Abstract
Unlike the case of traditional covalent polymers, the entanglements that determine properties of supramolecular polymers are defined by very specific, intermolecular interactions. Recent work using modular molecular platforms to probe the mechanisms underlying mechanical response of supramolecular polymers is reviewed. The contributions of supramolecular kinetics, thermodynamics, and conformational flexibility to supramolecular polymer properties in solutions of discrete polymers, in networks, and at interfaces, are described. Molecule-to-material relationships are established through methods reminiscent of classic physical organic chemistry.
Keywords: Supramolecular polymers, mechanochemistry, mechanism
A little history
A wonderful and well-known case study in the development of science is found in the birth of polymer chemistry in the early 20th century.1-3 Scientists of the day were intrigued and inspired by Nature's ability to create the stuff from which useful materials could be made—cotton, rubber, and silk, for example—but even the most fundamental molecular picture of what that “stuff” was had yet to be established. The majority view of early 20th–century scientists was that these materials were colloidal aggregates of small molecules held together by “partial valences”—in other words, that small molecules grouped and organized into larger, non-covalent superstructures that created the whole material and its properties. The “partial valence” view was widely and firmly held, both by organic chemists who had yet to see the accepted synthesis of a molecule of molecular weight greater than approximately 4,200 Da, and by physical chemists who were captivated by the ubiquity of colloidal aggregates in other contexts.
In marked contrast, Staudinger's classic 1920 paper “Über Polymerisation”4 voiced the view that asyet uncharacterized molecules of high molecular weight were the primary constituents of these materials, that the bonds within these molecules were the same “primary valences” responsible for small molecule structure, and that the high molecular weight was itself fundamentally responsible for the properties of the materials. In 1922, Staudinger coined the term “makromoleküle”5 to describe the large molecules he believed were implicated by observations made on rubber. While in the minority with his view, Staudinger was not alone. Among the notable proponents of the macromolecular theory was Carothers, who in 1928 was just beginning his meteoric career at DuPont. In Enough for One Lifetime, Carother's biography, author Matthew Hermes notes that “Carothers believed he could recognize…nonbonded aggregation…, and that is not what he observed when considering rubber and cotton and silk.”6 Staudinger and Carothers were criticized, both publicly and privately, for their shared hypothesis; in the 1920's, macromolecules were a conceptually charming idea, but their existence was “known” to be too impractical to be realized in either Nature or the laboratory.7
Of course, good science wins out in the end. Staudinger's careful work on the chemical derivatives of synthetic polymers (“polymer analogous reactions”) began to convert skeptics even before Carothers' “proof by synthesis” of condensation polymers erased any remaining doubt that macromolecules were a molecular reality. Nonetheless, the controversy had its price; Staudinger did not win an overdue Nobel Prize until 1953, by which time Carothers' clinical depression (exacerbated, if only partially, by what he perceived to be a lack of appreciation for his polymer work) had long since led to his suicide.
A recent twist
And so it was that a paradigm truly shifted, and an increasingly plastic world of consumer products firmly reinforced the relationship between the structure of big molecules and the properties of polymeric materials. But, while misapplied decades ago and subsequently less present in the collective scientific psyche, the concept of polymer-like properties through non-covalent aggregation was not devoid of merit; covalent polymerization may be sufficient, but it is not necessary (For an excellent perspective on the historical context of supramolecular polymerization, see the review by Zimmerman et al.8). For example, in the 1980's, Rehage and Hoffman published beautiful studies of the viscoelasticity of aqueous worm-like micellar aggregates,9, 10 which were known to form gels at low concentrations.11-13 Non-covalent aggregates of small, amphiphilic molecules generated mechanical properties typically associated with high molecular-weight polymers.
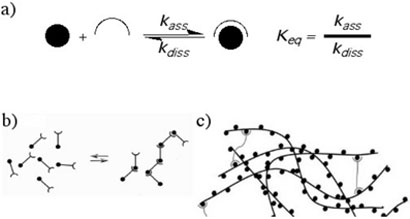
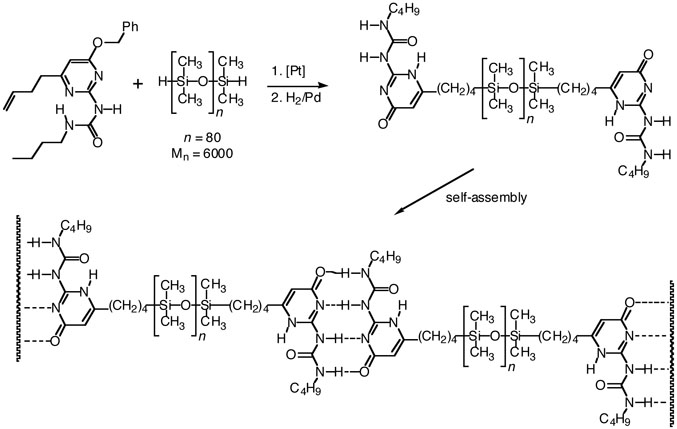
The broad concept was extended to main-chain supramolecular14 polymers15, 16 (Figure 1), in which molecular recognition events between end groups define the main chain of a linear polymeric assembly. Fouquey et al. reported the concept and rational design of supramolecular polymers based on hydrogen bond-mediated molecular recognition in 1990,17 and other conceptually similar examples were known18 or followed19-21 soon thereafter. In a groundbreaking paper in 1997, Sijbesma et al. covalently appended ureidopyrimidinone (UPy) units to the ends of short hydrocarbons, siloxanes, and PEO/PPO copolymers (Figure 2).22 The UPy units have a strong propensity for self-dimerization (Keq > 106 M−1in aprotic solvents), and once appended they have a remarkable impact on material properties. For example, end-grafted UPy groups convert oligomeric siloxanes from a thick fluid into a viscoelastic thermoplastic. When extended to three-dimensional networks, supramolecular cross-linking via the UPy units leads to a plateau modulus six times that found in polymers with the same number of potential covalent cross-linking groups.22 The technological utility of supramolecular approaches to polymer science was established, and subsequent intense effort in the field reinforced the potential of SP's.23-25
Figure 1.
Schematic representation of (a) a reversible assembly motif and its related equilibrium and kinetic rate constants, and (b) linear and (c) networked supramolecular polymers formed from that motif.
Figure 2.
The attachment of ureidopyrimidinone end groups to a Newtonian fluid polydimethylsiloxane creates a supramolecular polymer with pronounced thermoplastic character.
Polymers = entanglements
One consequence of the profuse activity is that many definitions of supramolecular polymers (SP's) exist. Among the productive definitions, and the one adopted here, is to define SP's in the context of entanglements;—intermolecular interactions that transfer mechanical forces from one molecule to the next. The term entanglement is quite general, and it includes topological entanglements (one polymer chain is physically wrapped around another), chemical entanglements (attractive intermolecular interactions between polymer chains), and surface adsorption (attractive intermolecular interactions between polymer chains and a particle surface, e.g. from a filler).
