Abstract
Background
There is substantial current interest in the cognitive deficits associated with schizophrenia, particularly those in the realm of memory. Yet the exact nature of these deficits remains a matter of some debate. This study sought to examine performance on two distinct aspects of memory performance: familiarity-based and source-based memory processes.
Methods
Eighteen medicated outpatients with schizophrenia and eighteen healthy adult control subjects performed an external source memory task. Key measures included the ability to distinguish old (previously experienced) items from new items, the ability to correctly identify the source (male voice or female voice) of previously experienced items, and the reaction time associated with these responses.
Results
Patients with schizophrenia showed an impaired ability to distinguish old from new items, but intact performance in correctly identifying the source of items recognized as old. Whereas control subjects showed a rapid response to items deemed unfamiliar, particularly in rejecting novel items, these responses were slowed in patients with schizophrenia. This was not attributable to a generalized diminution in processing speed, as reaction times to correctly recognized old items (regardless of source accuracy) did not differ between the two groups.
Conclusions
Patients with schizophrenia demonstrated impaired familiarity-based and intact source-based memory performance. In addition, the reaction time for novelty detection, an important component of familiarity-based memory, was significantly delayed in patients compared to controls, while the response times for source-based decisions were completely overlapping. Considered together, these findings suggest a deficit in the familiarity-based aspect of episodic memory in at least some patients with schizophrenia.
Keywords: schizophrenia, memory, source memory, episodic memory, novelty detection, response time
1. Introduction
Schizophrenia is a complex neuropsychiatric syndrome affecting approximately 24 million people worldwide. Although schizophrenia is characterized most dramatically by positive symptoms such as hallucinations and delusions, the subtle cognitive deficits associated with this syndrome, particularly in the realm of verbal memory, may have a greater impact on overall functional outcome (Green, Kern et al. 2000). As currently available treatments have only a limited impact on memory performance, many patients remain functionally impaired despite adequate control of primary symptoms (Jobe and Harrow 2005). There is therefore substantial interest in meeting this therapeutic need through the development of cognitive enhancing approaches (both pharmacological and psychotherapeutic) for patients with schizophrenia. Improved definition of the memory impairment seen in this disorder represents an important first step along this path.
While there are several types of memory, most of the emphasis in schizophrenia research has been on memory for events or experiences, also known as episodic memory. Within episodic memory there is an important distinction between remembering whether something has occurred and recollecting the specific details of that event. The former task can be accomplished based on a feeling of familiarity, while the latter task requires the recollection of contextual information. For example, remembering whether one has heard a piece of news is a familiarity-based process, whereas remembering the source of that information, called source monitoring, is a context-dependent memory process.
Several lines of research suggest that these two processes are separable and perhaps independent (Yonelinas 2001; Dobbins, Rice et al. 2003). Familiarity-based memory decisions occur more rapidly and are best represented by a signal detection model (Yonelinas and Jacoby 1994; Hintzman, Caulton et al. 1998). That is, familiarity appears to be assessed along a continuum, with cut-off criteria used to determine whether to accept or reject any particular piece of information as being previously experienced. Recollection-based (or source-based) memory is a slower process, which in some models is posited to occur only after the familiarity assessment has been completed (Atkinson and Juola 1974). This process is often construed as a search through the memory banks, evaluating whether the information at hand matches contextual details stored from previous experience. Importantly, familiarity and source-based memory appear to be subserved by distinct neural substrates, as demonstrated by both event-related potentials (Curran 2000; Duzel, Vargha-Khadem et al. 2001; Duarte, Ranganath et al. 2004) and functional magnetic resonance imaging (Henson, Homberger et al. 2005; Yonelinas, Otten et al. 2005).
The degree to which these two processes are impaired in patients with schizophrenia remains a matter of some debate. Based largely on a series of papers indicating differentially greater deficits in context-dependent memory performance in patients with schizophrenia (Huron, Danion et al. 1995; Rizzo, Danion et al. 1996; Brebion, Smith et al. 1997; Vinogradov, WillisShore et al. 1997; Kazes, Berthet et al. 1999), many would now argue for a specific source memory deficit in this population. The literature is far from uniform on this issue, however. First, it appears that simple recognition memory is more impaired in patients with schizophrenia than previously appreciated (Pelletier, Achim et al. 2005). In addition, a number of recent papers (Moritz, Woodward et al. 2005; Moritz, Woodward et al. 2006; Ragland, McCarthy et al. 2006; Weiss, Goff et al. 2006), have failed to find a specific deficit in source-based memory in schizophrenia.
This study sought to tease apart the relative contribution of familiarity-based and source-based memory processes, and examine their neural correlates using functional magnetic resonance imaging. To do this, we adopted a standard source memory paradigm in which subjects heard words spoken by two different external sources (male and female) and after a brief delay were asked to remember which source said which word. In this manuscript we present a detailed analysis of the behavioral data (both response accuracy and reaction times) from this experiment. Our a priori hypotheses were that patients would show intact familiarity-based memory and impaired source memory. We also hypothesized that the reaction times associated with new-old detection would be equivalent between the two groups, while those associated with source discrimination would be slower in the patients with schizophrenia, representing difficulty with this cognitive process, the employment of an alternative cognitive strategy, a speed-accuracy trade-off, or some combination of these three explanations.
