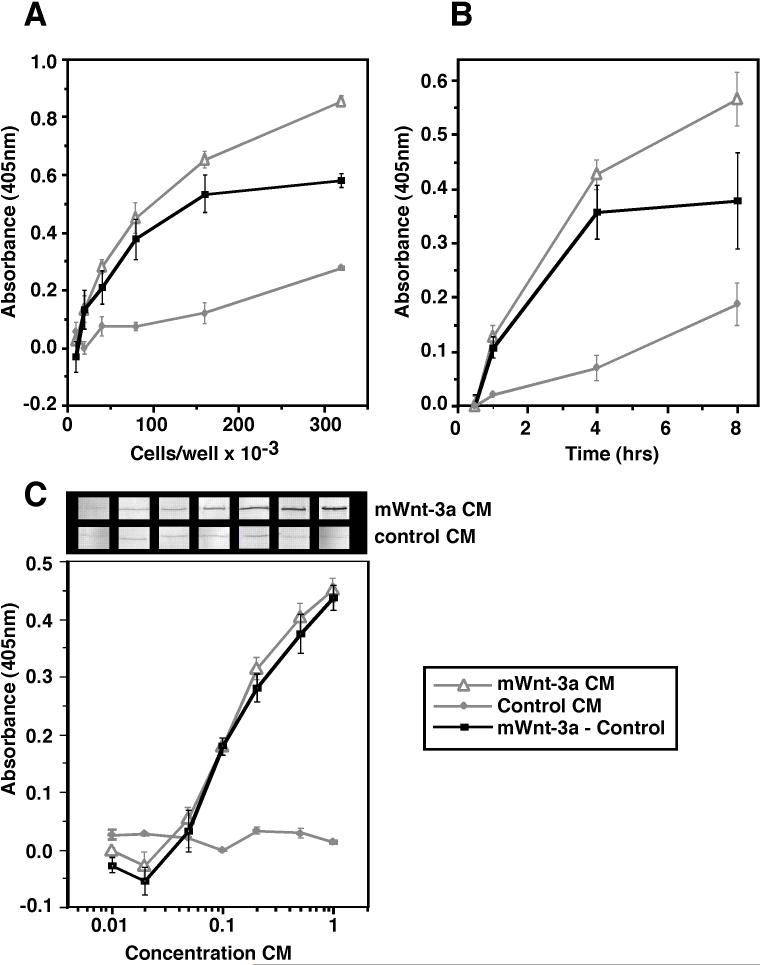
Previously reported assays for the accumulation of β-catenin by western blot have revealed qualitative differences in protein levels (van Leeuwen et al., 1994; Papkoff et al., 1996; Shimizu et al., 1997). In order to study the influence of Sfrps on Wnt-3a activity, we developed a sensitive enzyme-linked immunosorbant assay to quantitate the accumulation of β-catenin in L cells. In this assay, L cells are plated on 96 well plates, incubated with Wnt-3a, fixed, and subjected to immunohistochemical analysis. Because L cells do not express cadherins, β-catenin is not sequestered into membrane bound fractions and thus, it was not necessary to fractionate the cytosolic pool of β-catenin away from the membrane bound pool before quantitation of β-catenin levels. Before generating a concentration curve for Wnt-3a activity, we first optimized this assay with respect to cell density (Fig 1A) and time of Wnt-3a incubation (Fig 1B). Cell density and time of incubation were optimized at 100,000 cells/well and 3 hrs, respectively. Although the accumulation of β-catenin is more robust at later time points, we chose a 3 hr incubation period to avoid indirect effects of cell proliferation. Finally, a concentration curve was generated via ELISA and compared to a Western blot utilizing the same concentrations of Wnt-3a. This curve exhibits a classic sigmoidal shape. While both the ELISA and Western blot assays show similar trends, the ELISA assay is clearly more quantitative and more sensitive (Fig 1C). Based on this concentration curve, we chose to use Wnt-3a concentrations of 2.6 nM (purified Wnt-3a) or 0.5X (Wnt-3a conditioned medium) for further studies with Sfrps as this concentration will enable us to easily monitor the effects of Sfrps on the accumulation of β-catenin.
