SYNOPSIS
Objectives.
Bundling human immunodeficiency virus (HIV) testing with tests for other infectious diseases such as hepatitis C, syphilis, or gonorrhea has been proposed as a method to recruit at-risk individuals into HIV testing. The objectives of this study were to determine (1) the types of at-risk clients who choose the rapid vs. standard HIV test when bundled with hepatitis and sexually transmitted infection (STI) tests, and (2) whether clients receiving a rapid HIV test are more likely to return on time for hepatitis and STI test results.
Methods.
We recruited individuals from drug treatment programs, methadone maintenance programs, needle-exchange programs, a community-based agency serving the gay and lesbian community, and the Center for Behavioral Research and Services' office-based testing facility at California State University, Long Beach from January 2005 through November 2007.
Results.
A total of 2,031 clients from a multiple morbidities testing program in Long Beach, California, were tested between January 2005 and November 2007. For clients receiving hepatitis and STI testing, the majority chose the standard HIV test. Clients who received a rapid HIV test returned in significantly fewer days than clients who received a standard HIV test. Injection drug users and sex traders were more likely to choose the standard HIV test and more likely to fail to return for test results on time.
Conclusion.
The rapid HIV test, in conjunction with hepatitis and STI tests, results in clients being more likely to return on time for hepatitis and STI results. Public health efforts should focus on acquainting high-risk clients with rapid HIV testing.
One problem with standard testing for human immunodeficiency virus (HIV) is the failure of those tested to return for their results.1,2 One study found failure-to-return rates of 10% to 27% of people tested.3 The standard HIV test may be defined as a test that requires that the specimen, either blood or oral fluid, be processed by a commercial or public health laboratory. Results from tests requiring laboratory processing are not immediately available to the client and may take anywhere from two to 14 days, depending on the laboratory.
Rapid HIV testing has proved to be a solution to this problem for many different types of clients and settings, including traditional sexually transmitted disease clinics,4 mobile clinics,5,6 and emergency departments.7 Rapid HIV testing technologies offer results within 20 to 40 minutes. However, for some at-risk clients, the availability of HIV testing, either rapid or standard, may not be enough to convince these difficult-to-reach clients to get tested for HIV. In addition to providing rapid HIV testing, one strategy has been to offer HIV testing bundled with other tests. In California, the State Office of AIDS (SOA) piloted the provision of hepatitis C testing in conjunction with HIV testing to injection drug users (IDUs).8
Rapid HIV testing is widely available within the U.S., but rapid tests for other infections such as hepatitis and syphilis, while available in other parts of the world, are not yet commercially available in the U.S. or the manufacturers have not yet submitted applications to the U.S. Food and Drug Administration. Bundling testing services may result in more at-risk people being tested for HIV; however, it also raises the possibility that clients may now fail to return for results of all of the tests.
This study investigated whether (1) at-risk individuals would select the rapid or standard HIV test if offered a choice, and (2) clients receiving the rapid HIV test in conjunction with hepatitis and sexually transmitted infection (STI) testing (for syphilis, gonorrhea, and chlamydia) would return on time for those STI results compared with clients receiving a standard HIV test in conjunction with hepatitis and STI testing. For purposes of this study, tests for hepatitis A, B, and C were included because of the variety of at-risk people being tested. While hepatitis A is not generally considered an STI, it has been found in samples of men who have sex with men (MSM) whose sexual practices include oral-anal contact. Hepatitis B is also not exclusively an STI, but may be transmitted parenterally among IDUs.
