Abstract
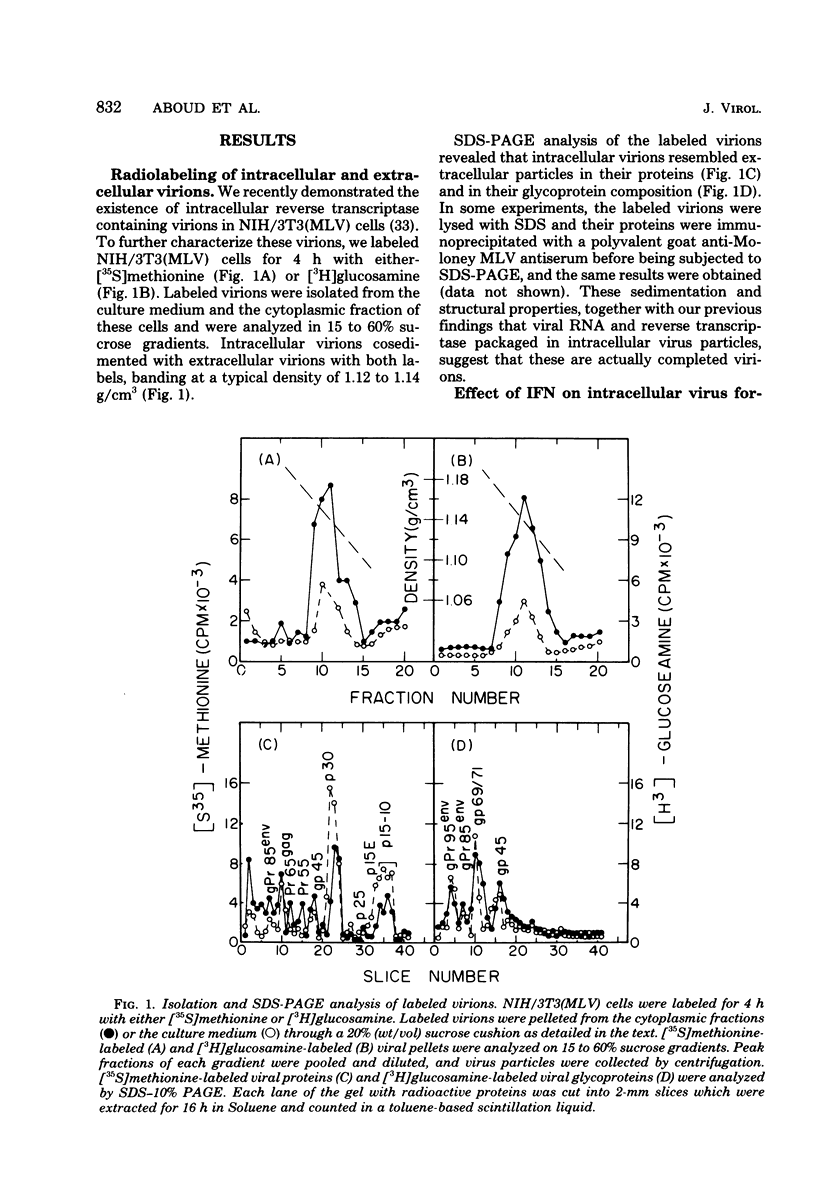
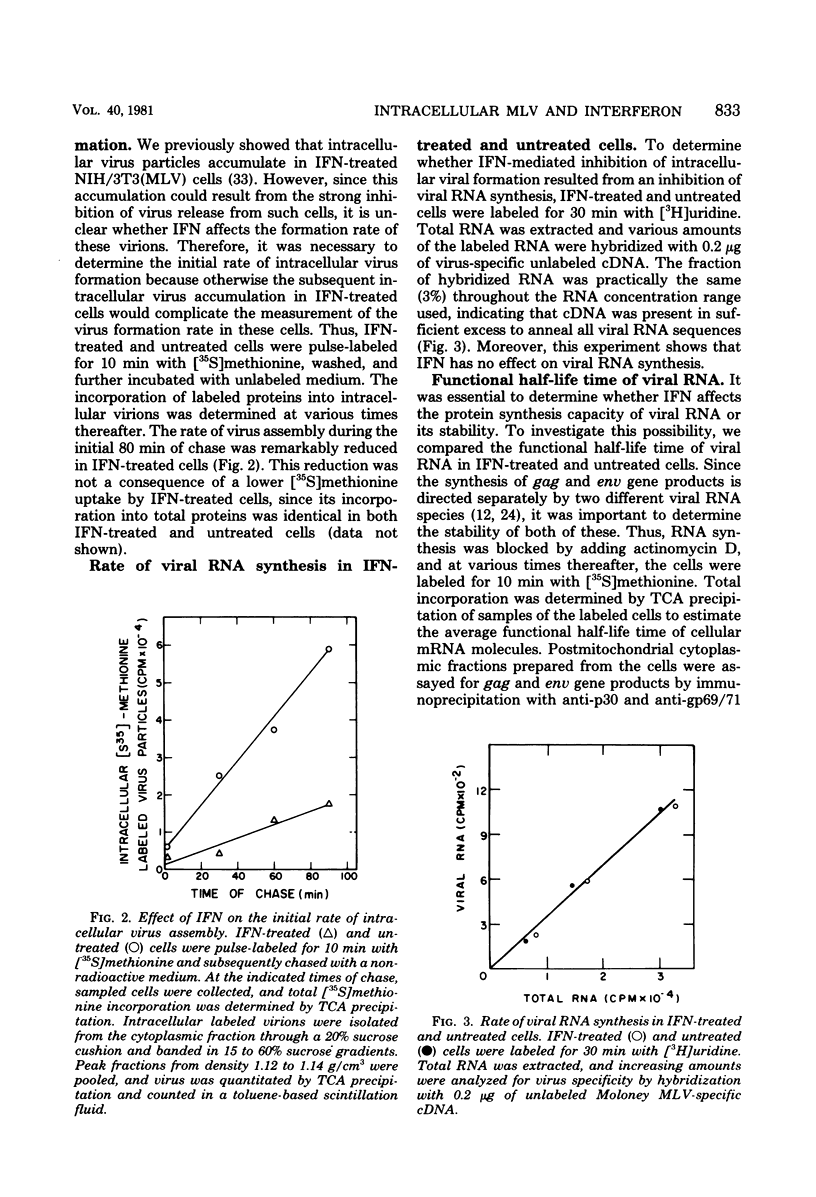
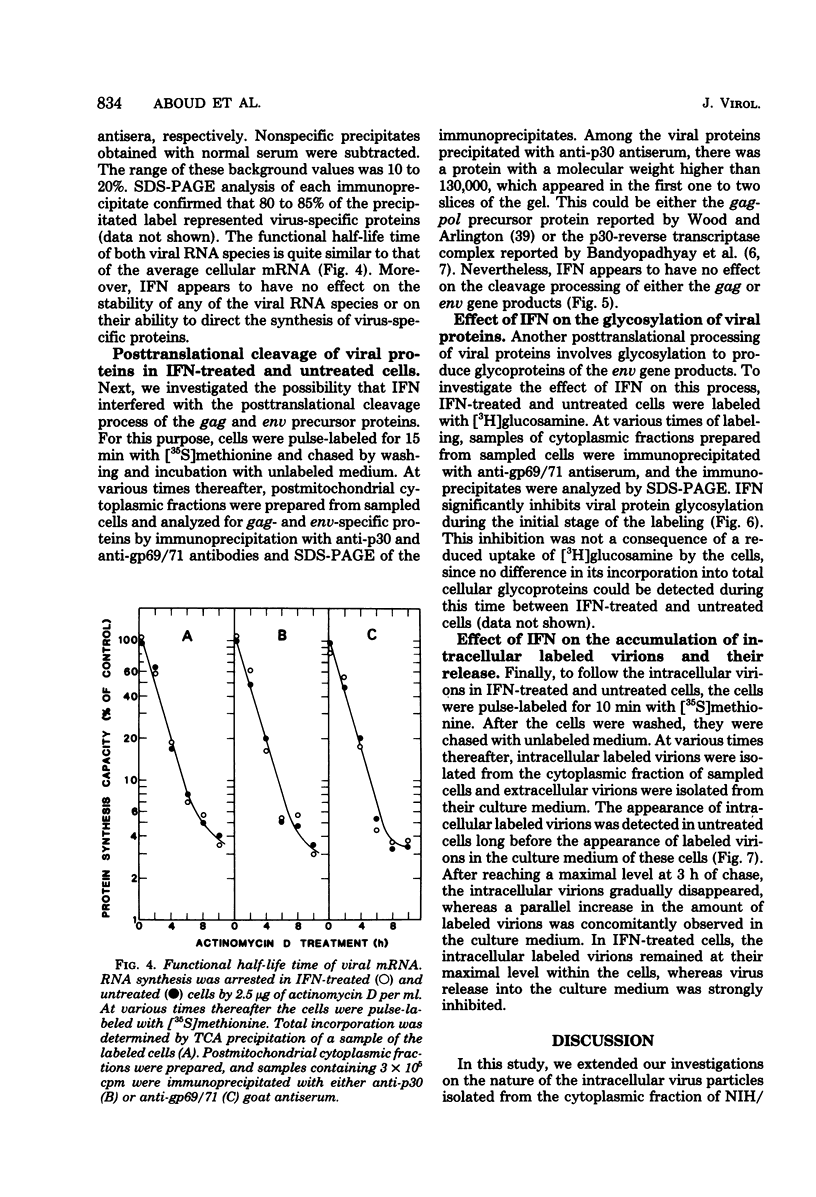
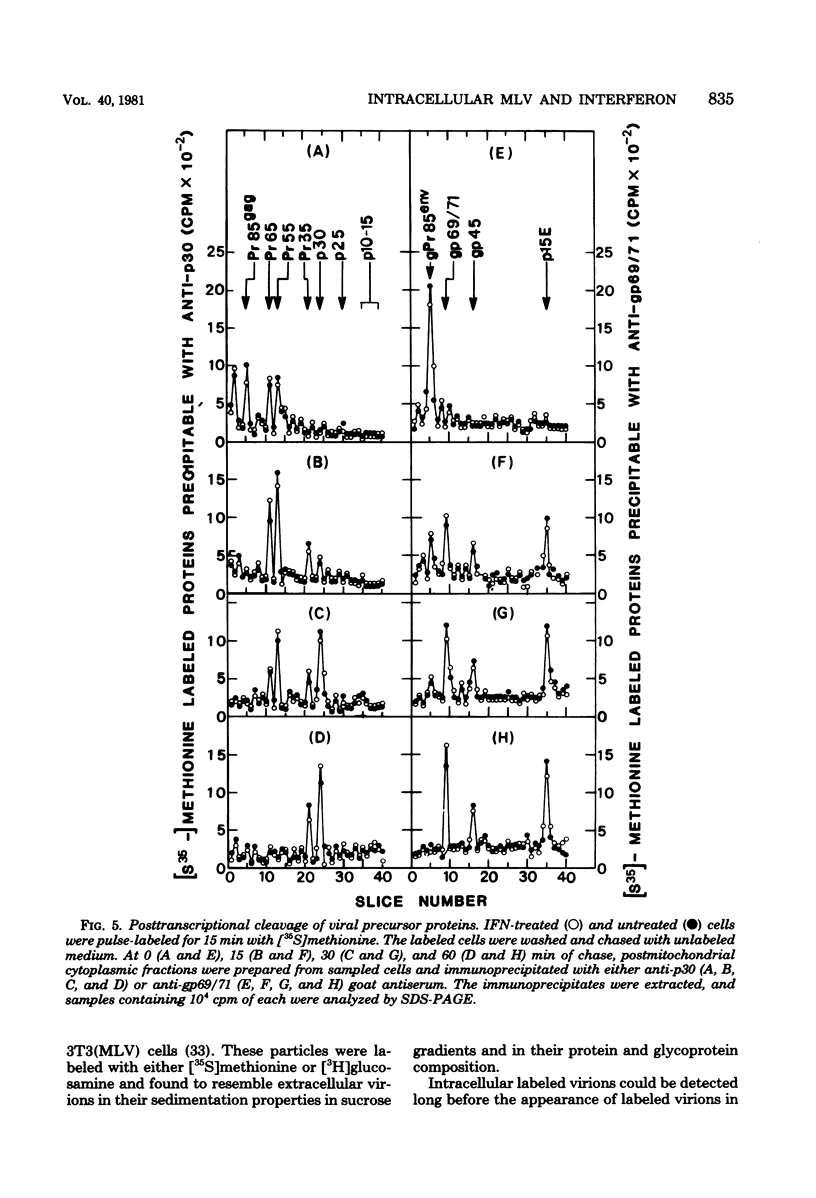
The effect of interferon on the biochemical properties and the maturation process of intracellular viral particles isolated from the cytoplasmic fraction of NIH/3T3 cells chronically infected with Moloney murine leukemia virus was investigated. By labeling these virions with either [35S]methionine or [3H]glucosamine, we demonstrated that they contain the same viral proteins and glycoproteins found in extracellular virions. Interferon treatment was found to reduce the rate of intracellular virus assembly. This effect was not a consequence of an interferon inhibition of viral RNA synthesis or its translation or a consequence of an interference with the posttranslational cleavage processing of viral precursor proteins, since all of these steps were not affected by interferon. However, the reduced rate of virus assembly could be attributed to the inhibition of viral protein glycosylation observed in interferon-treated cells. Nevertheless, despite this reduced rate, virus particles accumulated in interferon-treated cells. This accumulation was probably due to the strong inhibition of their final release from such cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Moldovan-Levin D., Salzberg S. Cytoplasmic viral DNA synthesis in exogenous infection of murine leukemia virus: effect of interferon and cycloheximide. J Virol. 1981 Feb;37(2):836–839. doi: 10.1128/jvi.37.2.836-839.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud M., Shoor R., Salzberg S. An effect of interferon on the uncoating of murine leukaemia virus not related to the antiviral state. J Gen Virol. 1980 Dec;51(Pt 2):425–429. doi: 10.1099/0022-1317-51-2-425. [DOI] [PubMed] [Google Scholar]