Supramolecular polymers, therefore, are reasonably viewed as systems in which specific intermolecular interactions create entanglements that would not exist in the absence of those interactions; the individual molecular constituents are too small, for example, to be physically entangled or to bridge between surfaces. As a consequence, SP properties are directly tied to the supramolecular interaction. For example, they might be responsive to external stimuli in a manner that permits them to be turned “on” and “off”;—toggling between polymeric and small molecular behavior and creating materials that are more easily processed or recycled. The responsiveness need not be limited to on/off states; it could also be tuned through synthesis and environment to create “smart” materials. In contrast to covalent polymers, the defining bonds are reversible and therefore self-repairing when broken. Finally, the supramolecular interaction is often dynamic and transient, permitting conformational changes and molecular relaxations on timescales not otherwise available to fixed, covalent structures.
An ongoing challenge for chemists is to relate behavior at the level of polymer physics to behavior of the small molecular constituents. For example, the mechanical properties of quadruple hydrogen-bonded UPy end-functionalized monomers are much better than those of SP's formed by weaker single or triple hydrogen bonding, but what is the ideal association constant or the ideal kinetics for equilibration, or the necessary number of reversible interactions? Pollino et al.26 showed that metal-ligand coordinative cross-links have a greater impact on viscosity than cross-links formed by hydrogen bonds, but to what extent does knowledge of small molecule interactions translate across length scales to the ultimate materials? The dynamic relaxation rates of polymer networks are often comparable to the dissociation rates of the defining interaction,22, 27 but in what circumstances does the reversibility of the interaction matter? Or more generally, what is the quantitative relationship between the thermodynamics and kinetics of the reversible association, the structure of the monomer, and the structure and properties of the reversible polymer? The answer to these questions, of course, likely depends on the specifics of the system and the desired application; the critical design criteria might differ, for example, for SP's at interfaces relative to SP's in the bulk. Nevertheless, to the extent that general relationships were established, materials properties could be controlled in a rational way through small-molecule synthesis.
To address these questions generally, our lab set out to develop modular SP systems that allow control over the thermodynamics, kinetics, and conformational flexibility of the interacting moieties within homologous series of compounds. The motivation was to find molecular toolboxes that could be transported readily to different material regimes. Using these molecular tools, we are able to probe the mechanisms behind the mechanics of single molecules, dilute SP solutions, interfaces, and associative networks. This review highlights those efforts.
The classic conundrum of physical organic chemistry
Inherent in this work is the nearly ubiquitous challenge of structure-activity studies: How do you change something, without changing something? Every structural change to a molecule has an influence on electronics, sterics, and intermolecular interactions, creating ambiguity in the interpretation. For the two examples discussed in this review;—insertion of methylenes to change the rates of ligand exchange in metal complexes, and perturbation of oligonucleotide sequence;—the dilemma is seen to be less severe than in other contexts, because minimal structural perturbations are made either outside the bonding structure defining the polymers (metal-ligand coordination), or they are hidden deep within the interior of structures that are effectively identical (DNA duplexes). The relevant physics, often captured in scaling laws28, 29 and statistical theories,1, 30, 31 are determined on length scales over which the finer details of molecular structure are often lost. As a somewhat counter-intuitive result, polymer physics provides a surprisingly fruitful arena for mechanistic chemistry.
Reversibility in linear SP's
The transient nature of the reversible, supramolecular bond is the most obvious difference between SP's and covalent polymers. A desirable approach to the study of SP dynamics would be to simply change the kinetics of a given intermolecular association and observe corresponding changes in the properties of the materials. While this strategy is potentially very informative, there exists a subtle, yet persistent, difficulty in distinguishing the contributions of the kinetics of a given molecular interaction (kdiss, Figure 1a) from those of its thermodynamics (Keq). In most reversibly–assembled systems, for example those based on hydrogen bonding, association occurs at or near the diffusion rate, and Keq and kdiss are strongly anti–correlated. For SP's, the inverse correlation of kdiss and Keq intrinsically frustrates efforts to determine the relative importance of the two contributions. High Keq leads to increased aggregation, higher SP molecular weights, and slower dynamics within the equilibrium polymer structure. At the same time, lower kdiss leads to slower reversible kinetics along the assembly. Dynamic properties, whether controlled by SP equilibrium structure or reversible kinetics, are slowed by both mechanisms.
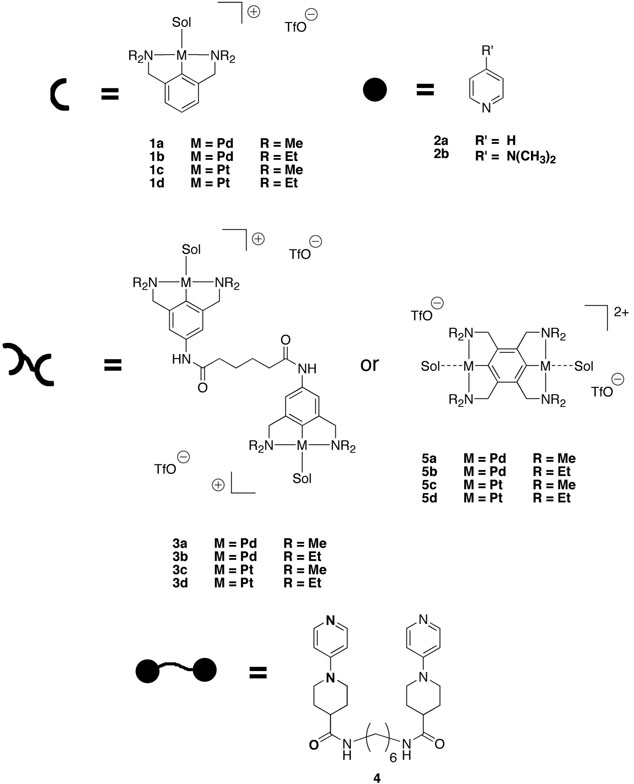
The independent control of association kinetics relative to thermodynamics is therefore desirable, and N,C,N-pincer metal–ligand coordination motifs such as 1•2 (Figure 3) have proven to be well–suited to that goal. Pincer compounds 1 and analogs have been synthesized and studied extensively by van Koten and co-workers,32-35 and they, with numerous other organometal coordination systems, have been used in SP's.24, 33 The reversible kinetics of interest are those of ligand exchange, and in Pd(II) and Pt(II) complexes, ligand exchange occurs through a sterically congested associative mechanism. Added bulk in the N–alkyl substituents R, therefore, slows the exchange while exerting a lesser effect on the relative energy of the roughly isosteric endpoints. For example, the association constants for 1a•2b and 1b•2b in DMSO are 1.6 and 1.3 × 103 M−1, respectively, but the rates of ligand exchange differ by nearly two orders of magnitude (70−100 s−1 for 1a•2b and 1.0 s−1 for 1b•2b).36
Figure 3.
N,C,N-pincer metal complexes and ligands for supramolecular polymerizations.
The 1•2 motif can be incorporated into supramolecular polymers such as the linear SP's 3•4, shown in Figure 3. A 1:1 mixture of 3a:4 (4.6 weight%) forms linear SP's in DMSO, as evidenced by an increased viscosity relative to solutions of the individual components.36 The viscosity is immediately reduced by the addition of an appropriate chain terminator, so the increased viscosity is not due to unspecific aggregation or ionic effects. One can now ask, and easily answer, a fundamental question: To what extent is the increased viscosity of the SP solution influenced by the reversibility of the metal-ligand bond? A similar solution of 3b•4 has the same viscosity, and so the equilibrium structure of the SP determines the viscosity of the SP solutions; the transience of the main chain does not contribute.36
Reversibility in SP networks
More recently, the kinetic control has been applied to SP networks (Figure 1c), for example poly(4–vinylpyridine) (PVP) that is crosslinked by bis(M(II)–pincer) compounds 3a–d (Figure 3).37, 38 The same simple steric effects in the pincer alkylamino ligands is used, both within the Pd complexes 3a-b and the slower, more strongly coordinating Pt complexes 3c-d.37, 38 As with the linear SP's, the independent control of kinetics is particularly significant; crosslinkers 3a and 3b are structurally identical components within the network, and so their similar thermodynamics (Keq = ~30 M−1 for 1a•2a and 1b•2a) ensure that the extent and nature of crosslinking is essentially the same in the two samples (or between Pt(II) pincer molecules 3c–d; Keq = 8 × 103 M−1 for 1c•2a and 4 × 103 M−1 for 1d•2a).
