Abstract
Following spinal cord injury (SCI), prolonged muscle spasms are readily triggered by brief sensory stimuli. Animal and indirect human studies have shown that a substantial portion of the depolarization of motoneurons during a muscle spasm comes from the activation of persistent inward currents (PICs). The brief (single pulse) sensory stimuli that trigger the PICs and muscle spasms in chronically spinalized animals evoke excitatory post-synaptic potentials (EPSPs) that are broadened to more than 500 ms, the duration of depolarization required to activate a PIC in the motoneuron. Thus, in humans, we investigated if post-synaptic potentials (PSPs) evoked from brief (<20 ms) sensory stimulation are changed after SCI and if they are broadened to ≥500 ms to more readily activate motoneuron PICs and muscle spasms. To estimate both the shape and duration of PSPs in human subjects we used peristimulus frequencygrams (PSFs), which are plots of the instantaneous firing frequency of tonically active single motor units that are time-locked to the occurrence of the sensory stimulus. PSFs in response to cutaneomuscular stimulation of the medial arch or toe of the foot, a sensory stimulus that readily triggers muscle spasms, were compared between non-injured control subjects and in spastic subjects with chronic (>1 year), incomplete SCI. In non-injured controls, a single shock or brief (<20 ms) train of cutaneomuscular stimulation produced PSFs consisting of a 300 ms increase in firing rate above baseline with an interposed period of reduced firing. Parallel intracellular experiments in motoneurons of adult rats revealed that a 300 ms EPSP with a fast intervening inhibitory PSP (IPSP) reproduced the PSF recorded in non-injured subjects. In contrast, the same brief sensory stimulation in subjects with chronic SCI produced PSFs of comparatively long duration (1200 ms) with no evidence for IPSP activation, as reflected by a lack of reduced firing rates after the onset of the PSF. Thus, unlike non-injured controls, the motoneurons of subjects with chronic SCI are activated by very long periods of pure depolarization from brief sensory activation. It is likely that these second-long EPSPs securely recruit slowly activating PICs in motoneurons that are known to mediate, in large part, the many seconds-long activation of motoneurons during involuntary muscle spasms.
Keywords: spasticity, motor unit, spinal cord injury, sensory processing, reflexes
Introduction
Involuntary muscle contractions, or spasms, are a key feature of the spasticity syndrome that develops in 65-78% of patients following injury to the spinal cord (Maynard et al., 1990; Biering-Sorensen et al., 2006). Studies in chronically spinalized animals have shown that the prolonged, involuntary activation of muscles during a spasm is due, in large part, to the uncontrolled activation of sodium and calcium persistent inward currents (PICs) in motoneurons located below the lesion (Eken et al., 1989; Li and Bennett, 2003, Heckman et al., 2005). In fact, the many seconds-long discharge of motoneurons produced from a brief cutaneous afferent stimulation is abolished when PICs are inactivated via hyperpolarization of the motoneuron. Importantly, PICs require depolarizations of ≥500 ms to fully activate (Li and Bennett, 2003; Li et al., 2004a; Moritz et al., 2007; see Discussion section), so prolonged depolarizing inputs are necessary to trigger PICs and spasms. The single-pulse cutaneous stimulation that triggers prolonged muscle spasms in chronic spinal animals activates an excitatory post-synaptic potential (EPSP) in the motoneuron that lasts for 500-1000 ms (Baker and Chandler, 1987; Bennett et al., 2001a; Li et al., 2004a). This NMDA-mediated EPSP (Bennett et al., 2001a), which is not present before injury, is capable of activating motoneuron PICs once they recover in the months following injury to produce the many seconds-long discharge of motoneurons during an involuntary muscle spasm.
Evidence for the depolarization of motoneurons from PIC activation during an involuntary muscle spasm has also been shown in patients with chronic spinal cord injury (SCI) (Gorassini et al., 2004; Nickolls et al., 2004). For instance, the amount of synaptic drive to a motoneuron during a muscle spasm, as reflected by the firing rate of a lower-threshold motor unit, is ∼50% lower than that required in recruiting the motoneuron at the onset of a muscle spasm. It is thought that the continued firing of the motoneuron at the lower level of synaptic input is possible due to the added depolarization provided by the motoneuron PIC (Gorassini et al., 2004). What remains to be shown in patients is whether the EPSPs evoked by brief, spasm-inducing sensory stimulation are sufficiently prolonged after SCI to more readily activate motoneuron PICs and muscle spasms.
The technique of peristimulus frequencygrams (PSFs) (Bessou et al., 1968) has recently been re-introduced for single motor unit recordings to estimate the amplitude and duration of sensory-evoked post-synaptic potentials (PSPs) in the motoneuron (Turker and Cheng, 1994). PSFs are plots of the instantaneous firing frequency of tonically active single motor units that are time-locked to the occurrence of the sensory stimulus. They provide an accurate measure of the falling phase of a PSP, to give its total duration, because the instantaneous frequency values can provide amplitude information concerning the underlying voltage trajectory of the motoneuron (Turker and Powers, 2005). By using PSFs we can measure if sensory inputs that readily trigger muscle spasms in subjects with chronic SCI are prolonged compared with similar stimuli evoked in non-injured controls. Specifically, we can estimate if the duration of a PSP is long enough (≥500 ms) to recruit slowly activating PICs that sustain motoneuron activity during a muscle spasm.
In this study, PSFs evoked from brief (<20 ms) cutaneo-muscular stimulation to the medial arch or second toe were compared in both non-injured and chronically injured subjects. Such stimuli were used as they readily evoke involuntary muscle spasms in patients with chronic SCI (Gorassini et al., 2004; Biering-Sorensen et al., 2006). Although PSFs avoid the count and synchronization errors associated with post-stimulus time histograms (PSTHs) in estimating the shape of PSPs (Turker and Powers, 2005), there are some limitations of PSF analysis. For example, factors such as accumulation of afterhyperpolarization (AHP) conductances and rate of change of membrane potential may skew the relationship between firing frequency and the underlying membrane potential at the soma to give a false indication of the PSP (see Turker and Powers, 2005 and Discussion section). Thus, in a set of parallel experiments performed in the sacral spinal cord of adult rats, we injected current profiles of varying shape into the soma of motoneurons to determine when firing rates deviated from the underlying membrane potential and to verify the shape of the PSP estimated from the human PSFs. Parts of this article have been published in abstract form (Norton et al., 2006).
Material and Methods
All human experiments were carried out with the signed, informed consent of the subject and all procedures were approved by the Health Research Ethics Board at the University of Alberta. All in vitro experiments were carried out in accordance with local animal care guidelines and with the approval of the University of Alberta Animal Welfare Committee.
Motor unit recordings in human subjects
Motor units were recorded from six neurologically intact control subjects and seven subjects with chronic incomplete SCI (iSCI) exhibiting involuntary muscle spasms (see Table 1 for Spasm frequency scores). Subjects with iSCI were chosen because they were able to maintain a steady background contraction that was required for the PSF technique. Both control and iSCI subjects were seated with their foot strapped to a footrest and their knee and ankle angles both at ∼120°. Two disposable cloth electrodes with full-surface solid adhesive hydrogel (3.3×2.2 cm2, Kendall Soft-E Mansfield, Massachusetts) were placed over the tibialis anterior (TA) muscle and were separated by at least 2 cm. An intramuscular electrode (described in Gorassini et al., 2002) was inserted in between the surface EMG electrodes to record single motor unit action potentials. Subjects were instructed to maintain a constant but weak contraction of their TA muscle with the aid of auditory feedback of the intramuscular EMG. Once steady firing of the motor unit was established, electrical stimulation (0.2 ms wide, 1-7 pulses, Table 2) using a constant current stimulator (Digitimer DS7A, Hertfordshire, UK) was applied either to the medial arch of the foot, using two small 2.2×2.2 cm2 cloth electrodes, or to the second toe, using custom-built cuff electrodes consisting of Tygon tubing slit open and sewn with silver wire. Such stimulation is likely to have activated cutaneous and muscle afferents directly, and possibly indirectly, due to a slight contraction of the adductor hallucis muscle. The strength of the applied stimulus, which was near painful threshold in controls but innocuous in iSCI subjects due to the injury, was increased until a stimulus locked reflex response was observable in the rectified and smoothed surface EMG signal (controls: 16-69 mA; iSCI: 35-99 mA, not significantly different at P = 0.8; Table 2). Approximately 300 stimuli were given per trial in control subjects; 30-100 stimuli were given per trial in iSCI subjects with rest periods every 2 min.
Table 1.
