Abstract
Tenascin-C is an adhesion-modulating matrix glycoprotein that has multiple effects on cell behavior. Tenascin-C transcripts are expressed in motile cells and at sites of tissue modeling during development, and alternative splicing generates variants that encode different numbers of fibronectin type III repeats. We have examined the in vivo expression and cell adhesive properties of two full-length recombinant tenascin-C proteins: TN-190, which contains the eight constant fibronectin type III repeats, and TN-ADC, which contains the additional AD2, AD1, and C repeats. In situ hybridization with probes specific for the AD2, AD1, and C repeats shows that these splice variants are expressed at sites of active tissue modeling and fibronectin expression in the developing avian feather bud and sternum. Transcripts incorporating the AD2, AD1, and C repeats are present in embryonic day 10 wing bud but not in embryonic day 10 lung. By using a panel of nine cell lines in attachment assays, we have found that C2C12, G8, and S27 myoblastic cells undergo concentration-dependent adhesion to both variants, organize actin microspikes that contain the actin-bundling protein fascin, and do not assemble focal contacts. On a molar basis, TN-ADC is more active than TN-190 in promoting cell attachment and irregular cell spreading. The addition of either TN-190 or TN-ADC in solution to C2C12, COS-7, or MG-63 cells adherent on fibronectin decreases cell attachment and results in decreased organization of actin microfilament bundles, with formation of cortical membrane ruffles and retention of residual points of substratum contact that contain filamentous actin and fascin. These data establish a biochemical similarity in the processes of cell adhesion to tenascin-C and thrombospondin-1, also an “antiadhesive” matrix component, and also demonstrate that both the adhesive and adhesion-modulating properties of tenascin-C involve similar biochemical events in the cortical cytoskeleton. In addition to these generic properties, TN-ADC is less active in adhesion modulation than TN-190. The coordinated expression of different tenascin-C transcripts during development may, therefore, provide appropriate microenvironments for regulated changes in cell shape, adhesion, and movement.
INTRODUCTION
Tenascin-C, the prototypic member of the tenascin gene family, is a matrix glycoprotein that is prominently expressed in developing tissues and in pathophysiological situations such as the matrix surrounding tumors (Chiquet-Ehrismann et al., 1986; Erickson, 1993). Many functional properties have been identified for tenascin-C in cell biological assays, including effects on cell adhesion, neurite outgrowth, cell migration, proliferation, and differentiation (reviewed by Erickson and Bourdon, 1989; Chiquet-Ehrismann, 1991; Sage and Bornstein, 1991; Chiquet-Ehrismann, 1995a). The effects of tenascin-C on cell adhesion are complex, in that substratum-bound tenascin-C supports attachment of some cell types but is nonadhesive or even repulsive for other cell types (Erickson and Taylor, 1987; Chiquet-Ehrismann et al., 1988; Bourdon and Ruoslahti, 1989; Spring et al., 1989; Prieto et al., 1992; Joshi et al., 1993). The nature of the attachment appears to be of low mechanical strength and often does not involve cell spreading (Lotz et al., 1989). Because some fragments of tenascin-C are more adhesive than the intact molecule, it appears that the latter contains both adhesive and counteradhesive domains (Friedlander et al., 1988; Spring et al., 1989; Prieto et al., 1992; Aukhil et al., 1993; Joshi et al., 1993; Götz et al., 1996). Tenascin-C in solution also has adhesion-modulating properties in that it prevents cell adhesion to fibronectin (Chiquet-Ehrismann et al., 1988) and disrupts preformed focal contacts in spread bovine aortic endothelial cells (Murphy-Ullrich et al., 1991).
The regulated expression and functional properties of tenascin-C have similarities with those of two other matrix glycoproteins, thrombospondin-1 (TSP-1) and SPARC/osteonectin (reviewed by Frazier, 1991; Sage and Bornstein, 1991; Lahav, 1993; Lane and Sage, 1994; Bornstein, 1995). These common properties have led these glycoproteins to be grouped functionally as so-called “antiadhesive” or “adhesion-modulating” glycoproteins and it has been proposed that they may play important roles in the coordinated regulation of cell adhesive, motile, and proliferative behavior at sites of tissue modeling during normal development and in pathophysiological situations (Chiquet-Ehrismann, 1991; Sage and Bornstein, 1991; Chiquet-Ehrismann, 1993, 1995b). To discover whether a common process of antiadhesion or adhesion modulation exists, it is necessary to determine whether these functional similarities are matched by mechanistic similarities.
One approach to this question is to examine cytoskeletal organization in adherent cells. In the case of TSP-1, various cell types attach but do not spread (for example, Tuszynski et al., 1987; Lawler et al., 1988; Varani et al., 1988), whereas other cells display a substantial degree of spreading (Asch et al., 1991, Stomski et al., 1992; Adams and Lawler, 1993, 1994; reviewed by Adams et al., 1995). Cell spreading on TSP-1 involves organization of filamentous actin (F-actin; Stomski et al., 1992; Adams and Lawler, 1994) yet does not correlate with organization of focal contacts or focal adhesions, the substratum contact structures that characterize cell adhesion to matrix components such as fibronectin and vitronectin (reviewed by Turner and Burridge, 1991; Hynes, 1992; Burridge and Chrzanowska-Wodnicka, 1996) but rather correlates with the assembly of radial microspikes that contain the 55-kDa actin-bundling protein fascin (Adams, 1995). Given that adhesion to TSP-1 involves formation of a distinctive type of substratum contact, it is important to determine whether adhesion to tenascin-C elicits similar cellular responses.
In structure, tenascin-C consists of six subunits, each of which is composed of multiple domains containing an amino-terminal interchain cross-linking domain, a series of epidermal growth factor (EGF)-like repeats, a series of fibronectin type III repeats, and a C-terminal fibrinogen homology domain (reviewed by Erickson, 1993; Chiquet-Ehrismann, 1995a). Several splice variants have been identified that differ in the number of fibronectin type III repeats spliced in between the fifth and sixth of the eight constant repeats. Initially, three alternatively spliced repeats termed A, B, and D were identified in chicken and human tenascin-Cs. Additional alternatively spliced repeats, including the repeat now termed repeat C, were also identified in human tenascin-C (Siri et al., 1991; Sriramarao and Bourdon, 1993) and in chicken, in which an additional repeat termed AD2 has been identified as well (Tucker et al., 1994). In chicken genomic DNA, these exons are ordered as A, B, AD2, AD1, C, and D. Chicken embryo fibroblasts produce a variety of tenascin-C transcripts that incorporate different combinations of the alternatively spliced repeats including AD2, AD1, and C. Transcripts containing repeat C are concentrated at sites of epithelial/mesenchymal interactions in chicken embryos (Tucker et al., 1994).
Several lines of evidence indicate that tenascin-C splice variants have different functional activities. Splice variants incorporating the ABD repeats bind less well to fibronectin (Chiquet-Ehrismann et al., 1991) and to the cell surface glycoprotein contactin/F11 (Zisch et al., 1992). Experiments using bacterially expressed protein domains have implicated various constant fibronectin type III repeats as playing a role in cell adhesion to tenascin-C (Spring et al., 1989; Prieto et al., 1992; Joshi et al., 1993; Yokosaki et al., 1996). In contrast, the alternatively spliced repeats ABCD have been implicated in the ability of tenascin-C to disrupt focal contacts (Murphy-Ullrich et al., 1991; Chung et al., 1996). The functional activities of the AD2AD1C repeats are currently unknown.
In this study, we have used a panel of cell lines to examine cell attachment and cytoskeletal organization in response to two full-length recombinant tenascin-Cs: TN-190, which contains only the constant repeats, and TN-ADC, which contains the three novel additional repeats AD2, AD1, and C. We have found that substratum-bound TN-ADC is more adhesive than TN-190 and in solution is less effective at causing detachment of cells spread on fibronectin in two of three cell lines tested. Cells that spread on either tenascin-C variant do not assemble focal contacts but exhibit clustered arrays of fascin microspikes. F-actin and fascin-containing substratum contacts remain when cells on fibronectin are treated with soluble tenascin-Cs. The significance of these results is discussed with respect to possible mechanisms for the adhesion-modulating properties of tenascin-C.
MATERIALS AND METHODS
Construction, Expression, and Isolation of Full-Length Recombinant Tenascin-Cs
A cDNA corresponding to the smallest naturally occurring avian splice variant, tenascin 190 (pCTN 190), was constructed as described (Fischer et al., 1995). To construct a tenascin including the recently discovered additional fibronectin type III repeats AD2, AD1, and C (Tucker et al., 1994), cDNA encoding repeats AD2, AD1, and C (nucleotides 1–800 of the EMBL data base entry X73833) was inserted into pCTN190, precisely between fibronectin type III repeats 5 and 6, by using a polymerase chain reaction (PCR)-based method called “splicing by overlap extension” (Horton et al., 1989), creating the plasmid pCTN ADC. This coding sequence was subcloned into the eukaryotic expression vector pCDNAI/neo (InVitrogen, San Diego, CA). Recombinant proteins were expressed in HT1080 cells and isolated and purified by affinity chromatography as described (Fischer et al., 1995). Schematic models of the variable repeats domain are shown in Figure 3D.
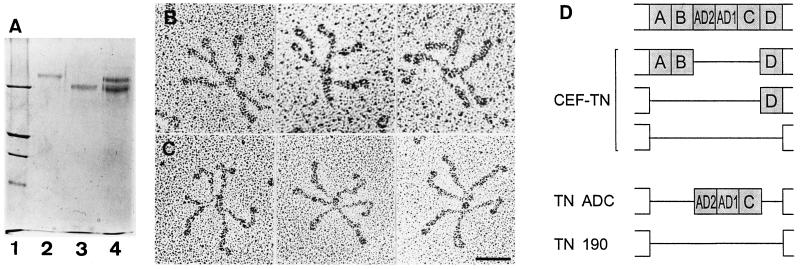
Figure 3.
Analysis of recombinant chick tenascin-C proteins. (A) SDS-PAGE analysis. Lane 1, molecular mass standards (from the top), 200 kDa, 116 kDa, 97 kDa, and 66 kDa; lane 2, recombinant tenascin-C variant that includes the fibronectin type III repeats AD2, AD1, and C (TN-ADC); lane 3, recombinant tenascin-190 (TN-190); lane 4, tenascin-C from chicken embryo fibroblast (CEF-TN; this contains a mixture of TN-190, TN-200, and TN-230 protein variants). All samples were resolved on a 6% polyacrylamide gel under reducing conditions. (B and C) Electron microscopy of recombinant tenascin-Cs. TN -190 (B) or TN-ADC (C) were sprayed onto mica and rotary-shadowed. Bar, 50 nm. (D) Schematic diagram showing ordering of the variable fibronectin type III repeats in chicken (top) and the repeat combinations in CEF-TN, TN-190, or TN-ADC proteins.
