Summary
Expression of genes with tight and precise temporal and spatial control is desired in a wide variety of applications ranging from cultured cells and transgenic animals to gene therapy. While current inducible systems, such as RU486 and chemical inducers of dimerization (CID), have improved earlier inducible models (Gossen et al., 1995) (Wang et al., 1994), no single system is perfect at present. One potential drawback of these systems is leakage of transgene expression, causing limitations of each system. We have developed an inducible model containing both RU486 and CID systems, which in addition to inducing caspase activation, has potential applicability specifically to other genes encoding proteins that require a dimerization event for activation. This Double-Inducible Gene Activation System generates two barriers for the target gene expression and protein activation thereby minimizing leakage.
Keywords: RU486, CID, keratin 14, inducible gene expression, double-inducible system
INTRODUCTION
A major goal of gene therapy and genetic engineering of animals and cultured cells is manipulation or replacement of gene expression. Towards that goal, exquisite, non-leaky temporal and spatial control of gene expression can minimize artifacts and increase utility by eliminating basal signaling and potential toxicities. Several strategies have been utilized to achieve this goal. Previous studies have demonstrated the ability of the mifepristone (RU486)-inducible system to control spatial and temporal transgene expression (Ngan et al., 2002). This system uses a chimeric transcription factor that can reversibly bind to a target gene promoter to allow for regulation of transgene expression upon administration of RU486 (Fig. 1a) (Bo et al., 2005). This type of on and off regulation can be achieved in any cells or tissues along with the use of a tissue-specific promoter (Tsai et al., 1998). Another inducible system includes a chimeric precursor, such as caspase 3, harboring a dimer binding domain (CBD), and a chemical inducer of dimerization (CID) that can bind CBD to bring two molecules of caspase 3 together to form a dimer, and subsequently initiate caspase-3 self-activation through internal proteolysis (Fig. 1b). The precursor will remain biologically inactive until its exposure to CID (Fan et al., 1999) (Shariat et al., 2001). Both inducible systems are specific, reversible, non-toxic, and have a high induction potential. However, a drawback of each system is the potential for leakage of transgene expression. In this study, we have generated a transgenic mouse line harboring a system that combines these two inducible models, called the “Double-Inducible Gene Activation System.” While the double-inducible activation system retains all of the advantages of both inducible systems, it compensates for each system’s drawbacks, resulting in highly inducible, efficient, and stringent gene expression.