2. Methods and Materials
2.1 Subjects
Eighteen outpatients (12 males and 6 females) with DSM-IV-defined schizophrenia (confirmed by the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer et al. 1995)) were recruited from our affiliated clinic in Boston. Patients had chronic mental illness (mean duration of illness 15.6 ± 11.4 years). All but one of the patients were taking a stable dose of antipsychotic medication (fourteen were on second generation antipsychotics, two on conventional antipsychotics, and one on a combination of second generation and conventional), and were not withdrawn from their medication for the purposes of the study.
Eighteen age-matched subjects (11 males and 7 females), recruited from the Greater Boston area by posted advertisement, served as a comparison group. Comparison subjects were free of any Axis I psychiatric condition (as determined by the SCID) and were not taking psychotropic medication. Neither patients nor comparison subjects had a history of major medical or neurological illness (e.g., seizure disorder, head trauma leading to altered mental state, or stroke). No subject met DSM-IV criteria for alcohol or other substance use disorder (excepting nicotine dependence) within the past three months.
There were no significant between-group differences in age, parental socioeconomic status, or mean parental education (Table 1). When compared to the patients with schizophrenia, comparison subjects had a higher level of attained formal education, better socioeconomic status, and higher overall verbal IQ as estimated by the North American Adult Reading Test (NAART) (Blair and Spreen 1989).
Table 1.
Demographic and disease-related characteristics
| Schizophrenia n = 18 | Control n = 18 | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 42.5 | 9.9 | 43.2 | 8.7 |
| Education (years) | 13.3 | 2.5 | 15.6 | 2.8 |
| Parental Education (years) | 13.4 | 2.7 | 13.2 | 2.6 |
| Socioeconomic Status a | 3.7 | 1.3 | 1.9 | 0.8 |
| Parental Socioeconomic Status a | 2.7 | 1.1 | 2.3 | 1.1 |
| Verbal IQ b | 102.2 | 13.1 | 110.7 | 10.5 |
| Right handed c | 16/18 | -- | 17/18 | -- |
| SAPS-Total Score (0-170) | 16.2 | 16.0 | -- | -- |
| SANS-Total Score (0-125) | 34.9 | 21.0 | -- | -- |
| Atypical Antipsychotic | 15/18 | -- | -- | -- |
| Smokers d | 11/18 | -- | 1/18 | -- |
Hollingshead social strata: 1=major business professional to 5=unskilled laborer
Estimate based on North American Adult Reading Test (Blair and Spreen 1989)
As determined by an Edinburgh Handedness score >40 (Oldfield, 1971)
Any self-reported tobacco use
Prior to enrollment of subjects, the protocol was approved by the institutional review boards of both the Massachusetts General Hospital and the Commonwealth of Massachusetts Department of Mental Health. All participants provided written informed consent after a complete description of the study and administration of a brief questionnaire to ensure capacity to consent.
2.2 Procedure
The experimental paradigm was adapted from a previously published source monitoring experiment (Wilding 1999). The stimuli consisted of 312 English words, divided into four groups of 78 words for counterbalancing purposes. These word groups were matched on word length (mean=5.5, range=4-9 letters), lexical frequency (mean=51 per million) (Kucera and Francis 1967), printed familiarity (mean score=530) (Coltheart 1981), and concreteness (mean score=545) (Coltheart 1981), with p-values >.10 for all pair-wise comparisons. The words were then counterbalanced across four lists so that they rotated through each experimental condition: presented during encoding spoken by male and female voices, and presented as a new word during the testing session.
Audio files were created using Wave Creator, Version 3.1.0.46 (Blaze Audio). All 312 words were recorded by one male and one female research assistant, with the resulting audio files normalized to ensure standardized volume. Corresponding visual word stimuli were generated using Presentation Version 0.76 (Neurobehavioral Systems, Inc., California), in black 72-point Times New Roman font on a white background.