Figure 1.
Development of quantitative assay to measure Wnt-3a induced accumulation of β-catenin in L cells. Optimization of the assay with respect to cell density is shown in panel (A). Cells were plated at increasing densities and incubated overnight prior to treatment with Wnt-3a. The following day, they were incubated with Wnt-3a conditioned media or control conditioned media at 0.5X concentration for 3 hours. Cells were then fixed and subjected to ELISA analysis as described in Materials and Methods. Next, we optimized the duration of Wnt-3a treatment as shown in panel (B). For these data, L-cells were plated at a density of 100,000 cells/well. The next day, they were incubated with Wnt-3a or control conditioned media at 0.5X concentration for the denoted time intervals prior to performing the ELISA. Finally, we sought to generate a concentration curve for Wnt-3a using our optimized conditions (panel C). L-cells were plated at a density of 100,000 cells/well and incubated overnight before treating with increasing concentrations (0.01X to 1.0X) of Wnt-3a or control conditioned media for 3 hours. We further compared samples analyzed by quantitative ELISA to those analyzed by western blot, which is the most frequently used qualitative assay for measurement of the accumulation of β-catenin (C). Though the data from the two assays are well correlated, the quantified data provided by the ELISA allows us to detect variations in the levels of soluble β-catenin accumulation at the three highest concentrations of Wnt-3a, while no distinction in β-catenin levels is readily detected in the Western blot. The gray lines with triangles denote values for Wnt-3a conditioned medium while the gray lines with diamonds denote values for control conditioned medium. The black lines with squares represent the product of subtracting the values for control conditioned medium from those for Wnt-3a conditioned medium. Error bars indicate standard error for 3 to 6 independent replicates.
Sfrp-1 and Sfrp-2 attenuate Wnt-3a activity, while Sfrp-3 does not
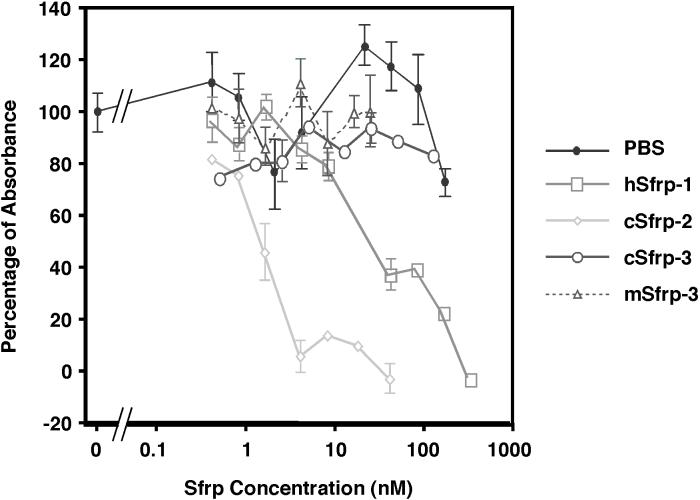
To assess the ability of the Sfrps to modulate Wnt-3a activity, L cells were treated with Wnt-3a alone or with Wnt-3a in combination with increasing concentrations of protein purified from Sfrp or mock transfected MDCK cells. Identical results were obtained with both purified mWnt-3a (2.6 nM) or mWnt-3a conditioned medium (at a 0.5X dilution). Sfrp-2 was the most potent inhibitor, achieving 50% inhibition at a concentration of 2.5 nM (Fig 2). Sfrp-1 also inhibited Wnt-3a activity, but was approximately 5 fold less potent (50% inhibition observed at 12.5 nM) (Fig 2). Commercially purchased mouse Sfrp-2 was less potent than our purified chick Sfrp-2 and inhibited Wnt-3a at doses comparable to those of Sfrp-1 (data not shown). Both chick and mouse Sfrp-3 were unable to inhibit Wnt-3a at concentrations up to 125 nM (Fig 2).