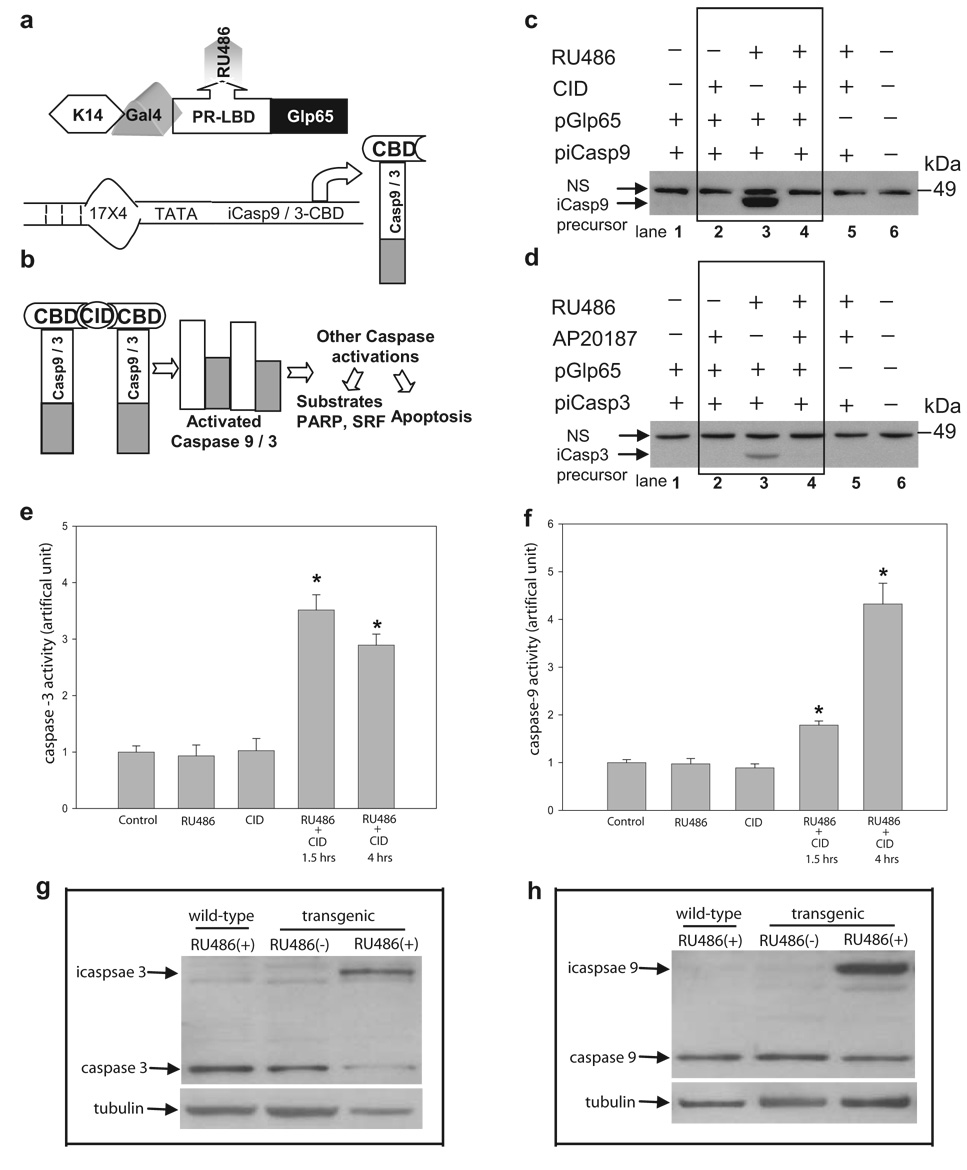
FIG. 1. Schematic diagram of the Double-Inducible Gene Activation System and its application in caspase-3 and caspase-9 inductions.
a: The chimeric transcription factor Glp65 with the ligand binding domain of the truncated human progesterone receptor (PR-LBDΔ), the GAL4 DNA binding domain, and the Glp65 transactivation domain of NF-κB is placed under the control of a keratin 14 (K14) promoter. CBD: CID binding domain b: The addition of CID forces aggregation of the chimeric caspase precursors, initiating self-activation. CID: chemical inducers of dimerization. c and d: Representative Western blots showed the target caspase-3 and caspase-9 gene inductions and activations. e and f: Caspase-3 and caspase-9 activity were measured under three different induction protocols. * P<0.05 compared to control. g and h: Induction of two transgenic caspases in mouse skin tissues compared to their endogenous ones.
We first evaluated this system in vitro in cell culture. Three plasmids were constructed. Chimeric transcription factor, Glp65 containing progesterone receptor ligand-binding domain (PR-LBDΔ) (Fig. 1a), and pTATA-HA-iCasp3 (myristoylated) or pTATA-HA-iCasp9 (Fig. 1a and 1b), carrying CBD, were cotransfected in CV1 cells (monkey kidney fibroblasts). Cell lysates were assessed by Western blot using an anti-HA antibody. As shown in figures 1c and 1d, RU486 induced the expression of iCasp9 (Fig. 1c, lane 3) or iCasp3 precursors (Fig. 1d, lane 3). These precursors remain inactive without the addition of CID. With the subsequent application of CID, caspase 3 and caspase 9 were activated as evidenced by the disappearance of the caspase precursors due to the HA tag carrying a caspase cleavage consensus site, DVPD (lanes 4 in Figs. 1c and 1d). No caspase precursors were induced with the application of CID alone (lanes 2 in Figs. 1c and 1d). Because of this caspase cleavage consensus site within HA tag, activation of caspase 3 and caspase 9 were further verified by using Clonetech ApoAlert Caspase Assay as shown in Figure 1e and 1f. Three-fold and two-fold increases in caspase-3 and capase-9 activity were observed in cells treated with both drugs for 1.5 hrs, but not in either RU486 or CID treated alone. A continue increase in caspase-9 activity was detected after 4hrs dual-drug treatment (Fig. 1f) compared to a slight decrease in caspase-3 activity suggesting a different activation pattern between the two caspases.