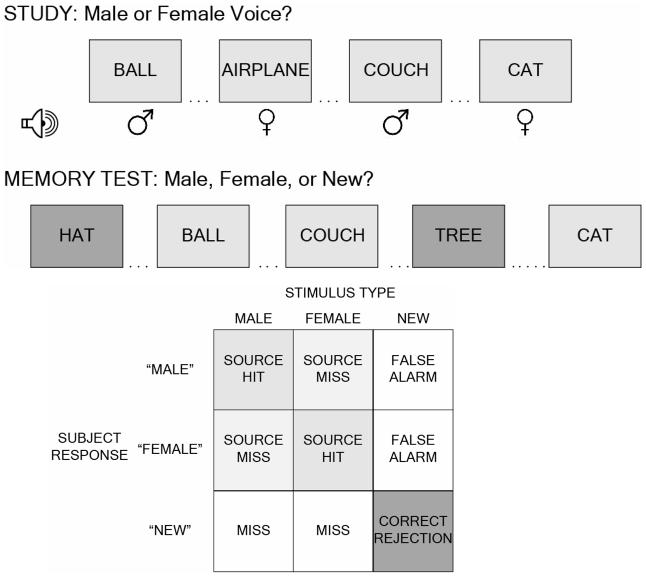
Prior to scan acquisition, each subject was given explicit instructions regarding the memory task, told that they would be tested on recollection of old words and the source of those words. They then performed a brief (six-word) sample experiment to show understanding of the instructions and the use of the button-box. The actual experimental session consisted of six interleaved encoding and testing sessions conducted while subjects were positioned in the MR scanner. During each encoding session, participants saw and heard 26 consecutive items (13 spoken by a male, 13 spoken by a female) and identified the gender of the voice using a keypad button. Words were rear-projected for 3000 msec onto a hemi-circular tangent screen and viewed through a mirror mounted on the head coil of the MR scanner. Each word was simultaneously presented aurally via pneumatic headphones at a clearly audible level using Presentation Version 0.80 (Neurobehavioral Systems, Inc., California). Immediately following this encoding session, participants were presented with a list containing both the 26 previously studied items and 26 new (not previously studied) items, and were asked to identify the original source of the voice (i.e., male, female, or new) (Figure 1). Words were presented one at a time visually for 3000 msec (with a 500 msec interstimulus interval), with subjects indicating their response (male, female, or new) by pressing one of three buttons on a keypad.
Figure 1.
Study design and analysis. (A) Schematic of the external source-monitoring paradigm employed. (B) Terminology used in describing events based on stimulus and response types
2.3 Statistical Analysis
Statistical analysis was performed using SPSS version 11.0 (SPSS Inc., Chicago). Accuracy data were examined by calculating standard measures of old-new recognition memory and source memory performance, with group means for these variables compared using an unpaired Student’s t-test. For old-new recognition accuracy, measures included the response accuracy for old items (percentage of old items labeled as either “male” or “female” -- hit rate), the incorrect characterization of new items as old (i.e., false alarm rate), and the difference between these values (i.e., hit rate minus false alarm rate, a.k.a. corrected recognition). Source memory accuracy was measured as the percentage of those items correctly identified as old that were correctly assigned to a source. For all analyses, items presented in either a male or female voice were combined, as neither hit rate nor source memory accuracy differed for these items in either group.
Reaction times (RTs) were recorded, trimmed to remove values less than 250 msec, and categorized by each of the five event types (source correct, source incorrect, source miss, correct rejection, and false alarm). RTs from each event type were pooled by group (control and schizophrenia) to create group-level RT distribution plots (Ratcliff and Murdock 1976; Leth-Steensen, Elbaz et al. 2000). As the resulting probability density distributions showed a pronounced right skew, the RT data were summarized by the median value, and between-group comparisons performed using the non-parametric median test (χ2 test for homogeneity of proportions), after applying the Yates Continuity Correction for 2×2 tables.
3. Results
Both groups demonstrated near-perfect performance on the gender identification task during encoding (control (mean accuracy ± standard deviation): 99.1 ± 1.5%, schizophrenia: 98.0 ± 2.5%; t=1.50, df=30, p=0.14) (Table 2). During the test, control subjects correctly identified a greater percentage of old items as old, by selecting either a “male” or “female” response (hit rate-control: 82.9 ± 14.9% vs. hit rate-schizophrenia: 70.5 ± 22.3%; t=1.98, df=34, p=0.056). There were no significant between-group differences in the response accuracy to new items (false alarm rate-control: 11.7 ± 18.0% vs. false alarm rate-schizophrenia: 15.5 ± 22.2%; t=-0.57, df=34, p=0.58). When considered together, the control subjects were significantly more accurate in old-new recognition (corrected recognition-control: 70.4 ± 19.9% vs. corrected recognition-schizophrenia: 55.4 ± 22.5%; t=2.12, df=34, p<0.05). However, when specifically examining the source accuracy of items deemed to be old (i.e., the percentage of correct source responses for old items labeled either “male” or “female”) there were no significant differences between the two groups (control: 77.5 ± 14.1% vs. schizophrenia: 73.3 ± 16.0%; t=0.83, df=34, p=0.41). Thus, once the between group differences in simple recognition are accounted for there was no evidence for a specific deficit in source memory.
Table 2.