Injury details for subjects with iSCI
| Subject code | Years post injury | ASIA score | Level of injury | Spasm freq. score | Baclofen (mg/day) | LEM | LT | PP |
|---|---|---|---|---|---|---|---|---|
| P1 | 5 | ASIA C | C5-C6 | 3 | 30 | 8 | 0 | 0 |
| P2 | 3 | ASIA C | C3-C5 | 2 | 120 | 20 | 2 | 2 |
| P3 | 23 | ASIA C | C5-C6 | 3 | - | 18 | 1 | 0 |
| P4 | 2 | ASIA C | T2-T4 | 3 | 100 | 22 | 1 | 1 |
| P5 | 4 | ASIA D | T7-T10 | 2 | 40 | 29 | 2 | 2 |
| P6 | 3.5 | ASIA D | C1-C3 | 1 | 40 | 40 | 2 | 2 |
| P7 | 3.5 | ASIA C | C3, C6 | 2 | 30 | 18 | 0 | 0 |
C = cervical, T = thoracic Spasm frequency score: 0 = no spasms, 1 = one or fewer spasms per day, 2 = between 1 and 5 spasms per day, 3 = 5 to <10 spasms per day, 4 = 10 or more spasms per day, or continuous contraction. LEM = lower extemity muscle score for the tested leg is based on a 0 - to 5-point manual muscle strength score for eight muscle groups (hip, knee and ankle flexors/extensors, hip abduction/adduction) with a maximum score of 40. LT = light touch and PP = pin prick for the area of skin over the medial malleolus just above the medial arch with 0 = absent, 1 = impaired and 2 = normal. Muscle and sensation scores are given for the upper extremity and hand in subject P2.
Table 2.
Summary of the properties of the PSF in control and iSCI subjects
| Subject (n) | Units (n) | Onset latency (ms) | Latency of inhibition (ms) | PSF/PSTH duration (ms) | Mean rate (Hz) | Current (mA) | Pulsesa (subjects) |
|---|---|---|---|---|---|---|---|
| Control (6) | 9 | 47±11 | 74±13 | 301±27 272±43 |
9.8±1.9 | 42±22 | 1 pulse (6) 3 at 300 Hz (1) 7 at 500 Hz (1) |
| iSCI (5) | 10 (all) | 61±27 | - | 1167±224 1051±292 |
6.1±2.0 | 44±19 | |
| iSCI (3) | 4 (vol) | 43±9 | - | 1096±166 1052±212 |
8.3±1.2 | 57±23 | 1 pulse (1) 3 at 300 Hz (2) |
| iSCI (4) | 6 (spont) | 72±28 | - | 1206±251 1049±434 |
4.5±0.9 | 36±13 | 1 pulse (3) 3 at 300 Hz (2) |
In the column ‘Units’, separate averages are given for all units recorded in iSCI subjects (all), units recorded during voluntary activation (vol) and units recorded during spontaneous unit discharge (spont). Onset latency is the time from stimulation to the first increase in firing rate of the motor unit in the PSF. Latency of inhibition is the time from stimulation to the first pause or reduction in motor unit firing after the stimulation. PSF and PSTH duration is the time to peak of the respective CUSUMs from the initial increase above pre-stimulus background. Mean rate is the background discharge rate of the unit before peripheral nerve stimulation.
Pulses indicates the number and frequency of the stimulation applied in (n) number of subjects. Two control subjects received a single pulse or train stimulation on different experiment days. All values are reported as means±SD.
Intramuscular and surface EMG signals were fed to an isolated, high-impedance amplifier (Intronix Technologies Corp., Bolton, ON, Canada). EMG signals were amplified by 5000 and high-pass filtered at 200 Hz for intramuscular EMG and band-pass filtered between 20 and 2.5 kHz for surface EMG. All signals were digitized at a sampling rate of 20 kHz using AxoScope hardware and software, Sunnyvale, California. Data were analysed off-line using custom cluster-cutting software (GetSpike, S.N. Baker, University of Newcastle upon Tyne, UK) and spike discrimination software (Clampfit, Axon Instruments or Spike3, Cambridge Electronic Design, Cambridge, UK). Once all units were selected for a single trial, waveforms were superimposed to compare the shape of each potential to ensure that the same waveform was analysed throughout a given trial.
Motor unit analysis: PSFs, PSTHs and related cumulative sums
Analysis was performed using custom-written scripts in MATLAB (Mathworks, Natick, MA, USA). To construct PSFs, the instantaneous firing rate of motor units were calculated and plotted against the time of occurrence of the stimulation. The instantaneous frequency value was placed at the end of the interspike interval where the frequency value was calculated as per Bessou et al., 1968 and Turker and Powers, 1999. The PSF was a collection of these single spike frequency occurrences at single points in time. Placing the frequency value at the end of the interspike interval in the motoneuron simulation work produced a PSF that best followed the trajectory of the simulated PSP because it best represented when a change in membrane potential occurred. The mean firing rate of the PSF was calculated by performing a running window average (every 10 ms). The PSTH was also calculated for the spike train using time bins that were 2 ms for experiments having ≥300 stimulation trials (non-injured controls) and 100 ms in width for experiments having ≤30 stimulation trials (iSCI) (Perkel et al., 1967). For the PSF and PSTH, the cumulative sum (CUSUM) of these measures was calculated by integrating both the PSF and PSTH (Ellaway, 1978). The estimated duration of the PSP in the motoneuron as a result of the cutaneomuscular stimulation was measured by examining both the duration of time the mean rate of the PSF was above baseline and the time to peak of the PSF CUSUM (Turker and Powers, 1999).
Intracellular recordings of sacral motoneurons in adult rats
Intracellular recordings in current clamp were made in vitro from motoneurons residing below the S2 transection in the whole sacrocaudal spinal cord of chronically injured (≥2 months), spastic rats (n = 14). A description of the intracellular recordings can be found elsewhere (Bennett et al., 2001b; Li and Bennett, 2003; Li et al., 2004a), hence only details specific to these experiments are described. However, it is important to note that the whole spinal cord from L4 and below (which was above the S2 lesion) was removed to limit any acute effects and to leave intact all dendrites of the recorded motoneurons. Antidromic stimulation of the fourth sacral (S4) and first caudal (Ca1) ventral roots was used to identify motoneurons. Only motoneurons with a stable penetration, resting potential below -60 mV, antidromic spike overshoot over 0 mV, and reliable repetitive firing were included in the study. Data were collected using an Axoclamp2b intracellular amplifier (Axon Instruments, Sunnyvale, California) running in discontinuous current-clamp mode (DCC, switching rate 7-10 kHz, output bandwidth 3.0 kHz). Motoneuron recordings were made in artificial cerebral spinal fluid composed of (in millimolars) 122 NaCl, 24 NaHCO3, 3 KCl, 2.5 CaCl2, 1 MgSO4 and 12 d-glucose mixed in distilled water (osmolarity of 298 mOsm) and saturated with 95% O2-5% CO2 to maintain a pH of 7.4.
Once a stable cell body penetration was established via a sharp microelectrode, a depolarizing current bias was applied to produce tonic firing in the motoneuron, typically at a frequency of 8-10 Hz. Various current injection profiles were then superimposed on this tonic, depolarizing current bias to reproduce the PSFs obtained from motor unit recordings in human subjects. Upwards of 30 trials were recorded for each current injection profile with a maximum of three different profiles tested in each motoneuron. Similar to the human motor unit data, PSFs from the multiple current injections were constructed and were aligned to the onset of the first ascending phase of the current injection profile. To compare the PSF to the underlying changes in mean membrane potential, or simulated PSP produced by the injected current, a hyperpolarizing current bias was applied to the cell to inactivate both spiking and voltage-dependent PICs. The PSF was then superimposed on the simulated (hyperpolarized) PSP to determine if firing rates of the motoneuron faithfully reflected the profile of the simulated PSP. In addition, we were able to determine which simulated PSP profile produced changes in the firing rate of the motoneuron that most closely resembled the PSF profiles recorded from human motor units.
Statistics
The PSF duration measured from each subject was averaged together for both the non-injured control and SCI groups. In some cases, two PSFs from different motor units were measured from the same subject when the units were recorded on different experiment days. When there were two PSF trials for a given motor unit, measurements from the two trials were averaged together. Data are shown as means±SD. Comparisons of PSF and motoneuron data were made using non-paired Student’s t-tests for data determined to be normally distributed using a Kolmogorov—Smirnov test. Regression analysis and the corresponding coefficient of determination (r2) was used to determine if the background firing rate of the motor unit could account for the differences in the measured duration of the PSF. Statistical significance was set to 0.95 (P≤0.05).