METHODS
Individuals were recruited from drug treatment programs, methadone maintenance programs, needle-exchange programs, a community-based agency serving the gay and lesbian community, and the Center for Behavioral Research and Services' (CBRS') office-based testing facility at California State University, Long Beach (CSULB) from January 2005 through November 2007. The majority of the testing took place in the CBRS mobile testing van, a large vehicle with two private counseling rooms, phlebotomy chairs, a laboratory, a centrifuge, and refrigeration facilities located on board.9
Eligibility
Eligibility for inclusion in the study required that participants be at least 18 years of age and meet the definition of a behavioral risk group (BRG) as defined by the Los Angeles County Office of AIDS Programs and Policy (OAPP). BRGs included MSM, women at sexual risk, IDUs, men who have sex with men and women, and transgender individuals. The funding agency allowed the program to test up to 15% of clients who did not fall into a BRG category, ensuring that most clients who wished to be tested received the testing. All tests were provided free to participants. HIV-positive clients who were already aware of their serostatus were tested for hepatitis and STIs. Clients who returned for standard test results (HIV, hepatitis, and/or STIs) received a $5 nonmonetary gift card as an incentive; the provision of a small nonmonetary incentive is standard for testing programs funded by OAPP (Personal communication, Paulina Zamudio, MPA, OAPP, December 2007).
Questionnaires
As part of their involvement in the project, participants completed a variety of questionnaires designed to elicit information on drug use, injection practices, sexual practices, and other risk factors related to acquiring hepatitis, syphilis, gonorrhea, chlamydia, and HIV. The following questionnaires were used:
Designer Drug Trailer, developed for use in conjunction with the RBA to elicit information on the use of so-called “designer” drugs such as ecstasy, ketamine, and gamma hydroxybutyric acid (GHB)
University of Rhode Island Change Assessment12
Sexual Addiction Screening Test (separate versions available for gay men, men who have sex only with women, and women)13,14
Coping Strategies Indicator15
Sexual Sensation Seeking, Non-Sexual Sensation Seeking Scale16
Procedure
All participants were screened using a brief form approved by the CSULB Institutional Review Board (IRB) to determine to which BRG they belonged. As noted previously, failure to fall into a BRG category did not mean that clients were denied testing. After screening clients, we obtained their informed consent using a form also approved by the CSULB IRB.
After obtaining informed consent, we administered all questionnaires, provided pretest counseling, and obtained an oral fluid sample to perform the rapid test using the OraQuick Advance® Rapid HIV-1/2 Antibody Test (OraSure Technologies, Bethlehem, Pennsylvania) for those individuals receiving the rapid test (per OAPP protocols, the client received counseling while the rapid test was processing). Phlebotomy (or if phlebotomy was not possible, a finger stick) was performed after the result of the rapid HIV test was known. Participants provided additional blood for serum banking as part of the protocol; sufficient blood was drawn during phlebotomy for testing, serum banking, and confirmatory HIV testing (if necessary).
Participants who chose the rapid HIV test received their HIV test results within 20 to 40 minutes, according to manufacturer protocols. Preliminary positive HIV results were provided to clients, who were then counseled on the meaning of the preliminary positive test result and the importance of returning for the confirmatory test result, as well as the results of the hepatitis and STI tests. Lastly, all participants were provided with the date on which they could receive their test results, usually within one week. Participants who tested positive for any infection were provided with referrals for additional medical follow-up, including liver function testing for those who were hepatitis C positive, and medical care and case-management services for those with a positive confirmatory test for HIV.17 Clients with positive STI results were immediately referred to the City of Long Beach Health Department for treatment.
Multiple morbidities testing
All participants were given the choice of receiving either the standard blood/oral fluid OraSure® test for HIV (with a one-week turnaround time for results) or the OraQuick Advance rapid HIV test, in addition to blood tests for syphilis and hepatitis A and B. According to OAPP guidelines, only participants with any IDU history could receive hepatitis C testing. All participants could also receive testing for gonorrhea and chlamydia by providing a urine sample.
All participants received pretest counseling for hepatitis A, B, and C as well as syphilis, gonorrhea, chlamydia, and HIV. All pretest protocols for hepatitis, syphilis, gonorrhea, and chlamydia followed the guidelines promulgated by the Centers for Disease Control and Prevention (CDC), and all pretest protocols for HIV testing (standard or rapid testing formats) followed the guidelines determined by OAPP and the SOA. All phlebotomists on the project were nationally certified by the National Accrediting Agency for Clinical Laboratory Sciences and were also certified by OAPP and the state of California to administer the OraQuick Advance rapid HIV test. Blood tests for hepatitis A, B, or C and syphilis seromarkers were conducted by a commercial laboratory.18–22 Specimens that tested positive for hepatitis C were automatically tested with the quantitative polymerase chain reaction (PCR) test for presence of hepatitis C virus (HCV). Clients also provided a urine specimen for gonorrhea and chlamydia testing.23 The laboratory provided results via fax, usually within two to three working days.
