Abstract
During embryonic development, complex events such as cellular proliferation, differentiation, survival, and guidance of axons are orchestrated and regulated by a variety of extracellular signals. Receptor tyrosine kinases mediate many of these events with several playing critical roles in neuronal survival and axonal guidance. It is evident that not all the receptor tyrosine kinases that play key roles in regulating neuronal development have been identified. In this study, we have characterized the spatial-temporal expression profile of a recently identified receptor tyrosine kinase, anaplastic lymphoma kinase (ALK), in embryonic chick by means of whole mount in situ hybridization in conjunction with immunohistochemistry. Our findings reveal that Alk is expressed in sympathetic and dorsal root ganglia as early as stage 19. In addition, mRNA is expressed from stage 23/24 (E4) until stage 39 (E13) in discrete motor neuron subsets of chick spinal cord along with a select group of muscles that are innervated by one of these particular motor neuron clusters. Interestingly, expression within the spinal cord is coincident with the onset and duration of motor neuron programmed cell death and during the period of musculature innervation and synapse formation. Hence, the data presented here identify ALK as a novel candidate receptor for regulating critical events in the development of neurons in both the central and peripheral nervous system.
Keywords: motor neuron, receptor tyrosine kinase, sympathetic ganglia, dorsal root ganglia, spinal cord
INTRODUCTION
Receptor tyrosine kinases (RTKs) comprise a distinct protein class with multiple members expressed in the developing central and peripheral nervous systems (CNS, PNS) where they regulate such key events as growth, cell proliferation, differentiation, cell survival, and synaptogenesis (Schlessinger, 2000). In particular, RTKs play a critical role in the survival of neurons from populations in which a large number undergo programmed cell death (PCD) during target innervation (Huang and Reichardt, 2001). Even with the discovery of numerous survival-promoting neurotrophins, the application of these ligands, individually or in concert, fails to save all motor neurons (MNs) from death; conversely, removal of specific neurotrophins or the RTKs through which they signal results in no greater than 40% PCD in MNs not originally intended to die (Gould and Oppenheim, 2004). This suggests that not all the factors that mediate motor neuron survival have yet been recognized. Thus, further identification of additional RTKs that play key roles in regulating neuronal development is still needed.
Anaplastic lymphoma kinase (ALK) is a recently identified RTK whose function in vivo remains speculative. ALK was originally identified as an oncogenic manifestation in anaplastic large cell lymphomas (ALCL) (Shiota et al., 1994). This non-Hodgkin's lymphoma has been attributed to a t(2;5) chromosomal translocation resulting in a nucleophosmin (NPM)-ALK fusion protein, known as p80 and composed of the amino portion of NPM and the intracellular carboxy portion of ALK, which gives rise to constitutively activated ALK signaling (Pulford et al., 2004). Identification of normal full-length human ALK revealed a single-pass transmembrane RTK belonging to the insulin receptor subfamily (Morris et al., 1997). Previous studies in mice have demonstrated an expression profile restricted to discrete areas of the developing CNS and PNS (Iwahara et al., 1997; Morris et al., 1997). In Drosophila, ALK is expressed in the developing CNS and is also involved in visceral mesoderm differentiation (Lee et al., 2003; Loren et al., 2003; Loren et al., 2001). ALK has also been identified in C. elegans where it is expressed at low levels in the nervous system and is proposed to inhibit or destabilize synapse differentiation (Liao et al., 2004). While these papers do not specifically address the function(s) of ALK, its conserved expression within the CNS suggests an important role(s) in neural development.
Investigations regarding the physiological function of mammalian ALK have yielded intriguing yet varied results. Chimeric fusions of ALK were among the first engineering attempts, in which the extracellular domain (ECD) of ALK was replaced with the IgG 2b Fc or epidermal growth factor receptor (EGFR) domain, to create a chimeric receptor that could dimerize and activate ALK signaling (Souttou et al., 2001). In the case of the ALK.Fc chimera, PC12 cells expressing the fusion protein underwent neurite outgrowth indicating ALK's potential involvement in neuronal differentiation (Souttou et al., 2001). Transfection of the EGFR/ALK hybrid into NIH3T3 cells followed by administration of epidermal growth factor (EGF) induced cell transformation suggesting a role for ALK in cell proliferation (Piccinini et al., 2002).
Two growth factors, pleiotrophin (PTN) and midkine (MK), have been identified as potential ligands for the ALK receptor (Stoica et al., 2001; Stoica et al., 2002). Interactions of PTN and MK with ALK in fibroblast, adrenal carcinoma, neuroblastoma and glioblastoma cell lines stimulated anti-apoptotic signaling and promoted cell survival (Bowden et al., 2002; Powers et al., 2002; Stoica et al., 2002). While PTN and MK have a demonstrated affinity for ALK in vitro, they also bind receptor protein tyrosine phosphatase-ζ/β (RPTP-ζ/β) (Maeda et al., 1999; Maeda et al., 1996) and syndecans (Kojima et al., 1996). Motegi and colleagues (2004) developed an agonist monoclonal antibody against the ECD of human ALK in order to solely investigate ALK-mediated activity. Their results indicated that ALK transmits both mitogenic and differentiation signals in SK-N-SH cells (Motegi et al., 2004).
The chick is a useful model system for the elucidation of ALK's in vivo function in the developing embryo given the ease of manipulations in ovo. To this end, we have determined the spatial-temporal expression pattern of Alk during development of both the spinal cord and PNS. In the present study, we describe the identification and expression of Alk transcript during chick development by means of whole-mount in situ hybridization using two separate riboprobes, each targeting a distinctly different region of the Alk gene. In conjunction with immunohistochemistry we demonstrate that Alk is expressed in discrete subsets of spinal cord motor neurons during the period of programmed cell death, and in a susbset of muscles, suggesting a possible role in neuromuscular synapse formation. In the PNS, Alk is robustly expressed in sympathetic ganglia throughout their genesis, differentiation and period of cell death and in progenitor cells in the dorsal root ganglia. Thus these expression patterns are highly suggestive of an important role for ALK in the genesis and differentiation of the nervous system and point to novel molecular interactions for motor neurons and cells in the PNS during key steps of development.