Figure 2.
Attenuation of Wnt-3a activity by Sfrp-1 and Sfrp-2, but not Sfrp-3. L cells were treated with either purified Wnt-3a (2.6 nM) or Wnt-3a conditioned media (0.5X) in combination with increasing concentrations of purified Sfrp1, 2 or 3 (data shown is for Wnt-3a conditioned media). The specific absorbance was calculated by subtracting the absorbance for control conditioned media from the absorbance for each experimental data point. The data were then normalized to a scale where the relative absorbance of Wnt-3a alone was set at 100% and control buffer/control conditioned media was set at 0%. Sfrp-2 was the best inhibitor with a half maximal concentration of 2.5nM, while Sfrp-1 half maximal concentration was at 12.5nM. Neither chick nor mouse Sfrp-3 was able to inhibit Wnt-3a. Error bars indicate standard error for three independent replicates.
The attenuation of Wnt-3a activity by Sfrp-1 is GSK-3-Dependent
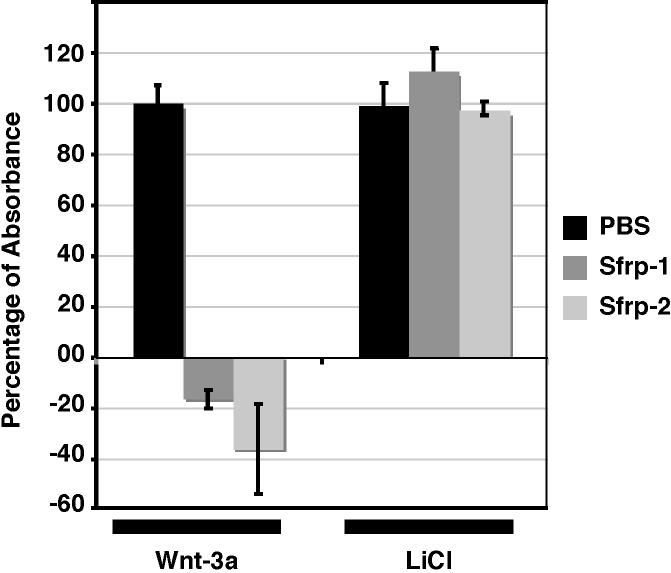
In addition to influencing TCF activity, β-catenin “independent” pathways have also been shown to antagonize the β-catenin dependent pathway by destabilizing β-catenin (Topol et al., 2003; Westfall et al., 2003). To further insure that we were not measuring secondary effects from the ability of Sfrps to modulate endogenous Wnts that signal through β-catenin independent pathways, we tested whether Sfrps were acting upstream of β-catenin. To do this, we inhibited GSK-3 activity by the addition of LiCl (Klein and Melton, 1996). We then tested the ability of Sfrp-1 and Sfrp-2 to influence the accumulation of β-catenin. Neither Sfrp was able to attenuate the accumulation of β-catenin (Fig 3), indicating that the inhibitory activity of Sfrp-1 is dependent on GSK-3 activity. Thus, it seems unlikely that our assay is detecting secondary effects of Sfrps on β-catenin via modulation of alternate pathways.
Figure 3.
To determine if Sfrp-1 acts upstream of GSK-3, L cells were treated with either Wnt-3a conditioned media (0.5X concentration) or 33mM of LiCl (or 33 mM NaCl as a control) in combination with 300 nM Sfrp-1 or Sfrp-2. Both Sfrp-1 and Sfrp-2 were able to inhibit Wnt-3a activity. LiCl inhibits GSK3 and results in an accumulation of β-catenin. Neither Sfrp-1 nor Sfrp-2 was able to inhibit β-catenin accumulation in LiCl treated cells. The relative percent absorbance was calculated as in Figure 2. Error bars indicate standard error for three independent replicates.