Results
PSFs in non-injured control subjects
Brief (<20 ms) electrical stimulation to the medial arch or toe of the foot of non-injured control subjects produced a cutaneomuscular reflex response in the TA muscle with modulations in EMG (Fig. 1) similar to that described previously (Gibbs et al., 1995; Nielsen et al., 1997). The PSF, which was used to estimate the underlying PSP in the motoneuron (Fig. 1, bottom graph), displayed an increase in firing rate above baseline from 70 to 370 ms after the stimulation, with an interposed cluster of firing rates that were near or slightly below the mean rate. Correspondingly, the firing probability of the motor unit (PSTH: middle graph) was also modulated for ∼300 ms after the stimulation but not always in the same direction as the firing rate in the PSF. For example, the second cluster of increased firing probability that followed the brief pause in firing (at second dotted vertical line) occurred when the firing rate of the unit was close to or slightly below the mean firing rate. In addition, the reduced firing probability that immediately followed this second cluster (just before the third dotted vertical line) was surprisingly associated with an increased firing rate of the unit. Likely, this second period of decreased firing probability is an artefact of the PSTH technique (Turker and Powers, 2005), whereas the increase in firing rate of the PSF during this period is a more accurate reflection of the underlying PSP in the motoneuron as shown in the ‘Simulated PSPs in motoneurons’ section.
Fig. 1.
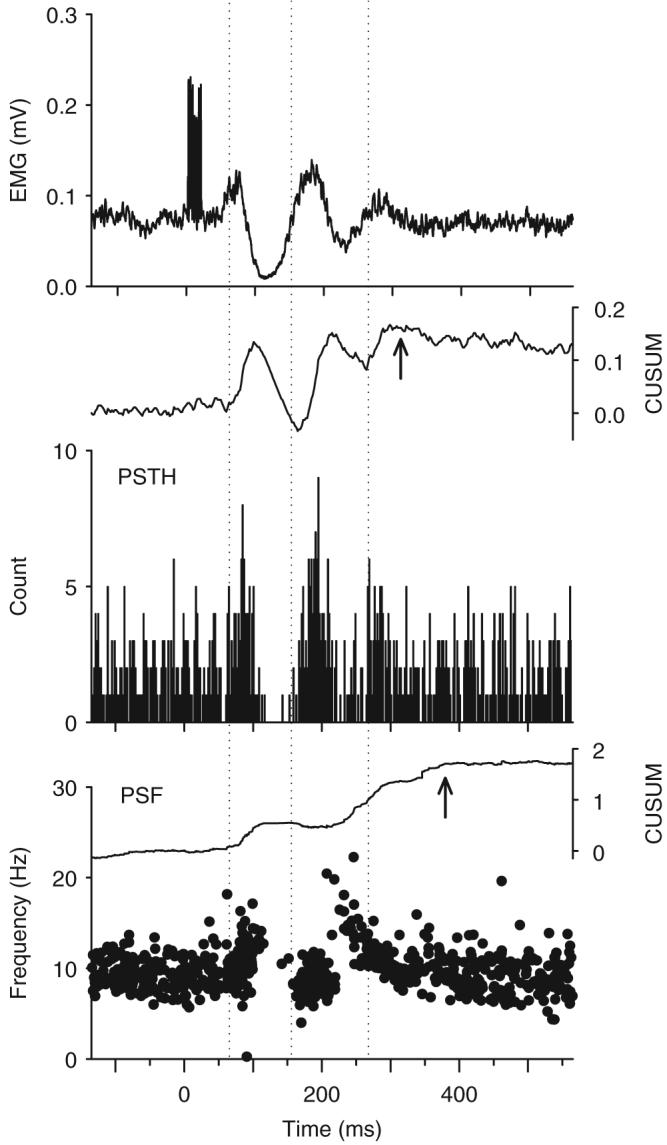
Example of a cutaneomuscular reflex response recorded in a non-injured control subject. Stimulation to the medial arch of the foot (7 pulses at 500 Hz, 16 mA) produced a polyphasic modulation in the rectified TA EMG (top trace) beginning at a latency of 70 ms (stimulation occurred at time 0). Likewise, a simultaneously recorded motor unit exhibited three main clusters of increased firing probability (PSTH: middle graph) at 70, 162 and 272 ms after the stimulation (marked by the dotted vertical lines). The firing rate of the unit, as depicted in the PSF (bottom graph), was above the mean rate for a period of 322 ms, as reflected in the time to reach the peak in the PSF CUSUM (at arrow in PSF CUSUM) from its initial increase. The peak of the PSTH CUSUM occurred earlier (at arrow in PSTH CUSUM), even though the firing rate was still above the mean pre-stimulation rate. Bin width of PSTH=2 ms. N=300 trials. CUSUM values are plotted as extra counts (PSTH) or frequencies (PSF) above background divided by the number of stimuli.
In addition to estimating the shape of the underlying PSP from the PSF, it was also possible to estimate its total duration from the CUSUM of the PSF and PSTH (upper graphs). The time from the initial increase in the PSF CUSUM (near the first dotted vertical line in Fig. 1) to its peak value (at arrow) was 322 ms in this subject and corresponded to the duration of time the mean rate was above pre-stimulus, baseline values. The time to reach the peak value of the PSTH CUSUM from its initial increase was shorter at 252 ms, even though the firing rate of the unit was still above the mean pre-stimulation rate. Because of this, the PSF and its CUSUM were used to estimate the duration of the underlying PSP in the motoneuron.
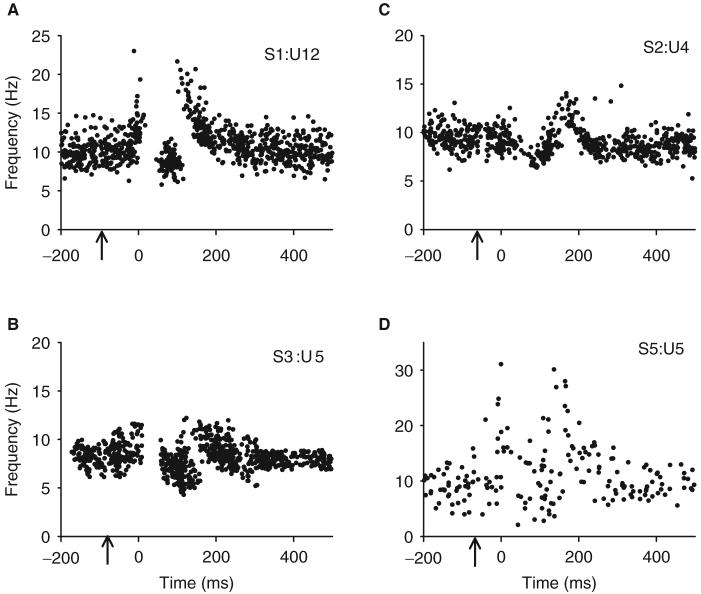
The general shape and duration of the PSFs in response to brief cutaneomuscular stimulation were very similar across the six control subjects regardless of the amplitude or number of stimulation pulses used (maximum duration <20 ms, Table 2). Figure 2 shows examples of four different PSFs recorded in four different subjects. In all cases, the shape of the PSF consisted of a 300 ms period of increased firing above baseline with an interposed series of firing rates that were close to or below the mean firing rate. As shown subsequently, a 300 ms EPSP with a brief intervening IPSP likely produced this characteristic PSF. Although the exact shape of the PSF varied from subject to subject, the overall duration of the PSF was very consistent at 301±27 ms (n = 9 units from six subjects), as measured from the PSF and its CUSUM. A consistent duration of the PSF occurred despite the fact that there were different levels of pre-stimulus background firing rates (Fig. 3), indicating that a transient activation of a PIC did not contribute to the total duration of the PSF (see ‘Discussion’ section). The onset latency of the pause in motor unit firing was also fairly consistent at 74±13 ms after the stimulation. Table 2 summarizes the various features of the PSFs.
Fig. 2.
(A-D) Examples of four different PSFs recorded from four different non-injured control subjects. Arrows indicate time of stimulation. Initial rising edge of PSF is aligned at time 0. Although the shapes of the PSF profiles vary between subjects, the duration of time that the firing rate was above the pre-stimulation mean rate was very consistent (301±27 ms, n=9 units from six subjects). S(n)=subject code; U(n)=motor unit code.
Fig. 3.
