Abstract
Aims
To investigate the association of arginine methylation with myocardial function and prognosis in chronic systolic heart failure patients.
Methods and results
Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA), as well as N-mono-methylarginine (MMA) and methyl-lysine, were simultaneously measured by tandem mass spectrometry in 132 patients with chronic systolic heart failure with echocardiographic evaluation and follow-up. Increasing ADMA and SDMA levels were associated with elevated natriuretic peptide levels (both P < 0.001), and increasing SDMA levels were associated with worsening renal function (P < 0.001). Higher plasma levels of methylated arginine metabolites (but not methyl-lysine) were associated with the presence of left ventricular (LV) diastolic dysfunction (E/septal E′, Spearman's r = 0.31–0.36, P < 0.001). Patients taking beta-blockers had lower ADMA levels than those not taking beta-blockers [0.42 (0.33, 0.50) vs. 0.51 (0.40, 0.58), P < 0.001]. Only increasing ADMA levels were associated with advanced right ventricular (RV) systolic dysfunction. Elevated ADMA levels remained a consistent independent predictor of adverse clinical events (hazard ratio = 1.64, 95% CI: 1.20–2.22, P = 0.002).
Conclusion
In chronic systolic heart failure, accumulation of methylated arginine metabolites is associated with the presence of LV diastolic dysfunction. Among the methylated derivatives of arginine, ADMA provides the strongest independent prediction of disease progression and adverse long-term outcomes.
Keywords: Heart failure, Arginine, Methylation, Diastolic function, Prognosis
Introduction
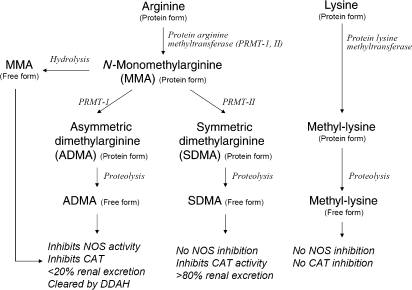
Altered nitric oxide (NO) bioavailability is a hallmark in the pathogenesis and progression of cardiovascular diseases. Recently, studies suggest that the synthesis of NO from its precursor l-arginine may be altered by the formation of a complex series of arginine methylation pathways in the presence of inflammation and oxidative stress through degradation of cellular proteins that contain arginine residues (Figure 1). In particular, two isoforms of methylarginine have been identified as potent endogenous NOS inhibitors:1 N-mono-methylarginine (MMA) and its methylation product, asymmetric dimethylarginine (ADMA). ADMA, which accumulates in much larger quantities than MMA, has emerged as a novel cardiovascular risk factor in the setting of diseases associated with endothelial dysfunction, including type 2 diabetes mellitus,2 coronary artery disease,3 end stage renal disease,4 and in heart failure.5 Systemic accumulation of ADMA has also been implicated in the pathogenesis of heart failure. In normal healthy volunteers, intravenous infusion of low-dose ADMA can result in a rise in systemic blood pressure and impairment of cardiac output.6 Indeed, patients with heart failure were found to have increased circulating levels of ADMA when compared with healthy controls.7,8
Figure 1.
Scheme of arginine metabolic pathways in high throughput mass spectrometry arrays. NOS, nitric oxide synthase isoforms; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine; DDAH, dimethylarginine dimethylaminohydrolase; PRMT, protein arginine methyltransferases; CAT, cationic amino acid transport.
In contrast, much less is known about the role of an alternative methylation product of MMA, symmetric dimethylarginine (SDMA), in the setting of heart failure. SDMA lacks NOS inhibitory activity and is largely cleared by the kidneys. However, all methylated arginine metabolites are thought to inhibit NO synthesis indirectly as l-arginine analogues via the competitive blockade of cationic arginine transport9—a process shown to be impaired in the setting of chronic heart failure.10 In contrast, other methylated amino acids products such as methyl-lysine may have limited effects on NO synthase or l-arginine transport.11
The relative contributions of these methylated derivatives of amino acids in the pathophysiology of human heart failure and their clinical significance are currently unknown. Observations from animal models have suggested that endogenous alterations to arginine metabolism through methylation pathways may play a pathologic role.12 Our group has recently reported the relationship between haemodynamic derangements and various simultaneously measured methylated arginine derivatives in patients presenting with acute cardiogenic shock.13 The objective of this study is to investigate the association of arginine methylation with clinical and paraclinical measures of myocardial function and prognosis in a small well-characterized cohort of chronic systolic heart failure patients.