MATERIALS AND METHODS
In situ Hybridization
Fertilized White Leghorn chicken eggs were obtained from SPAFAS (Charles River) and incubated at 37°C on mechanized inclined racks that slowly raise and lower the eggs, creating a simulated “rocking” motion. Embryos were dissected and staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1992). Chick embryos from a range of selected stages, including 19, 22, 23, 24, 25, 35, and 39, were fixed in 4% paraformaldehyde in 1X phosphate buffered saline, pH 7.4 (PBS), and subjected to whole-mount in situ hybridization (Nieto et al., 1996; Wilkinson and Nieto, 1993). Briefly, two separate digoxygenin-labeled antisense riboprobes were generated, one targeting a portion of ALK's intracellular domain sequence (∼800 n.t. – corresponding to nucleotides 3720−4527 in human ALK; NCBI accession no. NM_004304) and one targeting a region within the extracellular domain sequence (∼700 n.t. – corresponding to nucleotides 2043−2714 in human ALK; NCBI accession no. NM_004304). The riboprobe targeting the intracellular portion was derived from a cloned fragment of Alk cDNA (provided by Dr. Douglas Clary, Sugen) obtained during a partial library screen using st 35 chick spinal cord. The extracellular domain probe was synthesized from a cloned fragment of Alk cDNA generated via RT-PCR using total RNA from st 25 chick spinal cord. Primers were designed using available predicted chicken ALK sequence from the Ensembl database (www.ensembl.org/Gallus_gallus) (forward 5’-ACTGGCTGTTCACAACATGTGGTG, reverse 5’-GATCTTCCTCCAGTAGCACCTTCCAG). Expression patterns obtained for the two probes were identical, and sense-control riboprobes yielded no overlapping signal. Embryos were dehydrated in a series of graded methanol washes for 15 min each (25%, 50%, 75%, and 100% in PBS, pH 7.4, containing 0.25% Tween-20 (PBST)) and then rehydrated by performing the methanol washes in reverse. After 1h incubation in 6% H2O2 followed by proteinase K (10 ug/mL) treatment, cRNA digoxygenin-labeled probes were hybridized in 50% foramide/5X SSC, pH 4.5/50μg/mL heparin/50 μg/mL yeast tRNA at 70°C overnight. After hybridization, embryos were washed three times in 50% formamide/5X SSC, pH 4.5/1% SDS for 30 min at 70°C, three times in Tris buffered saline, pH 7.5 (TBS), with 0.25% Tween-20 for 5 min at room temperature, three times in 50% formamide/2X SSC, pH 4.5, for 30 min at 60°C, and blocked in 10% normal goat serum (NGS) in TBS, pH 7.5, containing 0.1% Tween-20 (TBST) for 90 min at room temperature. Detection of probe hybridization was achieved with α-digoxygenin-AP antibody (Roche, Indianapolis, IN; 1 093 274) (1:3000) in 1% NGS in TBST at 4°C overnight and subsequent incubation with the chromogenic substrate, nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) (prepared solution, Sigma-Aldrich, St. Louis, MO; B-1911). All embryos were then post-fixed in 3.7% formaldehyde and prepared for either cryosectioning or vibratome slicing.
Immunohistochemistry
Following in situ hybridization (without Proteinase K treatment), embryos were embedded in either optimal cutting temperature (OCT) compound (Tissue-Tek) when cryosectioning or 5% agarose/8% sucrose-PBS when vibratome-sectioning and then sectioned (14 μm for cryosections, 100 μm for vibratome slices). Stage 25 embryo slide-mounted cryosections were blocked with 10% NGS in TBS, pH 7.5, for 1h at room temperature and incubated overnight at 4°C with α-Isl1 or α-Lim1/2 primary antibody (1:10) in 0.4% Triton-X and 10% NGS in TBS, pH 7.5. Isl1 monoclonal antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA; 40−2D6) was raised in mouse against residues 86−175 of chicken Isl1 expressed in E. coli. The antibody has been well characterized for its recognition of the transcription factor in MNs within the medial portion of the lateral motor column (Tsuchida et al., 1994). Lim1/2 monoclonal antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA; 4F2) was raised in mouse against residues 1−360 of rat Lim2 expressed in E. coli. The antibody has been well characterized for its identification of Lim1 in MNs within the lateral portion of the lateral motor column (Tsuchida et al., 1994). Slides were rinsed four times in 10% NGS and incubated for 1h at room temperature with a biotinylated goat anti-mouse IgG secondary antibody (Vector, Burlingame, CA; BA-9200) (1:600) followed by fluorescein avidin (Vector, Burlingame, CA; A-2011) (1:600) for 1h at room temperature, rinsed, and mounted in Prolong Anti-fade (Molecular Probes, Eugene, OR; P-7481). Stage 23 embryo vibratome slices were blocked in 10% NGS for 2h at 4°C and incubated overnight at 4°C with α-Tuj1 (1:1000) and α-phoso-histone H3 (1:750) or α-Isl1 (1:10) and α-phospho-histone H3 (1:750) primary antibodies in 0.4% Triton-X and 10% NGS. Tuj1 monoclonal antibody (Berkeley Antibody Company, Berkeley, CA; MMS-435P) was raised in mouse against rat microtubules (peptide epitope, CEAQGPK). It is well characterized for recognition of neuron specific Class IIIβ-tubulin (Lee et al., 1990). H3 polyclonal antibody (Upstate, Charlottesville, VA; 06−570) was raised in rabbit against an N-terminal peptide (ARKpSTGGKAPRKQL) corresponding to amino acids 8−21 of chicken histone H3. The antibody has been characterized as a mitosis marker, recognizing phosphorylated histone H3 (Rifkin et al., 2000). Slices were washed six times in 10% NGS for 10 min and incubated for 2h at room temperature with goat anti-mouse IgG alexafluor 568 and goat anti-rabbit IgG alexafluor 488 secondary antibodies (Molecular Probes, Eugene, OR; A-11004 & A-11008, respectively) (1:2000), rinsed, and mounted in Prolong Anti-fade.