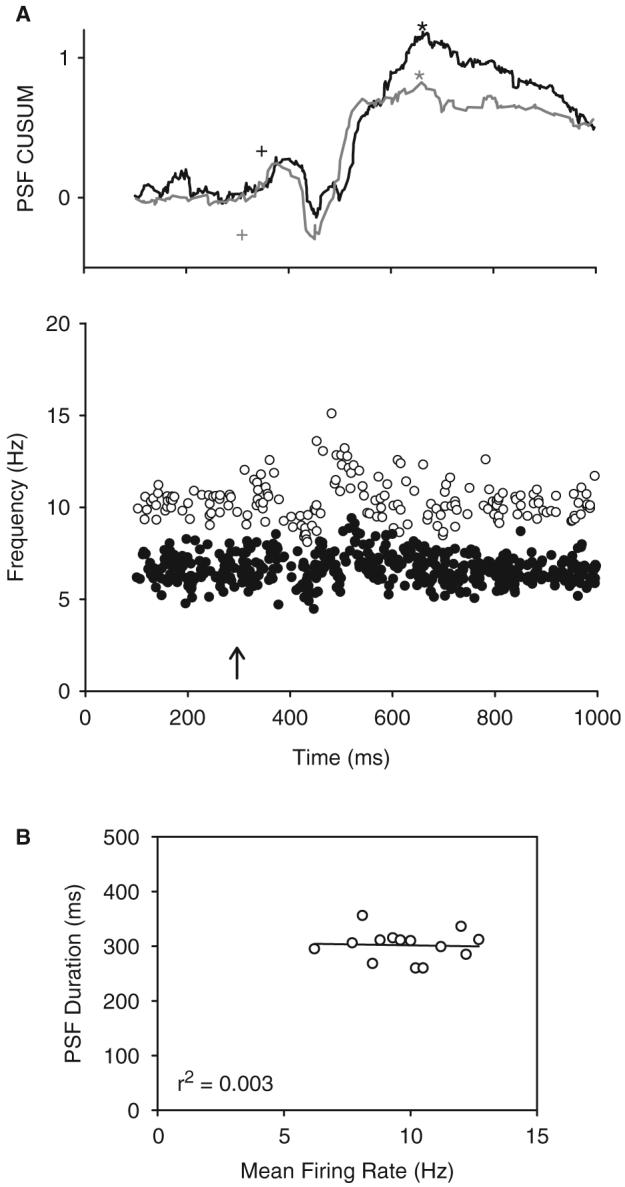
The effect of background firing rate on the PSF. (A) PSFs (bottom graph) and associated CUSUMs (top graph) from a single motor unit firing at a low (6.6 Hz: solid circles and black lines) and high (10.2 Hz: open circles and grey lines) pre-stimulus mean rate. Note that time to the peak of the CUSUM (asterisk) was similar for both trials with the onset of the rise in the PSF occurring at 76 ms during slow firing (black cross) and 40 ms during fast firing (grey cross). (B) When comparing all 13 units from the six non-injured control subjects (multiple PSFs from the same unit were not averaged here), there was no relationship between mean pre-stimulation firing rate and PSF duration. r2=coefficient of determination.
Simulated PSPs in motoneurons
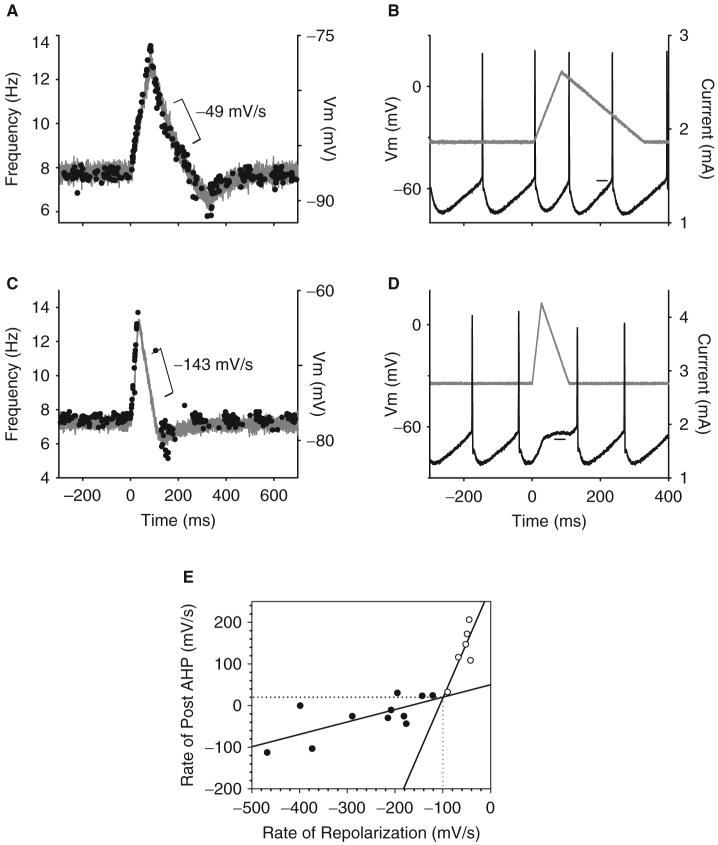
To determine the shape of the PSP that produced the PSF in control subjects, different profiles of current were injected into the soma of adult motoneurons to simulate different profiles of PSPs and their corresponding PSFs (see ‘Material and methods’ section). We first investigated if the pause and reduced firing rates that occurred shortly after the initial acceleration in the PSF was mediated by a fast repolarization of the membrane potential (e.g. IPSP), or, if it was a result of the accumulation of AHP conductances that can occur when a motoneuron accelerates its rate above the mean rate (see ‘Discussion’ section for details). As shown in Fig. 4A, when the repolarization phase of the simulated PSP (grey trace) decreased at a rate that was slower than -100 mV/s after a prior depolarization, there was no pause in firing of the motoneuron during this period (tested in six cells, n = 6 rats, noted by open symbols in Fig. 4E). It was only when the rate of repolarization was faster than -100 mV/s (Fig. 4C) that the mean interspike interval was broadened to produce a pause in firing during cell repolarization (tested in 11 cells from 11 rats, closed symbols in Fig. 4E). Such increases in interspike interval were produced because the trajectory of the membrane potential following the AHP (Fig. 4D) was flattened during the fast repolarizing current to prevent a spike from occurring (horizontal line marks period of post-AHP flattening). Flattening of the membrane potential after the AHP did not occur during a slow repolarizing current injection (horizontal line in Fig. 4B), so the firing rate of the motoneuron closely followed the trajectory of the simulated PSP (Fig. 4A). The relationship between the rate of repolarization of the simulated PSP and its effect on the membrane potential trajectory after the AHP is plotted in Fig. 4E for all cells and reveals that a pause in firing occurred when the rate of rise of the post-AHP membrane potential was slowed to 20.1 mV/s (see horizontal dashed line) by rates of repolarization that were -101 mV/s or faster (see vertical dashed line; details in Fig. 4 legend).
Fig. 4.
In vitro intracellular recordings of an adult rat motoneuron in response to injected current. (A) Triangular current injection with a slow speed of repolarization (grey trace in B) produced a slow decrease in membrane potential (-49 mV/s) recorded during cell hyper-polarization (simulated PSP: grey trace). The firing frequency of the motoneuron (PSF) closely followed the profile of the simulated PSP, even during the repolarization phase. (B) Corresponding example of membrane potential during cell firing reveals that the post-AHP trajectory (horizontal line) is not flattened during the period of cell repolarization and, thus, no pause in cell firing occurred. (C) In the same cell, a fast rate of repolarization (-143 mV/s) produced a pause in motoneuron firing as a result of flattening the trajectory of the membrane potential after the AHP during the period of fast repolarization (horizontal line in D) to broaden the interspike interval. (E) Relationship between the rate of decrease of the simulated PSP during cell repolarization (as shown in A and C) and the slope of the post-AHP membrane potential (as shown in B and D). Open symbols denote trials where no pause in firing was produced (as in A) withan average repolarization slope of -57±18 mV/s and a post-AHP slope of 130±60 mV/s; closed symbols denote when a pause in motoneuron firing occurred (as in C), with an average repolarization slope of -252±114 mV/s and post-AHP slope of 25±48 mV/s (corresponding values significantly different, P<0.001). Intersection of regression lines fit for no-pause and pause data occurs at 20.1 mV/s on y-axis and -101 mV/s on x-axis (see dotted lines).