Next, we assessed the effectiveness of our system in vivo in two bigenic mouse lines, K14-Glp65/iCasp3 and K14-Glp65/iCasp9. The bigenic mice were generated by breeding two individual mouse lines. The first expressed the chimeric factor Glp65 driven by the epidermal-specific keratin 14 (K14) promoter. The second transgenic mouse line carried a transgene consisting of either inducible caspase-3 or caspase-9 precursors preceded by GAL4 binding sites (Figs. 1a and 1b). The breeding of these two lines generated mice harboring both RU486 and CID regulators. The bigenic mice between 8 and 15 weeks of age were subjected to RU486 treatment using a 21-day-release pellet (945 µg/pellet) that was surgically inserted beneath the skin in the region of the posterior scapula. The addition of RU486 activates the regulator and allows it to bind to the GAL4 DNA binding site of the target gene (either icasp3 or icasp9) and induces target gene expression. After seven days of RU486 treatment, CID was applied at 50 µg per day intraperitoneally to induce self-activation of caspase precursors. Placebo pellets lacking RU486 and CID-diluent were used for control groups (Shariat et al., 2001). Five days following CID, reddening of the surface layers of the skin and thickness was observed on the skin covering the ears. We did not observe this phenotype in either control mice or in bigenic mice that only received RU486 treatment.
