SYNOPSIS
Objective.
We assessed the cost-effectiveness of determining new human immunodeficiency virus (HIV) diagnoses using rapid HIV testing performed by community-based organizations (CBOs) in Kansas City, Missouri, and Detroit, Michigan.
Methods.
The CBOs performed rapid HIV testing during April 2004 through March 2006. In Kansas City, testing was performed in a clinic and in outreach settings. In Detroit, testing was performed in outreach settings only. Both CBOs used mobile testing vans. Measures of effectiveness were the number of HIV tests performed and the number of people notified of new HIV diagnoses, based on rapid tests. We retrospectively collected program costs, including those for personnel, test kits, mobile vans, and facility space.
Results.
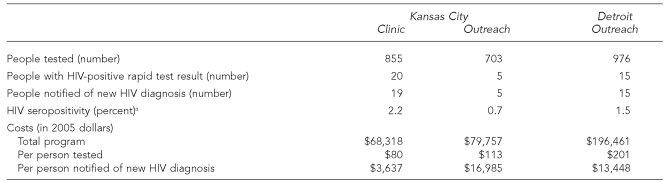
The CBO in Kansas City tested a mean of 855 people a year in its clinic and 703 people a year in outreach settings. The number of people notified of new HIV diagnoses was 19 (2.2%) in the clinic and five (0.7%) in outreach settings. The CBO in Detroit tested 976 people a year in outreach settings, and the number notified of new HIV diagnoses was 15 (1.5%). In Kansas City, the cost per person notified of a new HIV diagnosis was $3,637 in the clinic and $16,985 in outreach settings. In the Detroit outreach settings, the cost per notification was $13,448.
Conclusions.
The cost of providing a new HIV diagnosis was considerably higher in the outreach settings than in the clinic. The variation can be largely explained by differences in the number of undiagnosed infections among the people tested and by the costs of purchasing and operating a mobile van.
The Centers for Disease Control and Prevention (CDC) estimates that approximately 1.1 million people in the United States are infected with human immunodeficiency virus (HIV), but that approximately 25% of them are unaware of their infection.1 Some have never been tested for HIV. Others do not learn of their infection after conventional HIV testing, which requires people to return for their results a week or two later. Between 16% and 22% of people who tested positive in CDC-supported testing sites from 1999 through 2004 did not return to learn their test results.2 People who are unaware of their HIV infection are at higher risk of transmitting HIV to others and are unable to benefit from HIV treatment.3
In 2003, in response to the continuing HIV epidemic in the United States, CDC launched the Advancing HIV Prevention (AHP) initiative.4 One key goal was to reduce barriers to the early diagnosis of HIV infection by offering rapid HIV tests outside medical settings, including those served by community-based organizations (CBOs). Offering HIV testing through CBOs, either in CBO-based clinics or in outreach settings such as health fairs, public parks, and homeless shelters, was expected to increase HIV testing among people who were at risk for HIV infection and who had limited access to testing in medical settings. Offering rapid HIV testing was also expected to increase the number of people who received test results.5
In this study, we assessed program costs and effectiveness, in terms of the cost per person notified of a new HIV diagnosis, associated with the implementation of rapid HIV testing at two CBOs. The Kansas City Free Health Clinic in Kansas City, Missouri, offered testing at the CBO's clinic and in outreach settings. The Community Health Awareness Group in Detroit, Michigan, offered testing in outreach settings only.
METHODS
HIV testing and outreach
The Kansas City Free Health Clinic provides general medicine, mental health, and dental services as well as conventional HIV testing and HIV primary care. Under the AHP demonstration project, the CBO initiated rapid HIV testing in its walk-in clinic and, for the first time, offered testing in outreach settings from a mobile van. The clinic provided rapid HIV testing at no charge to low-income and uninsured people. Rapid HIV testing was offered from May 10, 2005, through March 31, 2006, in the clinic and in outreach settings. The outreach settings were health fairs, public parks, homeless shelters, substance-abuse treatment centers, soup kitchens, motels, bars and nightclubs, and areas frequented by commercial sex workers.
The Community Health Awareness Group in Detroit, which serves people who are infected with HIV or at risk for infection, previously had used a mobile van to deliver substance-abuse treatment services. Under the AHP demonstration project, the CBO began a new program offering free rapid HIV testing from its mobile van. Rapid HIV testing was offered from April 24, 2004, through March 28, 2006, at locations such as street corners, needle-exchange programs, substance-abuse and mental-health treatment centers, homeless shelters, soup kitchens, and bathhouses and bars frequented by men who have sex with men (MSM).