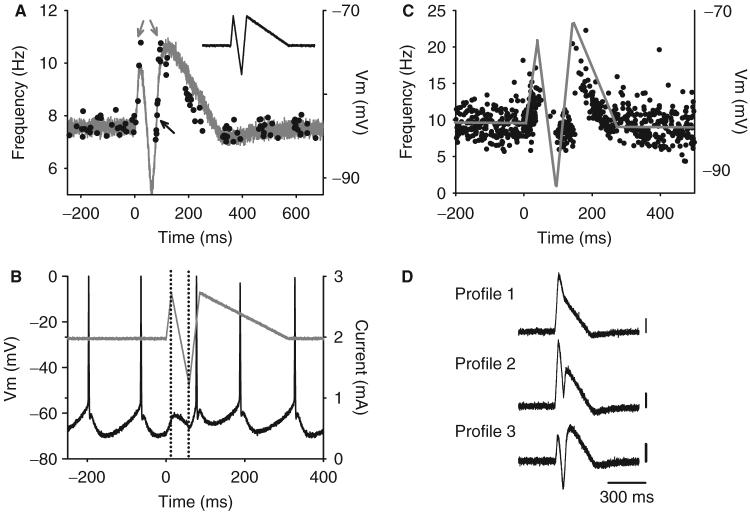
We next determined if the complex PSF recorded in control subjects was produced by a 300 ms EPSP with an intervening IPSP containing a deceleration in mean membrane potential of at least -100 mV/s. Such a simulated PSP as this (Profile 3 in Fig. 5D) reproduced the PSF recorded in control subjects (n = 7 cells from seven rats). As shown earlier, the initial increase in firing rate during the first depolarizing phase of the simulated PSP (grey trace in Fig. 5A) was followed by a pause in firing of the motoneuron during a fast repolarization because the membrane potential after the AHP was deflected downwards to prevent the cell from reaching spiking threshold (between dotted vertical lines in Fig. 5B). Shortly after the current and ensuing membrane potential began to increase again, the motoneuron resumed firing to produce rates that were close to the mean background rate (black arrow in Fig. 5A). Following this, the firing rates reached a second peak that was similar to the rates reached at the onset of the PSF (grey arrows), which then subsequently decreased in parallel to the slow repolarizing trajectory of the simulated PSP. Figure 5C displays a PSF profile from a control subject and the estimated PSP (grey line) that likely produced it based on the motoneuron simulation, i.e. a broad 300 ms EPSP with a brief intervening IPSP. Features of the PSF from motor unit recordings and motoneuron simulations are summarized in Table 3.
Fig. 5.
Simulated PSP that replicates PSF recorded in non-injured controls. Same format as in Fig. 4. Firing response (A) and corresponding membrane potential (B) of rat motoneuron to a simulated PSP profile containing a 300 ms EPSP with a fast (-440 mV/s) intervening IPSP. Note in B that the pause in motoneuron firing is due to a downward deflection of the membrane potential-during the hyperpolarizing current injection (marked by dotted vertical lines). In A, the firing rate of the motoneuron follows the trajectory of the slow repolarization of the simulated PSP (-59 mV/s in A; -37.7±13.3 mV/s in all seven cells). (C) PSF profile of non-injured control subject and the estimated PSP (grey trace) that likely produced it based on the intracellular data in A.(D) Different profiles of simulated PSPs used to replicate the human PSF data. Calibration bar=10mV.
Table 3.
Comparison between components of the PSFs recorded in rat motoneurons and the motor unit PSFs recorded in non-injured control subjects
| Mean background rate (Hz) | Post-pause rate(Hz) | Initial peak rate (Hz) | Second peak rate (Hz) | |
|---|---|---|---|---|
| Motoneuron PSF | 7.5±1.7 | 8.0±1.7 | 10.1±2.4 | 10.7±2.2 |
| Human motor unit PSF | 9.8±1.9 | 9.6±2.0 | 18.7±7.6 | 19.5±7.4 |
PSFs in subjects with iSCI
Cutaneomuscular reflex responses evoked in the TA muscle of subjects with iSCI were very different from non-injured controls, even though similar electrical stimulation was used in both groups (Table 2). As shown for a representative subject in Fig. 6, a PSF lasting near 1 s, with no intervening pauses or reduced firing rates, was produced after stimulation to the medial arch of the foot at an intensity that was below the threshold to evoke an involuntary muscle spasm. Relatively few stimulation trials (<30) were needed to produce a well-modulated PSF and PSF CUSUM. The rectified EMG and PSTH also displayed a similar duration of increased activity/counts; however, decreases in EMG and firing probability that occurred immediately after the initial increase (from 200 to 300 ms) occurred when firing rates in the PSF were well above background.
Fig. 6.
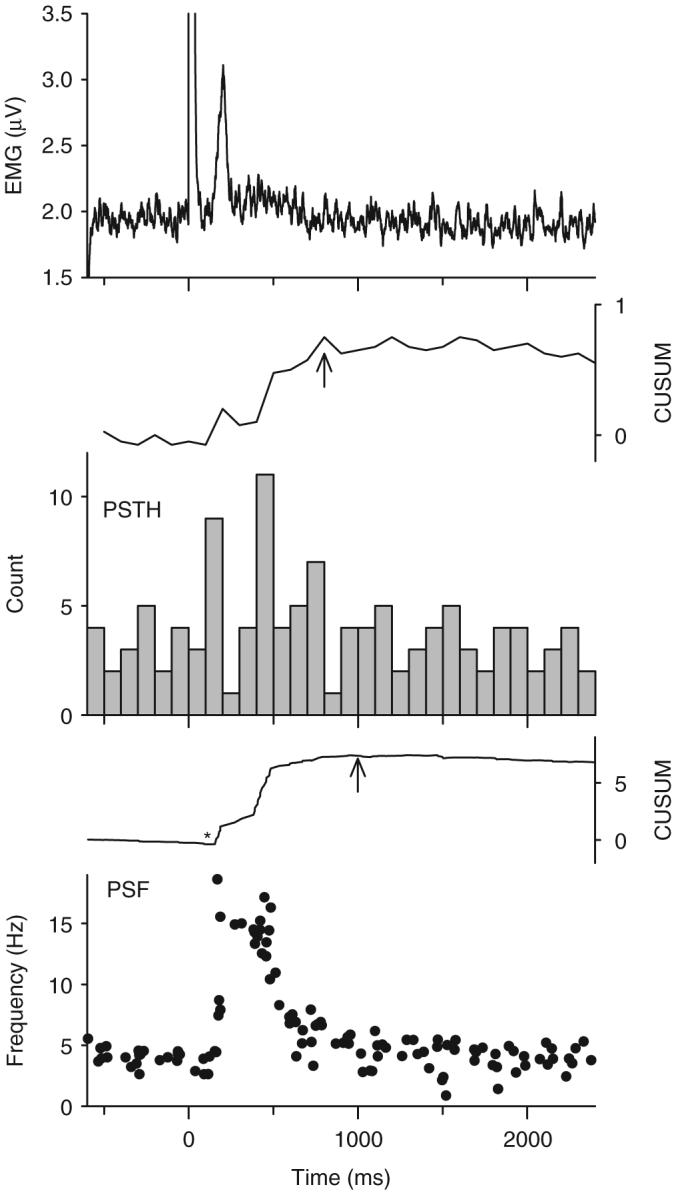
Example of a cutaneomuscular reflex response in iSCI subject P5. Same format as in Fig. 1. Stimulation to the medial arch of the foot (3 pulses, 100 Hz, 35 mA at time 0) produced a long-duration increase in mean firing rate of the unit as shown in the PSF (bottom graph), which was initiated 130 ms after the stimulation (asterisk in PSF CUSUM). The duration of the PSF, as measured by the time to peak of the PSF CUSUM (at arrow), was 850 ms. The rectified EMG (top graph) showed a similar duration of modulation. The PSTH (middle graph) showed less striking modulation as the PSF with a decrease in firing probability immediately following the first increase (near 200 ms) when rates in the PSF were high. Bin width of the PSTH=100 ms. N=25 trials.