- Aboud M., Weiss O., Salzberg S. Rapid quantitation of interferon with chronically oncornavirus-producing cells. Infect Immun. 1976 Jun;13(6):1626–1632. doi: 10.1128/iai.13.6.1626-1632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery R. J., Norton J. D., Jones J. S., Burke D. C., Morris A. G. Interferon inhibits transformation by murine sarcoma viruses before integration of provirus. Nature. 1980 Nov 6;288(5786):93–95. doi: 10.1038/288093a0. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Chang E. H., Levy C. C., Friedman R. M. Structural abnormalities in murine leukemia viruses produced by interferon-treated cells. Biochem Biophys Res Commun. 1979 Apr 27;87(4):983–988. doi: 10.1016/s0006-291x(79)80003-0. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Levy C. C. Effect of RNA tumor virus-specific protein p30 on reverse transcriptase. Intraspecies and interspecies interaction between reverse transcriptase and p30. J Biol Chem. 1978 Nov 25;253(22):8285–8290. [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Sobis H., de Somer P. Influence of interferon on virus-particle synthesis in oncornavirus-carrier lines. II. Evidence for a direct effect on particle release. Int J Cancer. 1974 Sep 15;14(3):335–340. doi: 10.1002/ijc.2910140306. [DOI] [PubMed] [Google Scholar]