Staff of both CBOs used various methods to recruit clients for testing in outreach settings, including posting signs on the van, distributing promotional flyers, and partnering with other agencies for referrals. Rapid HIV testing was performed using an OraQuick®Rapid HIV-1 Antibody Test or OraQuick Advance® Rapid HIV-1/2 Antibody Test (OraSure Technologies, Bethlehem, Pennsylvania) on either oral fluid or whole-blood specimens. To be eligible for rapid testing, people had to be capable of providing written informed consent. Using standardized forms, CBO staff members collected information on demographic characteristics, risk behaviors, and HIV testing history from all people tested. They provided pretest counseling and posttest risk-reduction counseling, regardless of test results. Oral fluid or whole-blood specimens were collected for confirmatory testing by Western blot from people whose rapid test results were preliminary positive.6,7 More details on CBO testing protocols are described elsewhere.8
Costs and effectiveness
We analyzed separate data on costs and effectiveness of rapid HIV testing in the Kansas City clinic, the Kansas City outreach settings, and the Detroit outreach settings. We obtained annual total program costs retrospectively for each intervention from a provider's perspective (e.g., we did not measure participants' costs), and expressed costs in 2005 U.S. dollars. The key cost-effectiveness measure was the mean cost per person notified of a new HIV diagnosis following a rapid test. This measure was obtained by dividing the annual total program cost by the number of people notified of new HIV diagnoses.
To estimate the total program cost, we identified the cost of each program element, such as personnel, facilities, equipment, and materials.9–12 Fixed costs (i.e., those that remain constant during a relevant period regardless of the number of people served) were those for program management (planning, administration, and supervision), training, travel, purchase and operation of mobile vans, durable goods, and equipment. Variable costs (i.e., those that vary with the number of people served) were those for recruitment, counseling and testing, and nondurable goods and supplies, such as test kits used for rapid testing, quality assurance, and confirmatory testing. The cost of rapid test kits was estimated based on a bulk purchase price ($8 for each test kit) paid by CDC.13 The cost of the confirmatory Western blot testing ($37.91 for the test kit and processing time) was based on data from a national commercial reference-testing laboratory.14
We calculated personnel costs based on the amount of time the CBO staff spent on each of the program activities, including recruitment, counseling, testing, training, and travel, as well as program planning, administration, and supervision. We multiplied the staff time associated with each activity by the compensation (wages plus benefits) received by the staff who performed these activities.
We estimated the recruitment cost for the Kansas City CBO by collecting data from a six-month recruitment log, which included the amount of time CBO staff spent on outreach, such as distributing promotional flyers. The recruitment cost included time spent to recruit people who later declined to be tested (62% of total). The Detroit site reported aggregate staff time for recruitment, which included the time devoted to the people who were tested and the people who declined testing. In-kind or nonmonetary incentives (e.g., transportation tokens or grocery vouchers) were provided to people who agreed to be tested in outreach settings, regardless of their HIV status.
To estimate the overhead (i.e., utilities and facility space) attributable to HIV testing, we multiplied the total cost the agency spent on overhead items by the proportion of time the staff spent on the AHP demonstration project. Travel costs included staff travel time and vehicle mileage to and from sites. To amortize the costs of office computers, mobile vans, and other equipment over the expected life of the equipment, we used a 3% discount rate.15 We included the cost of renting facility space, but excluded costs related to program evaluation.
This project was determined to be a public health program activity by CDC and, therefore, review by CDC's Institutional Review Board was not required.
RESULTS
The CBO in Kansas City tested a mean of 855 people a year in its clinic and 703 people a year in outreach settings, and notified 19 (2.2%) people in its clinic and five (0.7%) people in its outreach settings of new HIV diagnoses (Table 1). The CBO in Detroit tested a mean of 976 people a year in outreach settings and notified 15 (1.5%) people of new HIV diagnoses.
Table 1. Mean annual rapid HIV testing outcomes and program costs in Kansas City, Missouri, and Detroit, 2004–2006.
aHIV seropositivity (percent) is the proportion of new HIV-positive rapid test results among people tested.