Two tests were used for hepatitis C, depending upon the condition of the participants' veins. If participants were able to undergo phlebotomy and a blood sample could be obtained in this manner, the Abbott HCV enzyme immunoassay (EIA) 2.020 was used (Abbott Laboratories, Abbott Park, Illinois). For participants for whom phlebotomy was not an option due to vein damage, we used the test from the Home Access Health Corporation (Hoffman Estates, Illinois), which requires only a finger-stick blood sample. These participants underwent the same pretest counseling for hepatitis C as the other participants; however, due to our inability to obtain a large blood sample from them, they did not undergo testing for hepatitis A or B, syphilis, or PCR for hepatitis C. As with the other clients, these participants had the option of either the rapid or standard HIV test. Test results were provided through the same format for the home test kits as for the results obtained through venipuncture; that is, participants returned to the testing site, and a counselor provided test results and posttest counseling to explain the results.
Statistical analysis
Bivariate tests of association were used to determine differences between clients choosing the rapid HIV test compared with those choosing the standard HIV test. For purposes of determining on-time return for hepatitis and STI results, clients who returned within seven days for results were considered to be on time, while those who returned later than seven days after their initial blood draw or who did not return at all were considered to have failed to return on time for test results.
Logistic regression analysis was used to develop a model for predicting failure to return on time for hepatitis and STI results for those clients receiving the full complement of multiple morbidities testing.
RESULTS
Who chose the rapid HIV test over the standard HIV test?
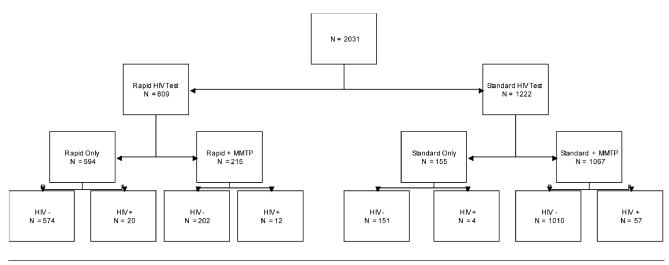
Individuals (n=2,031) in Long Beach were tested through a publicly funded HIV and STI testing program. The Figure shows a flow chart of clients receiving the various types of tests. A total of 809 clients chose the rapid HIV test, with 1,222 choosing the standard HIV test. Of those choosing the rapid HIV test, 594 chose to be tested only for HIV and declined any hepatitis or STI tests; of those choosing the standard HIV test, 155 chose to be tested only for HIV and declined any hepatitis or STI testing. The clients who received only HIV testing were not included in our analysis, as they did not receive any of the multiple morbidities testing. Our research question became, how do the 215 clients who chose the rapid HIV test plus the hepatitis and STI testing differ from the 1,067 who chose the standard HIV test plus hepatitis and STI testing? (For comparison, we will refer to these two groups as the rapid vs. standard group.)
Figure. Flow of clients through standard and rapid HIV testing (n=2,031).