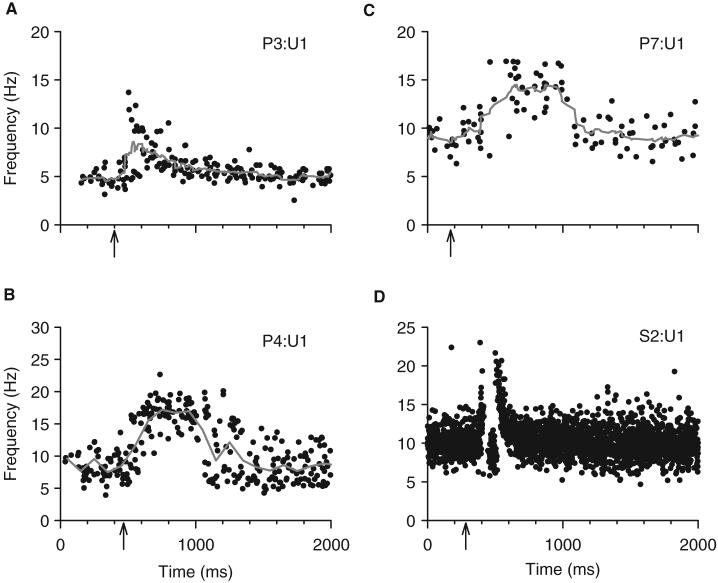
A similar PSF was produced in all seven incomplete SCI subjects tested as shown in Fig. 7 that displays the activity profiles for three representative TA motor units from three different subjects. A striking finding in all subjects was the complete lack of evidence for IPSP activation (e.g. pauses in firing). Also, there was a prolonged duration of the PSF compared with that in non-injured controls (see Fig. 7D for comparison). The average duration of the PSF, as determined from the PSF and its CUSUM, was 1167±224 ms (n = 10 TA units in five iSCI subjects, Table 2). The duration of the change in the PSTH gave similar values (1051±292 ms) but tended to give a false indication of IPSP activation (reduced firing probability after the initial increase), likely as a result synchronization of motoneuron firing. Similar long PSFs were recorded from the hamstrings and first flexor digitorum muscles in two other iSCI subjects in response to thigh and digit stimulation, respectively (data not shown).
Fig. 7.
Examples of different PSF profiles recorded from three iSCI subjects during spontaneous (A) and voluntary (B and C) motor unit activation. The grey lines show the mean PSF for each unit. Arrows mark the time of stimulation. (D) A PSF from a non-injured control subject is plotted on the same time scale for comparison. P(n)=iSCI subject code; U(n)=motor unit code.
In Fig. 7B and C, the background firing rate of the units was produced during a voluntary background contraction, whereas the PSF in Fig. 7A was obtained during spontaneous (involuntary) unit activity with mean firing rates that were lower (4.5±0.9 Hz) than the rates produced during voluntary muscle contractions (8.3±1.2 Hz, P<0.001, Table 2). The PSF measured from units that were activated during a voluntary contraction were slightly shorter in duration (1096±166 ms) compared with spontaneously activated units (1206±251 ms), but the difference was not statistically significant (Fig. 8). Moreover, there was no relationship between background firing rate and PSF duration when examining all units (r2 = 0.01). Overall, the PSF in iSCI subjects was ∼4 times longer in duration than the PSF in non-injured control subjects (non-injured control values are re-plotted in Fig. 8 for direct comparison, closed circles, P<0.00001), even for PSFs measured at matched levels of background firing rate (<10 Hz).
Fig. 8.
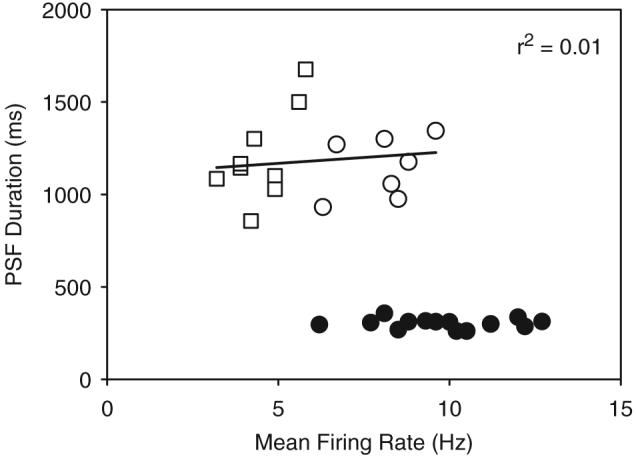
Relationship between the mean background firing of a motor unit recorded before cutaneomuscular stimulation (x-axis) and the duration of the PSF (y-axis) in subjects with iSCI: voluntary unit activity=open circles; spontaneous unit activity=open squares; comparative data from non-injured controls=closed circles. The regression line that fits through all iSCI data points gave a coefficient of variation (r2) of 0.01 (not significant).
EPSPs in chronically injured spastic rats
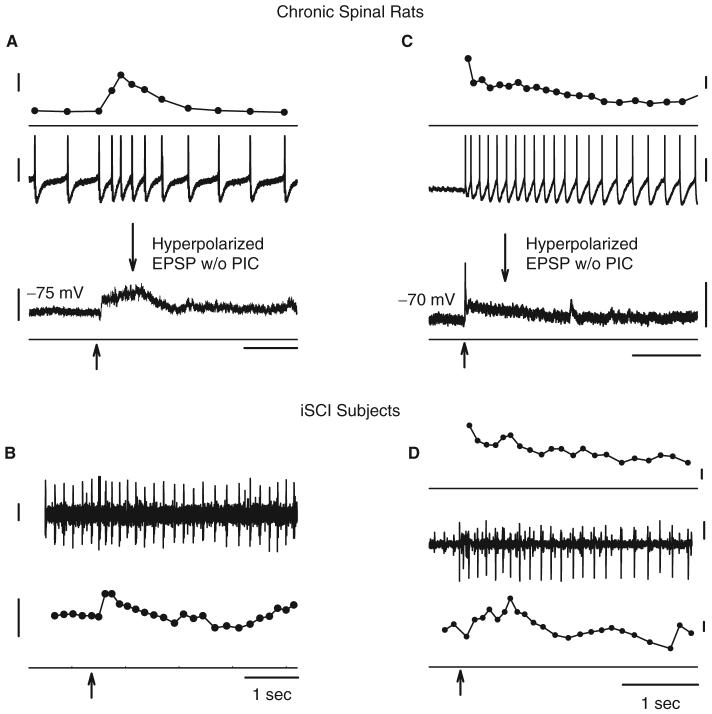
Similar to the estimated EPSPs measured from the PSFs in iSCI subjects, long-duration EPSPs were recorded in motoneurons of chronically spinalized rats in response to single-shock sensory stimulation (Fig. 9A). The profile of the EPSP during reflex activation of the motoneuron was revealed by hyperpolarizing the motoneuron to inactivate the voltage-dependent PICs (see bottom trace of Fig. 9A). Similar to the PSFs recorded in human subjects, the EPSP recorded in chronically injured rats had very long durations, lasting 960±270 ms (Li et al., 2004a). Likewise, there was no evidence of IPSP activation in contrast to the inhibition of reflex activity recorded in intact, non-lesioned rats (Bennett et al., 2004). When the motoneuron was made to tonically fire by a constant depolarizing current injection (middle trace, Fig. 9A), the firing rate of the unit in response to the dorsal root stimulation (top trace) clearly reflected the underlying EPSP profile. To compare, Fig. 9B shows a similar firing frequency response of a tonically firing motor unit to a single cutaneomuscular stimulation in an iSCI subject.
Fig. 9.
Recordings from rat motoneurons (A and C) and human motor units (B and D) during single-shock sensory stimulation (at arrows). (A) Low threshold (2× sensory threshold) stimulation to dorsal root of chronic (2 months) spinal rat produces a 1-s-long increase in firing rate of the tonically firing motoneuron (firing rate top trace; membrane potential middle trace) as a result of a sensory-evoked EPSP revealed by hyperpolarizing the motoneuron (bottom trace). (B) A similar change in firing rate of a tonically firing motor unit is shown for a single stimulation trial in iSCI subject P6 (intramuscular EMG top trace; firing rate bottom trace). (C) Prolonged activation of a motoneuron recruited by a 1s-long EPSP (see hyperpolarized response in bottom trace) due to activation of PIC. (D) Newly recruited motor unit continues to discharge in iSCI subject P3 (surface EMG, middle trace) after firing rate in a tonically firing motor unit returns to pre-recruitment level (bottom trace), an indication of PIC activation. The firing response of the newly recruited unit is shown in the top trace. Calibration bars: firing rate 2 Hz; membrane potential 20 mV; EMG 100 μV; time 1s.
Following SCI, PSPs evoked from brief sensory stimuli should be long enough (>500 ms) to activate PICs in the motoneuron. Therefore, a motoneuron that is recruited by a 1-s-long EPSP should continue to fire in a self-sustained manner following a brief sensory stimulation due to the activation of a PIC. An example of this is shown in Fig. 9C where a single-shock stimulation to the dorsal root recruited the motoneuron to prolonged discharge (top and middle traces) by a 1-s-long EPSP that was revealed during cell hyperpolarization (bottom trace). The continued firing of the motoneuron following the EPSP is indicative of the activation of a PIC. Likewise, in iSCI subjects when the stimulation current was increased to induce a small muscle spasm, motor units that were recruited by the stimulation continued to fire for many seconds after the stimulation (Fig. 9D), potentially as a result of PIC activation (see ‘Discussion’ section).