Methods
Study design and subject population
The Assessment of Doppler Echocardiography on Prognosis and Therapy (ADEPT) study is a single-centre, prospective research study approved by the Cleveland Clinic Institutional Review Board. This sub-study initially assessed 242 patients with stable (>3 months duration) but symptomatic [New York Heart Association (NYHA) functional classes II–III] heart failure between 1 May 2001 and 30 June 2003 for inclusion. Eligible subjects were 18–75 years of age, with left ventricular ejection fraction (LVEF) ≤35%. Subjects were excluded if they had significant primary valvular diseases, or significant hepatic or renal dysfunction. Significant hepatic dysfunction was defined as serum aminotransferase levels above 2x the upper limit of normal. Significant renal dysfunction was defined as estimated glomerular filtration rate (eGFR) ≤30 mL/min/1.73 m2. Plasma samples were available from 138 patients for the analysis. We excluded six patients due to renal and hepatic dysfunction, leaving the final sample size of 132 patients. Among the subjects enrolled, 40% were about to initiate beta-blocker therapy at the time of evaluation.
Plasma samples were obtained after informed consent and were processed and stored at −80°C until analysis. Plasma aminoterminal pro-B-type natriuretic peptide levels (NT-proBNP) were analysed by the Roche Elecsys commercial assay (Roche Diagnostics Inc., Indianapolis, IN, USA).
Comprehensive transthoracic echocardiography was performed to measure chamber dimensions, systolic function, and diastolic indices [including pulse-wave Doppler, colour M-mode, and tissue Doppler imaging (TDI)] as previously described. eGFR was calculated by the four-variable Modification of Diet in Renal Disease (MDRD) Study equation.14 Clinical events (death, cardiac transplantation, or heart failure hospitalization) were followed for 33.0 ± 16.4 months, and the last follow-up date was 24 March 2006. We used a combination of telephone follow-up as well as chart review from our medical records to ensure that collected follow-up data were accurate and complete. Heart failure hospitalization was defined by an admission with clinical or radiographic heart failure, which was adjudicated by an investigator who was blinded to echocardiographic results.
Sample preparation and analysis of arginine and lysine methylation metabolites
Plasma (100 µL) was combined with an equal volume of 10 µM [13C6] arginine in water (internal standard) and mixed by vortexing. The solution was immediately diluted with 550 µL of acetonitrile. The resulting suspension was centrifuged at 3000 rpm for 15 min at 4°C. The supernatant was then transferred via pipette to a labelled 13 × 100 mm glass test tube and concentrated to dryness using a vortex evaporator. The residue was dissolved in 200 µL of 50% methanol water. An aliquot of the sample solution was injected onto a high-performance liquid chromatography (HPLC) column and the levels of NO metabolites and NOS inhibitors were quantified by LC/ESI/MS/MS analysis using an upgraded ABI 365 triple quadrupole mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) with Ionics EP 10+ redesigned source (Concord, Ontario, Canada) and electrospray ionization (ESI) needle connected to an Aria LX4 series multiplexed HPLC system with Flux pumps (Cohesive Technologies, Franklin, MA, USA). The amino acids were separated on a 250 × 4.6 mm Rexchrom S5-100-P phenyl column (Product number 728207 Regis Chemical, Morton Grove, IL, USA) equipped with a 1 mm × 15 mm Optimize technologies Opti-Guard Phenyl guard column (Optimize Technologies, Oregon City, OR, USA). The solvents used were 0.1% formic acid and 10 mM ammonium formate in water (solvent A) and 0.1% formic acid and 10 mM ammonium formate in methanol (solvent B). The gradient used was as follows: the column was first equilibrated with 100% A at 800 µL/min and held at this composition for 0.5 min after the injection; a linear gradient was then run to 50% B (50% A) over the next 3 min and held at 50% B for 3.5 min at a flow rate of 800 µL/min. At 6.5 min, the flow rate was increased to 1000 µL/min and the solvent composition was changed to 100% B in a linear fashion over 1 min. A linear gradient was then run to 100% A at 1000 µL/min over 0.5 min and held at this composition and flow rate for 6 min. The 8.5 min duration data window was started at 3.5 min after the injection. Mass spectrometric analyses were performed online using ESI tandem mass spectrometry in the positive ion mode with multiple reaction monitoring using parent→daughter ion transitions and retention times unique for each analyte monitored. Cone potentials and collision energy were optimized for each analyte. The actual masses for each parent to daughter ion transition are as follows: ADMA (203.2→70.3); SDMA (203.2→70.2); MMA (189.3→70.2); and methyl-lysine (161.3→84.2). The imprecision of measurement for each analyte was less than 10% (ADMA: 8.1%; SDMA: 6.5%; MMA: 6.5%, and methyl-lysine: 5.3%). The accuracy of each measurement was greater than 97% (ADMA: 99.2%, SDMA: 99.1%; MMA: 97.8%; and methyl-lysine: 99.5%). The biological variation of each analyte is as follows: ADMA: 0.09–8.07 µM; SDMA: 0.06–6.17 µM; MMA: 0–0.43 µM; and methyl-lysine: 0.48–16.36 µM.