Characterization of Recombinant Tenascin-Cs
The purified recombinant tenascin-Cs were analyzed by SDS-PAGE under reducing conditions using 6% polyacrylamide gels (Laemmli, 1970) and by transmission electron microscopy. For rotary shadowing and electron microscopy, proteins were processed as described (Chiquet-Ehrismann et al., 1988).
Cell Culture
The cell lines used in this study included C2C12 murine skeletal myoblasts (Blau et al., 1985), the S27 variant subline that is deficient in proteoglycans (Gordon and Hall, 1989; a gift from Dr. Zach Hall, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD), G8 murine skeletal myoblasts (Christian et al., 1977), A10 rat aortic smooth muscle cells (Kimes and Brandt, 1976), A549 human lung carcinoma cells, C32 human melanoma cells, COS-7 green monkey kidney cells (Gluzman, 1981), G361 human melanoma cells, HT1080 human fibrosarcoma (Rasheed et al., 1974), MDCK canine kidney cells, and MG-63 human osteosarcoma cells. Most cell lines were cultured in DMEM containing 10% fetal calf serum; however, the myoblast cell lines were cultured in DMEM containing 20% fetal calf serum. All cells were maintained in a humidified 10% CO2 atmosphere at 37°C.
Cell Adhesion Assays
Fibronectin was isolated from horse serum (Life Technologies, Gaithersburg, MD) by affinity chromatography using a gelatin-agarose column (Sigma, St. Louis, MO). After washing the column with PBS, bound fibronectin was eluted with 4 M urea in PBS. Fibronectin-containing fractions were dialyzed overnight against PBS and then stored frozen at −70°C. Chick tenascin-C (CEF-TN) was purified from the conditioned medium of confluent cultures of primary chicken embryo fibroblasts grown in DMEM containing 10% FCS (Life Technologies), as described by Fischer et al. (1995). For cell adhesion assays, fibronectin, CEF-TN, or recombinant tenascin-Cs were diluted to 50 nM and allowed to adsorb to glass coverslips at 4°C overnight. Coverslips were then blocked with 1 mg/ml heat-denatured bovine serum albumin (BSA) in PBS for 1 h at room temperature and finally washed with PBS. The cell lines were trypsinized from stock cultures, washed once in DMEM containing 10% fetal calf serum and twice in serum-free medium, and resuspended at a concentration of 2 × 105 cells/ml, and a 30-μl aliquot was added to each coverslip. Cell attachment was carried out at 37°C for 1.5 h. Nonadherent cells were removed by washing in PBS and adherent cells were fixed either in 3.7% formaldehyde or in absolute methanol at −20°C and processed for immunofluorescence as described below. Cells were also scored for round or spread morphology. Cells with protrusive actin-rich processes were scored as irregularly spread (for example, see Figure 6, c and f); smooth-edged polygonal cells which had a larger spread area were scored as fully spread (see Figure 6a). In a separate series of experiments, polyclonal antiserum to tenascin-C (Chiquet-Ehrismann et al., 1986) or fibronectin (Ehrismann et al., 1981) was added to the coverslips at the same time as the cells. To examine the effects of tenascin-Cs on cell adhesion to fibronectin, tenascin-Cs were added in solution at a final concentration of 35 nM at the time of plating cells on fibronectin substrata. At the end of the assay period, residual adherent cells were either counted or processed for immunofluorescence. Statistical significance was calculated using a two-tailed t test.
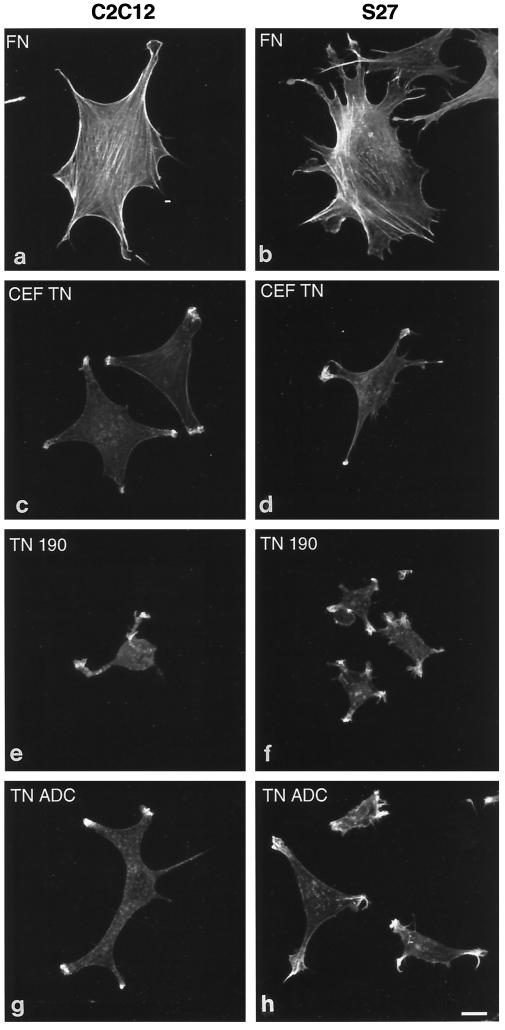
Figure 6.
Confocal microscopy of actin microfilament organization in C2C12 and S27 cells adherent on fibronectin or tenascin-C. Cells were plated onto coverslips coated with 50 nM fibronectin (a and b), 50 nM CEF-TN (c and d), 50 nM recombinant TN-190 (e and f), or 50 nM recombinant TN-ADC (g and h) for 90 min in serum-free medium. After incubation cells were fixed, stained with TRITC-phalloidin, and photographed on a confocal microscope. Bar, 5 μm.
Immunofluorescent Staining of Adhesion Assays
Immunofluorescence experiments were carried out as previously described (Adams, 1995). The staining reagents included tetramethylrhodamineisothiocyanate (TRITC)-phalloidin, mouse monoclonal antibody VIN 11.5 (both from Sigma), or mouse monoclonal antibody to human fascin (Yamashiro-Matsumura and Matsumura, 1986; a gift from Dr. George Mosialos, Harvard Medical School). The distribution of primary antibodies was visualized by using fluorescein isothiocyanate-conjugated goat anti-mouse IgG (ICN Immunobiologicals, Thame, Oxfordshire, United Kingdom). Samples were examined under epifluorescence by using a Zeiss Axiophot microscope and photographs were taken by using Kodak TMAX 100 film. For laser confocal microscopy, a Leica TCS 4D was used. Optical sections ranged from 0.8 μm to 3 μm and were recorded in the line average mode with picture size of 512 × 512 pixels. Images from optical sections were captured electronically and processed digitally. Figures were arranged and labeled by using Micrografx Designer 4.1 and printed by using Fujix Pictrography 3000.
Production of cDNA Probes for Tenascin-C by PCR
A cDNA probe corresponding to a portion of the EGF-like repeats domain of chicken tenascin-C, which is included in all TN-C transcripts, was prepared by reverse transcription-coupled PCR (RT-PCR) amplification (Perkin Elmer-Cetus, Norwalk, CT) from whole embryonic day (E) 10 brain poly(A) RNA (Micro Fast Track, Invitrogen) as template. The primers (5′-AAATGCATCTGCGAGGGC-3′ and 5′-GGAAGCTTGTTATTGCAGTCCTTCGG-3′) generated a 540-bp product (TN-EGF). RT-PCR was also used to make a 258-bp cDNA probe corresponding to the novel alternatively spliced fibronectin type III repeat, AD1 (primer pair: 5′-CGAATTCACGCTCACACTCACAAATGT-3′ and 5′-CGAATTCCTGTCATGACAAAAGCAGTG-3′) and a 246-bp DNA probe corresponding to the novel AD2 repeat (primer pair: 5′-CGAATTCGAACCTCTCCTTAGCAAACT-3′ and 5′-CGAATTCTGTGGTTGCCAGTGCTGTCA-3′). The identity of each of these probes was confirmed by restriction map analysis, and the specificity of each probe for the corresponding repeat was demonstrated empirically by Southern blot analysis as described above. A cDNA probe corresponding to the novel C repeat has been described previously (Tucker et al., 1994). A 955-bp pUC19 restriction fragment was used as a negative control for spurious hybridization and probe trapping.
RT-PCR and Southern Blot Analysis
Poly(A) RNA was prepared from tissues dissected from E10 embryos using the MicroFast Track kit (InVitrogen). RT-PCR (RT-PCR kit, Perkin Elmer-Cetus-Cetus) was carried out using random primers in the reverse transcriptase reaction. Primer pairs that spanned the novel repeats AD2, AD1, and C (Tucker et al., 1994) were then used. Products were separated on a 1% agarose gel, denatured for 45 min in 1.5 M NaCl and 0.5 M NaOH, neutralized in 1 M Tris(hydroxymethyl)aminomethane hydrochloride, pH 7.4, and 1.5 M NaCl, and transferred to nitrocellulose by capillary action. The filters were baked, prehybridized in 5× SSC, 5× Denhardt’s solution, 100 μg/ml salmon sperm DNA, and 50% deionized formamide for 3 h at 42°C and then hybridized in the same solution with the addition of 10% dextran sulfate and 32P-labeled cAD2 (5 × 105 cpm/ml) for 3 h. Filters were then rinsed in 0.5× SSC at room temperature for 20 min and at 40°C for 20 min and then exposed to x-ray film at −70°C (Sambrook et al., 1989). To control empirically for probe specificity, a lane overloaded with repeat C PCR product was also hybridized with cAD2 probe.
In Situ Hybridization
The cDNA probes described above were used for in situ hybridization on frozen sections of chick embryos, using methods adapted from those described previously (Tucker et al., 1994, 1995). Chick embryos (Univeristy of California at Davis, Department of Avian Sciences) were fixed in 4% paraformaldehyde in PBS overnight, rinsed in PBS, and then cryoprotected overnight in 25% sucrose in PBS. Embryos were embedded in TBS brand tissue freezing medium (Fisher Scientific, Pittsburgh, PA), and sections were cut at 12- to 14-μm intervals in a Bright cryostat. Sections were collected, air-dried on subbed slides, and then incubated for 1 h at room temperature in prehybridization buffer composed of 5× SSC, 5× Denhardt’s solution, 100 μg/ml salmon sperm DNA, and 20 μM 2-mercaptoethanol; all reagents were obtained from Sigma. The cDNA probes were labeled with 35S-labeled dCTP (New England Nuclear, Boston, MA) by using random primers (Promega, Madison, WI) and unincorporated nucleotides were removed with a G-50 Sephadex spin column (Worthington Biochemicals, Freehold, NJ). The slides were dipped in absolute ethanol, allowed to dry, and incubated in hybridization buffer, which corresponded to prehybridization buffer with the addition of 50% deionized formamide and 0.1% sarkosyl and contained 5 × 105 cpm/slide of the appropriate probe, at 42°C overnight. The slides were then rinsed with 1× SSC at room temperature and at 42°C, dried, dipped in LM-1 emulsion (Amersham, Arlington Heights, IL), and exposed for an appropriate length of time. Processed slides were counterstained with the nuclear dye bisbenzimide (H33258, Boehringer-Mannheim, Indianapolis, IN), and viewed under dark-field illumination.
