SYNOPSIS
Objectives.
Partner counseling and referral services (PCRS) provide a unique opportunity to decrease transmission of human immunodeficiency virus (HIV) by notifying sex and drug-injection partners of HIV-infected individuals of their exposure to HIV. We incorporated rapid HIV testing into PCRS to reduce barriers associated with conventional HIV testing and identify undiagnosed HIV infection within this high-risk population.
Methods.
From April 2004 through June 2006, HIV-infected people (index clients) were interviewed, and their partners were notified of their potential exposure to HIV and offered rapid HIV testing at six sites in the United States. The numbers of index clients participating and the numbers of partners interviewed and tested were compared by site. Descriptive and bivariate analyses were performed.
Results.
A total of 2,678 index clients were identified, of whom 779 (29%) provided partner locating information. A total of 1,048 partners were elicited, of whom 463 (44%) were both interviewed and tested for HIV. Thirty-seven partners (8%) were newly diagnosed with HIV. The number of index clients interviewed to identify one partner with newly diagnosed HIV infection ranged from 10 to 137 at the participating sites.
Conclusions.
PCRS provides testing and prevention services to people at high risk for HIV infection. Incorporating rapid HIV testing into PCRS and identifying previously undiagnosed infections likely confer individual and public health benefits. Further evaluation is needed to determine the best methods of identifying partners with previously unrecognized HIV infection.
Partner notification has long been practiced to inform the sex and drug-injection partners of people infected with human immunodeficiency virus (HIV) of their possible exposure to HIV and sexually transmitted diseases (STDs).1 In 1998, the Centers for Disease Control and Prevention (CDC) published guidance on partner notification for HIV, called partner counseling and referral services (PCRS), which has the objective of reducing HIV transmission. Because people who are aware of their HIV-infected status are less likely to participate in behaviors that transmit HIV,2 PCRS is designed to reduce HIV transmission by providing HIV counseling and testing services to people at high risk for infection, identifying previously undiagnosed infection, and linking HIV-infected people to care and treatment.3
PCRS has been used by health departments to interview partners of people with known HIV infection, notify them of their possible exposure to HIV, and arrange for them to be tested.4 An HIV-infected person is defined as an index client when he or she is reported to the health department. In traditional PCRS, index clients are located and interviewed by a disease intervention specialist to elicit information about current and past partners.1 Partners may be notified of their exposure to HIV by the index client, the disease intervention specialist, or both of them together. PCRS is comprehensive and includes elicitation, notification, and testing of partners as well as education, risk-reduction counseling, and assessment of the need for referral to psychosocial services, case management, and medical care and treatment. Participation in PCRS by index clients and partners is completely voluntary.
CDC launched the Advancing HIV Prevention (AHP) initiative in 2003, aimed at reducing barriers to early diagnosis of HIV infection.5 Preventing HIV transmission by working with HIV-infected people and their partners and implementing new models for diagnosing HIV infections outside medical settings are two key AHP initiative strategies. Incorporating rapid HIV testing into PCRS makes use of both of these strategies.
Although PCRS has been used as a strategy to prevent the transmission of HIV for many years, the incorporation of rapid HIV testing is new and offers partners the opportunity to be tested for HIV in the field. When conventional HIV testing methods are used in conjunction with PCRS, disease intervention specialists collect venous blood or oral fluid specimens in the field, which are then processed in a laboratory. Because of the variability in the time it takes laboratories to process specimens, it may take from one to 14 days for test results to be available. Moreover, this type of field-based specimen collection has not been used by all health departments, so in many instances, partners are referred for testing at health department clinics.
Under the AHP initiative, CDC funded a project to demonstrate the feasibility of conducting rapid HIV testing in conjunction with traditional and alternative approaches to conducting PCRS. The primary objective of this project was to decrease barriers to early diagnosis of HIV infection among partners of HIV-infected people by demonstrating new models of PCRS that included rapid HIV testing in the field and other settings.
METHODS
In September 2003, six health departments—Chicago Department of Public Health, Colorado Department of Public Health and Environment in Denver, Los Angeles County Department of Public Health, Louisiana Office of Public Health in New Orleans, San Francisco Department of Public Health, and Wisconsin Division of Public Health in Madison—received funding through CDC's AHP initiative for a demonstration project incorporating rapid HIV testing into PCRS.