Statistical analysis
Continuous variables were summarized as mean ± SD if normally distributed. Non-normally distributed continuous variables were summarized as median and inter-quartile range (IQR). Categorical variables were summarized as proportions and frequencies. Normality was assessed by the Shapiro–Wilk W-test. The Spearman's rank correlation method was used as a non-parametric measure of association for correlations between plasma ADMA, SDMA, MMA, or methyl-lysine levels and echocardiographic and clinical indexes. The plasma levels of the arginine metabolites were compared between the patients taking beta-blocker at baseline and those not using the Wilcoxon rank sum test. Kaplan–Meier survival plots were calculated from baseline to time of all-cause mortality, cardiac transplantation, or heart failure hospitalization. The log-rank test was used to test the difference in survival curves across groups. The Cox proportional hazards model was used to assess the clinical risks of increasing continuous standardized increments of methylated arginine metabolites. The proportional hazards assumption was verified with log(time) vs. log[−log(survival)] plots. The functional forms of the covariates were assessed by checking the martingale residuals. The results were adjusted for known clinical risk factors of adverse events using a multivariable Cox model. All P-values reported are from two-sided tests and a P-value <0.05 was considered statistically significant.
The sample size of 132 participants provided about 85% power to detect a hazard ratio of 1.5 per standard deviation of the plasma levels of the arginine metabolites with a two-sided type I error rate of 5%. The power calculations assumed an event rate of 40% among the study population. The statistical analyses were performed using JMP 5.1, SAS 9.1.3 (SAS Institute, Cary, NC, USA), and PASS (Kaysville, UT, USA). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics of study population
Baseline subject characteristics for the overall study cohort were typical of an ambulatory chronic systolic heart failure population as illustrated in Table 1. Overall, patients with higher plasma ADMA and SDMA levels were likely to be older [Spearman's r = 0.23 (P = 0.007) and 0.24 (P = 0.006), respectively], while patients with elevated SDMA levels were likely to have lower eGFR [Spearman's r = −0.30 (P < 0.001)]. However, such relationships were less apparent for MMA or methyl-lysine (Table 2).
Table 1.
Baseline subject characteristics (n = 132)
| Variable | Value |
|---|---|
| Demographics | |
| Mean age (years) | 57.8 ± 13.3 |
| Male gender, n (%) | 101 (77) |
| Mean BMI (kg/m2) | 28.2 ± 5.0 |
| Heart failure history | |
| NYHA class III or IV, n (%) | 41 (32) |
| Ischaemic aetiology, n (%) | 54 (42) |
| Co-morbidities | |
| Hypertension, n (%) | 71 (55) |
| Diabetes mellitus, n (%) | 37 (29) |
| Echocardiographic indices | |
| LV ejection fraction (%-units) | 25.6 ± 6.0 |
| LV end-diastolic dimension (mm) | 6.4 ± 0.9 |
| LV end-diastolic volume index (mL/m2) | 112.0 ± 35.5 |
| Diastolic stage >III, n (%) | 42 (36) |
| Medications | |
| ACE inhibitor and/or ARBs, n (%) | 121 (95) |
| Beta-blockers, n (%) | 77 (60) |
| Spironolactone, n (%) | 36 (30) |
| Loop diuretics, n (%) | 101 (78) |
| Digoxin, n (%) | 76 (63) |
| Median NT-proBNP (IQR) (pg/mL) | 1099 (469, 3036) |
| eGFR (mL/min per 1.73 m2) | 73.0 ± 20.9 |
| l-Arginine (μM) | 42 (36, 52) |
| Endogenous arginine metabolites (μM) | |
| Median ADMA (IQR) | 0.44 (0.36, 0.54) |
| Median SDMA (IQR) | 0.29 (0.23, 0.38) |
| Median MMA (IQR) | 35 (29, 46) |
| Median methyl-lysine (IQR) | 1.35 (0.91, 3.48) |
BMI, body mass index; NYHA, New York Heart Association; LV, left ventricle; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; NT-proBNP, aminoterminal pro-B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine; IQR, inter-quartile range.