Immunohistochemistry
Sections adjacent to those used for in situ hybridization were stained with antibodies to tenascin-C or cellular fibronectin. Sections were rinsed in PBS, blocked in 0.5% BSA (Sigma) in PBS, and then incubated overnight in a humid chamber with either a mouse monoclonal antibody to chick tenascin-C (M1, hybridoma supernatant diluted 1:1 in BSA/PBS; Chiquet and Fambrough, 1984) or a mouse monoclonal antibody to human cellular fibronectin, clone FN-3E2 (Sigma) diluted 1:50 in BSA/PBS. Sections were then rinsed in PBS and incubated in a TRITC-labeled rabbit anti-mouse IgG secondary antibody (Accurate Chemical and Scientific, Westbury, NY) for 2 h. After washing in PBS, the slides were mounted in PBS/glycerol and viewed under an epifluorescence microscope.
RESULTS
Tenascin-C Variants containing the Novel Repeats AD2, AD1, or C Colocalize with Fibronectin in the Developing Avian Feather Bud and Sternum
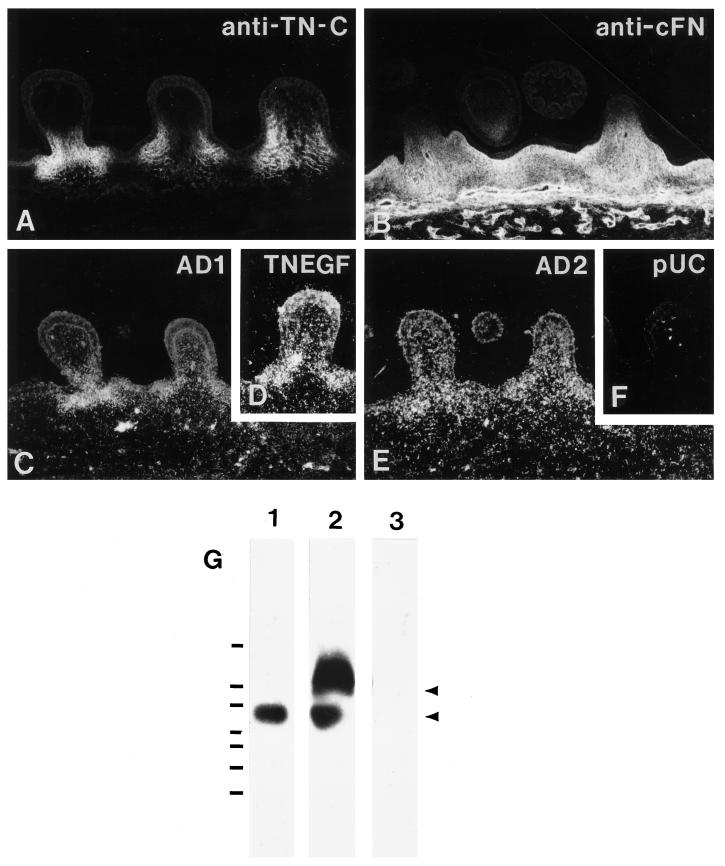
The expression of tenascin-Cs containing the AD2, AD1, or C repeats was examined in vivo, in two structures within the avian embryo that are known to express tenascin-C: developing feather buds (Tucker, 1991) and sternum (Mackie and Tucker, 1992; Tucker, 1993; reviewed by Mackie, 1994). First, sections made through the E10 dorsal feather tract were stained with antibodies to fibronectin or tenascin-C. Whereas tenascin-C immunoreactivity was restricted to areas of mesenchyme at the base of the developing feather buds (Figure 1A), fibronectin immunoreactivity was widespread and uniformly distributed throughout the dermis including the base of the feather buds, thus overlapping with sites of tenascin-C expression (Figure 1B; Tucker, 1991; Jiang and Chong, 1992). Adjacent sections were then used for in situ hybridization with a series of cDNA probes corresponding to the individual repeats AD2, AD1, or C or a universal tenascin-C probe corresponding to a portion of the EGF repeats. The universal tenascin-C probe TN-EGF hybridized within the mesenchyme at the base of the feather buds (Figure 1D). The AD2 and AD1 probes also hybridized within this region, the AD1 probe displaying a more restricted distribution (Figure 1, C and E).
Figure 1.
Localization of tenascin-C and fibronectin in feather buds. (A) Antibody to tenascin-C stains the mesenchyme at the base of the feather buds. (B) Antibody to cellular fibronectin stains all of the dermal extracellular matrix and the underlying blood vessels. (C–F) In situ hybridization on adjacent cross-sections through the dorsal feather tract of an E10 chick. (C) Tenascin C probe specific to fibronectin type III repeat AD1. (D) Universal tenascin-C probe TN-EGF. (E) Probe to repeat AD2. (F) pUC negative control probe. All the tenascin-C probes hybridize in the mesenchyme at the base of the feather buds. Bar, 100 μm. (G) RT-PCR detection of AD2AD1C-containing tenascin-C transcript in embryonic tissues. Primers corresponding to the beginning of repeat AD2 and the end of repeat C were used to amplify products from mRNA isolated from E10 chicken lung (lane 1) or wing (lane 2). Lane 3 was loaded with PCR product corresponding to repeat C. The blot was probed with cAD2. A single band of about 550 bp (i.e., two fibronectin type III repeats) was detected in amplification products from lung. In contrast, two bands corresponding to products containing 2 and 3 fibronectin type III repeats were detected in wingbud (arrows). DNA standards from bottom to top are 100 bp, 200 bp, 300 bp, 400 bp, 600 bp, 800 bp, and 2000 bp.
To assess whether tenascin-C transcripts in the wing bud include the AD2AD1C repeat combination, mRNA isolated from E10 tissues was examined by RT-PCR and Southern blotting. Although embryonic lung contained transcripts encoding the AD2 repeat in tandem with the C repeat, no intervening AD1 repeat was detected (Figure 1G, lane 1). In contrast, tenascin-C transcripts that incorporated the AD2, AD1, and C repeats were detected in wing bud (Figure 1G, lane 2). The cAD2 probe did not hybridize with purified PCR product amplified from the C repeat (Figure 1G, lane 3), indicating the specificity of the hybridization reaction. Thus, tenascin-C transcripts containing the AD2, AD1, and C repeats are present in certain embryonic tissues.
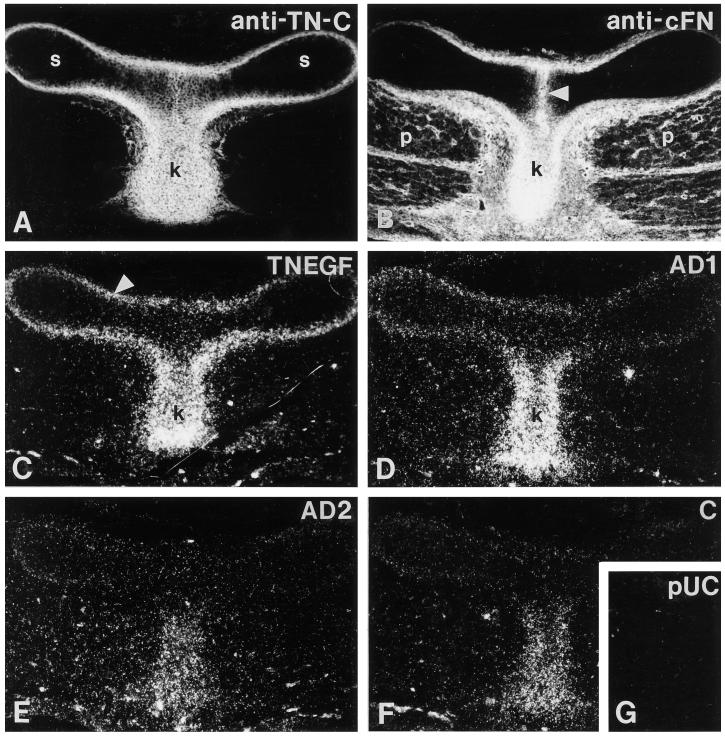
Tenascin-C protein in E10 sternum and keel was distributed throughout the keel and was also present in the perichondrium surrounding the sternal anlage (Figure 2A). Fibronectin protein colocalized with tenascin-C in the keel and perichondrium and was also present at the fusion point between the two sternal anlage and in the mesenchyme surrounding the pectoral muscles (Figure 2B). When matched cross-sections were hybridized with the set of tenascin-C probes, the universal TN-EGF probe was found to hybridize throughout the keel and in the perichondrium of the sternum, thus showing good correspondence with the sites of protein localization (Figure 2C). In contrast, the AD1, AD2, or C probes hybridized only within the keel, a more intense signal being obtained with the AD1 probe (Figure 2, D–F). Thus, in these two developing structures, the AD2-, AD1-, or C-containing splice variants are expressed at sites of active tissue modeling and high fibronectin expression by a subset of the cells that express tenascin-C.
Figure 2.
Localization of tenascin-C and fibronectin in sternum and keel. Cross-sections through sternum and keel of an E10 chick were processed for immunohistochemistry (A and B) or in situ hybridization (C–G). (A) Antibody to tenascin-C stains the perichondrium surrounding the sternal anlages (s) and the keel (k) intensely. (B) Antibody to cellular fibronectin stains the fusion point between the two sternal anlage (arrowhead), matrix surrounding the pectoral muscles (p) and the keel (k). (C) Universal probe TN-EGF hybridizes in sternal perichondrium (arrowhead) and in the keel (k). (D) AD1 probe. (E) AD2 probe. (F) C probe. All three probes hybridize in the keel. (G) pUC negative control. Bar, 100 μm.
Production of Recombinant Tenascin-Cs
Full-length chicken TN-190 protein has previously been expressed in stably transfected HT1080 cells (Fischer et al., 1995). This system was used to express a tenascin-C variant that included the AD2/AD1/C repeats. This recombinant protein has been termed TN-ADC (Figure 3D). SDS-PAGE analysis of the recombinant proteins under reducing conditions indicated that recombinant TN-ADC had an apparent molecular mass of about 230 kDa, clearly larger than recombinant TN-190, which migrated with an apparent molecular mass of about 190 kDa. As expected, purified CEF-TN contained three proteins of 190 kDa, 200 kDa, and 230 kDa, corresponding to splice variants containing 8, 10, and 11 fibronectin type III repeats, respectively (Figure 3A; Chiquet-Ehrismann et al., 1988; Jones et al., 1989; Spring et al., 1989). The 200-kDa and 230-kDa proteins contain various combinations of the six possible alternatively spliced repeats (Tucker et al., 1994; Figure 3D).
The recombinant tenascin-Cs were also examined by electron microscopy after rotary shadowing to determine their states of oligomerization. Both TN-190 and TN-ADC were detected as six-armed oligomers. The arms of the TN-ADC hexabrachions were longer than those of TN-190, consistent with the insertion of three additional fibronectin type III repeats (Figure 3, B and C). Thus, recombinant TN-ADC is correctly assembled into hexabrachions by HT1080 cells.