Sfrp-2 attenuates Wnt-3a activity in vivo
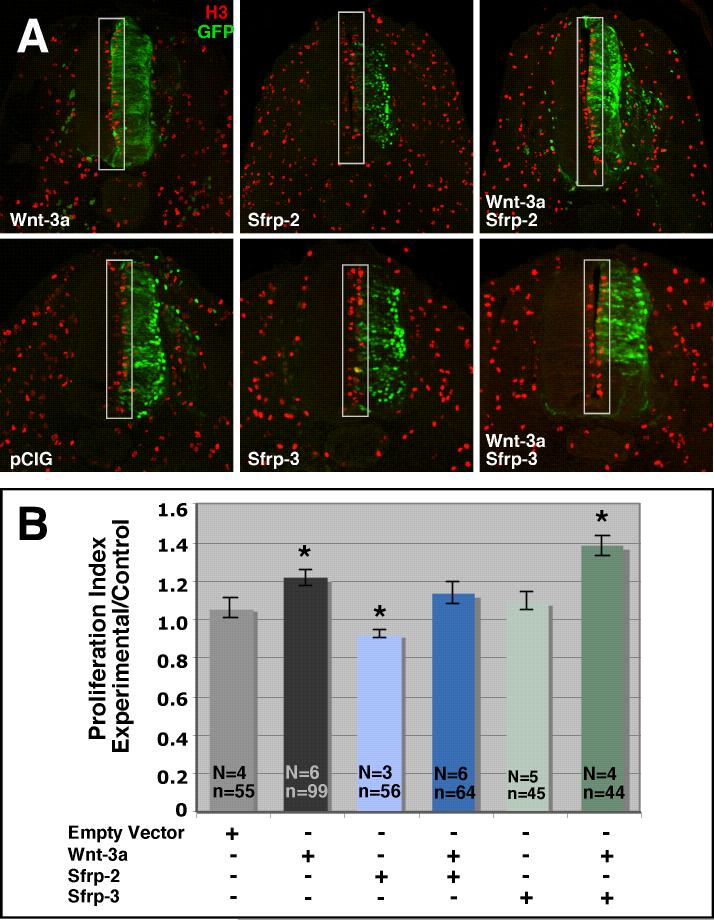
As Sfrp-2 was the most robust inhibitor of Wnt-3a activity in L cells, we sought to test the ability of Sfrp-2 to inhibit Wnt-3a in vivo. To do this, we expressed Wnt-3a and Sfrp-2, alone or in combination, in the neural tube of HH12-14 chick embryos via electroporation. Because Sfrp-3 failed to inhibit Wnt-3a activity in L cells, parallel experiments using Sfrp-3 and Wnt-3a were carried out as a negative control. Electroporation of the neural tube at these stages was chosen because we and others have previously shown that ectopic expression of Wnt-3a is directly responsible for quantifiable increases in proliferation (data not shown; Megason and McMahon, 2002). Transfection efficiency for each construct was assessed by the expression of green fluorescent protein (GFP). Sfrp-2 and Sfrp-3 expressing cells were marked by nuclear localized GFP while Wnt-3a expressing cells were marked by membrane localized GFP (Fig 4). In order to assess the ability of Sfrp-2 and Sfrp-3 to block Wnt-3a induced proliferation, sections were stained with anti-phosphohistone H3, which marks mitotic cells (Hendzel et al., 1997; Wei et al., 1999) (Fig 5A). Mitotic cells were counted on the left (control) and right (experimental) sides of the neural tube (Fig 5A). A proliferation index was established by dividing the number of mitotic cells on each side of the neural tube by the area of that side of the neural tube. As Wnt signaling does not alter the size of individual cells in the neural tube (data not shown), dividing the number of proliferative cells by the area of the neural tube provides a mechanism of normalizing proliferation back to the relative cell number on each side of the neural tube. Because the proliferation index is likely to vary according to age and axial level, we divided the proliferation index for the experimental side of the neural tube by the proliferation index for the control side of the neural tube in order to facilitate comparison of data from derived from different embryos. If electroporation of a construct has no effect on proliferation, we expect a ratio of 1. Constructs that cause an increase in proliferation are expected to yield ratios greater than 1 while constructs that inhibit proliferation are expected to yield ratios less than 1. Whereas the proliferation index ratio for embryos electroporated with empty vector (pCIG) was 1.06 +/- 0.05, embryos electroporated with Wnt-3a had a proliferation index ratio of 1.21 +/- 0.04 (Fig 5B). Consistent with previously reported data (Megason and McMahon, 2002), comparison of the two data sets shows that there is a statistically significant increase in the number of mitotic cells in Wnt-3a electroporated embryos (p≤ 0.003; Fig 5B). Similarly, embryos electroporated with Sfrp-2 exhibited a statistically significant decrease in proliferation (proliferation index ratio = 0.92 +/- 0.02) as compared to embryos electroporated with pCIG alone (p≤ 0.02). Embryos co-electroporated with both Wnt-3a and Sfrp-2 show no significant change in proliferation as compared to control (pCIG) embryos (p ≤ 0.4). The ratio