HIV = human immunodeficiency virus
MMTP = multiple morbidities testing program
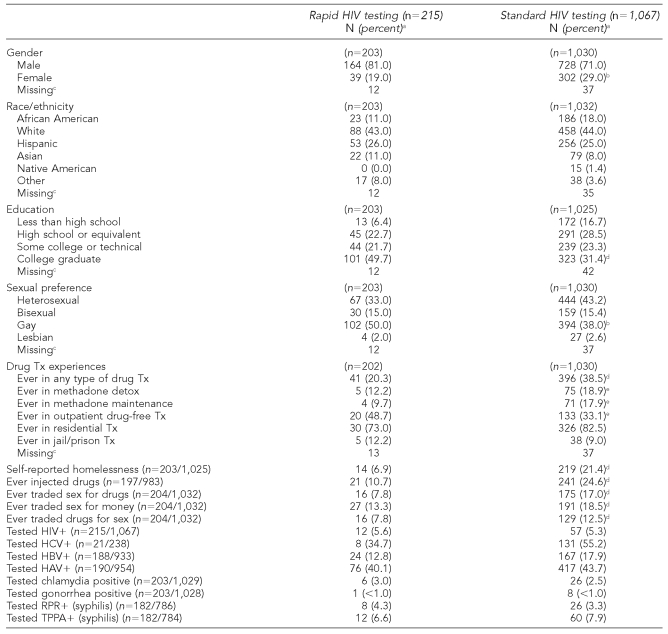
Of the 1,282 total clients who were tested for HIV as well as hepatitis and STIs, only 215 (16.7%) chose the rapid HIV test over the standard HIV test. Demographics comparing clients who chose the rapid HIV test with those who chose the standard HIV test can be found in the Table. Compared with the standard group, the rapid group was more likely to be male, more highly educated, more likely to have self-identified as gay (χ2 [2, n=1,003] = 6.18, p=0.045), less likely to report that they were homeless, and less likely to report having ever injected drugs.
Table. Characteristics of clients choosing rapid HIV testing compared with clients choosing standard HIV testing among those clients who also received hepatitis and STI testing (n=1,282).
aPercentages are based on number of people who answered the question.
bStatistically significant at p<0.05
c“Missing” indicates number of people who did not respond to the question.
dStatistically significant at p<0.001
eStatistically significant at p<0.01
HIV = human immunodeficiency virus
STI = sexually transmitted infection
Tx = treatment
HCV = hepatitis C virus
HBV = hepatitis B virus
HAV = hepatitis A virus
RPR = rapid plasma reagin
TPPA = treponema pallidum particle agglutination assay
There were no differences between rapid and standard groups on whether they tested positive for HIV, hepatitis A virus, hepatitis B virus, HCV, syphilis, gonorrhea, or chlamydia, and there were no differences in race/ethnicity between the rapid and standard groups.
Failure to return on time for hepatitis and STI results
Clients receiving the rapid HIV test returned in significantly fewer days for STI and hepatitis results (mean [M] = 9.49, standard deviation [SD] = 4.99) compared with clients who received standard HIV testing (M=14.67, SD=37.27, t [1,077] = 4.05, p<0.0001). Failure to return on time for both groups was associated with having ever injected drugs (χ2 [1, n=1,004] = 16.25, p<0.0001), having ever had sex for money (χ2 [1, n=1,049] = 20.87, p<0.0001), having ever had sex to get drugs (χ2 [1, n=1,049] = 21.19, p<0.0001), having a higher perception of risk for HIV (χ2 [1, n=1,029] = 16.18, p=0.0028), and being in one of the IDU BRGs (χ2 [1, n=7] = 25.08, p=0.0007). For clients receiving standard HIV testing, 18% never returned for their HIV and other test results. For both the rapid and standard groups, there was no difference between clients who returned on time and clients who did not return on time with respect to positive test results for hepatitis, syphilis, HIV, gonorrhea, or chlamydia.
Three variables were included in the logistic regression model predicting failure to return on time for test results. The final model included having ever injected drugs (odds ratio [OR] = 1.62, 95% confidence interval [CI] 1.19, 2.20), having ever traded sex for money (OR=1.84, 95% CI 1.29, 2.62), and having a higher perception of risk for HIV (OR=1.25, 95% CI 1.06, 1.47).
DISCUSSION
This study investigated whether individuals choosing the rapid HIV test within the context of multiple morbidities testing were more likely to return for hepatitis and STI test results on time than clients who chose the standard HIV test. Individuals who chose rapid HIV testing in conjunction with hepatitis and STI testing were younger, better educated, less likely to be homeless, and less likely to have ever injected drugs. It appears that individuals with less risky behaviors chose the rapid HIV test in this sample, and these clients also were significantly more likely than the standard group to return on time (within seven days) for the results of the other tests. However, there was no difference in seropositivity for hepatitis or STIs between the rapid and standard groups.