The addition of 2% (by functional group) 3b to a 100 mg mL−1 DMSO solution of PVP gives rise to a clear, thick, deep yellow solution whose viscosity is ~2000 times greater than that of PVP alone (33 Pa•s vs. 0.016 Pa•s). The viscosity does not increase upon the addition of the same quantity of monomeric 1b, and the viscosity of 3b•PVP reverts back to that of a free–flowing solution with the addition of the stronger ligand 2b, which competes the metal away from the PVP side groups. The increased viscosity of 3b•PVP networks is therefore attributed to some combination of two broad mechanistic possibilities brought about by interchain cross-linking. On one extreme, the viscous response could be dominated by the motion of equilibrium structures that are effectively intact on the timescale of the viscous response. As observed with the linear SP's described above, in this scenario the kinetics of metal–ligand dissociation are invisible in the materials properties, and only the number and connectivity of the cross-links are responsible for material response. Alternatively, the viscosity of the new equilibrium polymer structure could be limited largely by the dynamics of the individual cross-links and the ability of individual molecular connections to rearrange within the transient network.
Crosslinker 3a provides a mechanistic probe of the underlying dynamics, because the ligand exchange kinetics for 3a are substantially faster than for 3b while the association thermodynamics are very similar. The effect of those kinetics is dramatic. At 5% crosslinker, the dynamic viscosity of 100 mg mL −1 3a•PVP is only 6.7 Pa•s—a factor of 80 less than that of the isostructural network 3b•PVP. The addition of four methylenes to each metal site represents a very minor perturbation to the overall network structure, and its effect on flow properties of the equilibrium structure is expected to be negligible. The association constants are not identical, but the effect of the thermodynamics would be to increase the viscosity of 3a•PVP relative to 3b•PVP, the opposite direction of that observed. The kinetics dominate even the extent of crosslinking; 5% 3a•PVP is less viscous, by a factor of 5, than is 2% 3b•PVP.
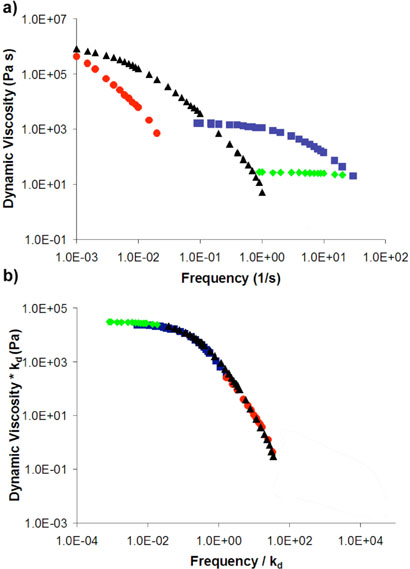
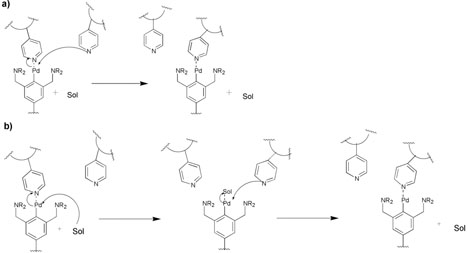
The difference of a factor of ~80 in the network viscosities is within experimental error of the difference in the ligand exchange rates, and a full spectrum of dynamic mechanical behavior is similarly well correlated. The frequency-dependent storage and loss moduli, G′ and G″, for multiple networks of either 3•PVP or the related 5•PVP, are reduced to a single master plot when scaled by the corresponding ligand exchange rates, measured on model systems (data for 5•PVP are shown in Figure 4). These scaled plots are similar to linear free energy relationships, in which rate or equilibrium constants have been replaced by material properties. That the relationships are quantitatively valid shows that the dynamics at the cross-links determine the dynamic mechanical response of the materials, but there are two molecular mechanisms by which cross-link dissociations might occur: direct displacement, in which a free pyridine of the PVP directly displaces the bound pyridine, and solvent–assisted exchange, in which a solvent molecule of DMSO would first displace the bound pyridine and, subsequently, a new pyridine would then displace the DMSO (Figure 5). The implications of these two mechanisms are subtly different. In the case of a direct displacement mechanism, the crosslink remains a part of the network even while it migrates. A solvent–assisted pathway, however, requires that stress relaxation (flow) occur while the crosslink is dissociated from the network.
Figure 4.
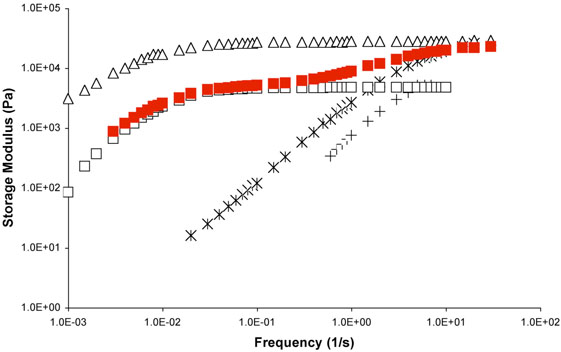
(a) Dynamic viscosity versus frequency for the networks 5•PVP, and (b) the same dynamic viscosity scaled by kdiss of the model complexes 1•2a versus the frequency of oscillation scaled by the same kdiss. Each of the networks consists of 5% (by metal functional group per pyridine residue) of  5a,
5a,  5b, (▲) 5c, and
5b, (▲) 5c, and  5d and PVP at 10% by total weight of network in DMSO at 20 °C. Data taken from ref. 38.
5d and PVP at 10% by total weight of network in DMSO at 20 °C. Data taken from ref. 38.
Figure 5.
Schematic of possible mechanisms of crosslink migration in 3•PVP networks in DMSO. (a) Direct displacement of one pyridine side chain by another. (b) Solvent-assisted exchange of pyridine side chains. Experiments support that the solvent-assisted mechanism is operative. Sol represents the DMSO solvent. Positive charges and counter ions are not shown. Reproduced with permission from J. Am. Chem. Soc. 2005, 127, 14488. Copyright 2005 American Chemical Society.
