Abstract
Cortical development is disrupted in presenilin-1 null mutant (Psen1−/−) mice. Prior studies have commented on similarities between Psen1−/− and reeler mice. Reelin induces phosphorylation of Dab1 and activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Psen1 is known to modulate PI3K/Akt signaling and both known reelin receptors (apoER2 and VLDLR) are substrates for Psen1 associated γ-secretase activity. The purpose of this study was to determine whether reelin signaling is disrupted in Psen1−/− mice. We show that while Dab1 is hypophosphorylated late in cortical development in Psen1−/− mice, it is normally phosphorylated at earlier ages and reelin signaling is intact in Psen1−/− primary neuronal cultures. γ-secretase activity was also not required for reelin induced phosphorylation of Dab1. Unlike reeler mice the preplate splits in Psen1−/− brain. Thus cortical development in Psen1−/− mice fails only after splitting of the preplate and is not due to an intrinsic failure of reelin signaling.
Keywords: presenilin-1, reelin, gamma-secretase, cortical lamination
INTRODUCTION
The presenilin-1 (Psen1) gene was first recognized because mutations in it and a related gene, presenilin-2 (Psen2), cause early onset familial Alzheimer’s disease (St George-Hyslop, 2000). Mice with null mutations in Psen1 (Psen1−/−) die during late intrauterine life or shortly after birth (Shen et al., 1997; Wong et al., 1997). The embryos are small with a generally hypomorphic caudal body as well as skeletal malformations and cardiovascular defects (Shen et al., 1997; Wong et al., 1997; Nakajima et al., 2004).
CNS development is disrupted in Psen1−/− embryos as well, in the telencephalon resulting in a cortical dysplasia that is invariably accompanied by some degree of intracerebral hemorrhage (Shen et al., 1997; Wong et al., 1997). The cellular and molecular basis for Psen1’s effects particularly on CNS development remain incompletely understood. However, cell migration defects in the developing neocortex of Psen1−/− mice have been reported in several studies (Shen et al., 1997; Hartmann et al., 1999; Handler et al., 2000; Yuasa et al., 2002; Louvi et al., 2004; Wen et al., 2005; Wines-Samuelson et al., 2005).
The reelin pathway is a key developmental pathway that influences cell migration and cortical histogenesis (Rice and Curran, 2001; D’Arcangelo, 2006). Hartmann et al. (Hartmann et al., 1999) first commented on similarities between the cortical dysplasia seen in Psen1−/− mice and reeler mice as well as reporting that Cajal-Retzius cells were depleted in Psen1−/− brain. Later studies documented Cajal-Retzius cell loss in Psen1−/− brain (Wines-Samuelson et al., 2005) as well as altered physiological characteristics of those that remain (Kilb et al., 2004).
Reelin acts by binding to two lipoprotein related receptors, the very low-density lipoprotein receptor (VLDLR) and apoER2 (Hiesberger et al., 1999; Trommsdorff et al., 1999). Reelin binding results in tyrosine phosphorylation of the adaptor protein Dab1 (reviewed in (Herz and Chen, 2006) which leads to recruitment and activation of Src family kinase members. Activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is one of the principle mechanisms of reelin’s downstream effects leading to phosphorylation and inactivation of glycogen synthase kinase 3β (GSK-3β).
Psen1 could potentially influence signaling through the reelin pathway by two mechanisms. Firstly, Psen1 is known to modulate signaling through the PI3K/Akt pathway. Indeed several studies have shown that as judged by Akt phosphorylation, basal PI3K activation is reduced in cells lacking Psen1 or both presenilins (Baki et al., 2004; Kang et al., 2005; Zhang et al., 2007), effects that occur independent of Psen1’s influence on γ-secretase activity. In addition, Psen1 is functionally best known for its role in regulating intramembrane proteolysis by affecting γ-secretase activity (Iwatsubo, 2004). Many γ-secretase substrates exist (Vetrivel et al., 2006) including apoER2 (May et al., 2003) and VLDLR (Hoe and Rebeck, 2005). In a number of settings γ-secretase cleavage results in an intracellular domain (ICD) being released that translocates to the nucleus and acts as a transcriptional regulator. The notch ICD represents the best studied of these (Lai, 2004) although transcriptionally active ICDs have been associated with the amyloid precursor protein (Raychaudhuri and Mukhopadhyay, 2007), ErbB4 (Sardi et al., 2006), LRP1 (Kinoshita et al., 2003) and the reelin receptor apoER2 (May et al., 2003). What role if any ICDs generated from the reelin receptors play in reelin signaling is unknown. However their existence suggests an additional mechanism whereby Psen1 might influence signaling through the reelin pathway.
This led us to investigate whether reelin signaling is altered in Psen1−/− brain. Here we show that while biochemical signaling through the reeler pathway is disturbed late in cortical development, it is intact at earlier stages. We also show that unlike reeler mice the preplate splits in Psen1−/− brain. These studies thus show that cortical development in the Psen1−/− neocortex fails only after splitting of the preplate and is not due to an intrinsic failure of reelin signaling in Psen1−/− cells.