Table 2.
Univariate correlations between plasma levels of methylated arginine and lysine metabolites and clinical and echocardiographic indices
| Variable | ADMA (μM) |
SDMA (μM) |
MMA (nM) |
Methyl-Lysine (μM) |
||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | |
| Age (years) | 0.23 | 0.007 | 0.24 | 0.006 | 0.07 | 0.412 | 0.01 | 0.943 |
| BMI (kg/m2) | −0.20 | 0.036 | −0.24 | 0.013 | −0.13 | 0.189 | −0.05 | 0.591 |
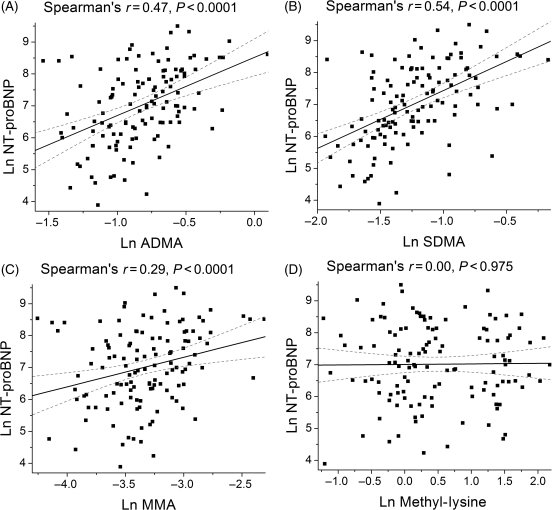
| NT-proBNP (pg/mL) | 0.47 | <0.001 | 0.54 | <0.001 | 0.29 | 0.001 | 0.00 | 0.975 |
| eGFR (mL/min per 1.73 m2) | −0.12 | 0.179 | −0.30 | <0.001 | −0.13 | 0.144 | 0.02 | 0.808 |
| Echocardiographic indices | ||||||||
| Calculated LV mass (g) | −0.12 | 0.162 | −0.10 | 0.284 | −0.17 | 0.059 | −0.05 | 0.558 |
| LA volume index (mL/m2) | 0.29 | 0.004 | 0.29 | 0.004 | 0.19 | 0.057 | 0.10 | 0.327 |
| Mitral E/A ratio | 0.26 | 0.004 | 0.28 | 0.002 | 0.17 | 0.066 | 0.14 | 0.118 |
| Mitral deceleration time (ms) | −0.24 | 0.009 | −0.20 | 0.028 | −0.10 | 0.264 | −0.05 | 0.623 |
| PV peak S/D ratio | −0.18 | 0.049 | −0.29 | 0.001 | −0.13 | 0.146 | −0.13 | 0.134 |
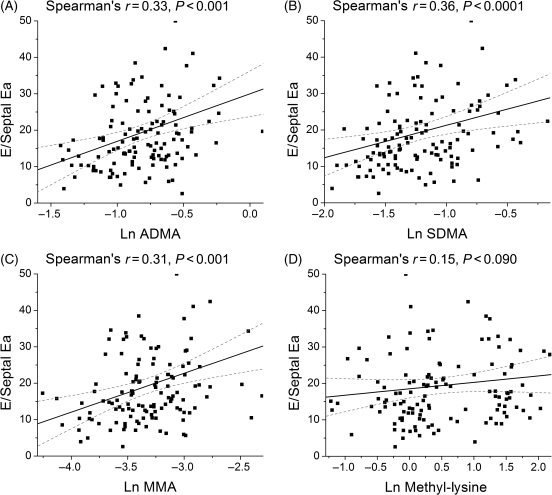
| Mitral E/septal Ea ratio | 0.33 | <0.001 | 0.36 | <0.001 | 0.31 | <0.001 | 0.15 | 0.090 |
| LV ejection fraction (%) | −0.21 | 0.021 | −0.15 | 0.110 | −0.11 | 0.248 | −0.01 | 0.945 |
| LV end-diastolic volume index (mL/m2) | 0.14 | 0.149 | 0.08 | 0.433 | 0.19 | 0.054 | 0.00 | 0.998 |
| RV systolic dysfunction class | 0.21 | 0.014 | 0.18 | 0.037 | 0.09 | 0.308 | −0.01 | 0.836 |
ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine; BMI, body mass index; NT-proBNP, aminoterminal pro-B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LV, left ventricle; LA, left atrial; PV, pulmonary vein; RV, right ventricular.