Cell Adhesive Behavior in Response to CEF-TN and Recombinant Tenascin-Cs
To evaluate and compare the adhesive activities of the natural and recombinant tenascin-Cs, a large panel of cell lines were tested for their ability to attach to tenascin C-coated substrata in short-term serum-free assays. Cells that attached were scored with respect to their morphology as described in MATERIALS AND METHODS. Attachment to tenascins was tested in parallel with adhesion to the well-characterized adhesive glycoprotein fibronectin. MDCK cells and A549 cells attached in small numbers to both fibronectin and CEF-TN and the attached cells remained round on both substrata. COS-7, C32, MG-63, and HT1080 cells underwent spreading on fibronectin; HT1080 cells and MG-63 cells did not adhere to CEF-TN; and COS-7 and C32 cells attached in small numbers (less than 5% of the input cells) and remained round. Thus, CEF-TN is clearly much less adhesive for these cell types than fibronectin. When the attachment of these six cell lines to recombinant TN-190 and TN-ADC were compared, TN-190 was found to be nonadhesive for all six cell lines, whereas about 5% of the input cells attached to TN-ADC. Only C32 cells showed some degree of spreading on TN-ADC. Because the attachment of these cells lines to TN-ADC was not significantly above their level of attachment to a BSA substratum, it appeared both splice variants were nonadhesive for these cell lines (our unpublished observations).
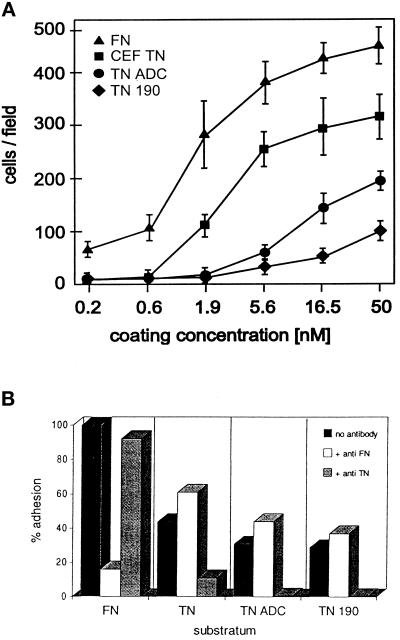
In contrast, the attachment assays revealed that CEF-TN had adhesive activity toward cells derived from murine skeletal muscle. C2C12 and G8 were derived from C3H or Swiss Webster mouse hindlimb muscle, respectively (Christian et al., 1977; Blau et al., 1985) and S27 is a proteoglycan-deficient subline of C2C12 that was derived by ethyl methanesulfonate mutagenesis (Gordon and Hall, 1989). These cells did not attach to BSA substrata, attached in large numbers to fibronectin substrata, and displayed intermediate levels of attachment on the tenascin-C preparations. Quantitative attachment assays demonstrated that C2C12 cells underwent concentration-dependent attachment to both fibronectin and tenascin-Cs. Of the three tenascin-C preparations, CEF-TN was the most adhesive in terms of cell number, TN-ADC had intermediate activity, and TN-190 had low activity at high coating concentrations (Figure 4A). Maximal levels of cell attachment to tenascin-Cs represented 48% of the numbers of cells attached to fibronectin in the case of CEF-TN, 40% in the case of TN-ADC, or 20% of cells attached to TN-190 (Figure 4A). The difference in cell attachment to TN-190 or TN-ADC at the 50 nM coating concentration was significant at p < 0.001. C2C12 cells attached on the various tenascin-C preparations underwent irregular partial spreading even at maximal coating concentrations: similar morphologies were observed with G8 and S27 cells.
Figure 4.
Quantitation of C2C12 cell adhesion to tenascin-Cs. (A) C2C12 cells were allowed to adhere for 1 h to the indicated concentrations of the various tenascin-C preparations or fibronectin, then fixed, stained, and counted. Each data point is the mean of triplicate experiments; bars indicate SEM. (B) Inhibition of cell adhesion to chick tenascin-C by antibodies. C2C12 cells were plated onto coverslips coated with 50 nM fibronectin (FN), CEF-TN (TN), recombinant TN-ADC, or recombinant TN-190 in the presence or absence of antiserum directed against fibronectin or tenascin-C. Solid bars, controls without antiserum; open bars, cells plated in the presence of anti-fibronectin serum; shaded bars, cells plated in the presence of antiserum to chick tenascin-C. Adherent cells were fixed and counted after a 90-min incubation at 37°C.
To quantify these observations, attachment and spreading on the different substrata were quantified for C2C12 and S27 cells, using maximally adhesive coating concentrations of fibronectin or tenascin-Cs. In terms of the total number of attached cells, attachment of C2C12 cells to CEF-TN was 44% of the level of cell attachment to fibronectin and attachment of S27 cells to CEF-TN was 86% of the level of adhesion to fibronectin. For both cell types, attachment to fibronectin corresponded to 90% of the input cells (Table 1). Next, the ratio of spread to attached cells was examined for C2C12 and S27 cells. In adhesion assays using CEF-TN or TN-ADC, between 74% and 98% of the attached C2C12 cells or S27 cells underwent irrregular spreading, whereas on TN-190, only about 53% of the cells that attached then spread irregularly (Table 1). Thus, the TN-ADC splice variant is more active with respect to cell spreading than is TN-190. Similar results were obtained with G8 cells (our unpublished observation).
Table 1.
Quantification of cell adhesion to tenascin-C variants
| Cell | Substratum
|
|||
|---|---|---|---|---|
| FN | CEF-TN | TN-190 | TN-ADC | |
| C2C12 | ||||
| % attached cellsa | 100 | 44 | 29 | 31 |
| Ratio spread to attached cells | 0.97 | 0.89 | 0.52 | 0.74 |
| S27 | ||||
| % attached cellsa | 100 | 86 | 60 | 77 |
| Ratio spread to attached cells | 1 | 0.98 | 0.54 | 0.84 |
C2C12 or S27 cells were plated on coverslips coated with 50 nM fibronectin (FN), 50 nM chicken embryo fibroblast tenascin-C (CEF-TN), 50 nM recombinant tenascin-C 190 (TN-190), or 50 nM recombinant tenascin-C ADC (TN-ADC) for 90 min in serum-free medium, and attached cells were then fixed and counted.
Numbers of attached cells on fibronectin were set as 100% and the numbers of attached cells on the tenascin-C variants are expressed as percentages relative to this value. Spreading was scored as described in MATERIALS AND METHODS.
To confirm that the observed cell attachment was directly to tenascin-C, attachment assays were carried out with C2C12 cells in the presence of antibodies to fibronectin or tenascin-C. A polyclonal antiserum to fibronectin had no effect on attachment to any of the tenascin-C substrata but reduced attachment to fibronectin by 87%. In contrast, an antiserum to chicken tenascin-C reactive with all splice variants decreased cell attachment to all the tenascin-C substrata by 76% to 95% and had no effect on cell attachment to fibronectin (Figure 4B). Thus, the cell attachment assays are measuring direct cellular responses to tenascin-C.
Cell Adhesion on Tenascin-C Correlates with a Distinctive Organization of Actin Microfilaments
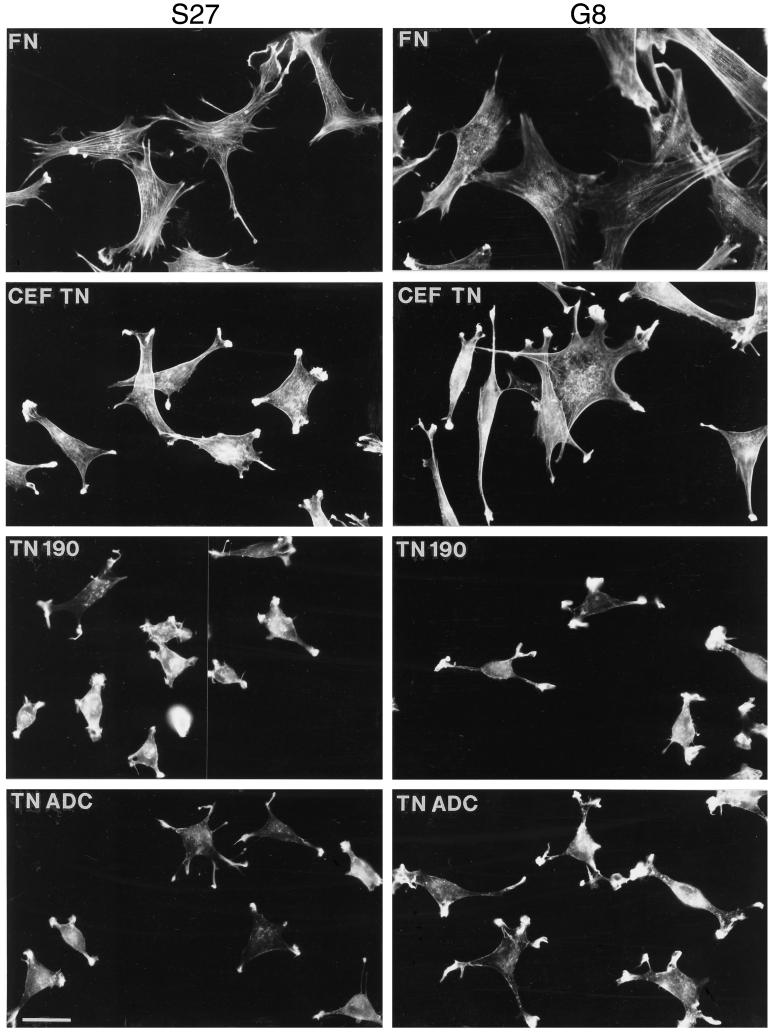
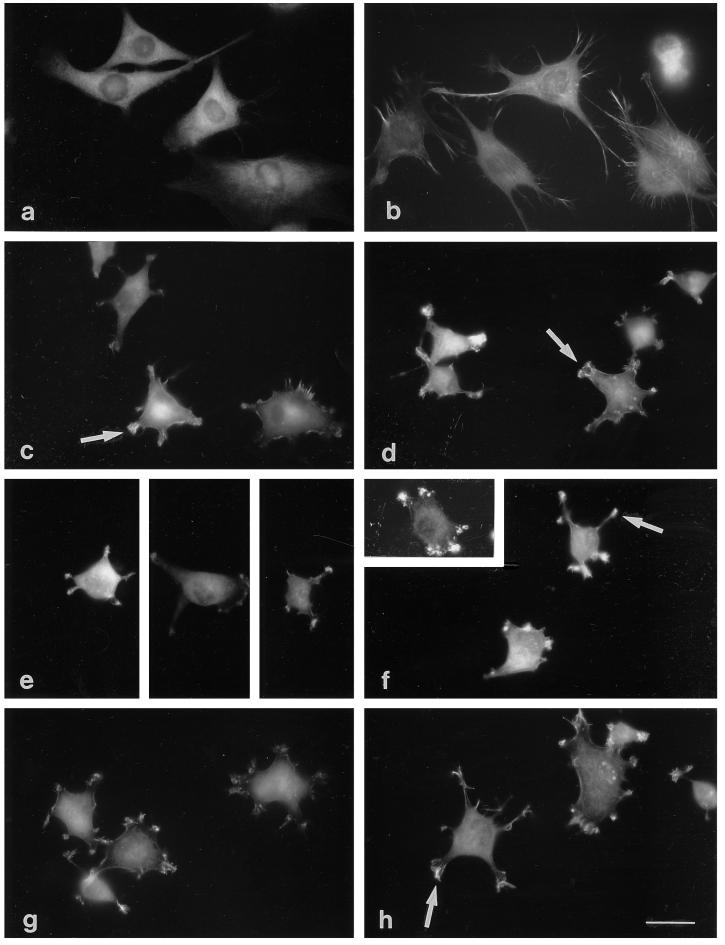
To examine the consequences of cell spreading on TN-C in detail, actin microfilament organization was examined in adherent G8, S27, and C2C12 cells. By using epifluorescence microscopy, it was apparent that S27 and G8 cells spread on fibronectin assembled longitudinal arrays of microfilaments (shown for S27 and G8, Figure 5; for C2C12, Figure 6 and see Figure 11). The G8 and S27 cells irregularly spread on CEF-TN and TN-ADC showed some organization of microfilaments, particularly at cell peripheries, but also tended to display a punctate F-actin staining pattern in the cell body (Figure 5). The more rounded cells on TN-190 displayed diffuse F-actin staining within the cell body. A striking feature of F-actin organization in S27 and G8 cells, observed in cells adherent on both TN-190 and TN-ADC, was the presence of large arrays of actin-rich ruffles and microspikes at points of cell spreading or at the tips of cellular protrusions (Figure 5).