Models of PCRS
The participating health departments incorporated rapid HIV testing into one or more of three different models for conducting PCRS. Three sites (Colorado, Louisiana, and Wisconsin) incorporated rapid HIV testing into the traditional model of PCRS, using disease intervention specialists employed by health departments to interview index clients and partners in their homes or workplaces or in the disease intervention specialists' vehicles or offices. At all three sites, disease intervention specialists received reports of HIV-infected individuals through routine surveillance or directly from HIV testing services.
Two sites (Chicago and Los Angeles) collaborated with staff at community-based organizations (CBOs) in addition to conducting traditional PCRS. In Chicago, trained counselors at 15 CBOs elicited partner information from newly diagnosed index clients identified through testing programs at the CBOs and provided this information to health department staff for use in conducting PCRS. Additionally, disease intervention specialists conducted traditional PCRS with individuals who had reactive HIV tests at six STD clinics, and these individuals were included in the project.
Los Angeles developed a model to build the capacity of CBOs to conduct PCRS and to improve referrals to the local health department. In Los Angeles, HIV medical outpatient clinics at three CBOs hired PCRS liaisons, who worked with the health department to conduct PCRS as one part of the comprehensive prevention services provided to HIV-infected people. Potential index clients with newly or previously diagnosed HIV infection were identified through counseling and testing programs, care and treatment services, support groups, and pharmacy visits. Clients who indicated that they wanted to participate in PCRS were interviewed and asked to provide information about their partners. Index clients were asked to bring their partners to the CBOs, where their partners could be notified, in the presence of a PCRS liaison, of their possible exposure to HIV infection and offered rapid HIV testing. Alternatively, index clients could choose to provide information about their partners directly to the health department so that a disease intervention specialist could conduct PCRS and HIV testing in the field. Promotional materials were developed to advertise PCRS within the clinic and in print media targeted to the communities served by the CBOs.
The San Francisco Department of Public Health developed a novel approach to providing PCRS, called the Partner Disclosure Assistance Program. Program materials were marketed through counseling and testing providers, medical providers, CBOs working with HIV-infected clients, and the local media. HIV-infected people were encouraged to contact Partner Disclosure Assistance Program staff by telephone or e-mail for assistance in disclosing their HIV-infected status to their partners. Field-based rapid HIV testing was made available to partners of index clients accessing this service.
At all sites, the time period for which index clients were asked to provide information about their partners (the contact period) was typically 12 months. However, if a previous negative HIV test result was available for an index client, the contact period could be shorter or longer than 12 months. Index clients were asked to disclose how many partners they had and the names of their partners during the contact period. Each site determined the amount of information required to initiate a partner investigation, depending upon preexisting criteria. At sites that used disease intervention specialists, surveillance records were reviewed to determine whether named partners were already known to be HIV-infected, and disease intervention specialists initiated notification of partners who did not have documentation of HIV infection. In Los Angeles, the PCRS liaisons did not have access to surveillance data, so index clients were asked to bring their partners to CBOs, where they could be notified and referred to follow-up services with the assistance of CBO staff.
Exclusion criteria
Partners were excluded from participation in the project if they were younger than 13 years of age or were already known to be infected with HIV. Additionally, partners who resided outside of the health department's geographical jurisdiction were not included in the project; however, their names were shared with the appropriate local or state health departments for PCRS.
Data collection
Following the preparatory phase for this project, interviews with index clients and partners and rapid HIV testing were conducted from April 2004 through June 2006. Demographic characteristics, information about risk behaviors, and HIV testing history were recorded on paper forms, either during interviews or afterward, using notes taken during interviews. In addition, HIV testing information, including types of specimens collected, test results, and receipt of test results, was collected. At sites other than Los Angeles and San Francisco, disease intervention specialists collected basic demographic data (such as age, gender, and race/ethnicity) from surveillance or laboratory reporting information about index clients who could not be located.