RESULTS
Dab1 is hypophosphorylated in the cerebral cortex of E18.5 Psen1−/− mice but normally phosphorylated in E14.5 to E16.5 brain
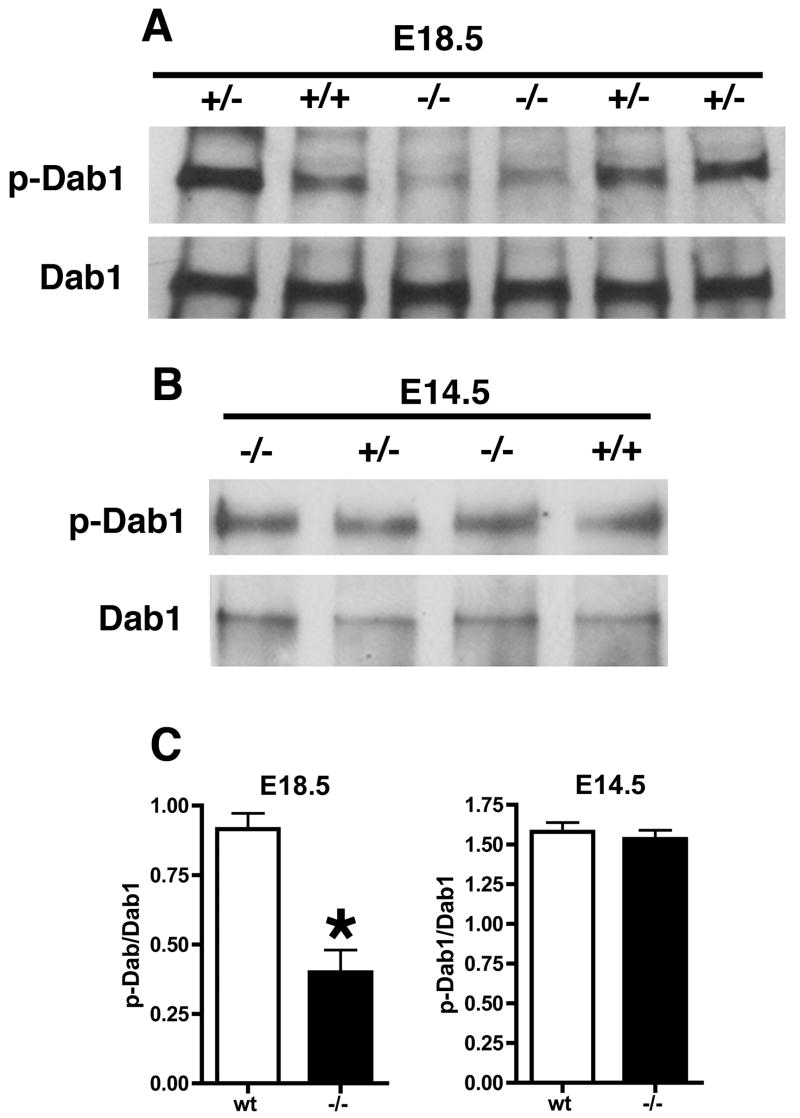
Dab1 is an adaptor protein that mediates downstream signaling through the reeler pathway. In reeler mice, throughout the period of neocortical development Dab1 levels are increased and the protein is hypophosphorylated (Rice et al., 1998). Therefore as an indicator of reelin pathway activation in Psen1−/− embryos we initially examined the phosphorylation status of Dab1 in E18.5 Psen1−/− embryos and controls by immunoprecipitating (IP) Dab1 from brain followed by Western blotting with an anti-phosphotyrosine antibody. As shown in Fig. 1A, Dab1 was hypophosphorylated in Psen1−/− compared to either wild type or Psen1+/− embryos while total Dab1 levels were unaltered in Psen1−/− brain with the ratio of p-Dab1/Dab1 significantly decreased in the E18.5 Psen1−/− embryos (Fig. 1C, p = 0.0065, unpaired t-test).
Fig. 1.
Decreased phosphorylation of Dab1 in E18.5 Psen1−/− mice but normal phosphorylation in E14.5 brain. Dab1 was immunoprecipitated from E18.5 (A) or E14.5 (B) brains of wild type (+/+) as well as heterozygous (+/−) and homozygous (−/−) Psen1 mutant mice. Representative Western blots from studies that were performed multiple times are presented probed with an anti-phosphotyrosine antibody. The ≈ 80-kDa Dab1 band is shown. Lower panels show the same blots reprobed for total Dab1. In (C) Dab1 phosphorylation is expressed as the ratio of p-Dab1 to total Dab 1. Results are presented ± the standard error of the mean and represent averages of n = 4 wild type (wt) and 2 Psen1−/− embryos at each age.
Thus reelin signaling appeared to be defective in Psen1−/− brain although unlike reeler mice (Rice et al., 1998), total Dab1 levels were not increased in Psen1−/− mice. However, to our surprise when we subsequently examined Dab1 phosphorylation at E14.5, Dab1 was phosphorylated in Psen1−/− brain to a similar level as in controls (Fig 1B) with no alteration in the level of total Dab1 and no change in the ratio of p-Dab1/Dab1 (Fig. 1C, p = 0.65, unpaired t-test). Similar results were obtained with extracts from E16.5 brain (data not shown).
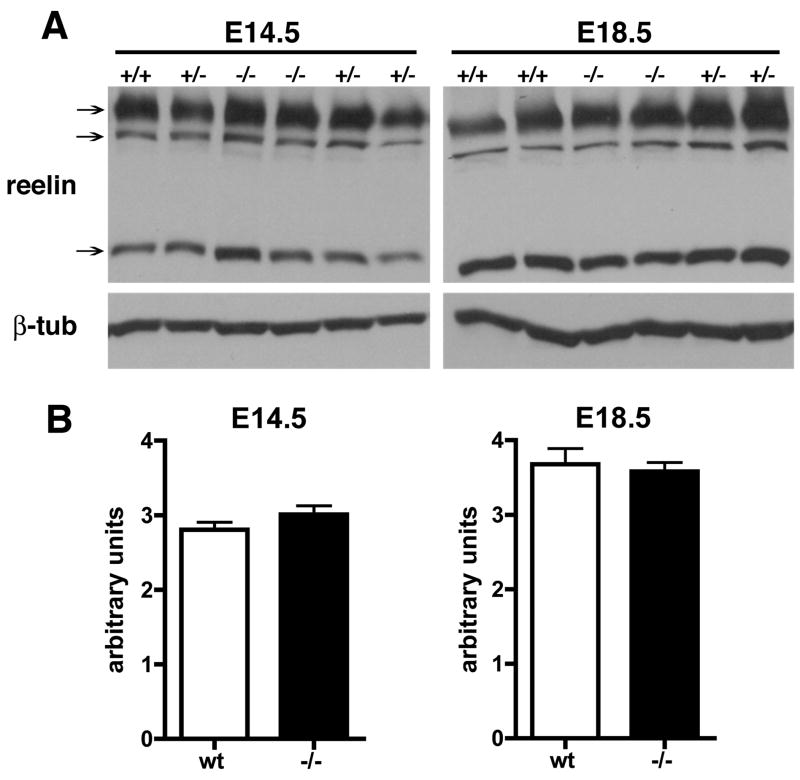
Since altered reelin levels might explain the Dab-1 hypophosphorylation at E18.5 we also examined reelin expression at E14.5 and E18.5 by Western blotting (Fig. 2). There were no differences in reelin levels between wild type (wt) and Psen1−/− at either age (p = 0.29, E14.5; p = 0.77, E18.5, unpaired t-tests). Thus Dab1 is hypophosphorylated in E18.5 Psen1−/− brain but normally phosphorylated at earlier embryonic ages and this change is not due to decreased levels of reelin.
Fig. 2.
No change in reelin levels in Psen1−/− brain. Reelin levels were determined by Western blotting (A) of extracts from E14.5 or E18.5 brain from wild type (+/+), heterozygous (+/−), or homozygous (−/−) Psen1 mutant mice. Arrows indicate the ≈180, 250 and 400 kDa reelin bands. Lower panel shows the same blot reprobed for β-tubulin. In (B) reelin levels were quantitated as a ratio of the sum of the three reelin bands to β-tubulin (n = 4 wild type and 2 Psen1−/− at each age). There were no differences between wild type (wt) and Psen1−/− at either age.