Figure 5.
Organization of actin microfilaments in S27 and G8 cells adherent on fibronectin or tenascin-C. Cells were plated onto coverslips coated with 50 nM fibronectin, 50 nM CEF-TN, 50 nM recombinant TN-190, or 50 nM recombinant TN-ADC for 90 min in serum-free medium. After incubation cells were fixed, stained with TRITC-phalloidin, and examined under epifluorescence. Bar, 10 μm.
Figure 11.
Cytoskeletal reorganization in response to soluble tenascin-Cs. C2C12 cells (a–h), COS-7 cells (i–l), or MG-63 cells (m–p) adherent on fibronectin (a, e, i, and m), on fibronectin in the presence of 35 nM CEF-TN (b, f, j, and n), in the presence of 35 nM TN-190 (c, g, k, and o), or in the presence of 35 nM TN-ADC (d, h, l, and p) were stained after 1 h with TRITC-phalloidin (e–p) or for fascin (a–d). Bar, 15 μm.
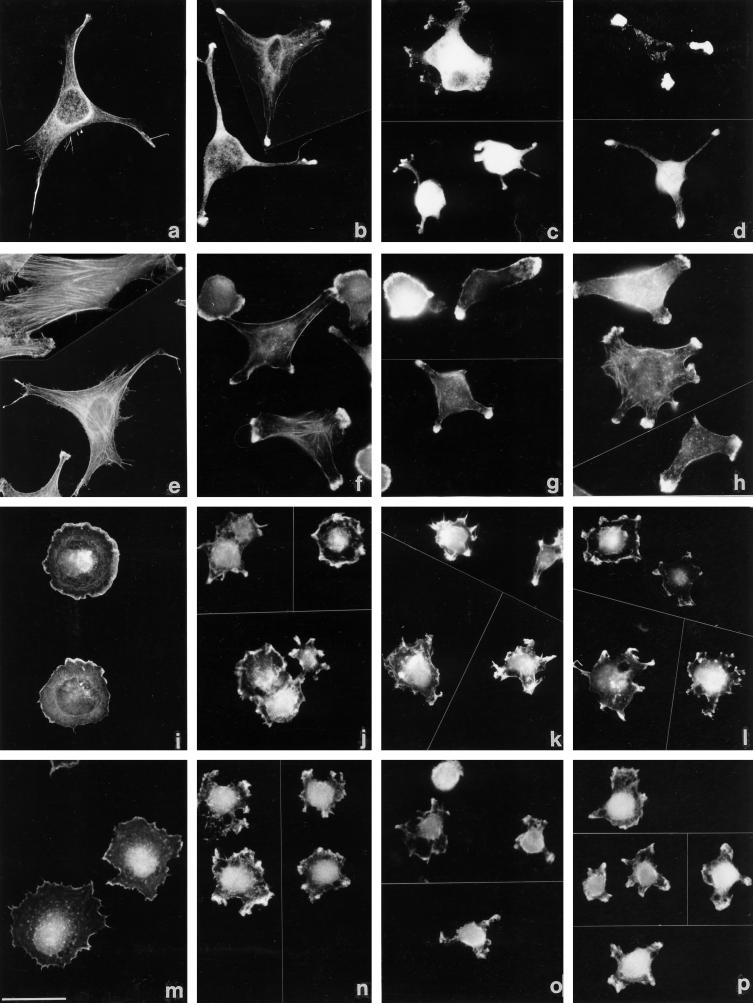
To enable actin organization to be analyzed in more detail, adherent C2C12 and S27 cells were examined by confocal microscopy. C2C12 cells spread on fibronectin organized prominent microfilament bundles that were located at the cell margins or ran longitudinally across the cell body (Figure 6a, see also Adams and Lawler, 1994). In contrast, cells adherent to CEF-TN spread less, tended to be irregular in shape, and displayed diffuse or punctate patterns of TRITC-phalloidin staining within the cell body and intense staining within areas of membrane ruffles and microspikes at the points of the spread cells (Figure 6c). Similar staining patterns were observed in C2C12 cells adherent on TN-190 or TN-ADC; however, the cells on TN-190 tended to remain round, with irregular cytoplasmic protrusions that bore areas of ruffles or microspikes at their tips (Figure 6e), whereas the cells on TN-ADC that had undergone spreading, assumed irregular angular morphologies and exhibited clustered arrays of microspikes and ruffles (Figure 6g).
S27 cells also spread extensively on fibronectin, although the overall cell morphology tended to be more irregular than that of C2C12 cells. These cells contained prominent arrays of actin microfilament bundles (Figure 6b). On CEF-TN, spreading was less extensive than on fibronectin. F-actin again localized as punctate spots rather that microfilament bundles, and microspikes and ruffles enriched in F-actin were apparent at points of substratum contact (Figure 6d). Similar TRITC-phalloidin staining patterns were observed in S27 cells adherent on CEF-TN, TN-190, and TN-ADC; however, it was clear that the S27 cells spread much less on a TN-190 substratum (Figure 6f) than they did on the TN-ADC substratum (Figure 6h). S27 differed from C2C12 cells in that many S27 cells displayed more elongated and prominent microspikes than C2C12 cells, irrespective of the substratum used (see Figure 6). The results of these experiments demonstrate for three cell lines that TN-190 is less adhesive than TN-ADC in terms of cell spreading and, furthermore, indicate that cell spreading on tenascin-C is irregular and involves formation of cortical actin microspikes rather than large microfilament bundles.
Cells Adherent on Tenascin-Cs Lack Focal Contacts and Display Fascin Microspikes
The organization of cortical F-actin in C2C12 cells adherent on tenascin-C substrata was reminiscent of the organization observed in cells adherent on TSP-1, which involves formation of prominent large arrays of actin microspikes at cell margins (Adams and Lawler, 1994). Therefore, the consequences of cell spreading on tenascin-C substrata were examined with respect to the formation of focal contacts and fascin microspikes, cell–substratum contact structures that distinguish the processes of cell adhesion on fibronectin or on TSP-1 (Adams, 1995).
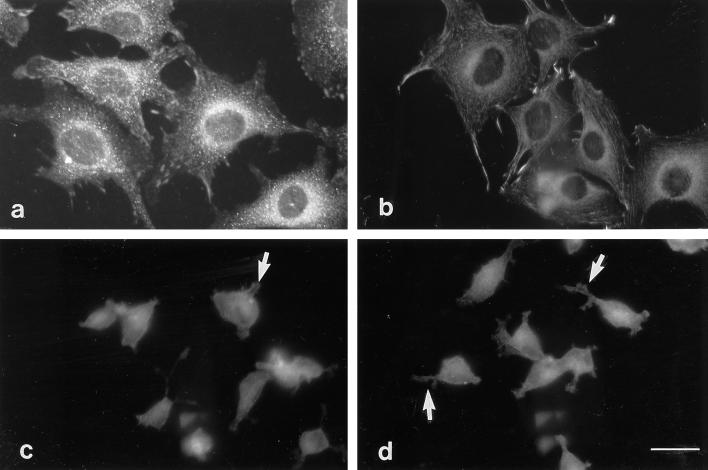
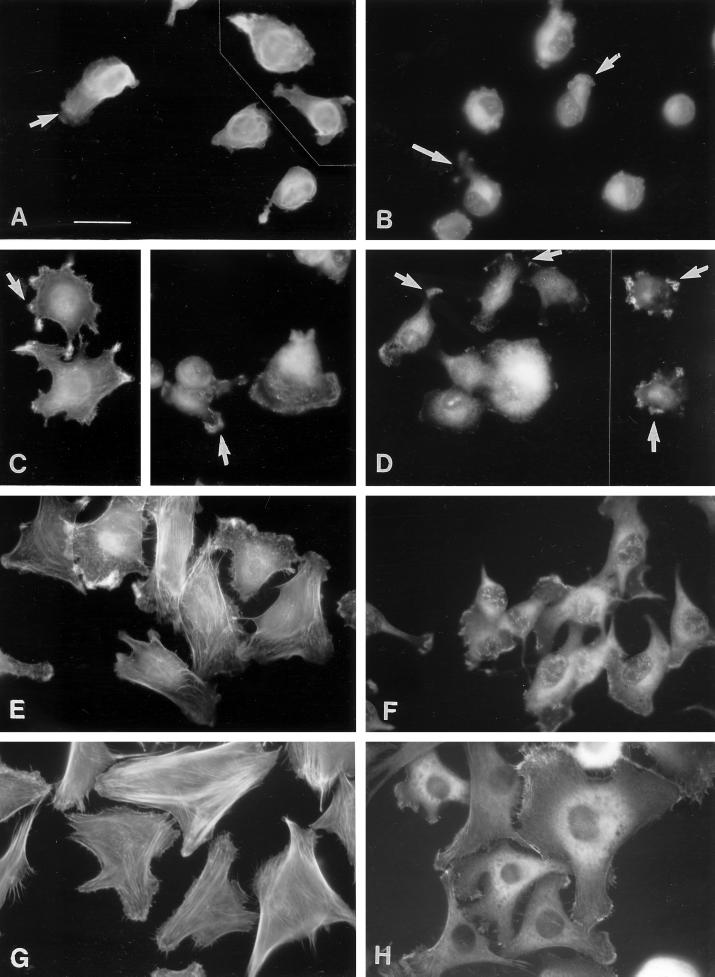
In these experiments, C2C12 and S27 cells were allowed to adhere to fibronectin or CEF-TN and then stained with an antibody to vinculin to visualize focal contacts (Geiger, 1979) or with an antibody to fascin (Yamashiro-Matsumura and Matsumura, 1986) to detect fascin microspikes. Both C2C12 and S27 cells formed abundant focal contacts when adherent on fibronectin (Figure 7, a and b). In contrast, no defined localization of vinculin was apparent in cells adherent on CEF-TN: vinculin did not localize to the cytoplasmic protrusions or regions of actin microspikes and ruffles but remained diffusely distributed throughout the cytoplasm (Figure 7, c and d). Thus, the cells adherent on CEF-TN did not organize focal contacts.