Whole-mount images were captured using Axiovision 3.1 software (Zeiss) in conjunction with an AxioCam HRC digital video camera (Zeiss). In situ hybridization and immunohistology sections were photographed using Kodak Elite Chrome 100 (fine grain) color slide film and a 35 mm camera (Nikon FX-35DX) attached to a Nikon Microphot-FXA fluorescence scope via the phototube assembly. Digital images of color slides were acquired using a Nikon Super Coolscan 4000 slide scanner and accompanying Nikon Scan software. Adobe Photoshop 7.0.1 was utilized to convert whole-mount images to black and white, uniformly modify image size for each photomicrograph within a figure, and to adjust image background color to near-white using the selective color adjustment function.
Limb Ablation
One-sided limb ablations were performed on chick hind limb buds at stage 17/18. Briefly, eggs were placed on their sides and a portion of each shell was cut away to expose and provide access to the developing embryo. A 5% solution of India ink was injected underneath the embryo to help visualize the location of limb buds. Once a hind limb bud had been identified, a pair of fine-tipped dissection forceps was used to clip and excise the prospective limb. After the surgical procedure, eggs were sealed with tape, and embryos were allowed to develop to stage 23/24 followed by in situ hybridization to determine impact upon Alk expression.
RESULTS
Expression of Alk mRNA in the developing PNS
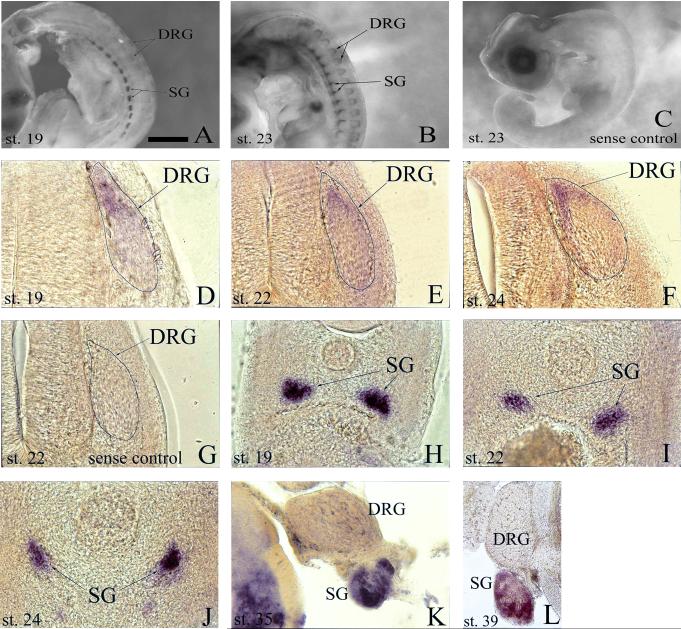
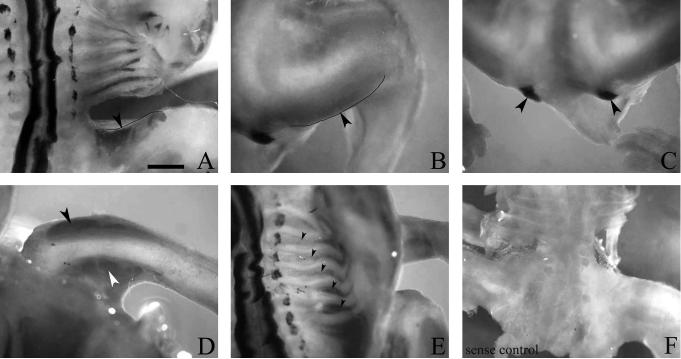
In the peripheral nervous system, prominent Alk expression is first detected in the cells of the sympathetic ganglia (SG) at st 19 (Fig. 1A, 1H). Inspection at later stages, including 22, 23, 24, 35, and 39, reveals the persistence of transcript in the SG with no obvious weakening of the signal intensity as far along as embryonic day 13 (E13), the latest stage investigated (Fig. 1B, 1I-1L). In the developing dorsal root ganglia (DRG), the onset of transcript becomes detectable at st 19 in the dorsal pole region (Fig. 1A, 1D). As development continues, transcript extends from the dorsal pole to the medial and lateral perimeter of the DRG and becomes more abundant with maximal expression observed at st 24/25 (Fig. 1A, 1B, 1D-F). By st 35 and beyond, in situ hybridization reveals no discernable Alk mRNA in the DRG (Fig. 1K, 1L).
Fig. 1.
Analysis of developmental expression of anaplastic lymphoma kinase (Alk) mRNA in chick peripheral nervous system by whole-mount in situ hybridization. A, B: Transcript is expressed in both sympathetic ganglia (SG) and dorsal root ganglia (DRG) at stages 19 and 23. C: No signal is detected in stage 23 whole-mounts using sense RNA probe as a negative control. D-F: Transverse sections (post-hybridization) reveal Alk expression in the progenitor zones of the DRG at stages 19 (D), 22 (E), and 24 (F). G: Transverse section (post-hybridization) of stage 22 embryo hybridized with sense RNA probe as a negative control. H-L: Transverse sections (post-hybridization) show presence of Alk in SG during stages 19 (H), 22 (I), 24 (J), 35 (K), and 39 (L). Scale bar: A, B, 1 mm; C, 1.5 mm; D, 65 μm; E-J, 120 μm; K, 175 μm; L, 350 μm.