Dab1 is normally phosphorylated in primary neuronal cultures from Psen1−/− embryos following stimulation with reelin
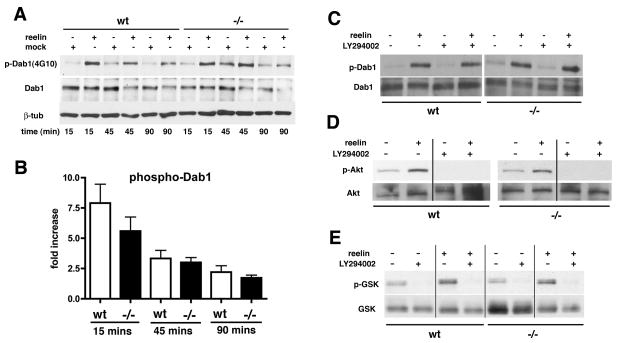
As an indication of the intrinsic responsiveness of Psen1−/− neuronal cells to reelin, we treated primary neuronal cultures from Psen1−/− and wild type littermate control embryos with recombinant reelin or control supernatants for varying time intervals and monitored Dab1 phosphorylation by Western blotting. After a 15 min exposure to reelin, an approximate five to seven-fold induction of Dab1 phosphorylation was observed in both wild type and Psen1−/− cultures (Figs 3A and 3B). This induction gradually subsided over 90 mins without any significant difference in responsiveness between wild type and Psen1−/− cultures (Fig 3B).
Fig. 3.
Treatment of Psen1−/− primary neuronal cultures with recombinant reelin induces normal Dab 1 phosphorylation. Primary neuronal cultures were treated with supernatants from reelin transfected or mock-transfected HEK cells. Results from a representative experiment are shown in panel A. The apparent increase in p-Dab1 in the Psen1−/− mock treated at 45 mins represents a loading effect. In (B) the fold increase in Dab1 phosphorylation is expressed as the ratio of p-Dab1 to total Dab1. Results are presented ± the standard error of the mean and represent averages from three-six independent experiments for each time point. Responses in wild type (wt) and Psen1−/− cultures were compared at each time point using unpaired t-tests. There were no significant differences at any of the time points (p values 0.10 to 0.46). In panels C–E, primary neuronal cultures were stimulated with reelin or control supernatant for 30 mins in the presence or absence of the PI3K inhibitor LY294002. Representative Western blots from studies that were performed multiple times are shown probed for phospho-Dab1/total Dab1 (C), phospho-Akt (p-Akt)/total Akt (D), and phospho-GSK-3β (p-GSK-3β/total GSK-3β (E).
Binding of reelin to VLDLR and apoER2 also stimulates PI3K, an activation that is dependent on Dab1 phosphorylation (Beffert et al., 2002). PI3K stimulation leads to phosphorylation of protein kinase B (Akt) on serine 473 which in turn phosphorylates glycogen synthase kinase-3β (GSK 3β) on serine 9 (Beffert et al., 2002). Interestingly, Psen1 has been reported to affect signaling through the PI3K/Akt pathway (Baki et al., 2004; Kang et al., 2005). Therefore as a measure of the intactness of downstream reelin signaling in Psen1−/− neurons we monitored Akt and GSK-3β phosphorylation following reelin stimulation. Reelin induced Akt (Fig. 3D) and GSK-3β (Fig. 3E) phosphorylation in both wild type and Psen1−/− cultures without any significant differences between genotypes. To test the dependence of Akt and GSK 3β phosphorylation on PI3K activation, cultures were stimulated with reelin in the presence or absence of the PI3K inhibitor LY294002. As shown in Fig. 3, the inhibitor blocked reelin induced Akt (Fig. 3D) and GSK-3β (Fig. 3E) phosphorylation in both wild type and Psen1−/− cultures while having no effect on reelin induced phosphorylation of Dab1 (Fig. 3C). Quantitation of duplicate experiments found no differences in the fold induction of Akt or GSK-3β phosphorylation between wild type and Psen1−/− neuronal cells (unpaired t-tests, p = 0.64 for Akt and p = 0.40 for GSK-3β, data not shown).
γ-secretase activity is not required for reelin induced phosphorylation of Dab1
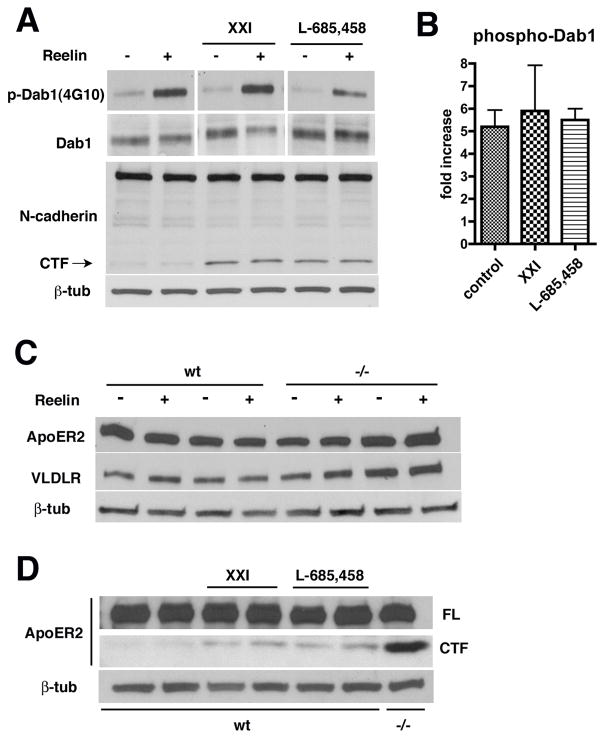
Both apoER2 (May et al., 2003) and VLDR (Hoe and Rebeck, 2005) are substrates for γ-secretase cleavage and the apoER2 cleavage has been reported to generate a transcriptionally active ICD (May et al., 2003). To determine whether γ-secretase activity affects reelin signaling we treated primary neuronal cultures from wild type embryos with γ-secretase inhibitors and examined whether reelin induced Dab1 phosphorylation in the presence of inhibitors. Primary neuronal cultures were treated overnight with either the γ-secretase inhibitor XXI or L-685,458 and then stimulated with reelin. Dab1 phosphorylation was induced in the presence of both inhibitors (Fig 4A). The fold increase in Dab1 phosphorylation was determined by the ratio of p-Dab1 to total Dab1 in control and inhibitor treated cultures (Fig 4B) and the three groups (n = 3, control and XXI treated; n = 2, L-865,458 treated) were compared using a one-way analysis of variance (ANOVA). No significant differences were found among the groups (F 2, 6 = 0.78, p = 0.49, no significant differences Tukey post-hoc tests). As a control for the adequacy of γ-secretase inhibitor treatment, the same samples were probed for N-cadherin to search for the N-cadherin’s γ-secretase cleaved C-terminal fragment (CTF) (Marambaud et al., 2002). As shown in figure 4A, an N-cadherin CTF accumulated in the presence of both inhibitors indicating that γ-secretase inhibition had been effective.