Experiments are consistent with the solvent–assisted pathway dominating direct displacement at millimolar concentrations of pyridine, and the dominance of the solvent–assisted mechanism in the networks is corroborated by the fact that the viscosity of the networks reflects the nucleophilicity of the solvent.38 DMF is less nucleophilic than DMSO, and network viscosities are higher in DMF than in DMSO. In CH2Cl2, the viscosity of 3b•PVP increases to the point that discrete, free-standing gels can be formed. Thus, mechanical properties are determined by the dissociation of the cross-linkers from the network, but it is the rate of dissociation rather than the fraction of time in the dissociated state (equal in 3a•PVP and 3b•PVP) that governs the properties.38
Experiments on SP networks formed from multiple types of cross-linkers show that the response to an applied stress occurs through sequential, individual dissociation and re–association events.39 Discrete contributions from each type of cross-linker are evident in the mechanical properties, rather than an average of the contributing species. For example, the dynamic viscosity and storage modulus G′ as a function of frequency were compared for five different networks: PVP with 5% of either 5b or 5c, 2.5% of either 5b or 5c, and 2.5% each of 5b and 5c, all at the same concentration of 10% by total weight in DMSO. Below ω = 0.1 s−1, the dynamic viscosity of the mixed network closely mimics that of the network consisting entirely of the slower component 1c•PVP at a concentration of 2.5% (Figure 6). As increases to values greater than 0.1 s−1, the dynamic viscosity of the mixed network increases and eventually plateaus at that of a network comprising PVP and 5% of the faster crosslinking component 5b. Similar effects are observed in the storage modulus and for different mixtures of networks (including those with three different cross-linkers). In all cases, the frequency onsets and magnitudes of the transitions are anticipated by the behavior of networks with a single cross-linker.
Figure 6.
Storage modulus, G′, versus frequency for  2.5% + 2.5% (5b + 5c)•PVP, (□) 2.5% 5c•PVP, (Δ) 5% 5c•PVP, (+) 2.5% 5b•PVP, and (*) 5% 5b•PVP. All networks 10% by total weight in DMSO, 20 °C. Reproduced with permission from Macromolecules 2005, 38, 10171. Copyright 2005 American Chemical Society.
2.5% + 2.5% (5b + 5c)•PVP, (□) 2.5% 5c•PVP, (Δ) 5% 5c•PVP, (+) 2.5% 5b•PVP, and (*) 5% 5b•PVP. All networks 10% by total weight in DMSO, 20 °C. Reproduced with permission from Macromolecules 2005, 38, 10171. Copyright 2005 American Chemical Society.
This behavior is essentially that of transient network models, in which the independent relaxations of stress–bearing entanglements determine the dynamic mechanical response of a network.40-48 Without considering either the exact structure of the networks, the detailed mechanism of relaxation,45, 49 or the extent of cooperativity in the associations, individual dissociation events clearly dominate the mechanical properties; no significant averaging or summation of different components is observed. The independence of the cross-links has significant consequences for the rational, molecular engineering of viscoelastic properties. When entanglements are defined by very specific interactions, the chemical control of properties follows. As long as the strength of the association is great enough to render associated a significant fraction of the crosslinkers, the dynamics of cross-link dissociation, rather than further details of their thermodynamics, are the key design criterion, and quite complex viscoelastic behavior can be engineered given suitable knowledge of the small molecules.
A macromolecular analog of the kinetic isotope effect
The ability to change dynamic response at the level of molecular associations creates the equivalent of a ‘macromolecular kinetic isotope effect’. The similarity with kinetic isotope effects in reaction mechanisms is phenomenological and not literal; actual isotopic substitutions, obviously, are not involved. Like the use of kinetic isotope effects in reaction mechanisms, however, contributions to rate determining material processes are revealed by kinetic differences in two isostructural systems. Structure is kept constant at two levels: the molecular structure of the individual SP constituents and the association constant Keq between molecular recognition partners. The former maintains chemical composition in the material, and the latter maintains structural composition in the extended polymer assembly.
The same mechanistic methodology can be applied to other regimes, for example the role of crosslinking in dense, surface-grafted polymer brush thin films.50 Cross-links are introduced by the simple addition to grafted PVP brushes of solutions containing the bis(PdII-pincer) compounds 3a or 3b. Because the association constants for pyridine coordination are similar, the uptake of 3a and 3b from equimolar solutions into the PVP brushes (at constant grafting density and molecular weight) should be effectively equivalent, producing samples with comparable structure (number and placement of crosslinks).
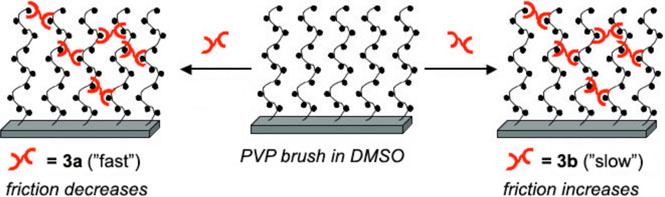
AFM studies on the thin (~50 nm) brush layers demonstrate that the dynamics of supramolecular cross-links contribute to the friction of the soft brush surfaces. When the faster 3a cross-linker is added, both the absolute friction values and the coefficient of friction (COF) drop to ~30% of those of the uncross-linked PVP control (Figure 7). When the slower cross-linker 3b is added, however, the absolute friction value and the COF increase dramatically; both the COF and the absolute friction values for PVP•3b are more than twice that of PVP alone. For both cross-linked samples, the friction is restored to its initial state by the addition of a competitive inhibitor.
Figure 7.
Reversible cross-linking of a surface-grafted polymer brush changes the mechanical properties (in particular, the friction as measured by atomic force microscopy) of the polymer brush surface. The kinetics of the cross-linking interaction have a profound effect on the friction, demonstrating that the cross-links function as stress-bearing entanglements in the friction experiment. See ref. 50.
The effect of cross-linking therefore includes kinetic contributions to friction that are similar to those observed in the networks. A combination of structural and dynamic factors is likely responsible for the significant but opposite effects from kinetically dissimilar cross-links. Stimulus-responsive polymer brush layers are of significant current interest,51-57 and the specific modulation of cross-linking kinetics is one method by which to exert control. As in the SP networks, discussed above, the cross-linking in the brushes is reversed by chemical competition, but responsiveness to other stimuli, such as temperature, could be engineered.
Big effects from small energies
Other surface chemistries are equally interesting. Polymer bridging between surfaces and particles, for example, mediates a range of fundamental processes in the material and life sciences, including: adhesion, tribology and polymer flow, microtubule formation and function, and cell surface interactions.28, 30 Bridges occur when a polymer chain is either physisorbed or covalently bound to two separate surfaces, and covalent polymer bridging has received extensive theoretical58 and experimental59 attention. When the potential bridges are linear SP's that can adjust their size and shape in response to the steric constraints imposed by the surfaces,60, 61 bridging structure and the resulting material properties reflect the chemistry of small–molecule self–assembly. Even sterically-induced entropic penalties as small as kT per chain are large enough to shift the chemical potential that drives polymerization and reduce the average SP molecular weight by ~40%. If mechanical properties scale, for example, as MW3.5, order-of-magnitude changes in properties might result from the fairly modest steric energetics.