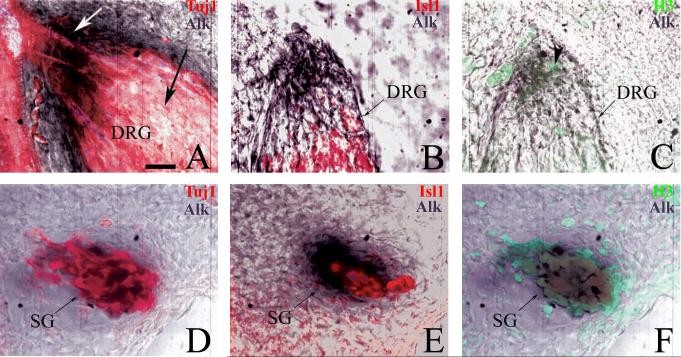
In the DRG, Alk+ cells were localized to the dorsal pole and periphery – areas known to be composed of mitotically active cells (Rifkin et al., 2000; Wakamatsu et al., 1997). From st 22 to 27, the DRG is spatially organized with an inner core composed primarily of post-mitotic neurons surrounded by a perimeter of dividing progenitor cells (Rifkin et al., 2000). To confirm that Alk is expressed by mitotically active cells, in situ hybridization was combined with IHC to stain for the mitosis marker, H3, and traditional neuronal markers, Tuj1 and Isl1. At st 23, there is no overlap of Alk+ and Tuj1+ cells in the DRG core nor is there apparent co-expression of Isl1 and Alk in this region either (Fig. 2A, 2B). In contrast, H3 staining reveals that a majority of Alk+ cells are located in the mitotically active dorsal pole with several H3+ cells co-expressing Alk (Fig. 2C). There are also Alk+ cells present in the progenitor zones that are neither H3+ nor Tuj1/Isl1+ suggesting that these cells are possibly preparing to enter the cell cycle, are in S-phase, or are finishing mitosis and have not yet differentiated.
Fig. 2.
Anaplastic lymphoma kinase (Alk) mRNA is expressed in mitotically active cells of the dorsal root ganglia (DRG) and sympathetic ganglia (SG). Stage 23 embryos were vibratome-sectioned after whole-mount in situ hybridization with Alk probe and immunostained with α-Tuj1 or α-Isl1 and α-H3. Alk = dark purple, Tuj1 and Isl1 = red, H3 = green. A, B: In the DRG, Alk is restricted to the progenitor zone (white arrow) with no transcript present in the core (black arrow). C: Multiple cells in the dorsal pole are Alk+ and co-express phoso-histone H3 (arrowhead). D-F: In the SG, the majority of Alk+ cells co-express Tuj1 (D) and H3 (F) with fewer also expressing Isl1 (E). Pseudo-coloration of confocal images was performed via Adobe Photoshop 7.0.1 in order to better visualize dual signals. Scale bar: A-F, 30 μm.
In the SG, Alk+ cells were distributed throughout the ganglia and co-expressed markers of both mitotically active and post-mitotic cells (Fig. 2D-F). Given that the SG is unique in that dividing cells also express markers of post-mitotic neurons (Avivi and Goldstein, 1999; Rothman et al., 1978), including neurofilament (NF), Tuj1, and Isl1, the most parsimonious conclusion is that both neurons and mitotically active sympathetic precursors express Alk. Expression persists as late as E13 indicating that Alk is clearly expressed by post-mitotic neurons in both the primary and secondary chain of SG.
Expression of Alk mRNA in the developing spinal cord
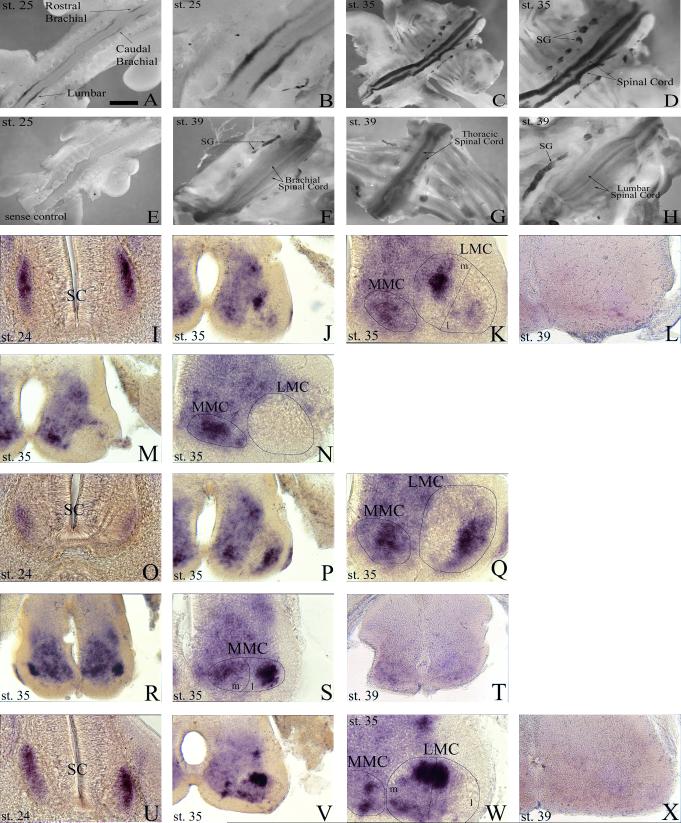
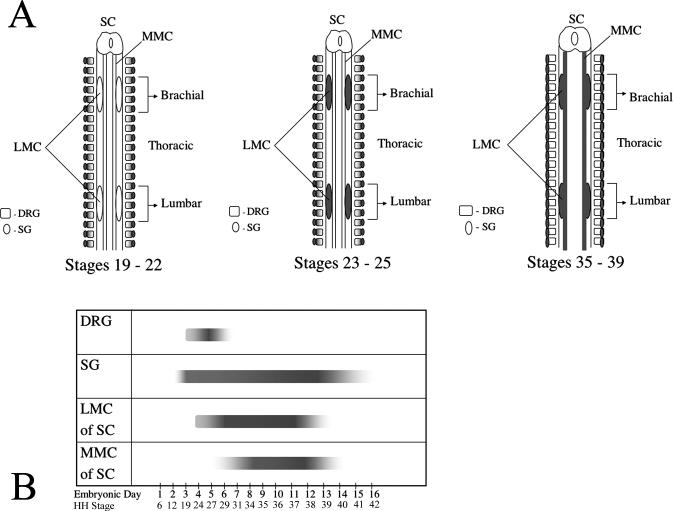
In the spinal column of chordates, MNs can be divided into two classes, those of the medial motor column (MMC) and those of the lateral motor column (LMC). The MMC is further divided into a medial portion that is present at all anterioposterior levels of the spinal cord and a lateral portion that is present only at thoracic levels. MNs of the medial MMC extend axons to axial muscles while those from the lateral MMC project axons to body wall muscles (William et al., 2003). The LMC also can be subdivided into medial and lateral subgroups, both of which are present only at limb (brachial and