HIV = human immunodeficiency virus
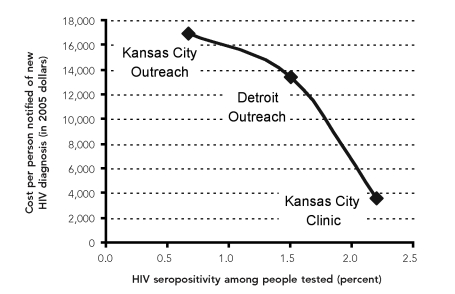
The overall annual cost of the rapid HIV testing program in Kansas City was $148,075: $68,318 a year in the clinic and $79,757 a year in outreach settings. We estimated the mean cost per person notified of a new HIV diagnosis at $3,637 in the clinic and $16,985 in the outreach settings. The overall annual cost of the program in the Detroit outreach settings was $196,461, and the mean cost per person notified of a new HIV diagnosis was $13,448. The wide variability in the cost per person notified of a new HIV diagnosis in large part reflects differences in the proportions of tested people whose results were positive (Figure).
Figure. Relationship between rapid HIV testing costs and HIV seropositivity among people tested in Kansas City, Missouri, and Detroit, 2004–2006.
HIV = human immundeficiency virus
In Kansas City, we estimated the mean cost of providing rapid HIV testing services to a person in the clinic at $80 and to a person in an outreach setting at $113. In Detroit, the estimated mean cost of providing rapid HIV testing to a person in an outreach setting was $201.
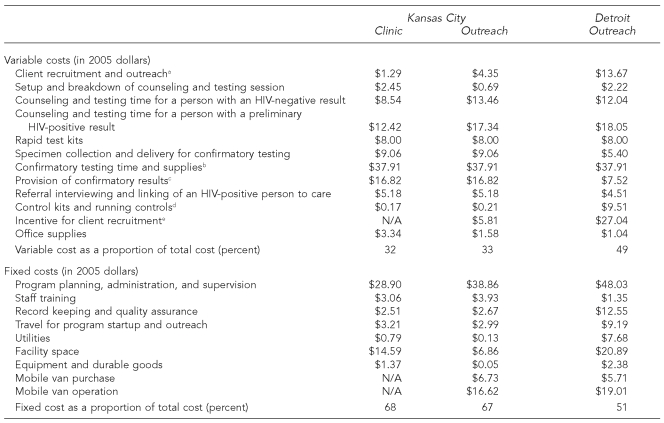
Fixed costs made up a large portion of the total program cost in both CBOs, ranging from 51% to 68% (Table 2). The key components of the fixed costs were program management, facility space, and the purchase and operation of testing vans; the costs related to the vans increased the fixed costs in outreach settings. The costs attributed to facility space and utilities were much higher in Detroit, in part because CBO staff in Detroit, compared with staff in Kansas City, devoted a greater proportion of their time (13.0% in Detroit vs. 1.9% in Kansas City) to the HIV testing project.
Table 2. Variable and fixed costs per client of rapid HIV testing services in Kansas City, Missouri, and Detroit, 2004–2006.
aIncludes determining eligibility and waiting for client to initiate the test.
bPreliminary HIV-positive result was confirmed by Western blot. Data on test kit cost and test processing time came from a national commercial reference-testing laboratory. (Source: Farnham PG, Hutchinson AB, Sansom SL, Branson BM. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep 2008;123[Suppl 3]:51-62.)
cIncludes time spent for prevention counseling.
dThe CBO in Detroit ran controls more frequently for quality assurance.
eBoth nonmonetary and in-kind incentives were offered in Detroit outreach settings.
HIV = human immunodeficiency virus
N/A = not applicable
CBO = community-based organization
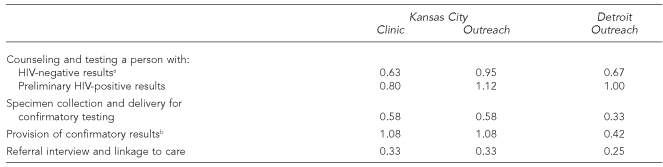
The costs of test kits, controls, incentives, and personnel time spent on client recruitment, outreach, counseling, and testing made up most of the variable costs. Across settings, the costs attributable to staff time spent for counseling and testing ranged from $8.54 to $13.46 for people whose test results were negative and from $12.42 to $18.05 for people whose test results were positive. Variations in these costs were due to the differences in staff wages in the two cities and the amount of time spent on counseling and testing (Tables 2 and 3). The Detroit CBO also incurred higher variable costs by providing nonmonetary and in-kind incentives to people who were being recruited for testing.
Table 3. Counseling and testing time in hours per person for rapid HIV testing services in Kansas City, Missouri, and Detroit, 2004–2006.
aIncludes eligibility determination, specimen collection, paperwork, test processing, and provision of results.
bIncludes time spent for prevention counseling.