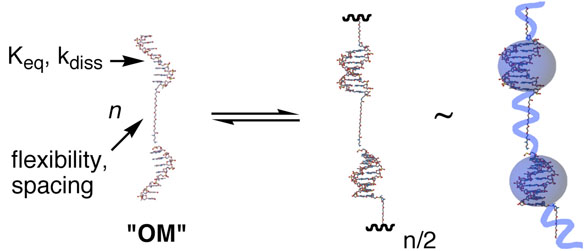
Oligonucleotide-base monomers (OM's) provide a utile molecular probe of SP brush properties brought about by bridging.62, 63 The OM's comprise oligonucleotide sequences that are covalently linked directly or through a synthetic spacer (Figure 8). Duplex formation creates a linear, polymeric assembly that resembles larger duplex DNA, but in which the main chain is defined by the reversible base pairing. The SP's formed from OM's are intrinsically modular: (a) the thermodynamics and kinetics of the association are determined by the variable base sequence; (b) the linear density of the reversible interactions, and the conformational flexibility along the polymer backbone, are each dependent on a spacer in which much variation is possible; (c) inter– and intra– chain interactions may be tuned by salt concentration in the buffer. Further, enzymatic covalent capture and characterization are possible. The equilibrium polymerization of the OM's has been characterized63 and provides a useful model system for delineating molecule–to–material relationships in SP's.
Figure 8.
Schematic representation of oligonucleotide-based monomers, OM's. Short oligonucleotides are linked by a spacer (left), and reversible base pairing defines the main chain of a reversible polymer that resembles beads on a string (far right), where the “beads” are rigid DNA duplexes and the “string” is the intervening spacer. The oligonucleotide sequence determines the thermodynamics and kinetics of the association, and the spacer determines the overall flexibility of the chain and spacing between reversible interactions. See ref. 62.
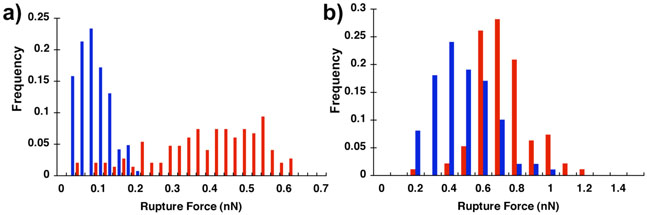
The bridging properties of OM reversible polymer brushes were examined using atomic force microscopy.64 On patterned gold substrates, SP brushes reversibly assembled in solution from different surface densities of a complementary oligonucleotide thiol anchor, and they were characterized in quasi-parallel. The adhesion between anchored SP surfaces was probed by AFM. Adhesive interactions in buffer between the tip and surface increase four-fold upon addition of a self-complementary OM to the solution. The SP–mediated difference in adhesion is attributed to the formation of multiple surface–to–tip bridges during contact, and the OM's provide tools by which to address whether the bridges are formed through molecular recognition of one brush layer with the other or by non–molecular recognition mediated adsorption of the brushes onto opposing surfaces. When similar, but non-complementary, brushes are displayed on opposing surfaces, for example, the adhesive interaction is much lower than that between the same tip and a complementary substrate, supporting direct SP bridging (~15–20 bridging contacts present with polymer that are not present without) as a dominant contributor to adhesion in these systems (Figure 9).
Figure 9.
a) Effect of self-assembled brush complementarity on adhesion measured between OMfunctionalized surfaces measured by atomic force microscopy. Greater adhesion is observed between complementary brushes (red) than brushes that are not complementary (blue). b) Effect of hybridization thermodynamics on adhesion measured between OM-functionalized surfaces measured by atomic force microscopy. Greater adhesion is observed between brushes formed with stronger hybridization thermodynamics (−10.3 kcal mol−1, red, vs. −9.4 3 kcal mol−1, blue). Data taken from ref. 64.
The modularity of the OM's allows additional structure-activity relationships to be explored.64 For example, the average hybridization energy of the defining SP associations can be lowered from −10.3 kcal mol−1 to −9.4 kcal mol−1 by replacing two C-G base pairs with A-T base pairs. The modest change in free energy cuts the adhesion roughly in half for the weaker OM surface brushes relative to the stronger (Figure 9). The magnitude of the change in adhesion indicates that the association thermodynamics influence adhesive properties both through the mechanical response of the individual associations (presumably related to their dissociation kinetics) and by influencing the number of adhesive contacts that are formed. Other details of OM structure also influence the mechanics of the interface. When a flexible spacer is introduced between the molecular recognition end groups, the adhesion between flexible SP brush surfaces is greater than that between rigid SP brush surfaces of comparable height, even though the individual associations in the flexible brushes are weaker than those in the rigid brush.
Bridging across larger gaps
The adhesive interaction has a long-range component. When a similarly functionalized AFM tip is held away from (not in contact with) an SP brush surface, bridges spontaneously form across ~5-10 nm gaps.65 Individual bridging events are observed upon retraction of the AFM tip, and the length of the bridges can be inferred from the force-vs.-distance retraction curves. The length distribution of the SP bridges approximates a Flory distribution,1 as expected for linear SP's. The actual distribution of bridge lengths, however, is skewed toward lengths that are much shorter than the equilibrium distributions in solution. Reversible bridging is therefore responsive to the spatial constraints of the intersurface gap, and shorter bridges are preferred to longer ones. Theoretical work by van der Gucht et al60, 61 predicts that polymer bridging between surfaces, similar to that observed in these single-molecule studies, creates a long-range attractive interaction between surfaces. The presence of an attractive force provides an interesting mechanism for self-repair, because surfaces that are mechanical disjoined might be slowly pulled into closer contact, where adhesion is greatest. The bridges formed across gaps and in surface-to-surface contact provide snapshots of different points in that process.
Conclusion: Whither structure?
There is an aesthetic to supramolecular chemistry that at first seems not to translate to SP's in the above context. The finer structural details of the molecular recognition groups—often a point of pride to the supramolecular chemist66, 67—are notably absent amid featureless kdiss's and Keq's. But we point out in conclusion that diversity in structure is as important as diversity in kinetics and thermodynamics, to the extent that structure impacts the environmental responsiveness of the recognition event. Recognition based on hydrogen bonding, for example, is sensitive and responsive to protic solvents,27, 68 while molecular encapsulation is sensitive and responsive to the presence of an incarcerated guest.27 The chemistry of these and other recognition motifs is then directly translated into the environmental responsiveness of SP's. Maximizing that response, for example by exploiting the details of phase transitions, represents one current interest in the field.
Among the additional interesting avenues for future research is the response of the defining interactions to an applied force. Although many mechanical properties of SP's have been successfully and quantitatively interpreted in terms of thermal rate and equilibrium constants, chemical behavior should be perturbed by the application of a mechanical stress. A recent resurgence in mechanochemical research69 provides technical and intellectual opportunities to correlate the mechanical response of individual molecules to that of SP's, and initial work to that end has recently begun in our group70 and others.71-73
The results presented here support the relevance of a small-molecule view of SP's, and they speak to the potential of reversible and specific intermolecular interactions as effectors of the entanglements or polymer bridges64, 65, 74 necessary to transmit force in materials. The sophistication available in the field of molecular recognition holds considerable promise as it is applied increasingly to areas of material science. Whether in cross-linked networks26, 75-84 or linear supramolecular polymers,17, 22, 85-103 the thermodynamics of the intermolecular interaction are a primary design consideration, but the kinetics of the interactions that create entanglements are particularly important under nonequilibrium conditions such as those imposed by a mechanical stress. In this regard, the behavior of small molecule model systems translates extremely well to macroscopic and nanoscale materials. The rational, molecular design and synthesis of materials and interfaces with very specific and customized properties is not only possible, it is readily accessible to the organic chemist; the languages of polymer physics and physical organic chemistry are closely connected.
Acknowledgment
We gratefully acknowledge the contributions of E. Fogleman, W. Yount, J. Xu, F. Kersey, H. Juwarker, J. Kim, D. Loveless, and S. Jeon, who were primarily responsible for the work reviewed here. We thank NIH (EB001037) and NSF (5003907) for funding.