To closely monitor the phenotypic changes of the skin after the activation of caspases, we tested the double induction system in neonates by delivering RU486 in utero. The RU486 was dissolved in sesame oil and delivered intraperitoneally for 5 days at a dosage of 100 µg/kg per day into pregnant female K14-Glp65/iCasp3 and K14-Glp65/iCasp9 bigenic mice at 14.5 days gestation. To counter the abortion side-effect of RU486, progesterone (Sigma) at 0.5 mg/mouse per day was applied along with RU486 (Cao et al., 2002). Transgenic pups were treated topically with 1 µg of CID on their dorsal anterior-posterior (AP) axes twice per day and monitored closely for phenotypic changes or visible signs of apoptosis. Control littermates were treated with only the CID diluent. After two days of CID induction, the skin of the CID-treated pups exhibited peeling and appeared dehydrated when compared to the skin of the control littermates. On day four, skin biopsies were taken from the back skin of the pups and fixed in 4% paraformaldehyde (Koster et al., 2004). The expression levels of induced caspase 3 and caspase 9 were evaluated by Western blot as shown in Figure 1g and 1h, strong expressions in both conditional proteins were detected exclusively in RU486-treated transgenic mice, but not in wild-type mice with RU486 or transgenic mice without RU486 treatment.
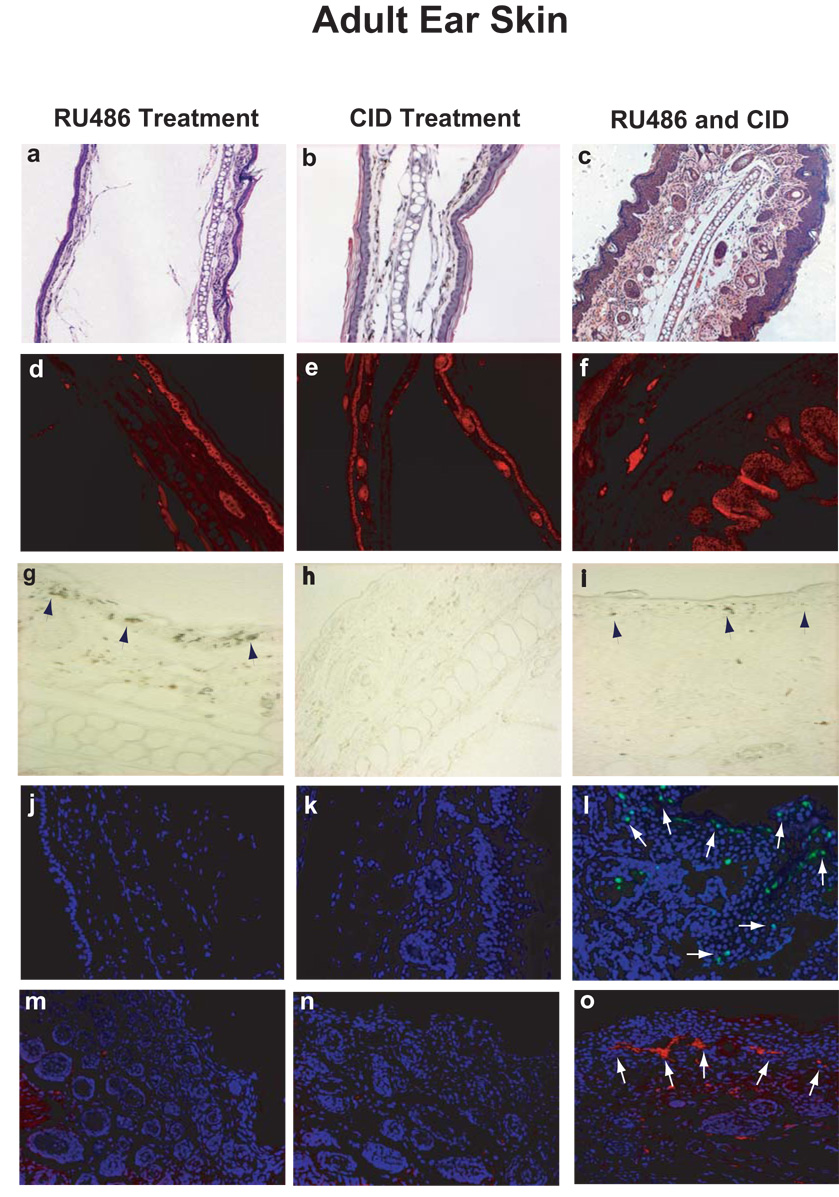
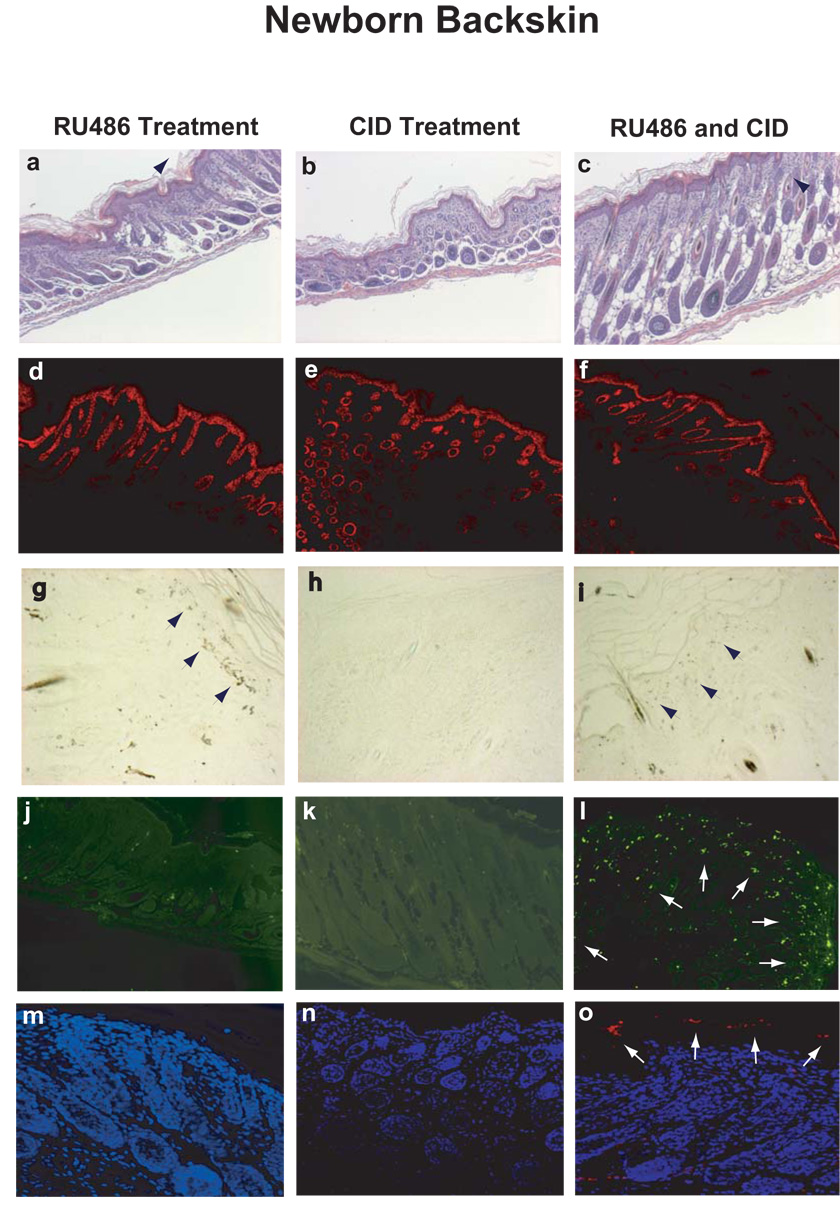
Induction of the two caspases was then further studied by immunohistochemical staining with an anti-HA antibody. The induced activation of caspases was probed by two antibodies exclusively against active forms of caspase 3 and caspase 9, respectively. Apoptosis was evaluated by TUNEL staining. The results with three different induction protocols from adult mouse ear skins and newborn mouse back skins were summarized in Figure 2 and Figure 3, respectively.