HIV testing
Testing was conducted using OraQuick® Rapid HIV-1 Antibody Tests and OraQuick Advance® Rapid HIV-1/2 Antibody Tests (OraSure Technologies, Bethlehem, Pennsylvania), which used whole-blood and oral fluid specimens. Test results were available in 20 minutes. Partners who accepted testing received a rapid HIV test during the PCRS session. In the rare instances when rapid testing was not available due to staffing, partners received a conventional OraSure® enzyme immunoassay (EIA) HIV test via venous blood or oral fluid specimens. Negative rapid and EIA HIV test results were considered conclusive. However, reactive rapid and EIA HIV tests required confirmatory testing using Western blot assays. Partners with reactive rapid test results had specimens collected for confirmatory testing. Testing was conducted in compliance with the confidentiality and testing requirements of each state and in compliance with national laboratory testing standards.
Data management and analysis
A contractor (Satyam Computer Services, Ltd., Hyderabad, India) entered data collected on paper forms into a Microsoft® Access database. One site (Colorado) entered data locally. We analyzed data at CDC using SAS version 9.16 and performed descriptive and bivariate analyses using Chi-square tests of significance. We calculated the number of index clients interviewed to identify one partner with newly diagnosed HIV infection—which we will refer to hereafter as the “number needed to interview”—by dividing the number of index clients interviewed by the number of partners with newly diagnosed HIV infection. We calculated confidence intervals using the adjusted Wald method.
Human subjects considerations
CDC determined that this project was a programmatic evaluation of the incorporation of rapid HIV testing into PCRS, an established and widely accepted public health activity, and thus did not require review by CDC's Institutional Review Board. Partners provided informed consent prior to being tested for HIV, in accordance with state and local laws and regulations.
RESULTS
Participation of index clients and partners by site
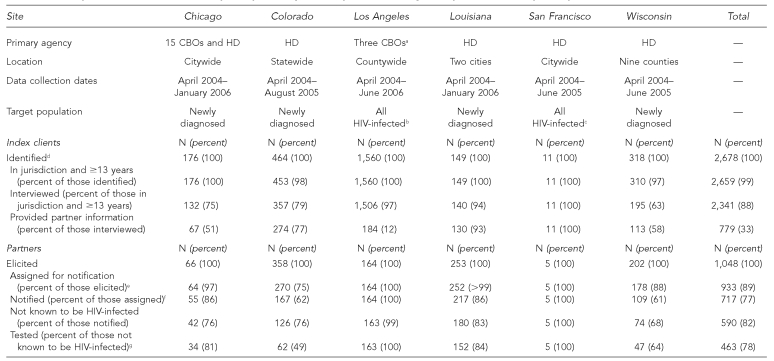
Of the 2,678 index clients included in this analysis, 2,341 (87%) agreed to be interviewed by a disease intervention specialist, CBO counselor, or PCRS liaison (Table 1). A number of reasons were provided for why index clients did not participate, including the following: a disease intervention specialist could not locate them (28%), index clients denied having sex or sharing drugs with others (23%), or index clients refused to provide a reason (38%). Among the 2,341 index clients who were interviewed, 779 (33%) provided names and locating information for partners. Index clients gave various reasons for not providing information about partners, including the following: their partners already knew that they were infected with HIV (61%), they used condoms during sex with their partners (19%), they were abstinent (16%), and they did not know the names of their partners (12%).
Table 1. Description of PCRS models and participation by site, Rapid HIV Testing to Improve PCRS Project, April 2004–June 2006.
aAt this site, the HD disease intervention specialist provided PCRS if requested by the index client.
bWhen data from Los Angeles are limited to index clients interviewed within three months of HIV diagnosis, 77 index clients were interviewed, 40 (52%) of whom provided partner information. From these index clients, 48 partners were elicited; 47 were not known to be HIV-infected, 30 of whom (64%) were tested.
cWhen data from San Francisco are limited to index clients interviewed within three months of HIV diagnosis, three index clients were interviewed, all of whom provided partner information. Nine partners were elicited, of whom three (33%) partners were tested.
dMethods for identifying index clients varied by site. Colorado, Louisiana, and Wisconsin included people identified by surveillance and testing programs; Chicago included people testing positive at CBOs and sexually transmitted disease clinics; Los Angeles included people identified through testing programs and comprehensives services for HIV-infected individuals at CBOs; and San Francisco included people responding to materials marketed to HIV-infected individuals.
eIncludes partners who were not known to disease intervention specialists, CBO staff, or PCRS liaisons to be HIV-infected, who had not been tested for HIV since the index client's diagnosis, and whose cases had been assigned for notification of exposure to HIV.
fIncludes partners who were informed of possible exposure to HIV.
gOf all partners tested, 451 people received a rapid HIV test, and 12 received a conventional enzyme immunoassay test.