HIV = human immunodeficiency virus
DISCUSSION
The CBOs in Kansas City and Detroit provided rapid HIV testing services, which resulted in previously unrecognized HIV infection being identified in 0.7% to 2.2% of the people tested. We estimated the cost of providing rapid HIV testing to people who received new HIV diagnoses at $3,637 in the Kansas City CBO clinic, $16,985 in the Kansas City outreach settings, and $13,448 in the Detroit outreach settings. The wide variation in the costs of identifying and notifying people with new HIV diagnoses in the clinic and in the outreach settings was primarily due to the varying proportions of people tested who had previously undiagnosed HIV infection. The mean overall cost of testing in the clinic and outreach settings, independent of underlying HIV seropositivity rates, varied because of the cost of purchasing and operating a mobile van for outreach testing, providing recruitment incentives, renting a facility, and paying staff (due to differences in wages).
The literature on the costs and the cost-effectiveness of HIV counseling and testing demonstrates that the costs of new HIV diagnoses vary according to the strategies used to recruit people for testing (e.g., outreach, partner notification, and social networks), testing technologies (e.g., rapid or conventional HIV testing), and costs included in the analysis (e.g., variable vs. fixed costs).16–21 We did not find published cost assessments of CBO-led HIV testing, either in clinics or outreach settings. However, several studies specified the costs of identifying new cases of HIV infection by recruitment strategy. For example, Golden and colleagues studied a peer-referral approach for HIV counseling and rapid testing among MSM in a sexually transmitted disease clinic in King County, Washington.20 They found that the cost per new HIV diagnosis ranged from $5,600 to $12,000 (adjusted to 2005 U.S. dollars) when the HIV seroprevalence rates were 4.4% and 1.3%, respectively. In two studies that used partner notification, the cost of a new HIV diagnosis was $3,800 (seroprevalence, 15%) in Colorado22 and $6,400 (seroprevalence, 14%) in Utah.23 In both of these studies, people with newly diagnosed HIV infection provided the names of sexual or needle-sharing partners to state health department staff, who then offered HIV testing to the partners.
In Kansas City, the proportion of people tested who received a new HIV diagnosis was larger in the clinic than in the outreach settings. This unexpected result led to a lower cost per person notified of new HIV diagnosis for the clinic. Because outreach testing sites were in relatively close proximity to the clinic—most of the sites were within a 15-minute drive—people who were aware of a recent HIV exposure or who frequently engaged in high-risk behaviors may have gone to the clinic for testing before they could be approached for testing in an outreach setting. Our analysis suggests that in communities such as the one served by the Kansas City CBO, where at-risk groups can and do go to a nearby clinic for HIV testing, the addition of outreach services may not have yielded many additional new HIV diagnoses. On the other hand, in Detroit, where the high-risk community was not served by such a clinic, the use of a mobile van in outreach settings provided a unique opportunity for testing.
The CBOs included in our analysis did not have prior experience in rapid HIV testing using mobile vans, although both CBOs had some experience in performing street outreach, offering HIV prevention, or delivering care and treatment for substance abuse in community settings. It is possible that programs become more successful over time in identifying locations where high-risk people congregate, and the HIV prevalence among individuals tested initially will be lower than when the program is more established. On the other hand, HIV prevalence among those tested may decline over time if the program is successful initially at targeting high-risk groups. To account for potential variability in HIV testing outcomes, we calculated the mean annual number of individuals notified of new HIV diagnoses during the entire two-year project period. To more accurately estimate costs, we collected cost data during the second year of program operations, when we expected the programs to be running more efficiently.
Limitations
The limitations of our study included the retrospective collection of cost data, raising the possibility of recall bias. Despite our efforts to track all program costs, we may have unintentionally excluded some costs. Also, we assigned the cost of the OraQuick test kit on the basis of bulk-purchase price ($8 per kit) available to CDC at the time. Current retail cost per test kit may range from $8 to $18.14 Other programs may have to pay a different price for rapid test kits; new testing technologies at varying prices may become available in the future. In addition, we analyzed the costs and effectiveness of the rapid HIV testing programs in only two CBOs, limiting our ability to generalize our results.
CONCLUSIONS
For these two CBOs, the variation in cost per person notified of a new HIV diagnosis was due primarily to differences in HIV seropositivity among people tested and in programmatic costs of providing testing in a clinic vs. outreach settings. On the basis of our results, CBOs that already offer HIV testing through a clinic may want to pilot HIV testing in outreach settings before investing in a mobile van to determine whether the number of new HIV diagnoses identified in outreach settings justifies the large investment.