of proliferation indices for embryos electroporated with both Wnt-3a and Sfrp-2 is also significantly less than for embryos electroporated with Wnt-3a alone (p≤ 0.03) and significantly greater than for embryos electroporated with Sfrp-2 alone (p≤ 0.002). By contrast, embryos electroporated with Sfrp-3 alone show no change in proliferation as compared to pCIG (p≤ 0.6). Furthermore, rather than attenuating Wnt-3a induced proliferation, co-electroporation of Sfrp-3 along with Wnt-3a, appeared to enhance proliferation in the neural tube (proliferation index ratio = 1.38 +/- 0.05).
Figure 4.
Design of electroporation constructs. Transcription of Wnt-3a and Sfrp-2/3 is driven by a β-actin promoter. The internal ribosomal entry site (IRES) allows for independent translation of the bicistronic mRNA into two proteins. Note that the cWnt-3a construct generates an eGFP variant that is localized to the membrane (*), while the Sfrp-2 and Sfrp-3 constructs encode an eGFP variant that is targeted to the nucleus (**).
Figure 5.
Ectopic Sfrp-2 attenuates ectopic Wnt-3a proliferative activity in vivo. HH12-14 embryos were electroporated with Sfrp-2, Sfrp-3 and/or Wnt-3a as indicated. Embryos were harvested 48 hours post-electroporation, sectioned and immunolabeled with anti-phosphohistone H3 antibodies to mark mitotic cells (A). The mitotic cells (in gray boxes) were counted for each side of the neural tube and normalized to the area of the appropriate side of the neural tube to generate a proliferation index. The ratios of experimental/control proliferation indices were graphed for comparison (B). Wnt-3a alone caused a significant increase in proliferation as compared to the pCIG control, while Sfrp-2 alone caused a significant decrease. Embryos co-electroporated with Wnt-3a and Sfrp-2 exhibited no significant change in proliferation. Electroporation of Sfrp-3 alone also failed to elicit a change in proliferation as compared to pCIG. Co-electroporation of Sfrp-3 with Wnt-3a shows an increase in proliferation. N=# of embryos analyzed; n= # of sections analyzed. Asterisks mark data sets that are significantly different than pCIG alone (p ≤ 0.02). Empty vector is pCIG with eGFP targeted to the nucleus.
Discussion
We have definitively demonstrated that Sfrp-1 and 2 inhibit Wnt-3a induced accumulation of β-catenin in L cells while Sfrp-3 does not. Our data agree with data from Wang et al, who showed that Sfrp-3 was unable to inhibit the induction of Xnr3 and siamois in animal cap explants obtained from Xenopus embryos injected with Wnt-3a mRNA (Wang et al., 1997b). In contrast to our data showing that Sfrp-3 is not able to inhibit Wnt-3a, Tzahor and Lassar reported that co-culture of Wnt-3a transfected fibroblasts with explants comprised of anterior paraxial mesendoderm and overlaying ectoderm promoted cardiogenesis while co-culture of Wnt-3a and Sfrp-3:IgG transfected fibroblasts with the explants did not (Tzahor and Lassar, 2001). And, whereas we show that Sfrp-2 can antagonize Wnt-3a, Lee et al showed that Sfrp-2 transfected fibroblasts were not able to attenuate the induction of Pax-3 by Wnt-3a in presomitic mesoderm explants (Lee et al., 2000).