PCRS = partner counseling and referral services
HIV = human immunodeficiency virus
CBO = community-based organization
HD = health department
The characteristics of participating index clients differed by site, as did the proportion of index clients who did not provide information about partners. Controlling for site, we found that index clients who did not provide partner information were more likely to be male (p<0.001) or older than 35 years of age (p<0.0001), and less likely to be black (p=0.03). The range in the proportion of index clients providing partner information was 12% to 100%. However, when data from the Los Angeles site were limited to index clients interviewed within three months of diagnosis, the range narrowed to 52% to 100%.
A total of 1,048 partners were elicited from index clients, of whom 933 (89%) were assigned to be notified by disease intervention specialists or CBO staff of their possible exposure to HIV. The primary reason why partners were not assigned to be notified was that they had already received an HIV test after the index client was diagnosed with HIV infection (86%). Of the 933 partners assigned for notification, 717 (77%) were notified of their possible exposure to HIV. Reasons why partners were not notified included the following: disease intervention specialists could not locate them (43%), they had moved out of the health department's jurisdiction (43%), or they were deceased (6%). Of the 717 partners who were notified, 463 (78% of those not known to be HIV-infected) were tested for HIV. The most common reason why notified partners refused testing was that they had been tested for HIV recently (51%). There was considerable variation among sites in the percentage of notified partners who agreed to be tested, ranging from 49% in Colorado to 100% in Los Angeles and San Francisco.
Demographic characteristics of index clients and partners
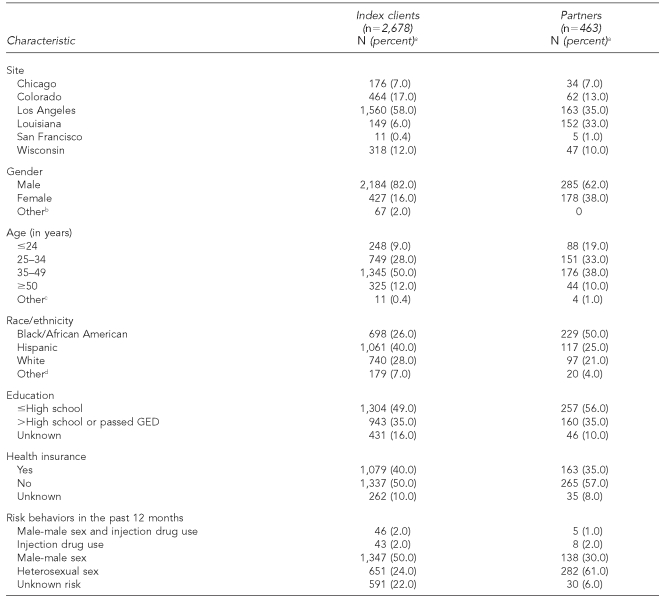
The majority of the 2,678 index clients identified by participating sites were male, and half were aged 35 to 49 years (Table 2). Overall, half of all index clients reported male-male sex in the past 12 months. Of the 463 partners who were tested for HIV, the majority were male. More than a quarter of these partners reported male-male sex in the past 12 months.
Table 2. Demographic characteristics of index clients identified and partners tested, Rapid HIV Testing to Improve PCRS Project, six sites, April 2004–June 2006.
aPercentages may not equal 100% due to rounding.
b“Other” gender response includes transgender, don't know, refused to answer, and missing.
c“Other” age response includes don't know, refused to answer, and missing.
d“Other” race response includes Native Americans/Alaska Natives, Asians, Native Hawaiians/Pacific Islanders, other race, don't know, and missing.