Figure 7.
Cells adherent on tenascin-C substrata do not assemble focal contacts. C2C12 (a and c) or S27 (b and d) cells were stained for vinculin after a 90-min adhesion to fibronectin (a and b) or CEF-TN (c and d). Focal contacts are present in the cells on fibronectin, but the cytoplasmic extensions and microspikes of cells adherent on CEF-TN show no localization of vinculin (small arrows in c and d indicate positions of protrusions). Bar, 10 μm.
In C2C12 and S27 cells spread on fibronectin, fascin remained diffusely distributed through the cytoplasm, as previously reported for other cell types (Adams, 1995; Figure 8, a and b). Some colocalization of fascin with actin microfilament bundles was detectable in S27 cells. In cells adherent on CEF-TN, fascin staining was diffuse within the cell body and appeared concentrated in the microspikes and ruffles present at the points of spread cells (Figure 8, c and d). In the more rounded cells attached to TN-190, fascin was prominent in regions of microspikes at the ends of cytoplasmic protrusions (Figure 8, e and f). The more extensively spread cells on TN-ADC displayed arrays of fascin microspikes at their margins, at points of substratum contact (Figure 8, g and h). Thus, it appears that cell adhesion to tenascin-C differs from adhesion to fibronectin in that it does not involve the assembly of focal contacts but does trigger the formation of fascin microspikes and ruffles at cell margins. These properties are shared by S27 cells and C2C12 cells and both TN-190 and TN-ADC elicit these responses. The fascin microspikes formed in response to tenascin-Cs differ in morphology from those assembled in response to TSP-1 (Adams, 1995) in that they are not formed circumferentially around cell margins but are present in clusters at discrete points along cell margins. Because these protrusions do not have the pronounced elongated shape of filopodia, we continue to term these structures microspikes.
Figure 8.
Presence of fascin-positive microspikes in cells adherent on tenascin-C substrata. C2C12 (a, c, e, and g) or S27 (b, d, f, and h) cells were stained for fascin after a 90-min adhesion to fibronectin (a and b), CEF-TN (c and d), TN-190 (e and f), or TN-ADC (g and h). The arrays of microspikes formed by cells adherent on the tenascin-C substrata stain positively for fascin (examples are indicated by arrows in c, d, f, and h). Bar, 10 μm.
Given that cells adherent on tenascin-C undergo irregular spreading in comparison with sister cells adherent on fibronectin, it was of interest to examine whether another situation of limited cell spreading would evoke fascin microspike formation. Therefore, C2C12 attachment assays were carried out by using substrata coated with low concentrations of fibronectin and the cells were fixed and stained for F-actin or fascin. On substrata coated with 1.25 μg/ml fibronectin, cells attached and tended to remain round, although examples of cells that had extended a smooth lamella on one side could be found. In all cells, F-actin and fascin appeared diffuse (Figure 9, A and B). On substrata coated with 2.5 μg/ml fibronectin, the extent of cell spreading was generally greater, although the cells still had a rounded shape. Formation of irregular F-actin-rich microspikes and ruffles was apparent at the margins of some cells (Figure 9C). These regions tended to stain positively for fascin (Figure 9D).
Figure 9.
F-actin and fascin distributions in C2C12 cells adherent on low concentrations of fibronectin. C2C12 cells were allowed to attach for 1 h to substrata coated with 1.25 μg/ml (A and B), 2.5 μg/ml (C and D), 5 μg/ml (E and F), or 20 μg/ml fibronectin (G and H) for 1 h then fixed and stained with TRITC-phalloidin (A, C, E, and G) or with antibody to fascin (B, D, F, and H). Arrows in A–D indicate examples of cells with F-actin- or fascin-containing membrane protrusions. Bar, 10 μm.
On substrata coated with 5 μg/ml fibronectin, the extent of cell spreading was greater and many cells had assumed a polygonal spread shape. Organization of F-actin into microfilament bundles was apparent in the central regions of the cells (Figure 9E) and fascin staining was diffuse throughout the cell body (Figure 9F). Full cell spreading was achieved on the surfaces coated with 20 μg/ml fibronectin and in these cells, as expected, large numbers of actin microfilament bundles were apparent and fascin staining was diffuse (Figure 9, G and H). Thus, although fascin-containing protrusions are present in poorly spread C2C12 cells attached to low concentrations of fibronectin, these structures are not present in more extensively spread cells and the very irregular protrusive morphologies bearing fascin microspikes that are observed on optimal coating concentrations of tenascin-C are not achieved.
Tenascin-Cs Cause Cytoskeletal Reorganization in Cells Adherent on Fibronectin
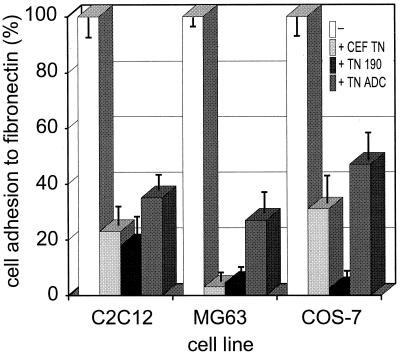
In addition to providing a less adhesive substratum than fibronectin, tenascin-C in solution has the property of preventing cell attachment to fibronectin (Chiquet-Ehrismann et al., 1988). To examine the activity of the recombinant tenascin-Cs in modulating cell adhesion to fibronectin, quantitative cell attachment assays were performed in which cells were allowed to adhere to fibronectin substrata in the presence or absence of the various tenascin-Cs. Three cell types were used in these assays: MG-63 and COS-7 cells, which do not attach to substratum-bound tenascin-C, and C2C12 cells, which undergo irregular spreading. For each cell line, all tenascin-Cs decreased cell adhesion to fibronectin, but TN-190 and TN-ADC displayed different levels of activity with respect to MG-63 and COS-7 cells. For C2C12 cells, CEF-TN decreased cell attachment by 80%, TN-190 decreased the number of attached cells by 83%, and TN-ADC decreased attachment by 65% (Figure 10). TN-190 inhibited MG-63 or COS-7 cell adhesion by around 95%, whereas TN-ADC was significantly less active and inhibited MG-63 cells attachment by 77% and COS-7 cell attachment by 57% (Figure 10). For each cell line, the differences in activity of TN-190 and TN-ADC were significant at p < 0.001. Thus, in assays using three cell types, both tenascin-C splice variants were capable of blocking cell attachment to fibronectin, yet TN-ADC was consistently less active on a molar basis than TN-190. A dot-blot binding assay was used to examine whether the distinct effects of TN-190 or TN-ADC were caused by differential binding to fibronectin. In assays using either 250 ng of fibronectin or tenascin-C as the solid-phase ligand, both splice variants bound fibronectin equivalently (our unpublished observation).
Figure 10.
Modulation of cell attachment to fibronectin by tenascin-Cs in solution. C2C12, MG63, or COS-7 cells were allowed to attach to fibronectin substrata for 60 min in the absence or presence of 35 nM tenascin-C added in solution, as indicated, and the percentage of input cells that attached was quantitated by direct counting. Each column is the mean of three assays; bars are the SEM.
A second set of assays examined cytoskeletal organization in cells adherent on fibronectin in the presence or absence of tenascin-C. Soluble tenascin-C has been shown to cause loss of actin microfilament bundles and focal contacts in preadherent endothelial cells and fibroblasts, without causing changes in cell attachment or spreading. This property has been linked to the A, B, C, and D fibronectin type III repeats (Murphy-Ullrich et al., 1991). To determine whether the alterations in cell adhesion caused by soluble tenascin-Cs in our assay systems involved alterations in the actin cytoskeleton or in fascin distribution, fibronectin adhesion assays were carried out in the presence of soluble tenascin-Cs under conditions that led to cell rounding but not complete cell detachment and then stained with TRITC-phalloidin or fascin antibody. Whereas control untreated C2C12 cells spread extensively on fibronectin and displayed prominent actin microfilament bundles and a uniform distribution of fascin (Figure 11, a and e), cells that had adhered in the presence of CEF-TN were less spread, contained fewer organized microfilament bundles, and exhibit diffuse F-actin staining. Many cells displayed actin-rich ruffles or microspikes at the periphery (Figure 11f). These F-actin-rich residual points of substratum attachment stained strongly for fascin (Figure 11b). Cells that had attached in the presence of an equimolar concentration of TN-190 tended to be more rounded and also displayed decreased F-actin organization and increased peripheral actin-containing ruffles (Figure 11g). Fascin staining appeared diffuse within the rounded cell bodies and was also concentrated at the tips of areas of ruffling or in irregular projections (Figure 11c). TN-ADC, like CEF-TN, did not cause such a large decrease in cell spreading as did TN-190 (Figure 11, compare h with g) but did cause a decrease in actin microfilament organization. Actin-rich ruffles were again present at residual points of substratum contact (Figure 11h). In these cells, intense fascin staining localized to the residual points of attachment and in the cell body (Figure 11d).
Similar changes in cell shape and cortical F-actin organization were observed in COS-7 and MG-63 cells when plated on fibronectin in the presence of soluble tenascin-Cs. These cell types spread but did not assemble prominent actin microfilament bundles when adherent on fibronectin (Figure 11, i and m). The addition of soluble tenascin-Cs resulted in decreased cell spreading of both COS-7 and MG-63 cells, with a somewhat greater effect on cell rounding being caused by TN-190 (Figure 11, k and o). The addition of CEF-TN, TN-190, or TN-ADC correlated with the formation of extensive peripheral F-actin-rich membrane ruffles, microspikes, and fingerlike protrusions by both COS-7 or MG-63 cells (Figure 11, compare j–l with i or n–p with m). Thus, in assays using three cell lines of different tissue origins, alterations in actin cytoskeletal organization occur in response to both recombinant tenascin-C variants.
DISCUSSION
Our studies offer several major novel conclusions. We have identified a mechanistic similarity between the processes of cell adhesion to tenascin-C and adhesion to TSP-1 in that cells adherent on tenascin-C form fascin microspikes and do not assemble focal contacts. This property is common to two tenascin-C splice variants, TN-190 and TN-ADC, and is observed in three cell lines capable of undergoing irregular spreading on tenascin-C substrata. Because the alterations in cell adhesion and cytoskeletal organization that occur when adherent cells are exposed to soluble tenascin-C also involve formation of fascin substratum contacts, our data also establish a biochemical similarity between the adhesive and adhesion-modulating properties of tenascin-C. This is the first report of expression and functional characterization of full-length tenascin-C containing the AD2, AD1, and C repeats. In addition to these generic responses of cells to tenascin-Cs, we obtained evidence for different levels of adhesive and adhesion-modulating activity in TN-190 and TN-ADC.