The majority of clients who were tested for hepatitis and STIs also chose the standard HIV test. These clients may have felt that because they needed to return for the majority of the test results within one week, the standard HIV test was the better choice. These individuals were less likely to return on time for their test results, were more likely to have ever injected drugs, and were more likely to have been engaged in trading sex for money or drugs. They also had a higher perception of risk for HIV, perhaps reflecting awareness that past or current injection drug use and sex trading were risky behaviors. This finding suggests that while bundling services may be an incentive to get these clients into testing services, the problem of getting them to return for test results remains.
Limitations
Several limitations to the current study must be considered. First, clients' reasons for selecting the standard HIV test over the rapid HIV test were not asked. We do not know whether clients selected standard HIV testing because of a lack of information about the rapid HIV test, because of familiarity with standard HIV testing, or because they may have heard negative things regarding the rapid HIV test (a higher likelihood of a false positive result, for example). We also do not know how many of the clients chose standard testing simply for convenience; that is, they knew they would have to return for the hepatitis and STI test results, so it may have seemed simpler to have all the tests done in the standard manner because they would have to make a return trip to receive results.
We also do not know whether clients who chose the rapid HIV test over standard testing did so because they were curious about the new technology or because they had been tested previously using the rapid HIV test. We do not know whether clients choosing the rapid HIV test may have been more anxious about finding out quickly if they were HIV-positive. Although questionnaires elicited the number of previous HIV tests clients may have had, the format of those previous tests (standard or rapid) was not asked.
Finally, it is possible that the length of the sessions overall had an impact on whether clients returned for test results. The sessions for the rapid and standard groups were identical except for the use of the rapid HIV test in the rapid group. Waiting for the rapid test specimen to produce a preliminary result added 20 minutes to these sessions. According to CDC and OAPP guidelines, this time is allocated for client counseling. It is possible that this additional counseling time had an impact on client behavior with respect to returning for the hepatitis and STI results. It is also possible that clients who chose the standard test did so because the overall testing session would be shorter.
CONCLUSION
There is an opportunity to educate current and former IDU clients about the rapid HIV test, as clients are less likely to choose this form of HIV testing if they are not familiar with it. If these clients could receive the rapid HIV test, it is possible that they would then experience receiving rapid test results, perhaps increasing the likelihood that they would return for other types of test results that are not currently available as rapid tests.
Footnotes
This research was supported in part by the Office of AIDS Programs and Policy of the Los Angeles County Department of Public Health, contract #H700939, and the California Community Foundation in Los Angeles.
REFERENCES
- 1.Ziek K, Goldstein MF, Beardsley M, Deren S, Tortu S. Factors associated with HIV testing and returning for test results in a sample of out-of-treatment drug users. J Drug Issues. 2000;30:675–86. [Google Scholar]
- 2.Molitor F, Bell RA, Truax SR, Ruiz JD, Sun RK. Predictors of failure to return for HIV test results and counseling by test site type. AIDS Educ Prev. 1999;11:1–13. [PubMed] [Google Scholar]