References
- 1.Flory PJ. Principles of Polymer Chemistry. Cornell University Press; Ithaca: 1969. [Google Scholar]
- 2.Furukawa Y. Inventing Polymer Science: Staudinger, Carothers, and the Emergence of Macromolecular Science. University of Pennsylvania Press; Philadelphia: 1998. [Google Scholar]
- 3.Morawetz H. Polymers: The Origin and Growth of a Science. Wiley; New York: 1985. [Google Scholar]
- 4.Staudinger H. Ber. Deut. Chem. Ges. 1920. p. 1073.
- 5.Staudinger H, Fritschi J. Helv. Chim. Acta. 1922;5:785. [Google Scholar]
- 6.Hermes ME. Enough for One Lifetime: Wallace Carothers, Inventor of Nylon. American Chemical Society and the Chemical Heritage Foundation; Washington D.C.: 1996. [Google Scholar]
- 7. “Dear colleague, drop the idea of large molecules; organic molecules with a molecular weight higher than 5000 do not exist. Purify your products, such as rubber, then they will crystallize and prove to be low molecular compounds!” —Heinrich Otto Wieland to Staudinger; see ref. 2, p. 67.
- 8.Zimmerman N, Moore JS, Zimmerman SC. Chem. Ind. 1998. p. 604.
- 9.Rehage H, Hoffmann H. Mol. Phys. 1991;74:933. [Google Scholar]
- 10.Rehage H, Hoffmann H. J. Phys. Chem. 1988;92:4712. [Google Scholar]
- 11.Candau SJ, Hirsch E, Zana R. J. Physique. 1984;45:1263. [Google Scholar]
- 12.Porte G, Appell J, Poggi Y. J. Phys. Chem. 1980;84:3105. [Google Scholar]
- 13.Hayashi S, Ikeda S. J. Phys. Chem. 1980;84:744. [Google Scholar]
- 14. Depending on one's perspective, the use of the term “supramolecular” may or may not be appropriate to many of the examples described in this review. For the purposes of this discussion, it is the specific, directional, and dynamic nature of the interactions that are important. Understanding the consequences of the interactions, regardless of their classification, on properties beyond the isolated molecules constitutes an objective that is often, although certainly not uniquely, described as “supramolecular”, and the term is used here in that context.
- 15.Lehn J-M. Macromol. Chem. Macromol. Symp. 1993;69:1. [Google Scholar]
- 16.Ciferri A. Supramolecular Polymers. 2nd. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 17.Fouquey C, Lehn J-M, Levelut A-M. Adv. Mater. 1990;2:254. [Google Scholar]
- 18.Broze G, Jerome R, Teyssie P, Marco C. Macromolecules. 1983;16:1771. [Google Scholar]
- 19.Alexander C, Jariwala CP, Lee CM, Griffin AC. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 1993;34:168. [Google Scholar]
- 20.Bladon P, Griffin AC. Macromolecules. 1993;26:6604. [Google Scholar]
- 21.Pourcain CB, Griffin AC. Macromolecules. 1995;28:4116. [Google Scholar]
- 22.Sijbesma RP, Beijer FH, Brunsveld L, Folmer BJB, Hirschberg JHKK, Lange RFM, Lowe JKL, Meijer EW. Science (Washington, D. C.) 1997;278:1601. doi: 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- 23.Corbin PS, Zimmerman SC. In: Supramolecular Polymers. 2nd. Ciferri A, editor. CRC Press; Boca Raton: 2005. p. 153. For representative examples of hydrogen-bonded supramolecular polymers, see: [Google Scholar]; Beijer FH, Kooijman H, Spek AL, Sijbesma RP, Meijer EW. Angew. Chem., Int. Edit. 1998;37:75. [Google Scholar]; Beijer FH, Sijbesma RP, Kooijman H, Spek AL, Meijer EW. J. Am. Chem. Soc. 1998;120:6761. [Google Scholar]; Boileau S, Bouteiller L, Laupretre F, Lortie F. New J. Chem. 2000;24:845. [Google Scholar]; Corbin PS, Zimmerman SC. J. Am. Chem. Soc. 1998;120:9710. [Google Scholar]; Ilhan F, Gray M, Rotello VM. Macromolecules. 2001;34:2597. [Google Scholar]; Norsten TB, Jeoung E, Thibault RJ, Rotello VM. Langmuir. 2003;19:7089. doi: 10.1021/la034809b. [DOI] [PubMed] [Google Scholar]; Sivakova S, Bohnsack DA, Mackay ME, Suwanmala P, Rowan SJ. J. Am. Chem. Soc. 2005;127:18202. doi: 10.1021/ja055245w. [DOI] [PubMed] [Google Scholar]; Sivakova S, Rowan SJ. Chem. Soc. Rev. 2005;34:9. doi: 10.1039/b304608g. [DOI] [PubMed] [Google Scholar]; Zimmerman SC, Zeng FW, Reichert DEC, Kolotuchin SV. Science. 1996;271:1095. doi: 10.1126/science.271.5252.1095. [DOI] [PubMed] [Google Scholar]; Kihara H, Kato T, Uryu T, Fréchet JMJ. Chem. Mater. 1996;8:961. [Google Scholar]; Geib SJ, Vicent C, Fan E, Hamilton AD. Angew. Chem., Int. Ed. 1993;32:119. [Google Scholar]; Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N. Nature. 1993;366:324. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]; Yamauchi K, Lizotte JR, Long TE. Macromolecules. 2002;35:8745. [Google Scholar]
- 24.Paulusse JMJ, Sijbesma RP. Chem. Commun. 2003. p. 1494. For representative examples of metal-ligand coordination in supramolecular polymers, see:; Fraser CSA, Jennings MC, Puddephatt RJ. Chem. Commun. 2001. p. 1310.; Wu XF, Fraser CL. Macromolecules. 2000;33:4053. [Google Scholar]; Al-Hussein M, de Jeu WH, Lohmeijer BGG, Schubert US. Macromolecules. 2005;38:2832. [Google Scholar]; Hofmeier H, Hoogenboom R, Wouters MEL, Schubert US. J. Am. Chem. Soc. 2005;127:2913. doi: 10.1021/ja042919e. [DOI] [PubMed] [Google Scholar]; Carlise JR, Weck M. J. Polym. Sci. Pol. Chem. 2004;42:2973. [Google Scholar]; South CR, Higley MN, Leung KCF, Lanari D, Nelson A, Grubbs RH, Stoddart JF, Weck M. Chem.-Eur. J. 2006;12:3789. doi: 10.1002/chem.200501028. [DOI] [PubMed] [Google Scholar]; Knapton D, Iyer PK, Rowan SJ, Weder C. Macromolecules. 2006;39:4069. [Google Scholar]; Beck JB, Ineman JM, Rowan SJ. Macromolecules. 2005;38:5060. [Google Scholar]; Rowan SJ, Beck JB. Faraday Discuss. 2005;128:43. doi: 10.1039/b403135k. [DOI] [PubMed] [Google Scholar]; Tew GN, Aamer KA, Shunmugam R. Polymer. 2005;46:8440. [Google Scholar]; Calzia KJ, Tew GN. Macromolecules. 2002;35:6090. [Google Scholar]; Vermonden T, van der Gucht J, de Waard P, Marcelis ATM, Besseling NAM, Sudholter EJR, Fleer GJ, Stuart MAC. Macromolecules. 2003;36:7035. [Google Scholar]