Memory accuracy measures in schizophrenia and comparison subjects
| Schizophrenia n = 18 | Control n = 18 | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Encoding Accuracy | 98.0% | 2.5 | 99.1% | 1.5 |
| Hit Rate (Probability “Old”: Old item) a |
70.5%* | 22.3 | 82.9%* | 14.9 |
| False Alarm Rate (Probability “Old”: New item) |
15.5% | 22.2 | 11.7% | 18.0 |
| Corrected Recognition Rate (Hit Rate - False Alarm Rate) b |
55.4%** | 22.5 | 70.4%** | 19.9 |
| Source Accuracy (Probability Correct Source: “Old”) |
73.3% | 16.0 | 77.5% | 14.1 |
Independent samples Student’s t-test: t = 1.98, df=34, p=0.056
Independent samples Student’s t-test: t = 2.12, df=34, p<0.05
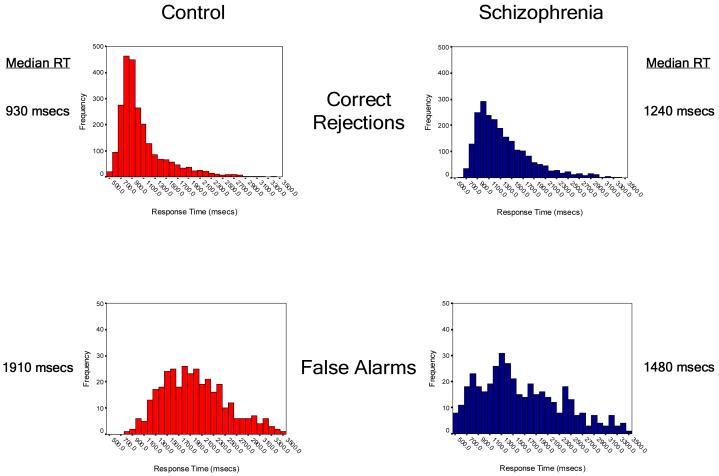
Reaction times for the 10,944 responses made during the experiment were split by group (control=5568 responses, schizophrenia=5376 responses) and then categorized by event type. The resulting probability distribution plots and summary statistics are shown in Figure 2. Control subjects were significantly faster than patients with schizophrenia in making a “new” response to new items (correct rejection: median RTs 930 msec vs. 1240 msec; χ2=502, df=1, p<0.0001), but were significantly slower when making an incorrect “old” response to these novel words (false alarm: 1910 msec vs. 1480 msec; χ2=36.2, df=1, p<0.0001). When considered together, the RT difference between correct rejections and false alarms was more than four-fold greater in control subjects when compared to patients with schizophrenia (980 msec vs. 240 msec).
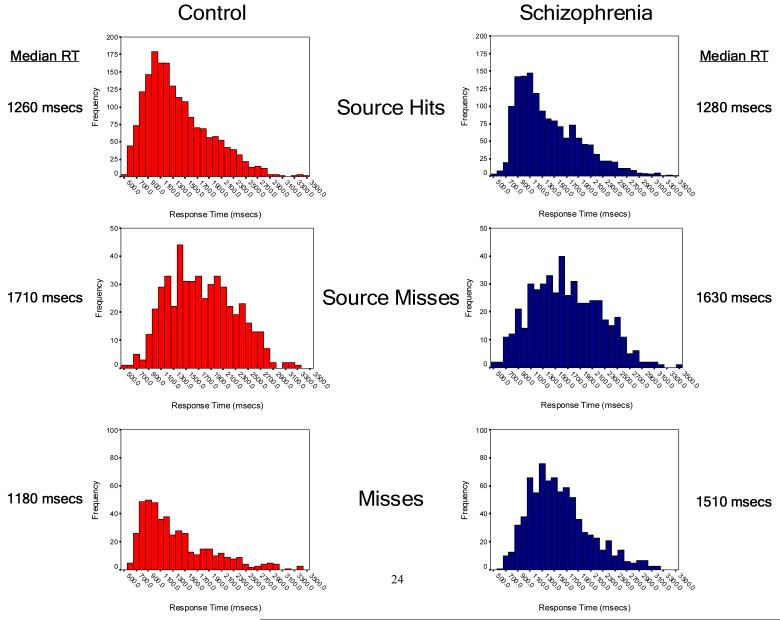
Figure 2.
Between-group comparisons of reaction times by event type. Histograms show the frequency of reaction times across the response window of 500msec to 3500msec following the onset of an event (display of a test word). The upper panel shows the reaction time profiles for responses to novel words, while the lower panel shows the reaction time profiles for words seen during the study period.