lumbar) levels of the spinal cord. Medial LMC MNs innervate ventrally derived limb muscles while lateral LMC MNs innervate dorsally derived limb musculature (Landmesser, 2001). To determine which divisions of MNs, if any, were expressing Alk during spinal cord development, transverse sections from embryos (st 24, 35 & 39) subjected to whole-mount in situ were collected (Fig. 3I-X) with select sections subsequently immunostained to identify MMC and LMC neurons based on specific transcription factor (Isl1 & Lim1) expression (Fig. 4).
Fig. 3.
Alk is dynamically expressed temporally and spatially in the spinal cord. A: Transcript is restricted to brachial and lumbar regions of the spinal cord at stage 25. B: Higher magnification of lumbar region from A. C: Expression of Alk mRNA is detected throughout the spinal cord at stage 35. D: Higher magnification of spinal cord from C. E: No discernable expression is detected in stage 25 whole-mounts using sense RNA probe as a negative control. F-H: Alk expression is waning in brachial (F), thoracic (G), and lumbar (H) regions of the spinal cord at stage 39. I-L: Transverse vibratome sections (post-hybridization) through the rostral brachial portion of the spinal cord reveal the presence of Alk at stages 24 (I) and 35 (J, K), but by stage 39 (L) transcript is virtually gone. M, N: “Break” in expression where LMC is devoid of transcript, creating a rostral and caudal separation. O-Q: Vibratome sections through the caudal brachial portion of the spinal cord reveal Alk mRNA at stages 24 (O) and 35 (P, Q). R-T: Thoracic sections demonstrate Alk is strongly expressed in spinal cord at stage 35 (R, S), but has greatly diminished by stage 39 (T). U-X: Alk expression in the lumbar level of the spinal cord at stages 24 (U) and 35 (V, W) with its virtual disappearance at stage 39 (X). LMC, lateral motor column; MMC, medial motor column; SC, spinal cord; SG, sympathetic ganglia. LMC and MMC were identified and demarcated based on position in the ventral portion of the spinal cord with medial (m) and lateral (l) halves representing an estimate of where separation occurs. K, N, Q, S, and W are higher magnification views of J, M, P, R, and V respectively. Scale bar: A, 1 mm; B, 450 μm; C, 1.7 mm; D, E, 1.3 mm; F-H, 1.5 mm; I, O, U, 100 μm; J, L, M, P, R, T, V, X, 160 μm; K, N, Q, S, W, 80 μm.
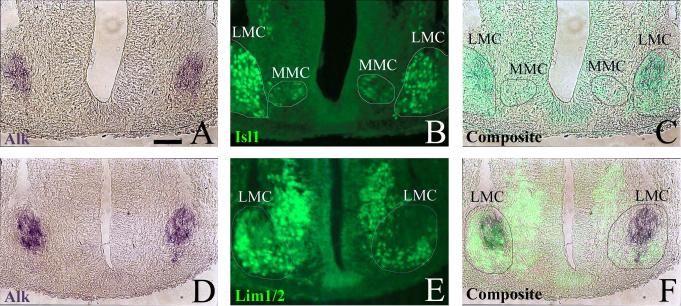
Fig. 4.
Alk is expressed by discrete subpopulations of motor neurons. A, B: Stage 25 embryo from Alk whole-mount in situ hybridization (dark purple) was cryosectioned and then immunostained with α-Isl1 (green). C: Alk and Isl1 staining are shown overlaid as a composite. D, E: Stage 25 embryo from Alk whole-mount in situ hybridization (dark purple) was cryosectioned and then immunostained with α-Lim1/2 (green). F: Alk and Lim1/2 staining are shown overlaid as a composite. Medial motor column (MMC) and lateral motor column (LMC) have been demarcated. The MMC and medial half of the LMC are known to express Isl1, however, the lateral half of the LMC does not. Conversely, the lateral LMC expresses Lim1 while the MMC and medial LMC do not. Positional identification of Alk and motor column markers indicate Alk is limited to cells of the LMC. Composite images were created using Adobe Photoshop 7.0.1. Scale bar: A-F, 50 μm.