Fig. 4.
γ-secretase activity is not need for reelin induced phosphorylation of Dab 1 in primary neuronal cultures. In (A) cultures were treated overnight with the γ-secretase inhibitors XXI or L-685,458 and then stimulated with reelin for 15 mins. Blotting for phospho-Dab1 and total Dab1 is shown. The same samples were blotted and probed for N-cadherin. A band for the full length N-cadherin is visible and in the γ-secretase inhibitor treated lanes a band that corresponds to the N-cadherin CTF is indicated. The N-cadherin blot was stripped and reprobed for β-tubulin (β-tub) as a loading control. In (B) the fold increase in Dab1 phosphorylation is expressed as the ratio of p-Dab1 to total Dab1. In (C) extracts from wild type and Psen1−/− neurons with or without reelin treatment were sequentially probed for apoER2, VLDLR and β-tubulin (β-tub). In (D) cultures were treated overnight with γ-secretase inhibitors and then stimulated with reelin followed by blotting for apoER2. Full-length (FL) apoER2 and the apoER2 CTF are shown. Last lane shows blotting for apoER2 in untreated Psen1−/− neurons. Reprobing for β-tubulin (β-tub) is shown in the lower panel.
As an additional indicator of the effectiveness of γ-secretase inhibition we also assessed apoER2 and VLDLR processing. Western blotting for apoER2 and VLDR showed that both receptors were equally expressed in wild type and Psen1−/− primary neuronal cultures and that their levels were not affected by treatment with reelin (Fig. 4C). As shown in figure 4D, a fragment accumulated in the γ-secretase inhibitor treated wild type cultures that migrated at the expected molecular weight for an apoER2 CTF (May et al., 2003) and a similar fragment appeared in Psen1−/− cultures. We were not able to detect a VLDLR CTF in γ-secretase inhibitor treated cultures. We suspect that this reflects a sensitivity problem related to the generally lower levels of VLDLR expressed in neurons (Trommsdorff et al., 1999) rather than a failure of the inhibitors to affect VLDLR cleavage. These studies thus argue that despite apoER2 and VLDLR being γ-secretase substrates, γ-secretase cleavage is not necessary for reelin activation of Dab1.
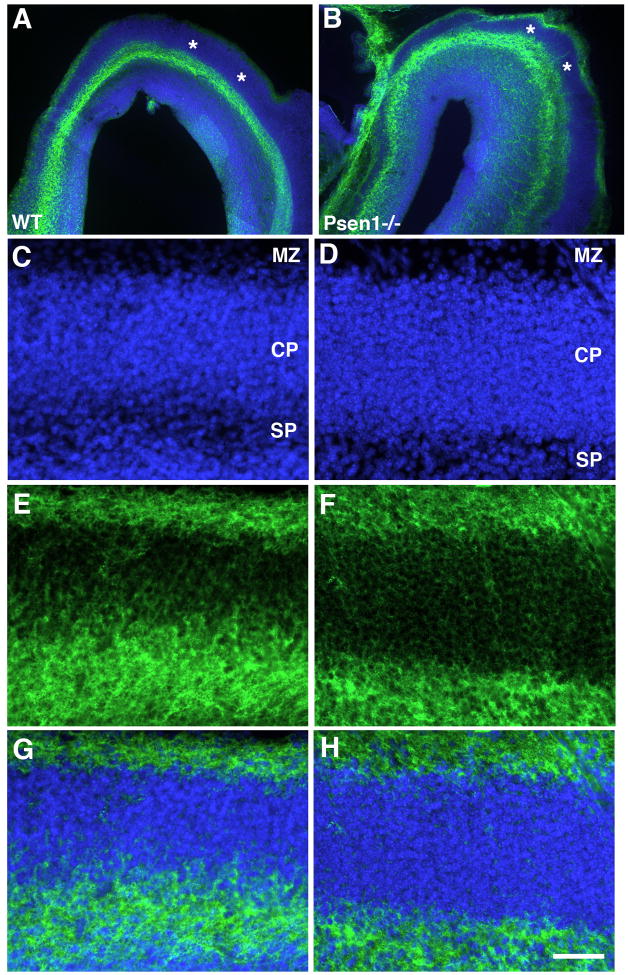
The preplate splits in Psen1−/− telencephalon
In developing mouse neocortex an initial wave of post-mitotic cells exits the ventricular zone, migrating radially and establishing a layer known as the preplate (Gupta et al., 2002; Ayala et al., 2007). A second wave of migrating cells splits the preplate into a superficial marginal zone and a deeper subplate. In reeler mice the preplate forms but never splits and an abnormal layer termed the superplate develops (Sheppard and Pearlman, 1997).
Splitting of the preplate can be assessed by chondroitin sulfate proteoglycan (CSPG) immunostaining which marks the preplate and its derivatives (Sheppard et al., 1991; Bicknese et al., 1994). CSPG immunostaining in the telencephalon from E15.5 wild type and Psen1−/− embryos is shown in Fig. 5. In both wild type and Psen1−/− brain, CSPG staining appeared in distinct layers corresponding to marginal zone and subplate labeling indicating that splitting of the preplate had occurred in the Psen1−/− telencephalon. We also found no difference between wild type and Psen1−/− embryos in the pattern of calretinin staining (Supplemental Fig. 1) which also labels the preplate and its derivatives (Weisenhorn et al., 1994; Fonseca et al., 1995). Thus unlike reeler mice, the preplate splits in Psen1−/− mice.
Fig. 5.
CSPG immunostaining reveals splitting of the preplate in Psen1−/− telencephalon. Horizontally cut Vibratome sections from E15.5 wild type or Psen−/− embryos were immunostained with CSPG (green) along with a DAPI nuclear stain (blue). In panels A and B, sections through the frontal poles are shown. Asterisks (*) indicate the unstained cortical plate. In panels C–H, sections from the lateral telencephalon of wild type (C, E, G) or Psen−/− embryos (D, F, H) are shown labeled for DAPI (C, D), immunostained for CSPG (E, F) or as a merged image (G, H) The marginal zone (MZ), cortical plate (CP) and subplate region (SP) are indicated. Scale bar: 200 μm for A–B and 50 μm for C–D.