Discussion
In this study, PSFs from tonically discharging single motor units revealed that brief (<20 ms) cutaneomuscular stimulation in subjects with chronic SCI produced a depolarization of the motoneuron that lasted for ∼1 s. In contrast, a similar stimulus in non-injured control subjects produced an EPSP lasting only 300 ms that was interrupted by a fast IPSP. This data is in agreement with previously published reports of inhibitory reflexes converting to facilitatory reflexes following chronic SCI (Jones and Yang, 1994; Crone et al., 2003; Xia and Rymer, 2005). We discuss how the processing of sensory reflex inputs by the spinal cord changes after injury to make motoneurons more susceptible to prolonged discharge during an involuntary muscle spasm.
Estimation of PSPs from PSFs
The PSF may not always faithfully reflect the profile of the underlying PSP given the effects that firing history may have on the discharge response of a motoneuron to a superimposed PSP (Turker and Powers, 2005). For instance, when a tonically firing motoneuron rapidly accelerates its discharge, the AHP conductance during these shortened interspike intervals may not decay to the same extent that occurs during the longer tonic firing intervals, resulting in an accumulation of AHP conductances that may artificially lengthen the interspike interval following the firing acceleration (Turker and Powers, 1999). Such AHP accumulation could explain the pause and subsequent reduced firing rates that followed the initial rate acceleration in the PSFs recorded in non-injured control subjects. However, when we removed PSF trials that contained an early acceleration in discharge at the onset of the PSF, the pause in motor unit firing and the cluster of firing rates that were slightly below the mean firing rate remained (data not shown). In addition, in simulated PSPs with slow repolarizations (< -100 mV/s), a pause in firing did not occur even though the firing rate of the motoneuron accelerated well above the mean background rate and indicates that the accumulation of AHP conductances was not pronounced enough to broaden subsequent interspike intervals. These findings, and the data from simulated PSPs with fast (> -100 mV/s) repolarizations, support the conclusion that the intermittent pause and reduced firing rates in the PSFs of non-injured controls were produced from a fast IPSP activated immediately after the onset of the PSF. Specifically, it was only when the velocity of the membrane potential following the AHP was reduced to <20 mV/s by a simulated fast repolarization that a pause in firing occurred (Fig. 4E). This finding is in agreement with recent studies in adult rat and cultured mouse motoneurons demonstrating that the rate of increase in membrane potential of a motoneuron must reach 20 mV/s or more before an action potential is triggered (Harvey et al., 2006; Kuo et al., 2006).
In addition to history-dependent effects on motoneuron discharge, transient activation of non-synaptic inputs, such as voltage-dependent PICs in the motoneuron, could also contribute to the shape and duration of the PSF that was modulated above background. To control for this, we measured the duration of the PSF modulation during different levels of voluntary motor unit discharge where one may expect PICs to be differentially activated, i.e. partially activated during low firing rates and fully activated during high firing rates (Elbasiouny et al., 2006). In addition, the slow but very regular spontaneous unit discharge that develops after chronic SCI is mediated from the regenerative activation of a sub-threshold, sodium-mediated PIC (NaPIC: Gorassini et al., 2004; Li et al., 2004a). Thus, background firing during spontaneous unit activity may occur with partial CaPIC activation, and, similar to the case of low background firing during voluntary contractions, modulation of the PSF during spontaneous unit firing may be a reflection of both synaptic reflex activation of the motoneuron and a transient activation of a PIC. However, in both non-injured and iSCI subjects, the duration of the PSF was not affected by the background firing rate of the motor unit (Figs 3 and 8; Turker and Powers, 1999). Even during low tonic motor unit discharge (5-7 Hz), PICs were likely to be fully activated or active in a stable manner so that further PIC currents were not recruited during the superimposed sensory activation of the motoneuron.
In summary, the PSF appears to be an accurate representation of the underlying PSP in contrast to the PSTH (and EMG), which is influenced by the synchronization of motoneuron firing following a rapid excitation. For example, at the onset of an excitatory stimulus that entrained the firing of the motor unit, a period of decreased firing probability followed, likely because of the refractory period of the motoneuron (e.g. Figs. 1 and 6). However, in the few trials where the motor unit discharged during this period, the firing rate was well above the mean rate, likely because the underlying PSP was well above the mean, pre-stimulus level (reviewed in Turker and Powers 2005). Thus, unlike the PSTH, the PSF provides information concerning the amplitude of the PSP given that the firing rate is linearly related to amplitude of the membrane potential at the motoneuron soma (e.g. Fig. 9 and Li et al., 2004a).
Mechanisms mediating the complex PSPs in non-injured controls
Cutaneomuscular reflexes similar to the ones evoked in non-injured controls in this study are likely mediated by both segmental and transcortical reflex pathways (Jenner and Stephens, 1982; Gibbs et al., 1995; Nielsen et al., 1997). PSF latencies in this study were consistent with both a segmental activation of spinal reflex pathways (E1 latencies ranging from 46 to 63 ms), especially during high background discharge rates (e.g., Fig. 3), and a transcortical activation of spinal reflex pathways (E2 latencies ranging from 61 to 82 ms, Gibbs et al., 1995). Thus, the 300 ms long depolarization of the motoneuron recorded in non-injured control subjects could have resulted from a temporally dispersed activation of the motoneuron from segmental, and then descending, synaptic inputs. In addition, activation of voltage-dependent receptors on upstream interneurons that possess a small degree of persistence, such as the NMDA or AMPA receptor (Edmonds et al., 1995), may also have contributed to the 300 ms depolarization of the motoneuron following a brief sensory stimulation.
Similar to motoneuron excitation, the inhibitory component of the PSF in non-injured controls could have resulted from segmental and/or descending activation of spinal inhibitory interneurons. As discussed subsequently, the inhibitory component of the cutaneomuscular reflex is abolished immediately after SCI (Bennett et al., 2004). This may occur either because segmental afferent inputs can no longer activate spinal inhibitory interneurons due to a lack of facilitation from descending inputs or because of the removal of direct activation of spinal inhibitory interneurons from descending inputs activated via transcortical reflex pathways. In non-injured control subjects, the average latency of the pause or deceleration in unit firing following cutaneomuscular stimulation was 74±13 ms (Table 2), which provides enough time for descending inputs to activate spinal inhibitory interneurons (Nielsen et al., 1997). Further studies are required to determine the origin of activation of the inhibitory component of the cutaneomuscular reflex.
Mechanisms mediating the prolonged PSPs in chronic SCI
In chronic and acutely spinalized rats, the 1-s-long EPSP evoked by a single-shock cutaneomuscular stimulation is mediated by the activation of NMDA receptors located on interneurons upstream from the motoneuron (Bennett et al., 2001a). For instance, addition of the NMDA receptor antagonist AP5 to the recording bath completely abolishes the long-lasting EPSP, leaving behind a short-latency, short-lasting (30 ms) monosynaptic EPSP. Given that the NMDA receptor also possesses a fair degree of persistence, especially in the face of reduced inhibition following spinal transection (see subsequently), it is likely that the prolonged EPSP recorded in motoneurons, and the PSFs recorded from iSCI subjects, are a result of prolonged activation of upstream excitatory interneurons. Interestingly, the duration of the EPSP following acute SCI is less than the duration following chronic SCI (740 ms versus 960 ms, respectively, Li et al., 2004a) and suggests that the NMDA receptor itself may become more persistent with time after injury. In addition, increases in the temporal dispersion of sprouted segmental afferent (Krenz and Weaver, 1998) or spared descending inputs (in the case of incomplete SCI), may increase the duration of the EPSP after chronic injury.
Mechanisms producing the reduction in inhibition after SCI
Immunohistochemical and pharmacological studies in animals have revealed that, in general, there is an increase in excitatory and a decrease in inhibitory spinal neurotransmission following acute or chronic SCI (Shapiro, 1997). For instance, increases in the number of glutaminergic inputs to motoneurons from terminals of intraspinal, but not primary afferent, neurons occur in the weeks following SCI as measured by vesicular glutamate transporter 2 and 1 labelling, respectively (Kitzman, 2006, 2007). Correspondingly, although the levels of vesicular gamma-aminobutyric acid (GABA) transporter (Kitzman, 2006) and glutamate decarboxylase 65 (Tillakaratne et al., 2000) terminal immuno-labelling remains constant after SCI, pharmacological antagonism of GABAA receptors with bicuculline and agonism of GABAB receptors with baclofen reveal reduced GABA-ergic activity and suppression of reflexes following SCI (Drew et al., 2004; Li et al., 2004b; Kakinohana et al., 2006). Moreover, levels of glycine are reduced following long-term SCI (Shapiro, 1997). It appears, therefore, that the pattern of increased excitatory neurotransmission onto excitatory interneurons/motoneurons combined with a reduction in inhibitory spinal mechanisms following SCI may abolish or reduce the reflex suppression of motoneurons in response to cutaneomuscular afferent stimulation that was observed in patients with chronic SCI. However, it is important to consider that the stimulation intensities used in this study were just above threshold to evoke a small reflex response in the TA muscle and that higher stimulation intensities could have activated IPSPs in some part of the 1-s-long EPSP.