Acknowledgments
The authors thank the following people for their assistance in data collection: Leleh Emami, Carla Gibson, Marquita Leverette, Etienne Orozzo, Rachel Pope, and Sandra Springer of the Kansas City Free Health Clinic in Kansas City, Missouri, and Adriana Graza, Lydia Meyers, Dr. Lisa Randall, and Donella Welton of the Community Health Awareness Group in Detroit.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Glynn M, Rhodes P. Estimated HIV prevalence in the United States at the end of 2003 (abstract T1-B1101); Programs and abstracts of the 2005 National HIV Prevention Conference; 2005 Jun 12–15; Atlanta. [cited 2008 Aug 26]. Also available from: URL: http://www.aegis.com/conferences/nhivpc/2005/t1-b1101.html. [Google Scholar]
- 2.Centers for Disease Control and Prevention (US) [cited 2008 Jun 9];HIV counseling and testing at CDC-supported sites—United States, 1999–2004. 2006 Available from: URL: http://www.cdc.gov/hiv/topics/testing/reports.htm.
- 3.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–50. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 4.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(15):329–32. [PubMed] [Google Scholar]
- 5.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20:1597–604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- 6.Notice to readers: protocols for confirmation of reactive rapid HIV tests. MMWR Morb Mortal Wkly Rep. 2004;53(10):221–2. [Google Scholar]
- 7.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–31. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 8.Rapid HIV testing in outreach and other community settings—United States, 2004–2006. MMWR Morb Mortal Wkly Rep. 2007;56(47):1233–7. [PubMed] [Google Scholar]
- 9.Levin HM, McEwan PJ. Cost-effectiveness analysis: methods and applications. 2nd ed. Thousand Oaks (CA): Sage Publications, Inc.; 2001. [Google Scholar]
- 10.Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 2nd ed. New York: Oxford University Press; 1997. [Google Scholar]
- 11.Gorsky RD. A method to measure the costs of counseling for HIV prevention. Public Health Rep. 1996;111(Suppl 1):115–22. [PMC free article] [PubMed] [Google Scholar]
- 12.Haddix AC, Teutsch SM, Corso PS, editors. Prevention effectiveness: a guide to decision analysis and economic evaluation. New York: Oxford University Press; 2003. [Google Scholar]
- 13.Rapid HIV test distribution—United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2006;55(24):673–6. [PubMed] [Google Scholar]
- 14.Farnham PG, Hutchinson AB, Sansom SL, Branson BM. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 2008;123(Suppl 3):51–62. doi: 10.1177/00333549081230S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 16.Ekwueme DU, Pinkerton SD, Holtgrave DR, Branson BM. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25:112–21. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 17.Farnham PG, Gorsky RD, Holtgrave DR, Jones WK, Guinan ME. Counseling and testing for HIV prevention: costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep. 1996;111:44–53. [PMC free article] [PubMed] [Google Scholar]
- 18.Toomey KE, Peterman TA, Dicker LW, Zaidi AA, Wroten JE, Carolina J. Human immunodeficiency virus partner notification: cost and effectiveness data from an attempted randomized controlled trial. Sex Transm Dis. 1998;25:310–6. doi: 10.1097/00007435-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Gorsky RD, MacGowan RJ, Swanson NM, DelGado BP. Prevention of HIV infection in drug abusers: a cost analysis. Prev Med. 1995;24:3–8. doi: 10.1006/pmed.1995.1002. [DOI] [PubMed] [Google Scholar]
- 20.Golden MR, Gift TL, Brewer DD, Fleming M, Hogben M, St. Lawrence JS, et al. Peer referral for HIV case-finding among men who have sex with men. AIDS. 2006;20:1961–8. doi: 10.1097/01.aids.0000247118.74208.6a. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha RK, Sansom SL, Richardson-Moore A, French PT, Scalco B, Lalota M, et al. Costs of voluntary rapid HIV testing and counseling in jails in 4 states—Advancing HIV Prevention demonstration project, 2003–2006. Sex Transm Dis. 2007 doi: 10.1097/olq.0b013e318148b69f. [published online ahead of publication] [DOI] [PubMed] [Google Scholar]
- 22.Spencer NE, Hoffman RE, Raevsky CA, Wolf FC, Vernon TM. Partner notification for human immunodeficiency virus infection in Colorado: results across index case groups and costs. Int J STD AIDS. 1993;4:26–32. doi: 10.1177/095646249300400106. [DOI] [PubMed] [Google Scholar]
- 23.Pavia AT, Benyo M, Niler L, Risk I. Partner notification for control of HIV: results after 2 years of a statewide program in Utah. Am J Public Health. 1993;83:1418–24. doi: 10.2105/ajph.83.10.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]