Possible reasons for the discrepancies between our L cells studies and other studies include the use of unknown concentrations of Wnts and Sfrps and the use of assays that are sensitive to both β-catenin dependent and β-catenin independent signaling. As many tissues and cultured cells express multiple Wnt ligands, the balance of these often opposing signals can influence readouts. For instance, the inhibition of a β-catenin dependent Wnt signal might yield a similar phenotype to the induction of a β-catenin independent Wnt signal. Recent evidence suggests that one mechanism for cross talk between the two pathways is that Wnt ligands act through the nemo-like kinase of the PCP pathway to inhibit β-catenin mediated signaling by phosphorylating TCF, thereby making it unresponsive to β-catenin (Ishitani et al., 1999; Rocheleau et al., 1999; Ishitani et al., 2003; Smit et al., 2004). While it is well established that Wnt-3a utilizes the β-catenin mediated signaling pathway in L cells (Shibamoto et al., 1998; Willert et al., 2003), we do not know if L cells express any endogenous Wnt family members that antagonize the β-catenin mediated pathway.
In contrast to previous studies, we utilized purified proteins at known concentrations for our L cell experiments and we utilized an assay that minimizes the measurement of secondary effects generated by the inhibition of endogenous Wnt ligands that signal via β-catenin independent pathways. Measurement of β-catenin levels rather than the activation of TCF/Lef reporters, which are sensitive to both β-catenin dependent and β-catenin independent signaling (Ishitani et al., 1999; Rocheleau et al., 1999; Ishitani et al., 2003; Smit et al., 2004), reduced the possibility of secondary effects obtained by the inhibition of β-catenin independent Wnt signaling. As it has also been reported that some Wnt ligands can antagonize the β-catenin dependent pathway via destabilization of β-catenin (Topol et al., 2003; Westfall et al., 2003), we also tested whether the inhibition of β-catenin accumulation measured in our assays required the presence of GSK-3, an upstream protein in the β-catenin dependent pathway. Sfrp-1 and Sfrp-2 were unable to inhibit the accumulation of β-catenin when GSK-3 was inhibited by the addition of LiCl. Thus, we have eliminated the possibility that the ability of Sfrp to inhibit accumulation of β-catenin is due to destabilization of β-catenin by an alternate signaling pathway. The most parsimonious interpretation of our results is that the inhibition of Wnt-3a activity occurs at the cell surface.
Sfrps have been proposed to act either by binding directly to Wnts (Leyns et al., 1997; Lin et al., 1997; Rattner et al., 1997; Wang et al., 1997a; Wang et al., 1997b; Lescher et al., 1998; Bafico et al., 1999; Dennis et al., 1999; Uren et al., 2000) or by dimerizing with Frizzled proteins (Bafico et al., 1999) to form non-functional complexes. Our data showing that Sfrp-1 and Sfrp-2 act upstream of GSK-3, are consistent with either model. If Sfrps inhibit by direct binding to Wnt-3a, our studies show specificity of binding to Wnt-3a. If they inhibit by complexing with Frizzleds, our studies reveal specificity of binding to Frizzleds. This latter model provides the possibility that the ability of Sfrps to inhibit particular Wnt ligands may depend more on the ability of Sfrp to bind to the Frizzled receptor than to the Wnt. Although we have obtained consistent results in both L cells and the chick neural tube, we cannot rule out the possibility that the ability of Sfrps to inhibit different Wnts varies with the expression of the Frizzled receptors is therefore, tissue specific.