- Billiau A. Effect of interferon on RNA tumor viruses. Tex Rep Biol Med. 1977;35:406–419. [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Maeyer-Guignard J., De Somer P. Trapping of oncornavirus particles at the surface of interferon-treated cells. Virology. 1976 Sep;73(2):537–542. doi: 10.1016/0042-6822(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Somer P. Influence of interferon on the synthesis of virus particles in oncornavirus carrier cell lines. IV. Relevance to the potential application of interferon in natural infectious diseases. J Infect Dis. 1976 Jun;133 (Suppl):A51–A55. doi: 10.1093/infdis/133.supplement_2.a51. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Friedman R. M. A large glycoprotein of Moloney leukemia virus derived from interferon-treated cells. Biochem Biophys Res Commun. 1977 Jul 11;77(1):392–398. doi: 10.1016/s0006-291x(77)80210-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Myers M. W., Wong P. K., Friedman R. M. The inhibitory effect of interferon on a temperature-sensitive mutant of Moloney murine leukemia virus. Virology. 1977 Apr;77(2):625–636. doi: 10.1016/0042-6822(77)90487-1. [DOI] [PubMed] [Google Scholar]
- Czarniecki C. W., Sreevalsan T., Friedman R. M., Panet A. Dissociation of interferon effects on murine leukemia virus and encephalomyocarditis virus replication in mouse cells. J Virol. 1981 Feb;37(2):827–831. doi: 10.1128/jvi.37.2.827-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Chang E. H., Ramseur J. M., Myers M. W. Interferon-directed inhibition of chronic murine leukemia virus production in cell cultures: lack of effect on intracellular viral markers. J Virol. 1975 Sep;16(3):569–574. doi: 10.1128/jvi.16.3.569-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. K., Banerjee D. K., Waechter C. J., Olden K., Friedman R. M. Interferon treatment inhibits glycosylation of a viral protein. Nature. 