Nascent Alk expression is first detected in the chick spinal cord at st 23/24 with definitive expression occurring at st 24/25. At these early stages, transcript is restricted to the brachial and lumbar levels of the spinal cord with an interruption or “break” (devoid of transcript) within the mid-brachial region, creating a rostral and caudal component of Alk expression at the brachial level (Fig. 3A, 3B, 3I, 3O, 3U). Expression occurs in the lateral edge of the ventral horn corresponding to MNs of the LMC found only at limb levels (Fig. 3I, 3O, 3U). Figure 4 confirms that these Alk+ cells express characteristic markers of LMC MNs. At st 25, the LMC is separating into a medial and lateral half with the former's MNs expressing Isl1 and the latter's expressing Lim1 (Shirasaki and Pfaff, 2002). Immunohistochemical staining with α-Isl1 (Fig. 4B) and α-Lim1/2 (Fig. 4E) indicates that the Alk+ cells (Fig. 4A, 4D) are located in the prospective medial division of the LMC with the majority of them co-expressing Isl1 (Fig. 4C, 4F).
At st 35, expression is no longer restricted to limb regions, and Alk mRNA is detected in MMC neurons at every level of the spinal cord (Fig. 3C, 3D, 3J, 3K, 3M, 3N, 3P-S, 3V, 3W) with the lateral MMC staining strongly at thoracic levels (Fig. 3S). It is worth mentioning that while Alk expression was not investigated thoroughly in cervical and sacral regions, Alk transcript throughout the MMC at these levels was diffusely and uniformly expressed (data not shown). Additionally, Alk is expressed by LMC MNs at brachial and lumbar levels, and the “break” in expression, seen in the st 24/25 brachial region, still remains (Fig. 3K, 3N, 3Q, 3W). There are also additional patches of intense, discrete expression in the spinal cord dorsal to the MMC and LMC (Fig. 3J, 3P, 3V). Based on their location, it is likely that a subset of interneurons, possibly V0 or V1, are expressing Alk, but further identification using interneuron markers would be needed. By stage 39, transcript signal diminishes throughout the spinal cord with expression in the brachial and lumbar regions virtually non-existent (Fig. 3F-3H, 3L, 3T, 3X). Figure 5 summarizes the spatial-temporal expression of Alk in the embryonic spinal cord, sympathetic ganglia, and dorsal root ganglia.
Fig. 5.
Spatial-temporal overview of anaplastic lymphoma kinase (Alk) mRNA expression in developing chick nervous system. A: Spatial schematic of Alk expression (shading) with emphasis on location of transcript during investigated time points. B: Temporal schematic of Alk expression (bars) with emphasis on span of transcript duration in the embryonic PNS and spinal cord. Solid bar ends denote the definitive identification of a start point regarding Alk expression. Diffuse bar ends denote that a definitive start or end point was not identified from the time points analyzed in this investigation. Depth of shading within the bars is a reflection of the intensity of Alk expression. SC, spinal cord; MMC, medial motor column; LMC, lateral motor column; DRG, dorsal root ganglia; SG, sympathetic ganglia.
Alk expression in hind limb and body wall musculature
In the course of whole-mount in situ hybridization, Alk expression was detected in st 35 hind limb and body wall muscles. Within the musculature of the thigh and shank, Alk transcript is present in the iliotibialis cranialis (Fig. 6A), iliotibialis lateralis (Fig. 6B), caudofemoralis (Fig. 6C), fibularis longus (Fig. 6D), and gastrocnemius externus (Fig. 6D); identification of hind limb muscles based on findings of Kardon (1998). Expression was also observed in the intercostals of the body wall (Fig. 6E). It is of interest to note that the intercostals are the target muscles that are innervated by MNs from the lateral MMC which also expresses Alk mRNA (Fig. 3S); Sharma et al. (2000). Whether or not the aforementioned thigh and shank muscles are targets innervated by Alk+ MNs of the lumbar LMC remains to be determined.
Fig. 6.
Alk is expressed by subsets of hind limb and body wall muscles. Stage 35 whole-mount in situ hybridization reveals Alk mRNA in the A: iliotibialis cranialis (arrowhead), B: iliotibialis lateralis (arrowhead), C: caudofemoralis (arrowheads), D: fibularis longus (black arrowhead), gastrocnemius externus (white arrowhead), and E: intercostals (arrowheads). F: No transcript was detected in stage 35 whole-mount using sense RNA probe as a negative control. Scale bar: A-C, E, 1 mm; D, 750 μm; F, 1.3 mm.
Limb-derived cues and the regulation of Alk
The onset of Alk expression in the spinal cord (st 23/24) overlaps temporally with the expression of ETS family genes. Lin et al. (1998) demonstrated that ETS transcription factors are potentially involved in the development of selective sensory-motor circuits in the spinal cord and that signals from the limb control the expression of Ets genes. Because of the temporal coincidence of Alk and Ets gene expression, we were curious if Alk was also regulated by limb-derived cues and if ALK or ETS factors might regulate the expression of each other. To test this, we ablated one hind limb from embryos at st 17/18 and allowed them to develop until st 23/24. The embryos were then hybridized with Alk antisense probe to determine whether limb cues were required for Alk expression. Unlike Ets genes, Alk mRNA is still present in the CNS and PNS at levels comparable to the control (intact) side of ablated embryos after limb removal (data not shown). These results provide evidence that Alk expression is not under the control of signals produced by the limb. Based on these data, it is also unlikely that there is an influence on Ets gene expression by ALK or vice versa.