Methylation metabolites of amino acids and cardiac function
The relation between plasma levels of methylated metabolites for arginine and lysine and echocardiographic indices of diastolic function are shown in Table 2. In particular, both plasma ADMA and SDMA levels correlated with echocardiographic estimates of increasing LV filling pressures (including mitral E/A, mitral deceleration time, and pulmonary vein S/D ratio). All three methylated arginine metabolites correlated with mitral E/tissue Doppler septal Ea (E/E′) ratios (Table 2; Figure 2). Similar results were observed when correlated with mean E/E′ ratio. In addition, plasma NT-proBNP levels were associated with elevated levels of ADMA, SDMA, and MMA, but not with methyl-lysine (Figure 3).
Figure 2.
Relation of plasma levels of methylated arginine metabolites and mitral E/tissue Doppler septal Ea (E/E′). [(A) ADMA; (B) SDMA; (C) MMA; (D) Methyl-lysine].
Figure 3.
Relation of plasma levels of methylated arginine metabolites and plasma levels of NT-proBNP [(A) ADMA; (B) SDMA; (C) MMA; (D) Methyl-lysine].
Methylation metabolites of amino acids and renal function
In the Spearman's correlation analysis, only increasing levels of SDMA were indicative of worsening renal function, as defined by decreasing eGFR [Spearman's r = −0.30 (P < 0.001); Table 2].
Methylation metabolites of amino acids and medication use
A subset of patients was recruited in the ADEPT study prior to the initiation of their beta-blocker therapy. Patients taking beta-blockers at baseline had significantly lower ADMA and MMA levels compared with those not taking beta-blockers (Table 3), whereas SDMA, l-arginine, and methyl-lysine levels did not differ significantly across beta-blocker use. No significant differences in metabolites monitored were present for patients taking vs. not taking ACE-I, ARBs, spironolactone, loop diuretics, or digoxin.
Table 3.
Arginine metabolite levels in patients taking vs. not taking beta-blocker at baseline
| Taking beta-blocker (n = 77) | Not taking beta-blocker (n = 52) | P-value | |
|---|---|---|---|
| ADMA (μM) | 0.42 (0.33, 0.50) | 0.51 (0.40, 0.58) | <0.001 |
| MMA (nM) | 33 (26, 44) | 38 (32, 50) | 0.014 |
| SDMA (μM) | 0.27 (0.22, 0.34) | 0.32 (0.23, 0.42) | 0.052 |
| Methyl-lysine (μM) | 1.42 (0.93, 3.88) | 1.23 (0.87, 2.04) | 0.120 |
Data expressed in median (inter-quartile range).
ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; MMA, N-mono-methylarginine.
Methylation metabolites of amino acids and clinical outcomes
Over a mean follow-up of 33 ± 16 months, there were 39 adverse clinical events (all-cause mortality, cardiac transplantation, or heart failure hospitalization) including 20 deaths.
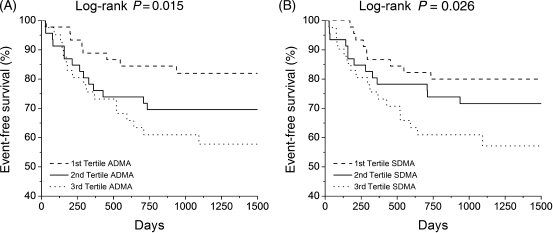
In multivariable Cox proportional hazard analysis, increasing ADMA levels conferred higher hazard ratios for death alone (1.84, 95% CI: 1.22–2.67, P = 0.004), death or cardiac transplantation (1.65, 95% CI: 1.19–2.21, P = 0.003), and death, cardiac transplantation, or heart failure hospitalization (1.64, 95% CI: 1.20–2.22, P = 0.002, Table 4), but increasing SDMA, MMA, and methyl-lysine levels did not. Risk for the occurrence of an adverse event associated with elevated ADMA levels remained significant after adjustment for age, LVEF, and eGFR (Table 4). In Kaplan–Meier analysis, increasing ADMA and SDMA levels predicted reduced event-free survival when comparing highest vs. lowest tertiles (ADMA: log-rank P = 0.015; SDMA: log-rank P = 0.026; Figure 4). Combining ADMA and SDMA values yielded similar results, although less robust than ADMA alone (data not shown).