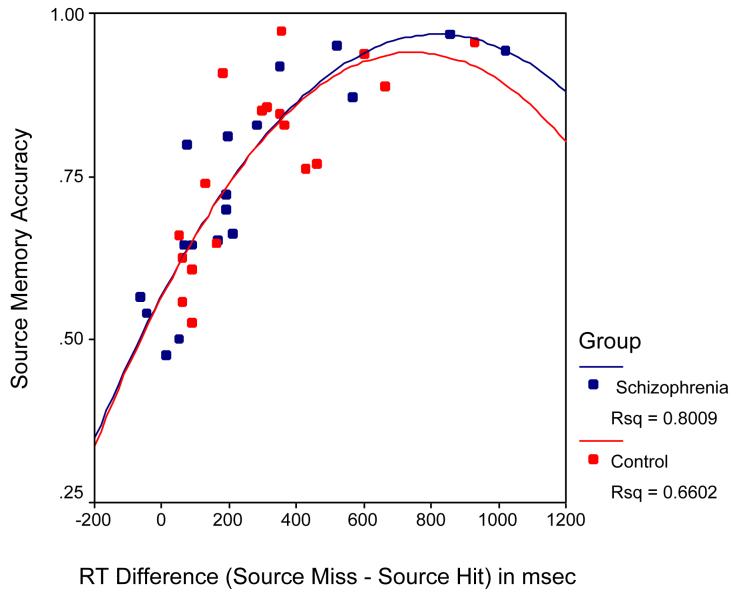
In responding to the old words previously seen and heard during the encoding period, control subjects were significantly faster in making an incorrect “new” response (miss: 1180 msec vs. 1510 msec; χ2=60.0, df=1, p<0.0001), but there were no between-group differences in RT when items were correctly identified as old, regardless of whether the source was identified correctly (source hit: 1260 msec vs. 1280 msec; χ2=0.61, df=1, p=0.44) or incorrectly (source miss: 1710 msec vs. 1630 msec; χ2=2.3, df=1, p=0.13). In addition, the RT difference between source hits and source misses was statistically equivalent when compared between groups. Interestingly, the degree of this RT separation between source hits and source misses was highly correlated with overall source accuracy at the individual subject level in both groups (Quadratic relationship, df=2,33; overall R-square = 0.74; omnibus F=47.6; p<0.0001) (Figure 3). Thus control subjects were significantly faster than patients with schizophrenia when making a “new” response, but showed an equivalent (or in the case of false alarms, slower) RT pattern to that of the patient group when making an “old” response.
Figure 3.
Relationship between source memory reaction time and source memory accuracy. Both groups showed faster reaction times to source hits when compared with source misses. At the individual subject level, the degree of reaction time difference between these two event types was highly correlated with overall source accuracy. As shown here, this second-order relationship was similar and significant in both groups.
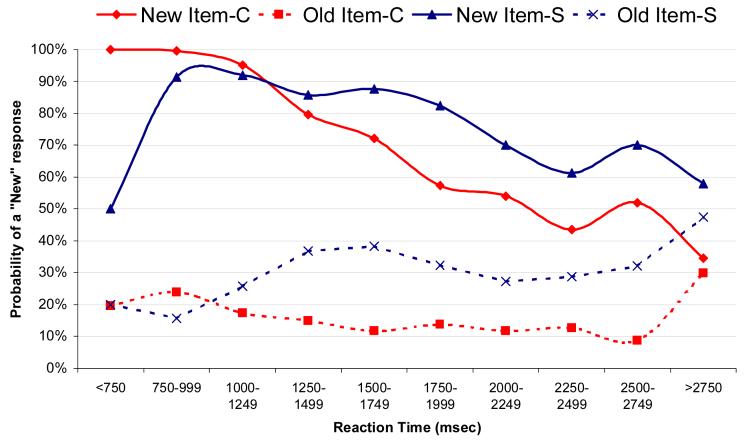
To follow up on this finding, we examined the response tendencies of both groups over the entire span of the 3500 msec response window (Figure 4). When a new item was presented, rapid responses (i.e., < 1000 msec) from the control group were highly accurate, with the proportion of accurate responses declining linearly with slower responses (increasing false alarm rate). Rapid responses to new items from the patients with schizophrenia tended to be inaccurate (50% false alarm rate) and actually showed an increase in accuracy with slower RTs. Responses to the old items followed similar patterns: to the extent that control subjects said “new” (i.e., forgotten item), these responses tended to be rapid, while patients with schizophrenia had a higher percentage of “new” responses to old items, and these occurred later in the response window.
Figure 4.
Modulation of response tendencies within the response window. Figure shows the likelihood of a “new” response as a function of the time at which the response occurred during the response window and the item type. When control subjects (red) make a rapid response (<1000 msec) to a new item they tend to say “new” (accurate response), with later responses becoming increasingly inaccurate (late false alarms). Rapid responses to new items made by the patients (blue), on the other hand, are highly inaccurate (50% false alarms) with their accuracy improving with slower responses. A similar pattern is seen with responses to old items: control subjects tend to make “new” responses early indicating a rapid realization that the item is unfamiliar, while patients show an increasing tendency to say “new” to an old item over the course of the response window.
Post-hoc bivariate correlations were performed to examine the relationship between psychopathology (as measured by the SAPS and SANS) and memory accuracy (as measured by corrected recognition and source accuracy). None of these correlations was significant at the 0.05 (two-tailed) level. Those patients experiencing some degree of hallucinatory activity (n=7 based on the SAPS Hallucinations Score) did have numerically poorer source memory accuracy than did non-hallucinating patients (n=11), but this comparison was not statistically significant (Source accuracy 78% vs. 66%, df = 16, t=1.58, p=0.13).
4. Discussion
There are two main findings from this study. First, patients with schizophrenia demonstrated impaired familiarity-based memory and intact source discrimination performance. Second, the reaction time for novelty detection, an important component of familiarity-based memory, was significantly delayed in patients compared to controls, while the response times for source-based decisions were completely overlapping. These findings, though in contrast with our a priori hypotheses, strongly suggest a primary deficit in the familiarity-based aspect of episodic memory in at least some patients with schizophrenia.