Our experiments have made use of recombinant TN-190 and a novel full-length recombinant tenascin-C, TN-ADC, that incorporates the newly identified AD2, AD1, and C fibronectin type III repeats and have examined the adhesive properties of these molecules in short-term in vitro assays. Since tenascin-C functions as a cell-type–dependent adhesion molecule, a large panel of cell lines were used to examine the activity of the tenascin-Cs in cell–substratum attachment assays. These experiments identified three cell lines that attached to CEF-TN and the recombinant proteins although, as predicted by the results of previous studies (Spring et al., 1989; Prieto et al., 1992; Aukhil et al., 1993; Joshi et al., 1993), the level of cell attachment in terms of cell number did not reach the level observed on a fibronectin substratum. Also in agreement with previous reports (Lotz et al., 1989; Spring et al., 1989; Prieto et al., 1992), CEF-TN and the recombinant proteins were essentially nonadhesive toward some of the cell lines tested.
Cell spreading on other extracellular matrix substrata depends on actin polymerization (Orlando and Cheresh, 1991; Adams, 1995); therefore, cell adhesion to tenascin-Cs was analyzed with respect to organization of the actin-based cytoskeleton. Cells partially spread on tenascin-C substrata assumed irregular shapes in comparison with sister cells spread on fibronectin substrata. These irregular shapes presumably result from a nonuniform distribution of cell–substratum adhesion points. Irregular shapes have also been described for cells adherent on TSP-1 substrata (Asch et al., 1991; Stomski et al., 1992; Adams and Lawler, 1993; Adams and Lawler, 1994). Irregular cell spreading on both CEF-TN and TN-ADC correlated with organization of F-actin into microfilament bundles, although the large longitudinal bundles typical of cells adherent on fibronectin were not observed. This organization of F-actin distinguished cells on TN-ADC or CEF-TN from the less-spread cells attached to TN-190, in which small points or dots of F-actin were observed against a diffuse background of staining. However, the most striking feature of F-actin organization, observed in cells adherent on CEF-TN, TN-190, or TN-ADC, was the presence of arrays of actin-rich microspikes or large ruffles at discrete points along the cell margins that represent sites of cell–substratum adhesion. The presence of these structures in irregularly spread cells contrasts with the membrane activity observed in cells initiating spreading on fibronectin, either at early time points in cells exposed to optimal fibronectin coating concentrations (Adams, 1995) or in the limited spreading achieved on low coating concentrations of fibronectin (this study).
To examine the nature of these contacts in more detail, the adherent cells were stained for markers of two types of substratum adhesion contacts. The most well-characterized type of cell–substratum contact is the focal contact, or focal adhesion, that forms the closest point of apposition between the ventral surface of cells and their substratum (Izzard and Lochner, 1976; Heath and Dunn, 1978). In well-spread cells, focal contacts are typically distributed over the entire ventral plasma membrane, at the termini of actin microfilament bundles. Focal contacts are rapidly assembled by cells spread on adhesive glycoproteins such as fibronectin, vitronectin, and collagen (reviewed by Burridge et al., 1988). Cells adherent on TSP-1 lack focal contacts and form substratum contacts characterized by fascin microspikes (Adams, 1995). Fascin is an 55-kDa actin-bundling protein that is found in several types of actin-containing structures in different organisms and that forms stable bundles with actin in vitro (reviewed by Edwards and Bryan, 1995). By using two cell lines, we found that cell adhesion to tenascin-C variants does not correlate with formation of focal contacts but does correlate with the formation of clusters of fascin microspikes and ruffles.
Because tenascin-C and fibronectin colocalize at sites of active morphogenesis during development, it appears likely that interactions between these proteins are involved in facilitating cell rearrangements. Our in situ hybridization data obtained from developing feather buds and sternum indicate that tenascin-C splice variants containing the AD2, AD1, or C repeats are expressed by subsets of the cells within these structures that express tenascin-C. Feather bud growth in organ culture is a tenascin-C-dependent process (Jiang and Chuong, 1992) and it is known that bud growth involves both cell proliferation and cell migration into the extending feather shaft (Wessels, 1965; Jiang and Chuong, 1992). Similarly, the keel, which forms a few hours after the fusion of two cartilagenous primordia give rise to the sternum, first appears as a mass of chondrogenic cells along the ventral midline of the sternal body that are amoeboid in shape and that appear to stream caudally toward the abdomen as the keel grows (Fell, 1939). Thus, the AD2, AD1, and C splice variants are expressed at sites of tissue modeling and may participate with other tenascin-C splice variants in modulating cell adhesive behavior in fibronectin matrices and in providing appropriate microenvironments to support regulated changes in cell growth and movement.
It is well-established that tenascin-C in solution modulates cell adhesive behavior on fibronectin substrata (Chiquet-Ehrismann et al., 1988). Even cell types that do not attach to tenascin-C are sensitive to adhesion modulation, presumably because the biophysical requirements for cell surface binding of tenascin-C in solution are less stringent than the requirements for formation of a mechanically strong substratum attachment. In endothelial cells and fibroblasts, disassembly of focal contacts is observed in the absence of alterations in cell spreading (Murphy-Ullrich et al., 1991). Peptides from the heparin-binding domain of TSP-1 also evoke this response and in both cases, activation of protein kinase G is required (Murphy-Ullrich et al., 1996). Our data establish a biochemical similarity between cell adhesion on tenascin-Cs and adhesion modulation by tenascin-Cs, in that both processes involve formation of cortical microspikes and ruffles that contain F-actin and fascin. Thus, as would be expected if the same sets of cell-surface binding proteins are ligated, we propose that both processes may involve similar intracellular signals and cytoskeletal organization events that then serve to promote or antagonize cell adhesion in a context-dependent manner, depending on the manner in which tenascin-C is presented to the cells.
S27 myoblasts differ from the C2C12 parental cell line by their deficiency in production of heparan sulfate and chondroitin sulfate proteoglycans (Gordon and Hall, 1989). S27 myotubes, unlike C2C12 myotubes, do not cause preferential accumulation of neurites containing synaptic vesicles in cocultured neurons (Lupa et al., 1990) and themselves display decreased clustering of acetylcholine receptors in response to soluble agrin (Ferns et al., 1993). The principal biochemical defect in S27 proteoglycan production appears to reside in decreased glycosaminoglycan chain length (Bowen et al., 1996). Several cell-surface proteoglycans are known to bind to tenascin-C. These include syndecan-1 (Salmivirta et al., 1991) and possibly heparan sulfate proteoglycans (Aukhil et al., 1993) and also chondroitin sulfate proteoglycans such as neurocan and phosphacan/receptor tyrosine kinase β (Barnea et al., 1994; Grumet et al., 1994). Although C2C12 and S27 cells attached equally well to fibronectin, S27 cells attached in much larger numbers to the tenascin-C variants and also tended to display larger arrays of fascin microspikes. Because the heparin-binding site of tenascin-C is located in the fibrinogen-like globular domain (Fischer et al., 1995), it would be expected that, as observed with S27 cells, alterations in proteoglycan-mediated adhesion would be apparent on both TN-190 and TN-ADC.
Several other cell-surface tenascin-C binding proteins have been identified in various cell types. These include F3/F11/contactin (Zisch et al., 1992), annexin II (Chung and Erickson, 1994), the integrin α2β1 on endothelial cells (Sriramarao et al., 1993), α8β1 on neurons and in 293 transfectants (Schnapp et al., 1995; Varnum-Finney et al., 1995), α9β1 in transfected cell lines (Yokosaki et al., 1994, 1996), αvβ3 on various cell types (Salmivirta et al., 1991; Joshi et al., 1993; Prieto et al., 1993), and also αvβ6 (Prieto et al., 1993; Yokosaki et al., 1996). Most of the mapped binding sites fall within the constant domains of the tenascin-C molecule (Zisch et al., 1992; Joshi et al., 1993; Varnum-Finney et al., 1995), but annexin II provides an example of a protein for which the binding site lies within the A, B, C, and D variable repeats (Chung et al., 1996). It will be of interest to identify the binding sites used by C2C12 and S27 cells.
In addition to the generic cellular responses to tenascin-C variants identified in our studies, the TN-ADC variant exhibited distinctive activities. On a molar basis, TN-ADC was quantitatively more active in supporting cell attachment and irregular cell spreading. This result was observed for both C2C12 cells and S27 cells and is the first report of a difference in adhesive properties between full-length tenascin-C splice variants. CEF-TN also supported a higher level of cell spreading than TN-190, presumably because this preparation contains a mixture of splice variants including ABAD2AD1C (Tucker et al., 1994). TN-ADC was also less active on a molar basis in terms of antiadhesive activity in preventing cell attachment to fibronectin. We did not detect differential fibronectin binding by TN-ADC or TN-190, thus this difference in activity may result from different mechanisms of interaction at the cell surface. For example, differences in cell spreading could result either from greater mechanical strength of initial cell attachment to TN-ADC (Lotz et al., 1989) or from activation of intracellular signaling pathways that are required to promote the cytoskeletal organization involved in cell spreading (reviewed by Schwartz et al., 1995; Yamada and Miyamoto, 1995). Activation of such pathways by soluble TN-ADC would also serve to limit the extent of cell rounding. Such mechanical and/or biochemical differences might result from direct interactions of the additional fibronectin type III repeats with cell surface binding proteins or from indirect effects of these repeats on the cell adhesive properties of tenascin-C. For example, the insertion of the AD2, AD1, and C repeats might have the effect of increasing the activity of an adhesive domain or decreasing the activity of an antiadhesive domain, conceivably by altering the accessibility of such sites to their cognate cell-surface receptors.
Cells adherent on TSP-1 tend to spread and assemble large arrays of radial fascin microspikes: cells on tenascin-C undergo more irregular cell spreading and localized microspike formation. It is possible that the ligation of cell-surface tenascin-binding proteins does not provide such a strong stimulus for microspike formation; however, it remains to be determined whether the more limited formation of microspikes is cause or consequence of irregular cell spreading. Although tenascin-C and TSP-1 are structurally distinct matrix macromolecules that interact with distinct arrays of cell surface receptors, these results establish a novel biochemical similarity in the processes of cell adhesion to tenascin-C and TSP-1. The identification of a biochemical response that differs from cellular responses to classical adhesive glycoproteins such as fibronectin offers a basis for further studies of the mechanisms by which cells respond to tenascin-C and other adhesion-modulating glycoproteins.