1980 Oct 2;287(5781):454–456. doi: 10.1038/287454a0. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Bolognesi D. P. Structure and morphogenesis of type-C retroviruses. Adv Cancer Res. 1978;28:63–89. doi: 10.1016/s0065-230x(08)60646-6. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Burke D. C. An interferon-sensitive early step in the establishment of infection of murine cells by murine sarcoma/leukaemia virus. J Gen Virol. 1979 Apr;43(1):173–181. doi: 10.1099/0022-1317-43-1-173. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Clegg C. The effect of mouse interferon on the transformation of NIH/3T3 mouse cells by murine sarcoma virus. Virology. 1978 Jul 15;88(2):400–402. doi: 10.1016/0042-6822(78)90298-2. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Fernie B., Maldarelli F., Hattman T., Wivel N. A. Effect of interferon on mouse leukaemia virus (MuLV). V. Abnormal proteins in virions of Rauscher MuLV produced in the presence of interferon. J Gen Virol. 1980 Jan;46(1):97–110. doi: 10.1099/0022-1317-46-1-97. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Fernie B., Maldarelli F., Wivel N. A. The errors in assembly of MuLV in interferon treated cells. J Supramol Struct. 1979;12(1):35–46. doi: 10.1002/jss.400120105. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Wivel N. A., Fernie B. F., Harper H. P. Effect of interferon on murine leukaemia virus infection. IV. Formation of non-infectious virus in chronically infected cells. J Gen Virol. 1979 Mar;42(3):467–480. doi: 10.1099/0022-1317-42-3-467. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Bakhanashvili M., Aboud M. Effect of interferon on mouse cells chronically infected with murine leukaemia virus: kinetic studies on virus production and virus RNA synthesis. J Gen Virol. 1978 Jul;40(1):121–130. doi: 10.1099/0022-1317-40-1-121. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Bakhanashvili M., Bari S., Berman I., Aboud M. Characterization of intracellular viral RNA in interferon-treated cells chronically infected with murine leukemia virus. J Virol. 1980 Sep;35(3):694–703. doi: 10.1128/jvi.35.3.694-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S., Levi Z., Aboud M., Goldberger A. Isolation and characterization of DNA-DNA and DNA-RNA. Biochemistry. 1977 Jan 11;16(1):25–29. doi: 10.1021/bi00620a004. [DOI] [PubMed] [Google Scholar]
- Sato T., Friend C., De Harven E. Ultrastructural changes in Friend erythroleukemia cells treated with dimethyl sulfoxide. Cancer Res. 1971 Oct;31(10):1402–1417. [PubMed] [Google Scholar]
- Sato T., Friend C., Stackpole C., De Harven E. Coating of Friend leukemia virus after treatment with specific antiserum. Cancer Res. 1972 Dec;32(12):2670–2678. [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., MacLeod R., Chang E. H., Myers M. W., Friedman R. M. The effect of interferon on de novo infection of Moloney murine leukemia virus. Cell. 1977 Feb;10(2):245–252. doi: 10.1016/0092-8674(77)90218-5. [DOI] [PubMed] [Google Scholar]
- Wood T. G., Arlinghaus R. B. Precursor polyproteins of Moloney murine leukemia virus. Biochim Biophys Acta. 1979 Nov 22;565(1):183–191. doi: 10.1016/0005-2787(79)90094-7. [DOI] [PubMed] [Google Scholar]