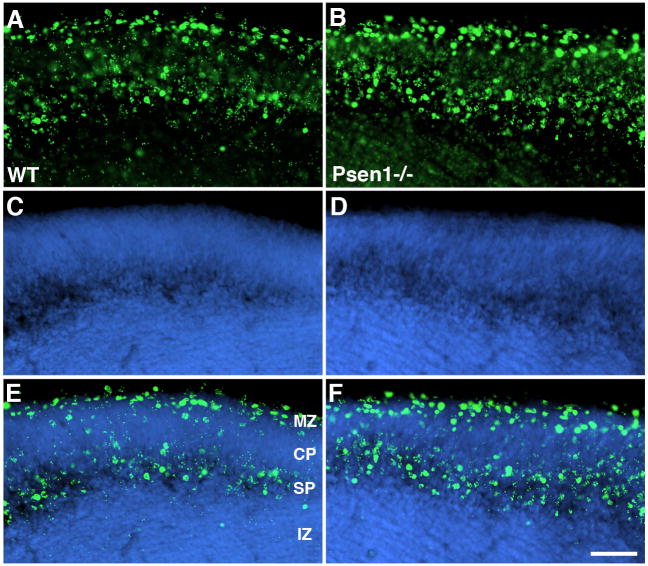
As an additional indicator of the status of cell migration in Psen1−/− telencephalon around the time of preplate splitting, we injected BrdU into pregnant female mice at E11.5 and examined BrdU immunostaining at E15. As shown in Fig. 6, in wild type and Psen1−/− embryos the most highly labeled cells (i.e. those cells that left the cell cycle first at E11.5) contributed to cells in the marginal zone and to cells in the subplate region. Some lightly BrdU labeled cells (indicative of cells that left the cell cycle and migrated later) could be seen in the central portion of the cortical plate. Thus in Psen1−/− embryos, latter generated cells continued to migrate into the cortical plate in a pattern that was not distinguishable between wild type and Psen1−/− brains, further indicating the intactness of neuronal migration at this stage of development in Psen1−/− brain.
Fig. 6.
No difference in BrdU labeling between wild type and Psen1−/− embryos. Pregnant female mice received one injection of BrdU at E11.5 and BrdU immunostaining was performed on sections of E15 brain. Coronal sections from the lateral telencephalon of wild type (A, C, E) or Psen−/− embryos (B, D, F) are shown immunostained for BrdU (A, B), labeled with DAPI (C, D), or as merged images (E, F). The marginal zone (MZ), cortical plate (CP), subplate region (SP) and intermediate zone (IZ) are indicated. Scale bar: 100 μm.
DISCUSSION
Here we investigated whether altered reelin signaling might play a role in the disrupted cortical lamination found in Psen1−/− mice. While our initial studies did indeed find Dab1 to be hypophosphorylated in Psen1−/− cortex at E18.5, subsequent studies found Dab1 to be normally phosphorylated at earlier ages. We also found that when primary neuronal cultures from Psen1−/− embryos were treated with recombinant reelin, Dab1 was phosphorylated normally and that reelin induced phosphorylation of Akt as well as phosphorylation of its downstream target GSK-3β.
Reelin signaling could be intact at the cellular level but nevertheless perturbed at the tissue level if migrating neurons in Psen1−/− brain were deprived of their source of reelin. Cajal-Retzius cells are present in Psen1−/− brain and express reelin (Kilb et al., 2004; Wen et al., 2005; Wines-Samuelson et al., 2005). However, both loss (Wines-Samuelson et al., 2005) as well as physiological dysfunction (Kilb et al., 2004) of Cajal-Retzius cells has been reported in Psen1−/− brain. Our finding that the preplate is split at E15.5 in Psen1−/− neocortex argues that signaling through the reelin pathway at the tissue level is intact up to this stage of cortical development. These findings are also consistent with the anatomical data which suggest that Cajal-Retzius cell loss is a relatively late event occurring well after the time of preplate splitting (Wines-Samuelson et al., 2005). Cajal-Retzius cell loss might explain the hypophosphorylation of Dab1 at E18.5. However even at this stage, losses of Cajal-Retzius cells are relatively mild (≈20%) (Wines-Samuelson et al., 2005), and as shown in this study reelin levels are not affected making it unlikely that altered availability of reelin explains the profound effects on Dab1 phosphorylation. It is also of note that recent studies have found that even massive losses of Cajal-Retzius cells do not lead to a reeler phenotype (Yoshida et al., 2006).
Multiple studies have addressed the issue of cortical development in Psen1 −/− mice (Shen et al., 1997; Handler et al., 2000; Yuasa et al., 2002; Louvi et al., 2004; Wen et al., 2005; Wines-Samuelson et al., 2005; Kim and Shen, 2008). That cortical development is altered in the absence of Psen1 is clear. However the timing of the onset of the developmental defect as well as its cellular and molecular nature remains less clear. Some studies have suggested that neural progenitor cells are depleted in Psen1−/− telencephalon as early as E12.5 (Shen et al., 1997) and that the cortical plate is thinned and disorganized at E14.5 (Handler et al., 2000). In the latter study an early migration defect was also supported by studies showing that progenitor cells labeled with BrdU at E10.5 were abnormally dispersed across the cortical layers in Psen1−/− telencephalon at E14.5 (Handler et al., 2000). Our own studies described here however found no systematic alteration in the distribution of BrdU labeled cells labeled at E11.5 and examined at E15.
Defects in cortical lamination can be generally divided into the preplate splitters and non-splitters (Gupta et al., 2002; Ayala et al., 2007), with reeler being the classic non-splitter (Rice and Curran, 2001) along with mice having null mutations in Dab1 (Howell et al., 1997) or combined mutations in the reelin receptors, VLDLR and apoER2 (Trommsdorff et al., 1999). Cdk5 null mutants were the first recognized preplate splitters (Gilmore et al., 1998; Kwon and Tsai, 1998) and combined mutations in Cdk5’s regulatory subunits p35 and p39 produce a similar phenotype (Ko et al., 2001).
Our results clearly place the Psen1 null mutant among the preplate splitters, thus arguing that the major defects in cortical development are relatively late events. Other studies have documented defects in both radial and tangential migration in Psen1−/− brain (Louvi et al., 2004). However these effects were only apparent at E16.5 or later also arguing for a relatively late effect of Psen1 on cell migration. Loss of radial glia occurs in Psen1−/− telencephalon (Louvi et al., 2004; Wen et al., 2005). However this loss is also only clearly evident at E16.5 or later. A relatively late migration defect is also supported by our prior BrdU labeling studies showing that cells labeled at E12.5 (one day later than in the present study) are abnormally dispersed across the cortex in the Psen1−/− telencephalon at E18.5 rather than being concentrated in the inner layers as in wild type mice (Wen et al., 2005). This pattern is most suggestive of a late migration defect in Psen1−/− brain in which later generated unlabeled cells fail to migrate past earlier generated labeled ones. The hypophosphorylation of Dab1 at E18.5 documented in this study can also be seen as further evidence of a late effect. Thus collectively most evidence supports a predominately late migration defect in the Psen1−/− telencephalon.