- 25.Gong CG, Gibson HW. Angew. Chem., Int. Ed. 1997;36:2331. For representative examples of supramolecular polymers based on inclusion complexes, see: [Google Scholar]; Gong CG, Ji Q, Glass TE, Gibson HW. Macromolecules. 1997;30:4807. [Google Scholar]; Harada A, Li J, Kamachi M. Nature. 1993;364:516. [Google Scholar]; Kamitori S, Matsuzaka O, Kondo S, Muraoka S, Okuyama K, Noguchi K, Okada M, Harada A. Macromolecules. 2000;33:1500. [Google Scholar]; Kelch S, Caseri WR, Shelden RA, Suter UW, Wenz G, Keller B. Langmuir. 2000;16:5311. [Google Scholar]; Okumura H, Kawaguchi Y, Harada A. Macromolecules. 2003;36:6422. [Google Scholar]; Okumura Y, Ito K, Hayakawa R. Polym. Adv. Technol. 2000;11:815. [Google Scholar]; Ooya T, Eguchi M, Yui N. J. Am. Chem. Soc. 2003;125:13016. doi: 10.1021/ja034583z. [DOI] [PubMed] [Google Scholar]; Shigekawa H, Miyake K, Sumaoka J, Harada A, Komiyama M. J. Am. Chem. Soc. 2000;122:5411. doi: 10.1021/ja026224u. [DOI] [PubMed] [Google Scholar]; Yamaguchi I, Osakada K, Yamamoto T. J. Am. Chem. Soc. 1996;118:1811. [Google Scholar]; Yoshida K, Shimomura T, Ito K, Hayakawa R. Langmuir. 1999;15:910. [Google Scholar]
- 26.Pollino JM, Nair KP, Stubbs LP, Adams J, Weck M. Tetrahedron. 2004;60:7205. [Google Scholar]
- 27.Castellano RK, Clark R, Craig SL, Nuckolls C, Rebek J. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12418. doi: 10.1073/pnas.97.23.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Gennes P-G. Introduction to Polymer Dynamics. Cambridge Univ. Pr.: 1990. [Google Scholar]
- 29.de Gennes PG. Scaling Concepts in Polymer Physics. Cornell University Press; Ithaca, NY: 1979. [Google Scholar]
- 30.Fleer GJ, Cohen Stuart MA, Scheutjens JMHM, Cosgrove T, Vincent B. Polymers at Interfaces. Chapman and Hall; London: 1993. [Google Scholar]
- 31.Flory PJ. Faraday Discuss. Chem. Soc. 1974;57:19. [Google Scholar]
- 32.Rietveld MHP, Grove DM, van Koten G. New. J. Chem. 1997;21:751. [Google Scholar]
- 33.Rodriguez G, Albrecht M, Schoenmaker J, Ford A, Lutz M, Spek AL, van Koten G. J. Am. Chem. Soc. 2002;124:5127. doi: 10.1021/ja0177657. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht M, van Koten G. Angew. Chem., Int. Ed. 2001;40:3750. doi: 10.1002/1521-3773(20011015)40:20<3750::AID-ANIE3750>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Slagt MQ, van Zwieten DAP, Moerkerk AJCM, Gebbink RJMK, van Koten G. Coord. Chem. Rev. 2004;248:2275. [Google Scholar]
- 36.Yount WC, Juwarker H, Craig SL. J. Am. Chem. Soc. 2003;125:15302. doi: 10.1021/ja036709y. [DOI] [PubMed] [Google Scholar]
- 37.Yount WC, Loveless DM, Craig SL. Angew. Chem., Int. Ed. 2005;44:2746. doi: 10.1002/anie.200500026. [DOI] [PubMed] [Google Scholar]
- 38.Yount WC, Loveless DM, Craig SL. J. Am. Chem. Soc. 2005;127:14488. doi: 10.1021/ja054298a. [DOI] [PubMed] [Google Scholar]
- 39.Loveless DM, Jeon SL, Craig SL. Macromolecules. 2005;38:10171. [Google Scholar]
- 40.Green MS, Tobolsky AV. J. Chem. Phys. 1946;14:80. [Google Scholar]
- 41.Cates ME. Macromolecules. 1987;20:2289. [Google Scholar]
- 42.Cates ME, Candau SJ. J. Phys.: Condens. Matter. 1990;2:6869. [Google Scholar]
- 43.Jongschaap RJJ, Wientjes RHW, Duits MHG, Mellema J. Macromolecules. 2001;34:1031. [Google Scholar]
- 44.Lodge AS. Trans. Faraday Soc. 1956;52:120. [Google Scholar]
- 45.Tanaka F, Edwards SF. Macromolecules. 1992;25:1516. [Google Scholar]
- 46.Turner MS, Cates ME. J. Phys. II France. 1992;2:503. [Google Scholar]
- 47.Turner MS, Marques C, Cates ME. Langmuir. 1993;9:695. [Google Scholar]
- 48.Yamamoto M. J. Phys. Soc. Jpn. 1956;11:413. [Google Scholar]
- 49.Leibler LM, Rubinstein M, Colby RH. Macromolecules. 1991;24:4701. [Google Scholar]
- 50.Loveless DM, Abu-Lail NI, Kaholek M, Zauscher S, Craig SL. Angew. Chem., Int. Ed. in press. [DOI] [PMC free article] [PubMed]
- 51.Granville AM, Boyes SG, Akgun B, Foster MD, Brittain WJ. Macromolecules. 2004;37:2790. [Google Scholar]
- 52.Kaholek M, Lee W-K, LaMattina B, Caster KC, Zauscher S. Nano Lett. 2004;4:373. [Google Scholar]
- 53.Kaholek M, Lee W-K, LaMattina B, Caster KC, Zauscher S. Polym. Mat. Sci. Eng. 2004;90:226. [Google Scholar]
- 54.Kizhakkedathu JN, Norris-Jones R, Brooks DE. Macromolecules. 2004;37:734. [Google Scholar]
- 55.LeMieux MC, Minko S, Usov D, Shulha H, Stamm M, Tsukruk VV. Polym. Mat. Sci. Eng. 2004;90:372. [Google Scholar]
- 56.Minko S, Stamm M, Goreshnik E, Usov D, Sidorenko A. Polym. Mat. Sci. Eng. 2000;83:533. [Google Scholar]
- 57.Motornov M, Minko S, Eichhorn K-J, Nitschke M, Simon F, Stamm M. Langmuir. 2003;19:8077. [Google Scholar]
- 58.Fleer GJ, Scheutjens JMHM. J. Colloid. Interface Sci. 1986;111:504. [Google Scholar]
- 59.Jeppesen C, Wong JY, Kuhl TL, Israelachvili JN, Mullah N, Zalipsky S, Marques CM. Science. 2001;293:465. doi: 10.1126/science.293.5529.465. [DOI] [PubMed] [Google Scholar]