The performance deficit observed in this study is best summarized by an increase in errors of omission (rather than errors of commission) in the patients with schizophrenia. Patients were less able to distinguish old from new words, and in cases of ambiguity, tended to say “new” more often than control subjects; this combination led to fewer words correctly recognized as being old. When words were recognized as old, the patients showed an equivalent ability to correctly identify the source of that information.
These findings must be considered in the context of a larger body of literature on source memory in schizophrenia. To our knowledge this is the sixth study to specifically examine the ability of patients to distinguish between two external sources of information (Harvey 1985; Brebion, Smith et al. 1997; Keefe, Arnold et al. 1999; Keefe, Arnold et al. 2002; Woodward, Menon et al. In Press). Of these, only one (Keefe, Arnold et al. 1999), in a population of largely unmedicated patients, showed evidence of a deficit in external source memory. Several other studies under the source memory umbrella have tested the ability of patients to distinguish self-generated from examiner-generated information (internal vs. external) or to distinguish between words imagined versus actually said by the patient (internal vs. internal). This literature is also decidedly mixed, with at least seven previous papers failing to find a significant performance difference between patients with schizophrenia and control subjects (Harvey and Serper 1990; Morrison and Haddock 1997; Keefe, Arnold et al. 1999; Moritz, Woodward et al. 2005; Moritz and Woodward 2006; Moritz, Woodward et al. 2006; Ragland, McCarthy et al. 2006). Thus, after controlling for differences in simple recognition memory, several studies have not uncovered an additional deficit in source memory performance. This is consistent with a recent meta-analysis of the extant literature of memory and schizophrenia (84 papers) showing no significant difference between the effect sizes for recognition and associative (including source) memory (Pelletier, Achim et al. 2005).
The heterogeneous nature of schizophrenia over its longitudinal course may help to explain the apparent inconsistencies within the source memory literature. A number of studies have found correlations between the degree of positive symptoms (especially hallucinations) and errant source decisions (Harvey 1985; Bentall, Baker et al. 1991; Brebion, Smith et al. 1997; Keefe, Arnold et al. 1999; Brebion, Gorman et al. 2005). Though the underlying mechanism for this connection remains unclear, neuroimaging research has shown that hallucinations are associated with sensory modality-specific increases in cortical activity, which may compete with exogenous sensory input for neural processing (please see (Weiss and Heckers 1999) for review). The decreased cortical responsiveness to external input may impair adequate feature binding, a key component of source-based recollection (Schacter, Norman et al. 1998). In contradistinction, it appears that patients with prominent negative symptoms show a very different pattern of memory deficit, predominated by slowed processing speed and errors of omission (“impaired memory efficiency”) (Brebion, Gorman et al. 2005). In the present study the overall level of psychopathology was low, with patients exhibiting predominantly negative symptoms. Thus the intact source memory performance of these patients may therefore relate to their relatively low degree of positive symptoms.
The lack of source memory deficits may also be explained in part by the nature of the encoding task, which required subjects to focus explicitly on the source information to be remembered (gender of the speaker). There is some evidence from the memory and aging literature that suggests that this type of focus may alleviate source memory deficits in patients with frontal lobe impairment (see for example, (Glisky, Rubin et al. 2001)). Further research using specific experimental manipulation of this task would be required to determine whether this factor plays a beneficial role in patients with schizophrenia as well. In addition, the verbal nature of the stimuli may have also minimized between-group differences, as the effect sizes associated with verbal materials have been shown to be significantly smaller than those associated with figural materials in previous recognition memory studies conducted in schizophrenia (Aleman, Hijman et al. 1999; Pelletier, Achim et al. 2005). This is particularly true for studies of source memory, where the effect size (Cohen’s d) for studies using verbal materials is less than half that of studies using figural materials (0.48 vs. 1.09) (Pelletier, Achim et al. 2005).
The integration of response accuracy and reaction time patterns, an approach with a rich history within the cognitive psychology literature (see for example (Murdock and Dufty 1972; Sternberg 1975; Ratcliff and Murdock 1976)), provides us with an additional perspective on the overall response strategy employed by each group. In the present study, control subjects appear to approach the task in a manner highly consistent with classic dual-process models (Atkinson and Juola 1974). As shown in Figures 2 and 4, they begin the task by excluding items that do not seem familiar, beginning most rapidly with truly novel words (median RT=930 msec), followed shortly thereafter by unfamiliar old words (median RT=1180 msec). Items that do meet some familiarity threshold are then passed on to a source decision, where presumably some type of scan of stored experiences is commenced. Source hits quickly find their match, whereas responses to both source misses and false alarms are made only after some delay, presumably due to an extended search process. Note that very few items are ultimately called “new” after an extended search; the overwhelming majority of new responses come within the earliest period of the response window.