DISCUSSION
In the present study, we report the first in-depth characterization of Alk expression in the developing spinal cord and peripheral nervous system. This analysis identified three CNS and PNS neural structures which express Alk in a temporally dynamic pattern that correlates with key developmental events in each neural component. Given the ease of conducting gain and loss of function experiments in the chick, this expression profile provides the necessary foundation for determining ALK's function during development of MNs, SG, and DRG in vivo.
Alk expression in the sympathetic ganglia is robust and temporally extensive
First detected at st 19 (∼E3), Alk in the SG has an intense and sustained expression lasting at least through st 39 (E13), the latest time-point examined. Thus the course of Alk expression in the SG encompasses the periods of proliferation, differentiation, and PCD in addition to the formation of both the primary and secondary chain of sympathetic ganglia. According to Rothman et al. (1978), proliferation takes place from E2−3 to E21 with fewer and fewer cells dividing as time progresses. Our findings show that at E4 a majority of Alk+ cells are mitotically active, co-expressing phospho-histone H3. Because traditional differentiation markers like Tuj1 and Isl1 can be expressed in cells that are still mitotically active in the SG, attempts to identify differentiated, post-mitotic Alk+ neurons in st 23 SG proved to be ambiguous. However, it has been determined that virtually all SG neurons have exited the cell cycle between E8 and E12 (Smet et al., 1986). Additionally, pyknotic neurons are found throughout development, but cell death is most prominent from E7 to E18 (Smet et al., 1986). Since Alk mRNA is transcribed during these developmentally significant times, ALK most likely participates in the genesis, maturation, and/or survival of sympathetic neurons. Thus, elucidating ALK's function during SG development will expand our understanding of the extracellular signals that regulate SG development which heretofore have focused primarily on the neurotrophins.
Restricted expression of Alk in the dorsal root ganglia to mitotically active progenitors
Hamburger et al. (1981) identified a region of small, immature cells that are mitotically active in the medial and dorsolateral perimeters of the DRG, from which, he proposed, the second wave of neurons is derived. These peripheral zones of proliferating progenitors are separate from the core that contains the first wave of early-differentiating post-mitotic neurons. Results from this study show that Alk expression is confined to the proliferative margins (rather than the interior neural core) from st 19 to 25 and is non-detectable by st 35. Within the progenitor zones, there are mitotically active Alk+ cells in addition to Alk+ cells that are neither dividing nor differentiated. These data suggest that ALK may be involved in regulating the proliferation of progenitor cells or their exit from the cell cycle in preparation for differentiation. In addition or alternatively, Alk might promote the survival of DRG progenitor cells. Since Alk is not expressed in the inner neural core of the DRG nor is it expressed during the period of PCD of post-mitotic neurons (Carr and Simpson, 1978; Hamburger et al., 1981), ALK is not a candidate receptor for mediating function in post-mitotic neurons. Thus, this is the first RTK identified to be expressed exclusively in DRG progenitor cells. Given our lack of understanding of the signals that regulate the mitogenesis, differentiation, or survival of DRG progenitor cells, further investigation of ALK's function in the DRG will be insightful.
Alk mRNA is differentially expressed in motor neuron subsets and in muscle subsets of the hind limb and body wall
This study demonstrates that Alk is expressed by discrete subpopulations of MNs. Collectives of Alk-expressing MNs are confined to the MMC and LMC of the developing spinal cord; although, Alk is first detected in only the LMC at st 23/24 with expression in the MMC following later in development. This temporal difference in expression is possibly explained by the differing birthdates of MNs in the LMC and MMC. The majority of brachial and lumbar LMC are born by st 22, whereas most of the MMC neurons are generated by st 24 (Hollyday and Hamburger, 1977; Prasad and Hollyday, 1991). Due to this time lag between the genesis of LMC and MMC MNs, a corresponding lag in the onset of Alk expression between these two groups would be expected.
Sectioning through E8.5−9 spinal cord at various axial levels reveals specific clusters of Alk+ cells within the LMC and MMC that change position upon progression through a particular column. Each spatial cluster, known as a motor pool, is a subdivision of MNs that collectively innervate a specific muscle (Hollyday, 1980; Landmesser, 1978). While several muscles, including the iliotibialis cranialis, iliotibialis lateralis, caudofemoralis, fibularis longus, and gastrocnemius externus, also expressed Alk, further study will be required to determine if they are innervated by the lumbar motor pools that also express Alk. In the thorax, MNs from the lateral half of the MMC in addition to their target musculature, the intercostals, both express Alk mRNA. Thus, ALK could participate in a matching mechanism to insure correct target innervation. One such mechanism could involve the guidance of axons and myotubes, via ALK and interactions with its ligand, to a common point where nerve and target are proximally “matched.”
Additionally, Alk expression in MNs overlaps temporally with the period of PCD from E5-E12, peaking at E8 (Okado and Oppenheim, 1984; Gould and Oppenheim, 2004). Furthermore, Alk expression coincides temporally with the establishment of columnar organization, but not columnar identity which has been determined prior to Alk expression (Okado and Oppenheim, 1984; Sockanathan et al., 2003; Tsuchida et al., 1994). It is also worth noting that Alk is expressed during the period of synapse formation (st 28−36) (Fredette and Ranscht, 1994). Therefore, if ALK is involved in synaptogenesis, it most likely does not have the role of destabilizing or inhibiting synapse differentiation, as is proposed by Liao et al. (2004) in C. elegans, since one would then anticipate a reduction in mRNA expression over this time period so that synapse stabilization could occur.
PCD in spinal motor neurons is profound, leading to the elimination of 50% of the MNs that are generated (Oppenheim, 1991). It is clear from the rich body of literature on MN cell death that not all MN survival can be mediated by currently identified neurotrophic factors. Thus, there may be as yet unidentified ligand/receptor interactions that regulate MN survival. Because of its noticeable transcript expression in MNs during the peak of PCD, ALK and its ligand(s) could play a major role in mediating the PCD of MNs.