FIG. 2. Microscopic and histological analysis of skin from adult mouse ear.
Skin sections from K14-Glp65/iCasp3 adult mouse ears were visualized by H&E stain (2a–c), and were immunostained by keratin 14 antibody (2d–f). Caspase-3 induction (2g) and activation (2i) were shown by immunohistochemical staining with anti-HA antibody. HA-positive dark brown cells were indicated by arrows. The skin apoptosis was evaluated by TUNEL assay (2j-1). Activated caspase 3 was detected by a specific antibody exclusively against active form of caspase 3 (red indicated by arrows) (2m–o).
FIG. 3. Microscopic and histological analysis of skin from newborn back skin.
Skin sections from K14-Glp65/iCasp9 newborn mice back skin were visualized by H&E stain (3a–c), and were immunostained by keratin 14 antibody (3d–f). Caspase-9 induction (3g) and activation (3i) were shown by immunohistochemical staining with anti-HA antibody. HA-positive dark brown cells were indicated by arrows. The skin apoptosis was evaluated by TUNEL assay (3j-1). Activated caspase 9 was detected by a specific antibody exclusively against active form of caspase 9 (red indicated by arrows) (3m–o).
Strong expression of caspase precursors was observed in RU486-alone treated group (Fig. 2g and Fig. 3g) but not in CID-only treated group (Fig. 2h and Fig. 3h). Less straining was found in dual-drug treated groups indicating caspase cleavage for HA tag (Fig. 2i and Fig. 3i). Meanwhile, after treatment with both RU486 and CID, the skin became hyperplasic as shown in figure 2c and figure 3c. We found significant increases in TUNEL positive nuclei (green) in mice with the two drug treatments (Fig. 2l and Fig. 3l), but not in either the RU486-only (Fig. 2j and Fig. 3j) or the CID-only (Fig. 2k and Fig. 3k) treated group, which displayed normal skin structures with no or basal level of apoptosis. Consistent with TUNEL assay, great increases in active caspase 3 and caspase 9 were detected by antibodies specific against caspase-3 and caspase-9 active forms in dual-drug treated skins (red stain in Fig. 2o and Fig. 3o). The apoptotic cells were predominantly localized at epidermal layers that corresponded with K14 expression in basal cells (Fig. 2d–2f and Fig. 3d–3f). These results suggested that the double-inducible gene activation system was efficient and highly regulated.
It must be noted that for some genes, even undetectable amounts of leakiness could be detrimental. Therefore, although leakage of basal expression was not detected in the RU486 or CID-treated groups, even minor amounts of leakiness were avoided with the double induction system. In addition to inducing caspase activation, the system has a more general applicability specifically to those proteins that require a dimerization event for activation. The transgenic caspase mouse lines are versatile too as they can be bred with a transgenic mouse line expressing any tissue-specific chimeric transcription factor to achieve a tissue-specific inducible-gene expression.
METHODS
Generation of Bigenic Animal Models
We generated three different transgenic mice lines to test the inducible systems. The first mouse line expressed a tissue-specific chimeric transcription factor (Glp65) that can be activated by RU486, which functioned as the first induction in our system. In this case, an epidermal-specific keratin 14 (K14) promoter was used to direct gene expression to the keratinocytes of the basal epidermis and hair follicles (K14-Glp65) (Fig. 1a) (Cao et al., 2002) (Kucera et al., 1996). The second mouse line expressed a target transgene. For this second induction, we generated caspase-3 and caspase-9 mice containing four copies of the 17-mer GAL4 binding site in the promoter region (Fig. 1a). Full length of caspase-3 cDNA with CID binding domain and HA-tag fragment was cloned by PCR from an expression vector pSH1/M-Fv2-Yama-E (Fan et al., 1999) and was then subcloned into p17×4 TATA-H2kd vector (Bo et al., 2005) by ClaI and BamH I to generate pTATA-HA-iCasp3 mice. The final DNA fragment for microinjection was cut out by SphI and EcoRI. The same strategy was applied for the generation of the inducible caspase-9 construct. By breeding these transgenic caspase lines to the K14-Glp65 mice, we generated bigenic mouse lines called K14-Glp65/iCasp3 and K14-Glp65/iCasp9, which harbored both RU486 and CID regulators as depicted in figure 1. With the application of RU486, the Glp65 fusion protein was exclusively expressed in skin keratinocytes, which subsequently initiated caspase precursor induction. In the presence of lipid-permeable dimeric ligand CID (AP20187, ARIAD Pharmaceuticals), which was applied intraperitoneally to adult mice or daubed topically to the back skin of neonates, the inactive caspase was forced to dimerize, leading to an autoproteolysis and self-activation (Fan et al., 1999; Mallet et al., 2002; Shariat et al., 2001).