The response pattern is strikingly different in the patients with schizophrenia. The initial responses associated with unfamiliar items (correct rejections and misses) are significantly delayed when compared to the control group, with between group differences in median reaction times of 306 msec and 330 msec respectively. When considered as a whole, the serial nature of the decision process suggested by the pattern seen in the controls is not apparent in the patients’ data, as responses associated with familiarity assessment now temporally overlap with those related to source discrimination. Though admittedly speculative, there may be a blunting or absence of a rapid and robust “novelty signal” in patients with schizophrenia, requiring some patients to rely on alternative cognitive strategies to engage in the task. This explanation of the data fits well with a number of recent theories regarding the role of novelty in the impaired cognitive processing seen in schizophrenia (Arnold 1999; Lisman and Otmakhova 2001; Kapur, Mizrahi et al. 2005).
It is important to note that these findings were not simply a result of an overall slowing in processing speed within the patient group. The reaction times associated with source decisions (source hits and source misses) were nearly identical to (and not statistically different than) those seen in the control cohort. In addition, the reaction time associated with false alarms was actually faster than that seen in the controls. This latter result is particularly interesting, as it highly consistent with a body of evidence demonstrating enhanced confidence in errant responses (and reduced confidence in correct responses) in patients with schizophrenia (Moritz, Woodward et al. 2003; Moritz, Woodward et al. 2005; Moritz and Woodward 2006).
There are two key limitations of the study that deserve mention. First, all of the patients in this study were on psychotropic medication. As the second-generation neuroleptics appear to have a small, and positive, effect on long-term verbal memory (Thornton, Van Snellenberg et al. 2006), it is unlikely that the between-group differences in memory performance are attributable to a medication effect. We cannot rule out the possibility that antipsychotic medication normalized the source memory performance in the patient cohort, though as previously demonstrated by Keefe and colleagues (Keefe, Poe et al. 2003), antipsychotic-induced benefits do not appear to be selective for source memory, as improvement in familiarity-based memory is seen as well. Second, the sample size for this study, conducted as part of a neuroimaging experiment, was relatively small. We cannot therefore rule out the possibility of type-II error as an explanation for the lack of between-group differences in source memory accuracy or reaction time. We believe this is unlikely for two reasons. First, we did find between-group differences in familiarity-based memory performance. As the effect sizes in the schizophrenia literature for abnormalities in this type of memory tend to be smaller than those associated with recollection-based memory tasks (Aleman, Hijman et al. 1999), it would seem improbable to have adequate power to detect the former, but not the latter type of abnormality. Second, given the number of events entered into the group-level RT analyses, they have exquisite power to detect small differences, and consequently a very low likelihood of type II error.
These limitations notwithstanding, our results provide further evidence for a specific deficit in familiarity-based memory processing, at least within a subset of patients with schizophrenia.
Acknowledgements
The authors would like to thank Ms. Tali Ditman and Mr. Michael Zussman for assistance with the paradigm development, and Ms. Lindsay Jubelt and Ms. Kaila Norman for assistance with data acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony P. Weiss, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Donald C. Goff, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Margaret Duff, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Joshua L. Roffman, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA.
Daniel L. Schacter, Department of Psychology, Harvard University, Cambridge, MA, USA.
References
- Aleman A, Hijman R, et al. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156(9):1358–66. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, et al. Memory impairment in schizophrenia: A meta-analysis. American Journal of Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Arnold OH. Schizophrenia - A disturbance of signal interaction between the entorhinal cortex and the dentate gyrus? The contribution of experimental dibenamine psychosis to the pathogenesis of schizophrenia: A hypothesis. Neuropsychobiology. 1999;40(1):21–32. doi: 10.1159/000026593. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Juola JF, Krantz DH, Atkinson RC, Luce RD, Suppes P. Contemporary developments in mathematical psychology. Vol. 1. W.H. Freeman; San Francisco: 1974. Search and decision processes in recognition memory. [Google Scholar]
- Bentall RP, Baker GA, et al. Reality monitoring and psychotic hallucinations. Br J Clin Psychol. 1991;30:213–222. doi: 10.1111/j.2044-8260.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Brebion G, Gorman JM, et al. A model of verbal memory impairments in schizophrenia: two systems and their associations with underlying cognitive processes and clinical symptoms. Psychological Medicine. 2005;35:133–142. doi: 10.1017/s0033291704002879. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, et al. Clinical correlates of memory in schizophrenia differential links between depression, positive and negative symptoms, and two types of memory impairment. American Journal of Psychiatry. 1997;154(11):1538–1543. doi: 10.1176/ajp.154.11.1538. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, et al. Discrimination accuracy and decision biases in different types of reality monitoring in schizophrenia. Journal of Nervous & Mental Disease. 1997;185(4):247–53. doi: 10.1097/00005053-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Coltheart M. MRC Psycholinguistic database User manual. 1981 [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Memory & Cognition. 2000;28(6):923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, et al. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, et al. Dissociable neaural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain Research: Cognitive Brain Research. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Duzel E, Vargha-Khadem F, et al. Brain activity evidence for recognition without recollection after early hippocampal damage. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(14):8101–8106. doi: 10.1073/pnas.131205798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, et al. Stuctured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Glisky EL, Rubin SR, et al. Source memory in older adults: an encoding or retrieval problem? J Exp Psychol Learn Mem Cogn. 2001;27:1131–46. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ”right stuff“? Schizophrenia Bulletin. 2000;26(1):119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD. Reality monitoring in mania and schizophrenia: the association between thought disorder and performance. J Nerv Ment Dis. 1985;173:67–73. doi: 10.1097/00005053-198502000-00001. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Serper MR. Linguistic and cognitive failures in schizophrenia: a mulitvariate analysis. Journal of Nervous & Mental Disease. 1990;178:487–494. [PubMed] [Google Scholar]