Influence of long EPSPs in triggering involuntary muscle spasms
The 1-s-long estimated EPSP recorded in chronically injured subjects should provide a long enough depolarization to securely recruit a slowly activating PIC in the motoneuron. In sacral motoneurons of chronic spinal rats, single-shock stimulation to tail afferents produced a 1-s-long EPSP that was capable of triggering sustained, multi-second discharge of the motoneuron as a result of PIC activation (Fig. 9). Interestingly, in some rats, single-shock stimulation only produces a brief, 30 ms mono-synaptic EPSP that fails to produce a long-lasting discharge of the motoneuron despite the presence of large PICs measured separately during voltage clamp (Li et al., 2004a). However, when a high frequency, 500ms train was applied to the dorsal roots of these animals, the resulting summated EPSP was then able to recruit the PIC to produce a multi-second long discharge of the motoneuron. Thus, depolarizing inputs to a motoneuron lasting <500 ms cannot securely recruit a PIC to produce self-sustained firing. This is in agreement with the observation that we rarely observed continued activity of motor units recruited by brief (<20 ms) sensory afferent stimulation in non-injured control subjects, given that the duration of uninterrupted depolarization was only ≤200 ms. In contrast, sustained unit activity is often observed in control subjects following a longer period (≥1 s) of sensory afferent stimulation (Gorassini et al., 1998; Collins et al., 2002). Likewise, continued activity of motor units recruited by brief sensory stimulation was observed in iSCI subjects likely as a result of PIC activation since the motor units recruited by the stimulation continued to fire even after the firing rate of a lower threshold, tonically firing motor unit returned to baseline values. This indicates that the newly recruited units continued to fire at a level of synaptic input, as reflected by the firing rate of the tonic unit, that was lower than the level needed to recruit the units initially, an indication that PICs sustained the activation of the motor units during the muscle spasm (Kiehn and Eken, 1997; Gorassini et al., 1998, 2004). Although cutaneous inputs may activate motoneurons in the same pool in a non-uniform manner (Stephens et al., 1976, 1978), the fact that the newly recruited units fired in a similar manner to the tonically firing units suggests that the two motoneurons were receiving similar synaptic drives.
In summary, the prolonged duration of depolarization from brief sensory stimulation after SCI increases the likelihood that PICs will be securely recruited to more readily trigger long-lasting involuntary muscle spasms once they recover after chronic SCI (Li and Bennett, 2003). It is likely that such self-sustained firing of the motoneuron occurs unchecked given the reduced presence of spinal inhibition following SCI. The anti-spastic medication baclofen, which primarily activates pre-synaptic GABAB receptors, likely works well to reduce the size and duration of sensory-evoked EPSPs to decrease the incidence of triggering muscle spasms, but it is ineffective in controlling the motoneuron PIC that mediates them (Li et al., 2004b). Most subjects in this study were on varying doses of oral baclofen (Table 1). Because baclofen administration was not systematically timed in these experiments, it is difficult to predict the effect that baclofen had on sensory-evoked EPSPs (i.e. PSFs) in our subjects and this will be the focus of future studies.
Acknowledgements
We gratefully acknowledge the excellent technical assistance provided by Jennifer Nevett-Duchcherer, Leo Sanelli and Xiaole Li. We like to thank Dr Randy Powers and Dr Kemal Turker for their advice on the PSF technique and Dr Colum MacKinnon for suggesting the use of the PSF technique in the first place. We are grateful to Dr Stuart Baker for the use of his Get-Spike Software. Funding was provided by the Canadian Institutes of Health Research and the National Institutes of Health, Grant NS048170.
Abbreviations
- AHP
afterhyperpolarization
- CUSUM
cumulative sum
- EPSP
excitatory post-synaptic potential
- IPSP
inhibitory post-synaptic potential
- iSCI
incomplete spinal cord injury
- PIC
persistent inward current
- PSF
peristimulus frequencygram
- PSTH
post-stimulus time histogram
- TA
tibialis anterior
References
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res. 1987;420:340–50. doi: 10.1016/0006-8993(87)91255-8. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Sanelli L. Role of NMDA in spasticity following sacral spinal cord injury in rats. Soc Neurosci Abstr. 2001a;31:933.11. [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001b;86:1955–71. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal injury. J. Neurophysiol. 2004;91:2247–58. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Bessou P, Laporte Y, Pages B. Frequencygrams of spindle primary endings elicited by stimulation of static and dynamic fusimotor fibres. J Physiol. 1968;196:47–63. doi: 10.1113/jphysiol.1968.sp008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering-Sorensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. 2006;44:708–22. doi: 10.1038/sj.sc.3101928. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal inhibition of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–88. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Gibb AJ, Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annu Rev Physiol. 1995;57:495–519. doi: 10.1146/annurev.ph.57.030195.002431. [DOI] [PubMed] [Google Scholar]
- Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurons. Prog Brain Res. 1989;80:257–67. doi: 10.1016/s0079-6123(08)62219-0. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurons: mode of activation and integration of synaptic inputs. J Physiol. 2006;570:355–74. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. EEG. 1978;45:302–4. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA. Cutaneomuscular reflexes recorded from the lower limb in man during different tasks. J Physiol. 1995;487:237–42. doi: 10.1113/jphysiol.1995.sp020874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett. 1998;247:13–6. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–58. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–8. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006;96:1171–86. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MG, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–56. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Jenner JR, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. J. Physiol. 1982;333:405–19. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AC, Yang JF. Reflex behavior during walking in incomplete spinal-cord-injured subjects. Exp Neurol. 1994;128:239–48. doi: 10.1006/exnr.1994.1133. [DOI] [PubMed] [Google Scholar]
- Kakinohana O, Hefferan MP, Nakamura S, Kakinohana M, Galik J, Tomori Z, et al. Development of GABA-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: a qualitative and quantitative electrophysiological and histopathological study. Neurosci. 2006;141:1569–83. doi: 10.1016/j.neuroscience.2006.04.083. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–8. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kitzman P. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in spastic rat. Exp Neurol. 2006;197:407–19. doi: 10.1016/j.expneurol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kitzman P. VGLUT1 and GLYT2 labeling of sacrocaudal motoneurons in the spinal cord injured spastic rat. Exp Neurol. 2007;204:195–204. doi: 10.1016/j.expneurol.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neurosci Lett. 1998;85:443–58. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol. 2006;574:819–34. doi: 10.1113/jphysiol.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–69. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004a;91:767–83. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. 2004b;92:2694–703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Waring WP., 3rd. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil. 1990;71:566–9. [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol. 2007;98:1042–7. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–70. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurons in man. J Physiol. 1997;501:473–84. doi: 10.1111/j.1469-7793.1997.473bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Gorassini MA. Estimation of post-synaptic potentials evoked by cutaneous stimulation in man. Proc Physiol Soc. 2006;3:PC61. [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. I: The single spike train. Biophys J. 1967;7:391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Neurotransmission by neurons that use serotonin, noradrena-line, glutamate, glycine, and [gamma]-aminobutyric acid in the normal and injured spinal cord. Neurosurg. 1997;40:168–77. doi: 10.1097/00006123-199701000-00037. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Garnett R, Buller NP. Reversal of recruitment order of single motor units produced by cutaneous stimulation during voluntary muscle contraction in man. Nature. 1978;272:362–4. doi: 10.1038/272362a0. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP, Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976;263:343–4. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJK, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60:219–30. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Turker KS, Cheng HB. Motor-unit firing frequency can be used for the estimation of synaptic potentials in human motoneurones. J Neurosci Methods. 1994;53:225–34. doi: 10.1016/0165-0270(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Turker KS, Powers RK. Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol. 1999;82:829–40. doi: 10.1152/jn.1999.82.2.829. [DOI] [PubMed] [Google Scholar]
- Turker KS, Powers RK. Black box revisited: a technique for estimating postsynaptic potentials in neurones. Trends Neurosci. 2005;28:379–86. doi: 10.1016/j.tins.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]