Immunofluorescence, Immunohistochemistry and Caspase Activity Assay
Histological changes were visualized by hematoxylin and eosin (H&E) staining. Keratinocytes in the basal epidermis and hair follicles were revealed by immunostaining with anti-keratin 14 antibody (Yuspa et al., 1989). Anti-HA and anti-tubulin antibodies (Santa Cruz) were applied for the Western blot and immunohistochemical stainings. Apoptosis was evaluated by TUNEL assays (Roche). Caspase-3 and caspase-9 activity was evaluated by ApoAlert Caspase Assay (Clonetech). Active caspase 3 and caspase 9 were visualized by antibodies (Santa Cruz) specific against their active isoforms.
ACKNOWLEDGEMENTS
We thank Renee Braun, Brenda Johnson, Ling Qian, Wei Yu and Li Chen for their excellent technical assistance. Correspondence and requests for materials should be addressed to Jiang Chang. jchang@ibt.tamhsc.edu.
Contract grant sponsors: National Institute of Health, contract grants numbers: P01-HL49953, R01-HL64356 (to RJS), and R01-HL72897 (to LW); American Heart Association Scientist Development Grant (to JC), contract grant number: 0335155N.
REFERENCES
- Bo J, Yu W, Zhang YM, Demayo FJ, Wei L. Cardiac-specific and ligand-inducible target gene expression in transgenic mice. J Mol Cell Cardiol. 2005;38:685–691. doi: 10.1016/j.yjmcc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Cao T, He W, Roop DR, Wang XJ. K14-GLp65 transactivator induces transgene expression in embryonic epidermis. Genesis. 2002;32:189–190. doi: 10.1002/gene.10063. [DOI] [PubMed] [Google Scholar]
- Fan L, Freeman KW, Khan T, Pham E, Spencer DM. Improved artificial death switches based on caspases and FADD. Hum Gene Ther. 1999;10:2273–2285. doi: 10.1089/10430349950016924. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera GT, Bortner DM, Rosenberg MP. Overexpression of an Agouti cDNA in the skin of transgenic mice recapitulates dominant coat color phenotypes of spontaneous mutants. Dev Biol. 1996;173:162–173. doi: 10.1006/dbio.1996.0014. [DOI] [PubMed] [Google Scholar]
- Mallet VO, Mitchell C, Guidotti JE, Jaffray P, Fabre M, Spencer D, Arnoult D, Kahn A, Gilgenkrantz H. Conditional cell ablation by tight control of caspase-3 dimerization in transgenic mice. Nat Biotechnol. 2002;20:1234–1239. doi: 10.1038/nbt762. [DOI] [PubMed] [Google Scholar]
- Ngan ES, Schillinger K, DeMayo F, Tsai SY. The mifepristone-inducible gene regulatory system in mouse models of disease and gene therapy. Semin Cell Dev Biol. 2002;13:143–149. doi: 10.1016/s1084-9521(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Desai S, Song W, Khan T, Zhao J, Nguyen C, Foster BA, Greenberg N, Spencer DM, Slawin KM. Adenovirus-mediated transfer of inducible caspases: a novel "death switch" gene therapeutic approach to prostate cancer. Cancer Res. 2001;61:2562–2571. [PubMed] [Google Scholar]
- Tsai SY, O'Malley BW, DeMayo FJ, Wang Y, Chua SS. A novel RU486 inducible system for the activation and repression of genes. Adv Drug Deliv Rev. 1998;30:23–31. doi: 10.1016/s0169-409x(97)00104-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, O'Malley BW, Jr, Tsai SY, O'Malley BW. A regulatory system for use in gene transfer. Proc Natl Acad Sci U S A. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]