- 3.Sullivan PS, Lansky A, Drake A HITS-2000 Investigators. Failure to return for HIV test results among persons at high risk for HIV infection: results from a multistate interview project. J Acquir Immune Defic Syndr. 2004;35:511–8. doi: 10.1097/00126334-200404150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Kassler WJ, Dillon BA, Haley C, Jones WK, Goldman A. On-site rapid HIV testing with same-day results and counseling. AIDS. 1997;11:1045–51. doi: 10.1097/00002030-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Liang TS, Erbelding E, Jacob CA, Wicker H, Christmyer C, Brunson S, et al. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care STDS. 2005;19:253–7. doi: 10.1089/apc.2005.19.253. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds GL, Fisher DG, Henry CL, Perez MJ. Mobile post-HIV test counseling. Int J STD AIDS. 2005;16:457. doi: 10.1258/0956462054094105. [letter re: Ellen JM, Liang TS, Jacob CA, Erbelding E, Christmyer C. Post-HIV test counseling of clients in a mobile STD/HIV clinic. Int J STD AIDS 2004;15:728-31] [DOI] [PubMed] [Google Scholar]
- 7.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33:147–55. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- 8.Stopka TJ, Marshall C, Bluthenthal RN, Webb DS, Truax SR. HCV and HIV counseling and testing integration in California: an innovative approach to increase HIV counseling and testing rates. Public Health Rep. 2007;122(Suppl 2):68–73. doi: 10.1177/00333549071220S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher DG, Reynolds GL, Jaffe A, Perez MJ. Hepatitis and human immunodeficiency virus co-infection among injection drug users in Los Angeles County, California. J Addict Dis. 2006;25:25–32. doi: 10.1300/J069v25n02_04. [DOI] [PubMed] [Google Scholar]
- 10.Dowling-Guyer S, Johnson ME, Fisher DG, Needle R, Watters J, Anderson M, et al. Reliability of drug users' self-reported HIV risk behaviors and validity of self-reported recent drug use. Assessment. 1994;1:383–92. [Google Scholar]
- 11.Needle R, Fisher DG, Weatherby N, Chitwood D, Booth R, Watters J, et al. Reliability of self-reported HIV risk behaviors of drug users. Psychol Addict Behav. 1995;9:242–50. [Google Scholar]
- 12.McConnaughy EA, DiClemente CC, Prochaska JO, Velicer WF. Stages of change in psychotherapy: a follow-up report. Psychotherapy. 1989;26:494–503. [Google Scholar]
- 13.Carnes P. Contrary to love: helping the sexual addict. Minneapolis: CompCare Publishers; 1989. [Google Scholar]
- 14.Carnes P. Don't call it love: recovery from sexual addiction. New York: Bantam Books; 1991. [Google Scholar]
- 15.Amirkhan JH. Criterion validity of a coping measure. J Pers Assess. 1994;62:242–61. doi: 10.1207/s15327752jpa6202_6. [DOI] [PubMed] [Google Scholar]
- 16.Kalichman SC, Rompa D. Sexual sensation seeking and sexual compulsivity scales: reliability, validity, and predicting HIV risk behavior. J Pers Assess. 1995;65:586–601. doi: 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds GL, Fisher DG, Jaffe A, Edwards J. Follow-up for medical care among drug users with hepatitis C. Eval Health Prof. 2006;29:355–66. doi: 10.1177/0163278706296003. [DOI] [PubMed] [Google Scholar]
- 18.DiaSorin Inc. Enzyme immunoassay for the detection of total antibodies to hepatitis A virus (anti-HAV) in human serum or plasma. Stillwater (MN): DiaSorin Inc.; 2005. Package insert. [Google Scholar]
- 19.Diagnostic Products Corporation. Immulite 2000: anti-HBc total antibodies to hepatitis B core antigen. Los Angeles: Diagnostic Products Corporation; 2006. Package insert. [Google Scholar]
- 20.Abbott Laboratories. Hepatitis C virus encoded antigen (recombinant c100-3, HC-31, and HC-34) Abbott HCV EIA 2.0. Abbott Park (IL): Abbott Laboratories Diagnostics Division; 2004. Package insert. [Google Scholar]
- 21.Arlington Scientific, Inc. (ASI) ASI RPR card test for syphilis. Springville (UT): ASI; 2006. Package insert. [Google Scholar]
- 22.Fujirebio Diagnostics Inc. SERODIA®-TP•PA: reagents for the detection of antibodies to Treponema pallidum. Malvern (PA): Fujirebio Diagnostics Inc.; 2006. Package insert. [Google Scholar]
- 23.Gen-Probe Inc. Gen-Probe APTIMA combo 2 assay. San Diego: Gen-Probe Inc.; 2007. Package insert. [Google Scholar]