Consistent with data obtained in our L cells assay, we have also determined that Sfrp-2, but not Sfrp-3, can inhibit the ability of ectopic Wnt-3a to induce proliferation in the chick neural tube. As the activity of endogenous Wnt-1 and Wnt-3a undoubtedly contributes to the total number of proliferative cells in electroporated neural tubes, it is possible that some of the diminution of proliferation that we observe in Sfrp-2 electroporated embryos and co-electroporated embryos is due to the inhibition of endogenous Wnt-1 by Sfrp-2. However, because the concentration of ectopic Wnt-3a far exceeds the concentrations of endogenous proteins, the ability of ectopic Sfrp-2 to inhibit proliferation in Wnt-3a electroporated embryos is likely to represent inhibition of Wnt-3a (and not just endogenous Wnt-1). We also observed that co-electroporation of Sfrp-3 and Wnt-3a resulted in increased proliferation as compared to Wnt-3a alone. Though we have not thoroughly analyzed the mechanism by which this occurs, we hypothesize that these data reflect the utilization of both β-catenin dependent and β-catenin independent Wnt signaling pathways in the neural tube.
In sum, the differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2 and Sfrp-3 suggests distinct roles in the neural tube. The expansion of the neural tube along the D/V axis is driven by a dorsal to ventral gradient of Wnt-1 and Wnt-3a activity in the chick neural tube (Megason and McMahon, 2002). This gradient is established around HH stage 17 and 21 of development (Megason and McMahon, 2002), and is preceded by the restriction of sfrp-1/2 expression to ventral domains of the neural tube (Esteve et al., 2000; Terry et al., 2000). While the regulation of the diffusion or movement of Wnt-1/3a protein is likely to represent one mechanism by which this activity gradient is established, our data lead us to predict that the expression of Sfrp-1 and Sfrp-2 in ventral domains plays a role in establishing and/or reinforcing this gradient.
By contrast, our data showing that Sfrp-3 does not inhibit Wnt-3a is not consistent with our previously predicted role for Sfrp-3 (Jin et al., 2001). In collaboration with Carol Erickson’s lab, we have previously shown that Wnt-3a stimulates the specification of melanoblasts at the expense of neuroblasts and glioblasts in avian neural tube explants (Jin et al., 2001). Interestingly, both Wnt-1 and Wnt-3a are expressed at developmental stages when neuroblasts and glioblasts are being specified (Hollyday et al., 1995). Because sfrp-3 transcripts are expressed in the dorsal neural tube during the specification of neuroblasts and glioblasts, but not during the specification of melanoblasts (Baranski et al., 2000; Jin et al., 2001), we speculated that Sfrp-3 would be able to inhibit both Wnt-1 and Wnt-3a during neuroblasts and glioblast specification to provide a permissive environment for the specification of these cells. Although there are multiple reports indicating that Sfrp-3 does indeed inhibit Wnt-1 (Finch et al., 1997; Leyns et al., 1997; Lin et al., 1997; Mayr et al., 1997; Salic et al., 1997; Wang et al., 1997b; Xu et al., 1998; Tzahor and Lassar, 2001), our data show that it does not inhibit Wnt-3a. Thus, we think it unlikely that Sfrp-3 is providing the sole cue that permits the specification of neuroblasts and glioblasts.
Acknowledgements
The authors would like to thank Dr. Hans Holtan of the Cell and Molecular Imaging Facility for his assistance with the confocal microscope. This work was funded by NIH MBRS (SO6 GM52588) and AREA (R15 HD4204501) grants to LWB as well as an NIH RIMI (P20 MD000262) grant to SFSU.