At the cellular level it has been suggested that neurons differentiate prematurely in Psen1−/− brain leading to a premature depletion of neural progenitor cells (Handler et al., 2000) although this effect has been described as being a region-specific effect that becomes normalized later. A similar effect has also been suggested to occur in a neural specific conditional knockout of Psen1 (Wines-Samuelson et al., 2005). The only quantitative analysis of this phenomenon (Wen et al., 2004), however, found no evidence for premature neuronal differentiation in single cell suspensions of Psen1−/− telencephalon suggesting that premature neuronal differentiation is not a general event in Psen1−/− brain and unlikely to explain the altered cortical development.
The causes of the late migration defect in Psen1−/− telencephalon remain unexplained. Similarities between Cdk5 and Psen1 null mutants have been commented on previously(Louvi et al., 2004). Interestingly, Cdk5 phosphorylates β-catenin and this phosphorylation has been reported to affect the amount of Psen1 that becomes bound to β-catenin (Kesavapany et al., 2001). In a latter study these same investigators showed that Cdk5 can phosphorylate a threonine in the Psen1 CTF and that this phosphorylation stabilizes the CTF (Lau et al., 2002). Both observations could place Psen1 in a linear pathway downstream of Cdk5 and notably Cdk5 null mutants show normal Dab1 phosphorylation early in cortical development, but hypophosphorylation late in development (Keshvara et al., 2002).
However, arguing against a Cdk5/Psen1 connection is the observation that Cdk5 and its activators are expressed primarily in post-mitotic neurons (Tsai et al., 1993; Zheng et al., 1998) while Psen1 is expressed in neural progenitor cells and indeed selective expression of Psen1 in neural progenitors cells is sufficient to rescue cortical development in Psen1−/− brain (Wen et al., 2005). By contrast Psen1 expression in post-mitotic neurons rescues none of the abnormalities in Psen1−/− brain (Wen et al., 2005) suggesting that the major effects of Cdk5 and Psen1 on cortical development occur in different cell types. In Cdk5 null mutants it has been proposed that the late hypophosphorylation of Dab1 may be caused by late migrating neurons failing to reach the source of reelin (Keshvara et al., 2002) and this remains a possible explanation for the hypophosphorylation of Dab1 late in development in Psen1−/− cortex even if Psen1 and Cdk5 do not interact in a linear pathway.
Functionally Psen1 is best known for its role in γ-secretase activity (Vetrivel et al., 2006). Among the known γ-secretase substrates are several members of the low-density lipoprotein receptor (LDLR) family including LRP1 (May et al., 2002), and the reelin receptors apoER2 (May et al., 2003) and VLDLR (Hoe and Rebeck, 2005). In a number of settings γ-secretase cleavage results in an ICD being released that translocates to the nucleus and acts as a transcriptional regulator. The notch ICD represents the best studied of these (Lai, 2004) and altered notch signaling has been described in Psen1−/− embryonic brain (Handler et al., 2000; Yuasa et al., 2002). However, transcriptionally active ICDs have been associated with the amyloid precursor protein (Raychaudhuri and Mukhopadhyay, 2007), ErbB4 (Sardi et al., 2006), LRP1 (Kinoshita et al., 2003) and apoER2 (May et al., 2003). However, the fact that reelin signaling is intact in Psen1−/− cortex suggests that in the case of apoER2, a γ-secretase generated ICD is unlikely to play a significant role in reelin induced cortical lamination.
It is perhaps more surprising that reelin induced activation of the PI3K/Akt pathway is intact in Psen1−/− neuronal cells given the reported effects of Psen1 and Psen1 FAD mutants on PI3K/Akt activation (Weihl et al., 1999; De Sarno et al., 2001; Vestling et al., 2001; Baki et al., 2004; Kang et al., 2005; Repetto et al., 2007; Zhang et al., 2007). Indeed several studies have shown that as judged by Akt phosphorylation, basal PI3K activation is reduced in cells lacking Psen1 or both presenilins (Baki et al., 2004; Kang et al., 2005; Zhang et al., 2007). However, ligand induced activation of Akt is more variably affected by the absence of presenilins and when affected has been attributed to altered receptor levels rather than interference with signal transduction (Kang et al., 2005; Repetto et al., 2007). While the studies reported here do not negate a role of Psen1 in PI3K/Akt signaling, they show that in the context of reelin signaling, Psen1 is not essential for PI3K/Akt activation in neuronal cells and that the failure of cortical development in Psen1−/− mice is a relatively late event that is not due to an intrinsic failure of reelin signaling.
EXPERIMENTAL PROCEDURES
Animals
Heterozygous Psen1+/− mice (Shen et al., 1997) were mated to produce Psen1−/− embryos with the day a vaginal plug was detected designated as E0.5. Psen1+/+and Psen1+/− littermates were used as controls. Since Psen1+/− mice exhibit no known developmental abnormalities, they were treated as wild type controls. All protocols were approved by the Institutional Animal Care and Use Committees of the James J. Peters Department of Veterans Affairs Medical Center and the Mount Sinai School of Medicine and were conducted in conformance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH publication 80–23).
Dab1 Immunoprecipitations
For immunoprecipitations (IPs), tissue was homogenized by sonication in 350 μl of 25 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton-X100 plus HALT protease inhibitor cocktail, (Pierce Biotechnologies, Rockford IL, USA) and phosphatase inhibitor cocktail I and II (Sigma-Aldrich, St. Louis MO, USA) (IP buffer). The suspension was gently mixed by rotation for 2 hrs at 4°C and then centrifuged at 14,000 × g for 10 mins at 4°C. The supernatant was saved and protein concentrations were determined using the BCA reagent (Pierce Biotechnologies) according to the manufacturer’s instructions. 300 μg of total protein from each sample was precleared with 20 μl of Protein G beads (Santa Cruz Biotechnology, Santa Cruz CA, USA) and immunoprecipitated with goat anti-Dab1 polyclonal antibody (1:10 final dilution; Santa Cruz Biotechnology) overnight at 4°C. Immune complexes were precipitated by addition of 20 μl of Protein G beads and the suspension rotated for 2 hrs at 4°C. The pellet was collected by centrifugation, washed four times with IP buffer and resuspended in 20 μl of IP buffer mixed with 20 μl of 4X Laemmli gel loading buffer. The final sample was boiled for 10 mins and then centrifuged at 14,000 × g for 10 mins at room temperature. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Billerica MA, USA). After blocking for 1h with 5% bovine serum albumin in 50 mM Tris HCl pH 7.6, 0.15M NaCl, 0.1% Tween-20 (TBST), the membranes were probed with a mouse monoclonal anti-phosphotyrosine antibody (1:5,000; PY20, Zymed/Invitrogen, Carlsbad CA, USA) diluted in blocking buffer. After extensive washes, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse IgG (1:10,000; GE Amersham, Piscataway, NJ, USA). The bands were visualized with ECL+ reagent (GE Amersham) and exposed to CLXposure film (Pierce). Total Dab1 was determined using a polyclonal rabbit anti-Dab1 antibody (1:3000; Rockland Immunochemical Inc., Gilbertsville, PA, USA).