In summary, our profiling studies in chick reveal that Alk mRNA is prominently and distinctly expressed in subsets of cells in the developing PNS, spinal cord, and musculature. The period of Alk expression coincides with key biological events such as cellular proliferation, neuronal differentiation, programmed cell death, and motor neuron innervation of muscles implicating ALK's potential role in numerous major developmental processes. Additional studies to elucidate the functional significance of ALK in the developing nervous system are currently underway.
ACKNOWLEDEMENTS
We thank Valerie J. Todd and Marta Chaverra for valued technical assistance and Dr. Roger Bradley and Judy Bononi for helpful advice regarding whole-mount in situ hybridization.
Grant Sponsor: National Institute of Health; Grant Number: NS35714
LITERATURE CITED
- Avivi C, Goldstein RS. Differential expression of Islet-1 in neural crest-derived ganglia: Islet-1 + dorsal root ganglion cells are post-mitotic and Islet-1 + sympathetic ganglion cells are still cycling. Brain Res Dev Brain Res. 1999;115(1):89–92. doi: 10.1016/s0165-3806(99)00054-1. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Stoica GE, Wellstein A. Anti-apoptotic signaling of pleiotrophin through its receptor, anaplastic lymphoma kinase. J Biol Chem. 2002;277(39):35862–35868. doi: 10.1074/jbc.M203963200. [DOI] [PubMed] [Google Scholar]
- Carr VM, Simpson SB., Jr. Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol. 1978;182(4):727–739. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Ranscht B. T-cadherin expression delineates specific regions of the developing motor axon-hindlimb projection pathway. J Neurosci. 1994;14(12):7331–7346. doi: 10.1523/JNEUROSCI.14-12-07331.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TW, Oppenheim RW. The function of neurotrophic factor receptors expressed by the developing adductor motor pool in vivo. J Neurosci. 2004;24(19):4668–4682. doi: 10.1523/JNEUROSCI.0580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Brunso-Brechtold JK, Yip JW. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981;1(1):60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Organization of motor pools in the chick lumbar lateral motor column. J Comp Neurol. 1980;194(1):143–170. doi: 10.1002/cne.901940108. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Hamburger V. An autoradiographic study of the formation of the lateral motor column in the chick embryo. Brain Res. 1977;132(2):197–208. doi: 10.1016/0006-8993(77)90416-4. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14(4):439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- Kojima T, Katsumi A, Yamazaki T, Muramatsu T, Nagasaka T, Ohsumi K, Saito H. Human ryudocan from endothelium-like cells binds basic fibroblast growth factor, midkine, and tissue factor pathway inhibitor. J Biol Chem. 1996;271(10):5914–5920. doi: 10.1074/jbc.271.10.5914. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19(2):175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425(6957):507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17(2):118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Liao EH, Hung W, Abrams B, Zhen M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 2004;430(6997):345–350. doi: 10.1038/nature02647. [DOI] [PubMed] [Google Scholar]
- Loren CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003 doi: 10.1038/sj.embor.embor897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loren CE, Scully A, Grabbe C, Edeen PT, Thomas J, McKeown M, Hunter T, Palmer RH. Identification and characterization of DAlk: a novel Drosophila melanogaster RTK which drives ERK activation in vivo. Genes Cells. 2001;6(6):531–544. doi: 10.1046/j.1365-2443.2001.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274(18):12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM). J Biol Chem. 1996;271(35):21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene. 1997;14(18):2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- Motegi A, Fujimoto J, Kotani M, Sakuraba H, Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J Cell Sci. 2004;117(Pt 15):3319–3329. doi: 10.1242/jcs.01183. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Okado N, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J Neurosci. 1984;4(6):1639–1652. doi: 10.1523/JNEUROSCI.04-06-01639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Piccinini G, Bacchiocchi R, Serresi M, Vivani C, Rossetti S, Gennaretti C, Carbonari D, Fazioli F. A ligand-inducible epidermal growth factor receptor/anaplastic lymphoma kinase chimera promotes mitogenesis and transforming properties in 3T3 cells. J Biol Chem. 2002;277(25):22231–22239. doi: 10.1074/jbc.M111145200. [DOI] [PubMed] [Google Scholar]
- Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277(16):14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- Prasad A, Hollyday M. Development and migration of avian sympathetic preganglionic neurons. J Comp Neurol. 1991;307(2):237–258. doi: 10.1002/cne.903070207. [DOI] [PubMed] [Google Scholar]
- Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199(3):330–358. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]
- Rifkin JT, Todd VJ, Anderson LW, Lefcort F. Dynamic expression of neurotrophin receptors during sensory neuron genesis and differentiation. Dev Biol. 2000;227(2):465–480. doi: 10.1006/dbio.2000.9841. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Gershon MD, Holtzer H. The relationship of cell division to the acquisition of adrenergic characteristics by developing sympathetic ganglion cell precursors. Dev Biol. 1978;65(2):322–341. doi: 10.1016/0012-1606(78)90030-1. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Shiota M, Fujimoto J, Semba T, Satoh H, Yamamoto T, Mori S. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9(6):1567–1574. [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Smet P, Rush RA, Straznicky C. The thoracic sympathetic neurons of the chick: normal development and the effects of nerve growth factor. Histol Histopathol. 1986;1(4):315–322. [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40(1):97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Souttou B, Carvalho NB, Raulais D, Vigny M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(12):9526–9531. doi: 10.1074/jbc.M007333200. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276(20):16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277(39):35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Watanabe Y, Nakamura H, Kondoh H. Regulation of the neural crest cell fate by N-myc: promotion of ventral migration and neuronal differentiation. Development. 1997;124(10):1953–1962. doi: 10.1242/dev.124.10.1953. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130(8):1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]