ACKNOWLEDGMENTS
We thank Theres Schultless for the electron microscopy of recombinant tenascins, George Mosialos for the gift of fascin antibody, and Zach Hall for the gift of S27 cells. J.C.A. gratefully acknowledges the support of a Senior Fellowship in Basic Biomedical Science from the Wellcome Trust (grant 038284). We are most grateful to the British/Swiss Joint Research Programme of the British Council and Swiss National Research Foundation for a travel grant that enabled these experiments to be initiated. This work was supported in part by funds provided by the National Science Foundation (BNS-9021124) and the University of California at Davis School of Medicine to R.P.T.
REFERENCES
- Adams JC. Formation of stable microspikes containing actin and the 55-kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J Cell Sci. 1993;104:1061–1071. doi: 10.1242/jcs.104.4.1061. [DOI] [PubMed] [Google Scholar]
- Adams J, Lawler J. Cell-type specific adhesive interactions of skeletal myoblasts with thrombospondin-1. Mol Biol Cell. 1994;5:423–437. doi: 10.1091/mbc.5.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Tucker RP, Lawler J. The Thrombospondin Gene Family. Austin, TX: R.G. Landes; 1995. and Berlin: Springer-Verlag. [Google Scholar]
- Asch AS, Tepler J, Silbiger S, Nachman RL. Cellular attachment to thrombospondin: cooperative interactions between receptor systems. J Biol Chem. 1991;266:1740–1745. [PubMed] [Google Scholar]
- Aukhil I, Joshi P, Yan Y, Erickson HP. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993;268:2542–2553. [PubMed] [Google Scholar]
- Barnea G, Grumet M, Milev P, Silvennoine O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase β is expressed in the form of proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994;269:14349–14352. [PubMed] [Google Scholar]
- Blau HM, Pavlath GK, Hardeman EC, Chiu C-P, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon MA, Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989;108:1149–1155. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signalling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Christian CN, Nelson PG, Peacock J, Nirenberg M. Synapse formation between two clonal cell lines. Science. 1977;198:995–998. doi: 10.1126/science.193191. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Fambrough DM. Chick myotendinous antigen I. A monoclonal antibody as a marker for tendon and muscle morphogensis. J Cell Biol. 1984;98:1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Anti-adhesive molecules of the extracellular matrix. Curr Opin Cell Biol. 1991;3:800–804. doi: 10.1016/0955-0674(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Tenascin and other adhesion-modulating proteins in cancer. Semin Cancer Biol. 1993;4:301–310. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Tenascins, a growing family of extracellular matrix proteins. Experientia. 1995a;51:853–862. doi: 10.1007/BF01921736. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Inhibition of cell adhesion by anti-adhesive molecules. Curr Opin Cell Biol. 1995b;7:715–719. doi: 10.1016/0955-0674(95)80114-6. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;53:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47:131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Matsuoka Y, Hofer U, Spring J, Bernasconi C, Chiquet M. Tenascin variants: differential binding to fibronectin and distinct distribution in cell cultures and tissues. Cell Regul. 1991;2:927–938. doi: 10.1091/mbc.2.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol. 1994;126:539–548. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RA, Bryan J. Fascins, a family of actin bundling proteins. Cell Motil Cytoskeleton. 1995;32:1–9. doi: 10.1002/cm.970320102. [DOI] [PubMed] [Google Scholar]
- Ehrismann R, Chiquet M, Turner DC. Mode of action of fibronectin in promoting chick myoblast attachment. J Biol Chem. 1981;256:4056–4062. [PubMed] [Google Scholar]
- Erickson HP. Tenascin-C, tenascin-R and tenascin-X—a family of talented proteins in search of functions. Curr Opin Cell Biol. 1993;5:869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol, 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Taylor HC. Hexabrachion proteins in embryonic chicken and human tumors. J Cell Biol. 1987;105:1387–1394. doi: 10.1083/jcb.105.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. The origin and developmental mechanics of the avian sternum. Phil Trans R Soc Lond B Biol Sci. 1939;229:407–463. [Google Scholar]
- Ferns MJ, Campaneli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Fischer D, Chiquet-Ehrismann R, Bernasconi C, Chiquet M. A single heparin-binding region within the fibrinogen-like domain is functional in chick tenascin-C. J Biol Chem. 1995;270:3378–3384. doi: 10.1074/jbc.270.7.3378. [DOI] [PubMed] [Google Scholar]
- Frazier WA. Thrombospondins. Curr Opin Cell Biol. 1991;3:792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Hoffman S, Edelman GM. Functional mapping of cytotactin: proteolytic fragments active in cell-substrate adhesion. J Cell Biol. 1988;107:2329–2340. doi: 10.1083/jcb.107.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chick gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV-40 transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gordon H, Hall ZW. Glycosaminoglycan variants in the C2 muscle cell line. Dev Biol. 1989;135:1–11. doi: 10.1016/0012-1606(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Götz B, Scholze A, Clement A, Joester A, Schutte K, Wigger F, Frank R, Spiess E, Ekblom P, Faissner A. Tenascin-C contains distinct adhesive, anti-adhesive and neurite outgrowth promoting sites for neurons. J Cell Biol. 1996;132:681–699. doi: 10.1083/jcb.132.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Katthikeyan M, Bourdon RK, Margolis RK, Margolis R. Interactions with tenascin and differential effects on cell adhesion of neuron and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue J. Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high voltage electron-microscope study. J Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. Cell-to-substratum contacts in living fibroblasts : an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976;21:129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Jiang T-X, Chuong C-M. Mechanisms of skin morphogenesis. I. Analyses with antibodies to adhesion molecules tenascin, N-CAM, and integrin. Dev Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jones FS, Burgoon MP, Hoffman KL, Crossin KL, Cunningham BA, Edelman GM. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing and binding regions. Proc Natl Acad Sci USA. 1989;86:1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Chung C-Y, Aukhil I, Erickson HP. Endothelial cells adhere to the RGD domain and the fibrinogen-like terminal knob of tenascin. J Cell Sci. 1993;106:389–400. doi: 10.1242/jcs.106.1.389. [DOI] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976;98:349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahav J, editor. Thrombospondin. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Lane T, Sage H. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of Arg-Gly-Asp, calcium and integrin receptors. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109:1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupa MT, Gordon H, Hall ZW. A specific effect of muscle cells on the distribution of presynaptic proteins in neurites and its absence in a C2 muscle cell variant. Dev Biol. 1990;142:31–43. doi: 10.1016/0012-1606(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Mackie EJ. Tenascin in connective tissue development and pathogenesis. Perspect Dev Neurobiol. 1994;2:125–132. [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP. Tenascin in bone morphogenesis: expression by osteoblasts and cell type-specific expression of splice variants. J Cell Sci. 1992;103:765–771. doi: 10.1242/jcs.103.3.765. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Hook M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin [published erratum appears in J. Cell Biol. (1992). 116, 833] J Cell Biol. 1991;115:1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Pallero MA, Boerth N, Greenwood JA, Lincoln TM, Cornwell TL. Cyclic GMP-dependent protein kinase is required for thrombospondin and tenascin mediated focal contact disassembly. J Cell Sci. 1996;109:2499–2508. doi: 10.1242/jcs.109.10.2499. [DOI] [PubMed] [Google Scholar]
- Orlando RA, Cheresh DA. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin αvβ3 and its ligand. J Biol Chem. 1991;266:19543–19550. [PubMed] [Google Scholar]
- Prieto AL, Andersson-Fisone C, Crossin KL. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol. 1992;119:663–678. doi: 10.1083/jcb.119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment of cytotactin/tenascin. Proc Natl Acad Sci USA. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Nellson-Rees WA, Toth EM, Arnstein P, Garcher MB. Characterization of a newly derived human sarcoma cell line. Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Salmivirta M, Elenius K, Vaino S, Hofer U, Chiquet-Ehrismann R, Thesleff I, Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem. 1991;266:7733–7739. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin α8β1 functions as a receptor for tenascin, fibronectin and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Siri A, Carnemolla B, Saginati M, Leprini A, Casari G, Baralle F, Zardi L. Human tenascin: primary structure, pre-mRNA splicing patterns and localization of the epitopes recognized by two monoclonal antibodies. Nucleic Acids Res. 1991;19:525–531. doi: 10.1093/nar/19.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J, Beck K, Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989;59:325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, Bourdon MA. A novel tenascin type III repeat is part of a complex of tenascin mRNA alternative splices. Nucleic Acids Res. 1993;21:163–168. doi: 10.1093/nar/21.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by α2β1 and α3β1integrins. J Cell Sci. 1993;105:1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- Stomski FC, Gani JS, Bates RC, Burns GF. Adhesion to thrombospondin by human embryonic fibroblasts is mediated by multiple receptors and includes a role for glycoprotein 88 (CD36) Exp Cell Res. 1992;198:85–92. doi: 10.1016/0014-4827(92)90152-x. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The sequential expression of tenascin mRNA in epithelium and mesenchyme during feather morphogenesis. Roux’s Arch Dev Biol. 1991;200:108–112. doi: 10.1007/BF00637191. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The in situ localisation of tenascin splice variants and thrombospondin-2 mRNA in the avian embryo. Development. 1993;117:347–358. doi: 10.1242/dev.117.1.347. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Adams JC, Lawler J. Thrombospondin-4 is expressed by early osteogenic tissues in the chick embryo. Dev Dyn. 1995;203:477–490. doi: 10.1002/aja.1002030410. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Spring J, Baumgartner S, Martin D, Hagios C, Poss PM, Chiquet-Ehrismann R. Novel tenascin variants with a distinctive pattern of expression in the avian embryo. Development. 1994;120:637–647. doi: 10.1242/dev.120.3.637. [DOI] [PubMed] [Google Scholar]
- Turner CE, Burridge K. Transmembrane molecular assemblies in extracellular matrix interactions. Curr Opinion Cell Biol. 1991;3:849–853. doi: 10.1016/0955-0674(91)90059-8. [DOI] [PubMed] [Google Scholar]
- Tuszynski GP, Rothman VL, Murphy A, Siegler K, Smith L, Smith S, Karczewski J, Knudsen KA. Thrombospondin promotes cell-substratum adhesion. Science. 1987;236:1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Varani J, Nickoloff BJ, Riser BL, Mitra RS, O’Rourke K, Dixit VM. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988;81:1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Venstrom K, Muller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor α8β1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels NK. Morphology and proliferation during early feather development. Dev Biol. 1965;12:131–153. doi: 10.1016/0012-1606(65)90025-4. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signalling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S, Matsumura F. Intracellular localization of the 55kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J Cell Biol. 1986;103:631–640. doi: 10.1083/jcb.103.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosaki Y, Monis H, Chen J, Sheppard D. Differential effects of the integrins α9β1, αvβ3 and αvβ6 on cell proliferative responses to tenascin. J Biol Chem. 1996;271:24144–24150. doi: 10.1074/jbc.271.39.24144. [DOI] [PubMed] [Google Scholar]
- Yokosaki Y, Palmer EL, Prieto AL, Crossin AL, Bourdon MA, Pytela R, Sheppard D. The integrin α9β1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- Zisch AH, D’Alessandri L, Ranscht B, Falchetto R, Winterhalter KH, Vaughan L. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J Cell Biol. 1992;119:203–213. doi: 10.1083/jcb.119.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]