References
- Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- Baranski M, Berdougo E, Sandler JS, Darnell DK, Burrus LW. The dynamic expression pattern of frzb-1 suggests multiple roles in chick development. Dev Biol. 2000;217:25–41. doi: 10.1006/dbio.1999.9516. [DOI] [PubMed] [Google Scholar]
- Brown AM, Wildin RS, Prendergast TJ, Varmus HE. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986;46:1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- Cauthen CA, Berdougo E, Sandler J, Burrus LW. Comparative analysis of the expression patterns of Wnts and Frizzleds during early myogenesis in chick embryos. Mech Dev. 2001;104:133–138. doi: 10.1016/s0925-4773(01)00369-0. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Dennis S, Aikawa M, Szeto W, d’Amore PA, Papkoff J. A secreted frizzled related protein, FrzA, selectively associates with Wnt-1 protein and regulates wnt-1 signaling. J Cell Sci. 1999;112:3815–3820. doi: 10.1242/jcs.112.21.3815. [DOI] [PubMed] [Google Scholar]
- Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H, Nusse R, van der Neut R, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson ME, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- Duprez D, Leyns L, Bonnin M, Lapointe F, Etchevers H, De Robertis EM, Le Douarin N. Expression of frzb-1 during chick development [In Process Citation] Mech Dev. 1999;89:179–183. doi: 10.1016/s0925-4773(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Esteve P, Morcillo J, Bovolenta P. Early and dynamic expression of cSfrp1 during chick embryo development. Mech Dev. 2000;97:217–221. doi: 10.1016/s0925-4773(00)00421-4. [DOI] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci U S A. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frowein J, Campbell K, Gotz M. Expression of Ngn1, Ngn2, Cash1, Gsh2 and Sfrp1 in the developing chick telencephalon. Mech Dev. 2002;110:249–252. doi: 10.1016/s0925-4773(01)00590-1. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hoang B, Moos M, Jr., Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Houston DW, Wylie C. Cloning and expression of Xenopus Lrp5 and Lrp6 genes. Mech Dev. 2002;117:337–342. doi: 10.1016/s0925-4773(02)00205-8. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233:22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech Dev. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, Hattersley G, Rosen V, Luyten FP, Dale L, Francis-West PH. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- Lee CS, Buttitta LA, May NR, Kispert A, Fan CM. SHH-N upregulates Sfrp2 to mediate its competitive interaction with WNT1 and WNT4 in the somitic mesoderm. Development. 2000;127:109–118. doi: 10.1242/dev.127.1.109. [DOI] [PubMed] [Google Scholar]
- Lescher B, Haenig B, Kispert A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev Dyn. 1998;213:440–451. doi: 10.1002/(SICI)1097-0177(199812)213:4<440::AID-AJA9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Wang S, Julius MA, Kitajewski J, Moos M, Jr., Luyten FP. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci U S A. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Mayr T, Deutsch U, Kuhl M, Drexler HC, Lottspeich F, Deutzmann R, Wedlich D, Risau W. Fritz: a secreted frizzled-related protein that inhibits Wnt activity. Mech Dev. 1997;63:109–125. doi: 10.1016/s0925-4773(97)00035-x. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci U S A. 1997;94:13636–13641. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–726. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–4748. doi: 10.1242/dev.124.23.4739. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem. 2004;279:17232–17240. doi: 10.1074/jbc.M307801200. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93:195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Terry K, Magan H, Baranski M, Burrus LW. Sfrp-1 and sfrp-2 are expressed in overlapping and distinct domains during chick development. Mech Dev. 2000;97:177–182. doi: 10.1016/s0925-4773(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Samos CH, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997a;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Moos M., Jr.w. Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts -3A, -5A, or -11. Biochem Biophys Res Commun. 1997b;236:502–504. doi: 10.1006/bbrc.1997.6995. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams CL. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell Signal. 2003;15:1071–1080. doi: 10.1016/s0898-6568(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Xu Q, D’Amore PA, Sokol SY. Functional and biochemical interactions of Wnts with FrzA, a secreted Wnt antagonist. Development. 1998;125:4767–4776. doi: 10.1242/dev.125.23.4767. [DOI] [PubMed] [Google Scholar]
- Young CS, Kitamura M, Hardy S, Kitajewski J. Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]