- 60.van der Gucht J, Besseling NAM, Fleer GJ. J. Chem. Phys. 2003;119:8175. [Google Scholar]
- 61.van der Gucht J, Bessling NAM, Stuart MAC. J. Am. Chem. Soc. 2002;124:6202. doi: 10.1021/ja0125422. [DOI] [PubMed] [Google Scholar]
- 62.Fogleman EA, Yount WC, Xu J, Craig SL. Angew. Chem., Int. Ed. 2002;41:4026. doi: 10.1002/1521-3773(20021104)41:21<4026::AID-ANIE4026>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 63.Xu J, Fogleman EA, Craig SL. Macromolecules. 2004;37:1863. [Google Scholar]
- 64.Kim J, Liu Y, Ahn SJ, Zauscher S, Karty JM, Yamanaka Y, Craig SL. Adv. Mater. 2005;17:1749. [Google Scholar]
- 65.Kersey FR, Lee G, Marszalek P, Craig SL. J. Am. Chem. Soc. 2004;126:3038. doi: 10.1021/ja0499501. [DOI] [PubMed] [Google Scholar]
- 66.Lehn J-M. Angew. Chem., Int. Ed. 1990;29:1304. [Google Scholar]
- 67.Hof F, Nuckolls C, Craig SL, Martin T, Rebek J., Jr. J. Am. Chem. Soc. 2000;122:10991. [Google Scholar]
- 68.Hirschberg J, Brunsveld L, Ramzi A, Vekemans J, Sijbesma RP, Meijer EW. Nature. 2000;407:167. doi: 10.1038/35025027. [DOI] [PubMed] [Google Scholar]
- 69.Beyer MK, Clausen–Schaumann H. Chem. Rev. 2005;105:2921. doi: 10.1021/cr030697h. [DOI] [PubMed] [Google Scholar]
- 70.Kersey FR, Craig SL. J. Am. Chem. Soc. 2006;128:3886. doi: 10.1021/ja058516b. [DOI] [PubMed] [Google Scholar]
- 71.Guan Z, Roland JT, Bai J, Ma S, McIntire T, Nguyen M. J. Am. Chem. Soc. 2004;126:2058. doi: 10.1021/ja039127p. [DOI] [PubMed] [Google Scholar]
- 72.Paulusse JMJ, Huijbers JPJ, Sijbesma RP. Chem. Eur. J. 2006;12:4928. doi: 10.1002/chem.200600120. [DOI] [PubMed] [Google Scholar]
- 73.Paulusse JMJ, Sijbesma RP. Angew. Chem., Int. Ed. 2004;43:4460. doi: 10.1002/anie.200460040. [DOI] [PubMed] [Google Scholar]
- 74.Zou S, Schonherr H, Vancso GJ. Angew. Chem., Int. Ed. 2005;44:956. doi: 10.1002/anie.200460963. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Y, Beck JB, Rowan SJ, Jamieson AM. Macromolecules. 2004;37:3529. [Google Scholar]
- 76.Brunsveld L, Folmer BJB, Meijer EW. MRS Bull. 2000;25:49. [Google Scholar]
- 77.Kato T, Kihara H, Kumar U, Uryu T, Fréchet JMJ. Angew. Chem., Int. Ed. 1994;33:1644. [Google Scholar]
- 78.Lange RFM, van Gurp M, Meijer EW. J. Polym. Sci., Part A, Polym. Chem. 1999;37:3657. [Google Scholar]
- 79.Loontjens T, Put J, Coussens B, Lange R, Palmen J, Sleijpen T, Plum B. Macromol. Symp. 2001;174:357. [Google Scholar]
- 80.Müller M, Dardin A, Seidel U, Balsamo V, Iván B, Spiess HW, Stadler R. Macromolecules. 1996;29:2577. [Google Scholar]
- 81.Park T, Zimmerman SC, Nakashima S. J. Am. Chem. Soc. 2005;127:6520. doi: 10.1021/ja050996j. [DOI] [PubMed] [Google Scholar]
- 82.Rieth LR, Eaton RF, Coates GW. Angew. Chem., Int. Ed. 2001;40:2153. doi: 10.1002/1521-3773(20010601)40:11<2153::AID-ANIE2153>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 83.Thilbault RJ, Hotchkiss PJ, Gray M, Rotello VM. J. Am. Chem. Soc. 2003;125:11249. doi: 10.1021/ja034868b. [DOI] [PubMed] [Google Scholar]
- 84.Vermonden T, van Steenbergen MJ, Besseling NAM, Marcelis ATM, Hennink WE, Sudhölter EJR, Cohen Stuart MA. J. Am. Chem. Soc. 2004;126:15802. doi: 10.1021/ja0458928. [DOI] [PubMed] [Google Scholar]
- 85.Bosman AW, Folmer BJB, Hirschberg JHKK, Keizer HM, Sijbesma RP, Meijer EW. Polym. Prepr. 2002;43:322. [Google Scholar]
- 86.Castellano RK, Rudkevich DM, Rebek J., Jr. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7132. doi: 10.1073/pnas.94.14.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dankers PYW, van Beek DJM, ten Cate AT, Sijbesma RP, Meijer EW. Polym. Mat. Sci. Eng. 2003;88:52. [Google Scholar]
- 88.Gohy J-F, Lohmeijer BGG, Varshney SK, Decamps B, Leroy E, Boileau S, Schubert US. Macromolecules. 2002;35:9748. [Google Scholar]
- 89.Gohy J-F, Lohmeijer BGG, Varshney SK, Schubert US. Macromolecules. 2002;35:7427. [Google Scholar]
- 90.Hilger C, Stadler R. Makromol. Chem. 1991;192:805. [Google Scholar]
- 91.Hirschberg JHKK, Ramzi A, Sijbesma RP, Meijer EW. Macromolecules. 2003;36:1429. [Google Scholar]
- 92.Hofmeier H, El-Ghayoury A, Schubert US. Polym. Prepr. 2003;44:711. [Google Scholar]
- 93.Hofmeier H, Gohy J-F, Schubert US. Polym. Mat. Sci. Eng. 2003;88:193. [Google Scholar]
- 94.Hofmeier H, Schmatloch S, Schubert US. Polym. Prepr. 2003;44:709. [Google Scholar]
- 95.Knapp R, Schott A, Rehahn M. Macromolecules. 1996;29:478. [Google Scholar]
- 96.Lohmeijer BGG, Schubert US. Angew. Chem., Int. Ed. 2002;41:3825. doi: 10.1002/1521-3773(20021018)41:20<3825::AID-ANIE3825>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 97.Meier MAR, Schubert US. Polym. Mat. Sci. Eng. 2003;88:443. [Google Scholar]
- 98.Sijbesma RP, Folmer BJB, Meijer EW. Polym. Prepr. 2002;43:375. [Google Scholar]
- 99.ten Cate AT, van Beek DJM, Spiering AJH, Dankers PYW, Sijbesma RP, Meijer EW. Polymer Preprints (American Chemical Society, Division of Polymer Chemistry) 2003;44:618. [Google Scholar]
- 100.Paulusse JMJ, Huijbers JPJ, Sijbesma RP. Macromolecules. 2005;38:6290. [Google Scholar]
- 101.Schmatloch S, Gonzalez MF, Schubert US. Macromol. Rapid Commun. 2002;23:957. [Google Scholar]
- 102.Schubert US, Eschbaumer C. Angew. Chem., Int. Ed. 2002;41:2892. doi: 10.1002/1521-3773(20020816)41:16<2892::AID-ANIE2892>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 103.Schubert US, Schmatloch S, Precup AA. Design. Mon. and Polym. 2002;5:211. [Google Scholar]