HIV = human immunodeficiency virus
PCRS = partner counseling and referral services
GED = General Educational Development test
HIV test results
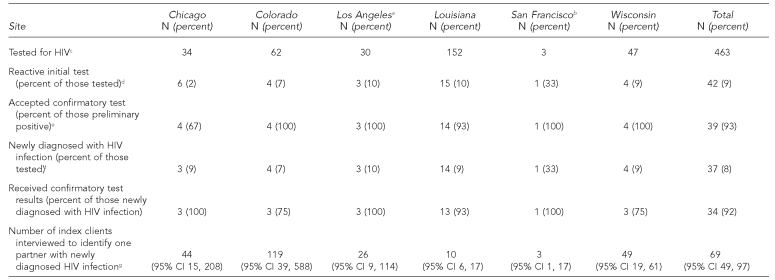
Overall, 463 partners were tested for HIV (Table 3). A total of 451 partners (97%) received rapid testing, and of these, 173 (38%) were tested in the field, 138 (31%) in a clinic or disease intervention specialist's office, and 131 (29%) at a CBO. The remaining nine partners (2%) were tested in a variety of other locations. Of the 463 partners tested, 42 partners (9%) had reactive rapid or EIA test results.
Table 3. HIV test results among partners, by site, Rapid HIV Testing to Improve PCRS Project, April 2004–June 2006.
aIncludes only partners referred by index clients interviewed within three months of their HIV diagnosis. When all partners are included, 163 partners were tested, 12 had a reactive initial test, and all 12 accepted a confirmatory test; 11 partners (7% of all partners tested) were newly diagnosed with HIV infection, all of whom received their confirmatory test results. Including all partners, the number of index clients interviewed to identify one partner with newly diagnosed HIV infection was 137 (95% CI 76, 256).
bIncludes only partners referred by index clients interviewed within three months of their HIV diagnosis. When all partners are included, five partners were tested, one had a reactive initial test, and this partner was newly diagnosed with HIV infection and received his or her confirmatory test results. Including all partners, the number of index clients interviewed to identify one partner with newly diagnosed HIV infection was 11 (95% CI 3, 10,000).
cOf all partners tested, 451 people received a rapid HIV test, and 12 received a conventional enzyme immunoassay test.
dPartners had reactive results on rapid HIV test (n=37) or enzyme immunoassay test (n=5).
eOf the three partners who are missing data for the confirmatory test, one partner refused to take the confirmatory test, one partner did not return for his/her confirmatory test, and the data for one partner is missing as to whether he/she received a confirmatory test.
fOf the two partners who accepted confirmatory tests and were not found to be newly confirmed positive, both had negative confirmatory test results.
gCalculated by dividing the number of index clients interviewed by the number of partners with newly diagnosed HIV infection.
HIV = human immunodeficiency virus
PCRS = partner counseling and referral services
CI = confidence interval
A total of 39 partners (93%) with reactive rapid or EIA test results received confirmatory HIV testing by Western blot. Information about whether one partner who had a reactive test result received confirmatory testing was missing, and two partners who had reactive test results did not have confirmatory testing conducted—one refused to have a confirmatory test performed and one did not return to have a confirmatory test conducted. Two partners who had reactive rapid test results had negative confirmatory test results and did not return for additional follow-up confirmatory testing, per testing recommendations,7 so we could not determine whether these people had false-positive test results. Assuming that these two test results were false-positive results, the specificity of the OraQuick rapid test in this project was 99.5% (95% confidence interval [CI] 98.2, 99.9). The manufacturer's information reports the specificity of the OraQuick tests as 99.8% (95% CI 99.6, 99.9). Of all partners tested, 37 partners (8%) were newly confirmed with HIV infection and 34 partners (92%) received their confirmed positive test results. The partners who did not receive their confirmatory test results could not be located.
Integrating test results with other PCRS performance characteristics, the mean number of index clients interviewed to identify one partner with newly diagnosed HIV infection was 69. This ranged by site from 10 in Louisiana to 137 in Los Angeles.
Perceived HIV status and HIV testing history
Partners were asked what they thought their HIV serostatus was at the time of the interview, before their test results were available. Prior to receiving their test results, 2% of partners (11% of newly diagnosed HIV-infected partners and 2% of HIV-uninfected partners) believed they were HIV-infected, and 23% were unsure if they were HIV-infected. Twenty-two percent of partners who were tested in this project had never been tested for HIV, an additional 47% had not been tested for HIV in the past 12 months, and 41% of all partners tested had no prior plans to be tested for HIV in the next six months.