Reelin Preparation
To obtain recombinant reelin containing or control supernatants, HEK 293 cells were transfected with the plasmid pCRL (D’Arcangelo et al., 1997) or with pcDNA3 (Invitrogen, Carlsbad CA) using the Fugene 6 reagent (Roche Applied Science, Indianapolis, IN, USA). Twenty-four hours post-transfection the cells were switched to Neurobasal medium (Invitrogen) for 48 hrs. Control and reelin containing supernatants were harvested, centrifuged at 14,000 × g for 15 mins and the supernatants frozen at −80°C. The presence of full length reelin was verified by Western blotting using the mouse monoclonal anti-reelin antibody G10 (Chemicon, Temecula, CA USA).
Treatment of Primary Neuronal Cultures with Reelin
Primary neuronal cultures were prepared from the cerebral cortex isolated from E15.5–16 embryos as previously described (Gama Sosa et al., 2007) and maintained in Neurobasal medium/B27 supplement (Invitrogen). Cultures were treated after 5 days in vitro (DIV) with reelin or control supernatant and lysed in a cold cell lysis buffer consisting of 10 mM Na phosphate buffer, pH 7.4, 150 mM NaCl, 2mM EDTA, 1%Triton X-100, 0.25% Na deoxycholate, and 0.5% SDS, supplemented with HALT protease and phosphatase inhibitor cocktails (Pierce) (Beffert et al., 2002). The lysates were centrifuged for 15 min at 14,000 rpm and the supernatant saved. When γ-secretase inhibitors were used, cultures were treated overnight with 1μM of inhibitor XXI (also known as compound E) or L-685,458 (Calbiochem, San Diego CA, USA). To inhibit PI3K activity cultures were pretreated with 50 μM LY294002 (Cell Signaling Technology, Danvers, MA USA) for 1 hour.
Dab-1 phosphorylation was assessed by Western blotting using the anti-phosphotyrosine monoclonal antibody 4G10 with the phospho-Dab1 (p-Dab1) band identified by size as described in Bock et al. (Bock et al., 2003). 10–15 μg of protein was transferred onto Nitrocellulose filters (Protran, BA85, 0.45 mm, Schleicher & Schuell/Perkin Elmer Life Sciences, Waltham, USA) and the filters were blocked for 1hr in PBS/5% non-fat dry milk (Upstate). The filters were incubated overnight with anti-phosphotyrosine monoclonal antibody 4G10 (Upstate Biotechnologies/Millipore; 1:1500 diluted in blocking solution) followed by HRP-conjugated goat anti mouse IgG (GE Amersham; 1:5000 in blocking solution). Signals were detected using the ECL+ reagent (GE Amersham) and exposure to CLXposure film (Pierce). Total Dab1 was determined independently using rabbit anti-Dab1 antibodies (Rockland; 1:3000). Levels of Akt and GSK3β and their phosphorylation status were analyzed by Western blot using mouse monoclonal or rabbit polyclonal anti p-Akt (Ser473), anti-total Akt, anti-p-GSK3β (Ser9) and anti-total GSK-3β (Cell Signaling Technology) as suggested by the manufacturer. Western blotting for apoER2 and VLDLR were performed with a rabbit polyclonal anti-apoER2 antiserum (1:1500, Novus Biologicals, Littleton, CO, USA) and a mouse monoclonal anti-VLDLR antibody (Santa Cruz Biotechnologies). A rabbit polyclonal anti β-tubulin antibody (1:1500) (Abcam Inc. Cambridge, MA, USA) was used as loading control. For densitometric quantification of bands, nonsaturated chemiluminiscence films were scanned and the images analyzed with ImageQuant TL software (GE Amersham).
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde in phosphate buffer saline (PBS) overnight and stored in PBS until processing. Immunohistochemical staining was performed as previously described (Wen et al., 2005). The primary antibodies utilized were a monoclonal IgM anti-chondroitin sulfate proteoglycan (CSPG) antibody (1:300; CS65, Sigma-Aldrich) and a rabbit polyclonal anti-calretinin antibody (1:500; Chemicon). Immunofluorescence staining was detected with species specific Alexa Fluor™ secondary antibody conjugates (1:400, Invitrogen) and nuclei were counterstained with 1 μg/ml 4-6-diamidino-2-phenylindole (DAPI). BrdU immunohistochemistry was performed as previously described (Wen et al., 2002).
Supplementary Material
Calretinin immunostaining reveals splitting of the preplate in Psen1−/− telencephalon. Coronally cut Vibratome sections from E15.5 wild type (A, C, E) or Psen−/− embryos (B, D, F) were immunostained for calretinin (red) along with a DAPI nuclear stain (blue). Sections through the lateral telencephalon are shown labeled for DAPI (A, B), immunostained for calretinin (C, D) or as a merged image (E, F). The marginal zone (MZ), cortical plate (CP), subplate region (SP) and intermediate zone (IZ) are indicated. Scale bar: 100 μm.