- Henson RN, Homberger M, et al. Further dissociating the processes involved in recognition memory: and fmri study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Hintzman DL, Caulton DA, et al. Retrieval dynamics in recognition and list discrimination: further evidence of separate processes of familiarity and recall. Memory and Cognition. 1998;26:449–462. doi: 10.3758/bf03201155. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM, et al. Impairment of Recognition Memory with, but Not without, Conscious Recollection in Schizophrenia. American Journal of Psychiatry. 1995;152(12):1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- Jobe TH, Harrow M. Long-term outcome of patients with schizophrenia: a review. Canadian Journal of Psychiatry. 2005;50:892–900. doi: 10.1177/070674370505001403. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, et al. From dopamine to salience to psychosis - linking biology, pharmacology and phenomenology of psychosis. Schizophrenia Research. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kazes M, Berthet L, et al. Impairment of consciously controlled use of memory in schizophrenia. Neuropsychology. 1999;13(1):54–61. doi: 10.1037//0894-4105.13.1.54. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Poe MP, et al. Source monitoring improvement in patients with schizophrenia receiving antipsychotic medications. Psychopharmacology. 2003;169:383–389. doi: 10.1007/s00213-003-1476-0. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Arnold MC, et al. Source monitoring deficits in patients with schizophrenia; a multinomial modelling analysis. Psychological Medicine. 1999;29(4):903–914. doi: 10.1017/s0033291799008673. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Arnold MC, et al. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophrenia Research. 2002;57:51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis W. Computational Analysis of Present-Day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, et al. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11(5):551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS. The contribution of metamemory deficits to schizophrenia. Journal of Abnormal Psychology. 2006;15:15–25. doi: 10.1037/0021-843X.15.1.15. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, et al. Investigation of metamemory dysfunctions in first-episode schizophrenia. Schizophrenia Research. 2006;81:247–252. doi: 10.1016/j.schres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, et al. Source monitoring and memory confidence in schizophrenia. Psychological Medicine. 2003;33:131–139. doi: 10.1017/s0033291702006852. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, et al. Confidence in errors as a possible basis for delusions in schizophrenia. Journal of Nervous & Mental Disease. 2005;193:9–16. doi: 10.1097/01.nmd.0000149213.10692.00. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Haddock G. Cognitive factors in source monitoring and auditory hallucinations. Psychological Medicine. 1997;27:669–679. doi: 10.1017/s003329179700487x. [DOI] [PubMed] [Google Scholar]
- Murdock BB, Dufty PO. Strength theory and recognition memory. Journal of Experimental Psychology. 1972;94:284–290. [Google Scholar]
- Pelletier M, Achim AM, et al. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;74:233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Ragland JD, McCarthy E, et al. Levels-of-processing effect on internal source monitoring in schizophrenia. Psychological Medicine. 2006;36:641–648. doi: 10.1017/S0033291706007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Murdock BB. Retrieval processes in recognition memory. Psychological Review. 1976;83:190–214. [Google Scholar]
- Rizzo L, Danion JM, et al. Patients with schizophrenia remember that an event has occurred, but not when. British Journal of Psychiatry. 1996;168:427–431. doi: 10.1192/bjp.168.4.427. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, et al. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory scanning: New findings and current controversies. Quarterly Journal of Experimental Psychology. 1975;27:1–32. [Google Scholar]
- Thornton AE, Van Snellenberg JX, et al. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. Journal of Psychopharmacology. 2006;20:335–346. doi: 10.1177/0269881105057002. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, WillisShore J, et al. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. American Journal of Psychiatry. 1997;154(11):1530–1537. doi: 10.1176/ajp.154.11.1530. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Goff D, et al. Front-hippocampal function during temporal context monitoring in schizophrenia. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.06.025. In Press. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of hallucinations: a review of the literature. Psychiatry Research-Neuroimaging. 1999;92(23):61–74. doi: 10.1016/s0925-4927(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Wilding EL. Separating retrieval strategies from retrieval success: An event-related potential study of source monitoring. Neuropsychologia. 1999;37:441–454. doi: 10.1016/s0028-3932(98)00100-6. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Menon M, et al. Source monitoring biases and auditory hallucinations. Cognitive Neuropsychology. doi: 10.1080/13546800701307198. In Press. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. Dissociations of processes in recognition memory: effects of interference and of response speed. Canadian Journal of Experimental Psychology. 1994;48:516–535. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, et al. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]