DISCUSSION
This project demonstrated that rapid HIV testing can be incorporated into PCRS. Three-quarters of partners notified of their possible exposure to HIV infection through PCRS who were not known to be HIV-infected were tested for HIV, which is similar to findings reported by others. In a review by Fenton and Peterman, 38% to 100% of those partners notified received HIV testing,8 and in a review by Hogben and colleagues, 42% to 97% of partners notified were tested.9 In a study of PCRS in Georgia, 54% of partners were tested.10
Offering rapid HIV testing in conjunction with PCRS provides potential advantages over conventional HIV testing. First, rapid HIV testing allows disclosure of test results within 20 minutes and may reduce the number of partners who fail to return for test results, which has been shown to be substantial in multiple settings. Among those receiving conventional testing, failure to return for test results among people at high risk of HIV infection has been found to be 10% to 27%.11 Among people tested for HIV at STD clinics in two studies, more than half of those tested did not return for their results in one study, and of those who had positive results, almost 60% did not return.12,13 In a study of HIV testing in outreach settings, failure to return for test results was found to be 18% to 43%.14 In this project, of the 463 partners who were tested, 451 received a rapid HIV test, and of these, 426 (94%) received their rapid test results. Only six partners (16%) with reactive rapid test results did not receive confirmatory testing or did not receive the results of their confirmatory tests. Because there are no published reports of return rates for conventional HIV testing in PCRS, we cannot determine if the difference we see is due to rapid HIV testing itself or to programmatic differences between PCRS and other settings in which HIV testing occurs.
Another advantage of providing rapid HIV testing in PCRS is that it may reduce the resources that need to be allocated to PCRS because disease intervention specialists do not need to spend as much time following up with people who have negative test results, although they would need to ensure that partners who have reactive test results receive confirmatory tests (and receive the results of those tests). A theoretical advantage of conducting rapid HIV testing with PCRS is that people who receive reactive rapid test results may reduce their risk behavior, and thereby reduce transmission to their partners, one to two weeks earlier than they might have, had they undergone conventional HIV testing.
Although this project was designed to evaluate the integration of rapid HIV testing into PCRS, information about the process measures of PCRS was also obtained. Eighty-eight percent of index clients agreed to be interviewed in this project, but only 33% of those who were interviewed provided information about their partners, and index clients who provided information about their partners differed from those who did not by age, gender, and race/ethnicity. The proportion of index clients who provided information about their partners in this project was lower than that found in previously published reports of PCRS.15–18 However, a substantial number of the participating index clients at the Los Angeles site were interviewed three or more months after being diagnosed with HIV; when we excluded these individuals from the analysis, we found that 69% of the remaining index clients at all sites had provided partner information, a proportion that is consistent with previous reports.
Although this project was not designed to evaluate the different PCRS models used by participating sites, examining the number needed to interview enables us to compare the performance at the sites. Three sites (Colorado, Louisiana, and Wisconsin) employed the traditional model of PCRS, and the number of index clients interviewed to identify one partner with newly diagnosed HIV infection ranged from 10 in Louisiana to 119 in Colorado. Studies of HIV PCRS have reported numbers needed to interview ranging from two to 13 in individual jurisdictions.19,20 One study of 28 jurisdictions found a median number needed to interview of nine in areas where men who have sex with men (MSM) accounted for less than half of the HIV infections and 36 in areas where MSM accounted for 50% or more of the cases.15 Although Louisiana performed at the level of the reported studies, Wisconsin and Colorado failed to achieve comparable results. This project was not designed to explain these differences, but this variation may be due to multiple factors, such as differences in HIV prevalence in each jurisdiction, variation in the proportion of index clients who are MSM, characteristics of the health department or its staff that were not captured through our data collection processes, or chance, given the small numbers of participants at these sites.
Los Angeles and Chicago developed alternative models of PCRS. Although the number needed to interview in the Los Angeles site was 137, this number was heavily influenced by the large number of previously diagnosed index clients interviewed at this site. When data were limited to the 77 index clients diagnosed within three months of the PCRS interview and the three partners who they referred that were newly diagnosed with HIV infection, the number needed to interview was much lower at 26. For people interviewed three or more months following diagnosis, the number needed to interview was 179, suggesting that providing PCRS to HIV-infected people more than three months after their diagnosis may not be as effective as providing PCRS to people who have been recently diagnosed with HIV. These results are similar to findings by Ahrens and colleagues, who reported a number needed to interview of 21 among newly diagnosed index clients, lower than that among previously diagnosed index clients.21 Chicago employed a mixed model, using both traditional and alternative PCRS. All three newly diagnosed partners were elicited from index clients participating in traditional PCRS. Although the numbers are small, this may indicate that at the Chicago site, traditional PCRS was more effective in identifying new HIV diagnoses than the alternative model.