Acknowledgments
Grant sponsor: National Institute on Aging; Grant numbers AG020139 and AG029361. National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD); Young Investigator Award (RDG)
References
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. Embo J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- Bicknese AR, Sheppard AM, O’Leary DD, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006;8:81–90. doi: 10.1016/j.yebeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Lesort M, Bijur GN, Johnson GV, Jope RS. Cholinergic- and stress-induced signaling activities in cells overexpressing wild-type and mutant presenilin-1. Brain Res. 2001;903:226–230. doi: 10.1016/s0006-8993(01)02428-3. [DOI] [PubMed] [Google Scholar]
- Fonseca M, del Rio JA, Martinez A, Gomez S, Soriano E. Development of calretinin immunoreactivity in the neocortex of the rat. J Comp Neurol. 1995;361:177–192. doi: 10.1002/cne.903610114. [DOI] [PubMed] [Google Scholar]
- Gama Sosa MA, De Gasperi R, Rocher AB, Perez GM, Simons K, Cruz DE, Hof PR, Elder GA. Interactions of primary neuroepithelial progenitor and brain endothelial cells: distinct effect on neural progenitor maintenance and differentiation by soluble factors and direct contact. Cell Res. 2007;17:619–626. doi: 10.1038/cr.2007.53. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3:342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Hartmann D, De Strooper B, Saftig P. Presenilin–1 deficiency leads to loss of Cajal-Retzius neurons and cortical dysplasia similar to human type 2 lissencephaly. Curr Biol. 1999;9:719–727. doi: 10.1016/s0960-9822(99)80331-5. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Regulation of ApoE receptor proteolysis by ligand binding. Mol Brain Res. 2005;137:31–39. doi: 10.1016/j.molbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–383. doi: 10.1016/j.conb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Kang DE, Yoon IS, Repetto E, Busse T, Yermian N, Ie L, Koo EH. Presenilins mediate phosphatidylinositol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling. J Biol Chem. 2005;280:31537–31547. doi: 10.1074/jbc.M500833200. [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Lau KF, McLoughlin DM, Brownlees J, Ackerley S, Leigh PN, Shaw CE, Miller CC. p35/cdk5 binds and phosphorylates beta-catenin and regulates beta-catenin/presenilin-1 interaction. Eur J Neurosci. 2001;13:241–247. [PubMed] [Google Scholar]
- Keshvara L, Magdaleno S, Benhayon D, Curran T. Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling. J Neurosci. 2002;22:4869–4877. doi: 10.1523/JNEUROSCI.22-12-04869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W, Hartmann D, Saftig P, Luhmann HJ. Altered morphological and electrophysiological properties of Cajal-Retzius cells in cerebral cortex of embryonic Presenilin-1 knockout mice. Eur J Neurosci. 2004;20:2749–2756. doi: 10.1111/j.1460-9568.2004.03732.x. [DOI] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003;278:41182–41188. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YT, Tsai LH. A novel disruption of cortical development in p35(−/−) mice distinct from reeler. J Comp Neurol. 1998;395:510–522. doi: 10.1002/(sici)1096-9861(19980615)395:4<510::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lau KF, Howlett DR, Kesavapany S, Standen CL, Dingwall C, McLoughlin DM, Miller CC. Cyclin-dependent kinase-5/p35 phosphorylates Presenilin 1 to regulate carboxy-terminal fragment stability. Mol Cell Neurosci. 2002;20:13–20. doi: 10.1006/mcne.2002.1108. [DOI] [PubMed] [Google Scholar]
- Louvi A, Sisodia SS, Grove EA. Presenilin 1 in migration and morphogenesis in the central nervous system. Development. 2004;131:3093–3105. doi: 10.1242/dev.01191. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Moriizumi E, Koseki H, Shirasawa T. Presenilin 1 is essential for cardiac morphogenesis. Dev Dyn. 2004;230:795–799. doi: 10.1002/dvdy.20098. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri M, Mukhopadhyay D. AICD and its adaptors - in search of new players. J Alzheimers Dis. 2007;11:343–358. doi: 10.3233/jad-2007-11311. [DOI] [PubMed] [Google Scholar]
- Repetto E, Yoon IS, Zheng H, Kang DE. Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J Biol Chem. 2007;282:31504–31516. doi: 10.1074/jbc.M704273200. [DOI] [PubMed] [Google Scholar]
- Rice D, Curran T. Role of reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Rice DS, Sheldon M, D’Arcangelo G, Nakajima K, Goldowitz D, Curran T. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Sheppard AM, Hamilton SK, Pearlman AL. Changes in the distribution of extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J Neurosci. 1991;11:3928–3942. doi: 10.1523/JNEUROSCI.11-12-03928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Pearlman AL. Abnormal reorganization of preplate neurons and their associated extracellular matrix: an early manifestation of altered neocortical development in the reeler mutant mouse. J Comp Neurol. 1997;378:173–179. doi: 10.1002/(sici)1096-9861(19970210)378:2<173::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop PH. Molecular genetics of Alzheimer’s disease. Biol Psychiatry. 2000;47:183–199. doi: 10.1016/s0006-3223(99)00301-7. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- Vestling M, Wiehager B, Tanii H, Cowburn RF. Akt activity in presenilin 1 wild-type and mutation transfected human SH-SY5Y neuroblastoma cells after serum deprivation and high glucose stress. J Neurosci Res. 2001;66:448–456. doi: 10.1002/jnr.10006. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Zhang YW, Xu H, Thinakaran G. Pathological and physiological functions of presenilins. Mol Neurodegener. 2006;1:4. doi: 10.1186/1750-1326-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihl CC, Ghadge GD, Kennedy SG, Hay N, Miller RJ, Roos RP. Mutant presenilin-1 induces apoptosis and downregulates Akt/PKB. J Neurosci. 1999;19:5360–5369. doi: 10.1523/JNEUROSCI.19-13-05360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenhorn DM, Prieto EW, Celio MR. Localization of calretinin in cells of layer I (Cajal-Retzius cells) of the developing cortex of the rat. Dev Brain Res. 1994;82:293–297. doi: 10.1016/0165-3806(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Wen PH, De Gasperi R, Gama Sosa MA, Elder GA. Neural progenitor cells do not differentiate prematurely in presenilin-1 null mutant mice. Neurosci Lett. 2004;371:249–254. doi: 10.1016/j.neulet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Wen PH, De Gasperi R, Sosa MA, Rocher AB, Friedrich VL, Jr, Hof PR, Elder GA. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1-null mutant mice. Development. 2005;132:3873–3883. doi: 10.1242/dev.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PH, Shao X, Shao Z, Hof PR, Wisniewski T, Kelley K, Friedrich VL, Jr, Ho L, Pasinetti GM, Shioi J, Robakis NK, Elder GA. Overexpression of Wild Type But Not an FAD Mutant Presenilin-1 Promotes Neurogenesis in the Hippocampus of Adult Mice. Neurobiol Dis. 2002;10:8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M, Handler M, Shen J. Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev Biol. 2005;277:332–346. doi: 10.1016/j.ydbio.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Nakajima M, Aizawa H, Sahara N, Koizumi K, Sakai T, Usami M, Kobayashi S, Kuroyanagi H, Mori H, Koseki H, Shirasawa T. Impaired cell cycle control of neuronal precursor cells in the neocortical primordium of presenilin-1-deficient mice. J Neurosci Res. 2002;70:501–513. doi: 10.1002/jnr.10430. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu R, Wang R, Hong S, Xu H, Zhang YW. Presenilins regulate the cellular level of the tumor suppressor PTEN. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calretinin immunostaining reveals splitting of the preplate in Psen1−/− telencephalon. Coronally cut Vibratome sections from E15.5 wild type (A, C, E) or Psen−/− embryos (B, D, F) were immunostained for calretinin (red) along with a DAPI nuclear stain (blue). Sections through the lateral telencephalon are shown labeled for DAPI (A, B), immunostained for calretinin (C, D) or as a merged image (E, F). The marginal zone (MZ), cortical plate (CP), subplate region (SP) and intermediate zone (IZ) are indicated. Scale bar: 100 μm.