The passive model implemented in San Francisco was unsuccessful. Few HIV-infected people participated in this program, despite the staff's efforts to improve outcomes through training sessions with health-care providers and CBO staff, follow-up interviews with these collaborators, and increased marketing to HIV-infected people and the general public. This result suggests that HIV-infected individuals should be actively sought out to provide effective PCRS.
This project accessed index client and partner populations that were predominantly male, aged 25 to 49 years, and members of racial/ethnic minority groups. All of these characteristics are highly represented in the HIV epidemic, suggesting that PCRS reaches people who may have a great need for testing. Furthermore, data from this project confirmed that HIV PCRS programs access a population with a high level of unrecognized HIV infection. Eight percent of partners who were tested received new HIV diagnoses. This percentage is comparable to other published findings, which have reported that 7% to 39% of partners tested through PCRS were infected with HIV.15,20,22 The proportion of people tested through PCRS who were newly diagnosed with HIV infection was substantially higher than that found among people tested at HIV counseling and testing centers (generally 1% to 2%).23
Nearly one-fourth of partners tested in this project reported that they had never been tested for HIV, and half had not been tested in the past 12 months. Furthermore, almost half reported that they had no plans to be tested in the next six months. These data suggest that many were either not aware of their risk for HIV infection or had structural, psychological, financial, or other barriers to being tested.24,25 We were able to provide testing to many people in need of these services in this project.
Limitations
This project had several limitations. Not all index clients provided partner information, not all partners were interviewed or tested, and those who participated differed from those who did not by gender, age, and race/ethnicity. Therefore, the results from this analysis are not representative of all partners of HIV-infected individuals. Additionally, the project was conducted during a limited time frame at only six sites. PCRS varies widely by health department jurisdiction, and these data do not necessarily represent the breadth of programs and experiences. Furthermore, our data do not include all HIV tests received by partners as a result of notification, but rather only those conducted by project staff; therefore, we have likely underestimated the numbers of partners tested for HIV. Finally, although our original intent was to collect data on linkage to HIV care and treatment for partners with newly diagnosed HIV infection, these data proved extremely difficult to capture for a number of reasons, including the time lag between diagnosis and follow-up medical appointments for HIV care.
CONCLUSIONS
PCRS provides access to HIV prevention, testing, and referral services for people at high risk of HIV infection, including people who are unaware that they are infected with HIV and, thus, more likely to transmit infection. Rapid HIV testing can be incorporated into PCRS. Rapid HIV testing also likely confers benefits to individual and public health because it reduces barriers to testing, minimizes failure to return for test results, and decreases time spent on locating and notifying those tested of their negative test results. Our results clearly show that a passive model was ineffective at identifying partners with unrecognized HIV infection. However, further evaluation of active models of PCRS will be needed to determine the best methods of identifying partners with previously unrecognized HIV infection. Frequent evaluation and quality improvement of all PCRS activities will help to achieve this goal.
Acknowledgments
The authors acknowledge Scott Kellerman, MD, MPH, for his contributions to the development and implementation of the partner counseling and referral services demonstration project.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) [cited 2007 Nov 2];HIV partner counseling and referral services—guidance, December 30, 1998. Available from: URL: http://www.cdc.gov/hiv/resources/guidelines/pcrs/pdf/pcrs.pdf.
- 2.Adoption of protective behaviors among persons with recent HIV infection and diagnosis—Alabama, New Jersey, and Tennessee, 1997–1998. MMWR Morb Mortal Wkly Rep. 2000;49(23):512–5. [PubMed] [Google Scholar]
- 3.Potterat JJ, Meheus A, Gallwey J. Partner notification: operational considerations. Int J STD AIDS. 1991;2:411–5. doi: 10.1177/095646249100200603. [DOI] [PubMed] [Google Scholar]
- 4.West GR, Stark KA. Partner notification for HIV prevention: a critical reexamination. AIDS Educ Prev. 1997;9(3) Suppl:68–78. [PubMed] [Google Scholar]
- 5.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(15):329–32. [PubMed] [Google Scholar]
- 6.SAS Institute Inc. SAS: Version 9.1. Cary (NC): SAS Institute Inc.; 2003. [Google Scholar]
- 7.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–31. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 8.Fenton KA, Peterman TA. HIV partner notification: taking a new look. AIDS. 1997;11:1535–46. doi: 10.1097/00002030-199713000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals: a systematic review. Am J Prev Med. 2007;33(2) Suppl:S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Toomey KE, Peterman TA, Dicker LW, Zaidi AA, Wroten JE, Carolina J. Human immunodeficiency virus partner notification. Cost and effectiveness data from an attempted randomized controlled trial. Sex Transm Dis. 1998;25:310–6. doi: 10.1097/00007435-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan PS, Lansky A, Drake A HITS-2000 Investigators. Failure to return for HIV test results among persons at high risk for HIV infection: results from a multistate interview project. J Acquir Immune Defic Syndr. 2004;35:511–8. doi: 10.1097/00126334-200404150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hightow LB, Miller WC, Leone PA, Wohl D, Smurzynski M, Kaplan AH. Failure to return for HIV posttest counseling in an STD clinic population. AIDS Educ Prev. 2003;15:282–90. doi: 10.1521/aeap.15.4.282.23826. [DOI] [PubMed] [Google Scholar]
- 13.Wiley DJ, Frerichs RR, Ford WL, Simon PA. Failure to learn human immunodeficiency virus test results in Los Angeles public sexually transmitted disease clinics. Sex Transm Dis. 1998;25:342–5. doi: 10.1097/00007435-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kinsler JJ, Cunningham WE, Davis C, Wong MD. Time trends in failure to return for HIV test results. Sex Transm Dis. 2007;34:397–400. doi: 10.1097/01.olq.0000249757.10209.b3. [DOI] [PubMed] [Google Scholar]
- 15.Golden MR, Hogben M, Potterat JJ, Handsfield HH. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis. 2004;31:709–12. doi: 10.1097/01.olq.0000145847.65523.43. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RE, Spencer NE, Miller LA. Comparison of partner notification at anonymous and confidential HIV test sites in Colorado. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:406–10. [PubMed] [Google Scholar]
- 17.Pavia AT, Benyo M, Niler L, Risk I. Partner notification for control of HIV: results after 2 years of a statewide program in Utah. Am J Public Health. 1993;83:1418–24. doi: 10.2105/ajph.83.10.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer NE, Hoffman RE, Raevsky CA, Wolf FC, Vernon TM. Partner notification for human immunodeficiency virus infection in Colorado: results across index case groups and costs. Int J STD AIDS. 1993;4:26–32. doi: 10.1177/095646249300400106. [DOI] [PubMed] [Google Scholar]
- 19.Golden MR. Editorial: HIV partner notification: a neglected prevention intervention. Sex Transm Dis. 2002;29:472–5. doi: 10.1097/00007435-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Partner counseling and referral services to identify persons with undiagnosed HIV—North Carolina, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(48):1181–4. [PubMed] [Google Scholar]
- 21.Ahrens K, Kent CK, Kohn RP, Nieri G, Reynolds A, Philip S, et al. HIV partner notification outcomes for HIV-infected patients by duration of infection, San Francisco, 2004 to 2006. J Acquir Immune Defic Syndr. 2007;46:479–84. doi: 10.1097/qai.0b013e3181594c61. [DOI] [PubMed] [Google Scholar]
- 22.Landis SE, Schoenbach VJ, Weber DJ, Mittal M, Krishan B, Lewis K, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med. 1992;326:101–6. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (US) [cited 2007 Dec 18];HIV counseling and testing at CDC-supported sites—United States, 1999–2004. 2006 :9. Available from: URL: http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/ctr04.pdf.
- 24.Spielberg F, Branson BM, Goldbaum GM, Lockhart D, Kurth A, Celum CL, et al. Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr. 2003;32:318–27. doi: 10.1097/00126334-200303010-00012. [DOI] [PubMed] [Google Scholar]
- 25.Inungu JN. Potential barriers to seeking human immunodeficiency virus testing among adults in the United States: data from the 1998 National Health Interview Survey. AIDS Patient Care STDS. 2002;16:293–9. doi: 10.1089/10872910260066723. [DOI] [PubMed] [Google Scholar]