Table 4.
Univariate and multivariable hazard ratios for predicting death, transplant, or heart failure hospitalization for plasma levels of methylated arginine metabolites
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| ADMA univariate modela | 1.64 (1.20–2.22) | 0.002 |
| ADMA multivariable modelb | 1.47 (1.03–2.11) | 0.037 |
| SDMA univariate modela | 1.21 (0.91–1.55) | 0.174 |
| MMA univariate modela | 1.26 (0.94–1.62) | 0.114 |
| All methylarginines univariate modela | 1.46 (1.09–1.94) | 0.012 |
| Methyl-lysine univariate modela | 0.97 (0.69–1.29) | 0.827 |
aHazard ratios per 1-SD increment [1-SD for ADMA = 0.14 µM; 1-SD for SDMA = 0.12 µM; 1-SD for MMA = 14 nM; 1-SD for all methylarginines (ADMA+SDMA+MMA) = 0.24 µM; 1-SD for methyl-lysine = 1.81 µM].
bMultivariable adjustments including age, eGFR, and LV ejection fraction (1-SD for LVEF = 5.99%; 1-SD for age = 13.3 years; 1-SD for eGFR = 20.9 mL/min per 1.73 m2). Hazard ratios per 1-SD increment ADMA = 0.14 µM.
Figure 4.
Kaplan–Meier analysis of event-free survival stratified according to plasma levels of ADMA (A) and SDMA (B).
Discussion
We observed a direct relationship between systemic accumulation of several methylated arginine metabolites and altered LV diastolic performance in a well-defined cohort of patients with chronic systolic heart failure. However, following adjustments for other co-morbidities, only plasma levels of ADMA were associated with advanced systolic dysfunction and independently predicted overall long-term adverse event rates. Taken together, our data point to the upregulation of arginine methylation pathways as being associated with the development of LV diastolic dysfunction as well as right ventricular (RV) systolic dysfunction. Alterations in the production and catabolism of endogenous NOS inhibitors remained the major mechanistic link contributing to disease progression in chronic heart failure.
Protein methylation has been recognized in a wide range of biological processes, which can act epigenetically to modulate gene expression such as post-translational modification. While arginine methylation has been considered primarily an irreversible and systemic vascular process, animal studies have pointed to a potential cardiac source of methylated arginine derivatives in the setting of heart failure progression. Free cellular ADMA and MMA (but not SDMA) are hydrolyzed by dimethylarginine dimethylaminohydrolases (DDAH), which is expressed in cardiac myocytes as well as in the vascular endothelium. Indeed, progressive decline of DDAH-2 (but not DDAH-1) expression has been observed in rapid-pacing dog models.12 Furthermore, increases in both eNOS and DDAH-1 gene and protein expression have been associated with ventricular unloading following left ventricular assist device implantation in end-stage human heart failure.15
The first new finding is the association between arginine methylation and LV diastolic more than systolic dysfunction. Historically, endogenous NO production is believed to be integral to the maintenance of normal LV relaxation and diastolic distensibility not only due to its acute haemodynamic benefits but also to its long-term inhibition of adverse ventricular remodelling.16–19 In patients with heart failure, exogenous, intracoronary administration of NO donors has been shown to improve LV stroke volume and work through reduced LV filling pressure and increased recruitment of LV preload reserve.20,21 Increased endomyocardial inducible and endothelial NOS gene expression has also been associated with augmented LV diastolic function and reduced diastolic stiffness.20,22 In addition, chronic inhibition of NOS has been shown in animal models to induce progressive myocardial interstitial and peri-vascular fibrosis through a signalling cascade involving angiotensin II, aldosterone, and transforming growth factor-β mediated by endothelin.23,24 While ADMA and MMA may provide direct endogenous NOS inhibition, SDMA may also inhibit l-arginine transport, thereby indirectly affecting NO production by limiting NOS substrate availability.25 Therefore, the relationship observed between all methylated arginine metabolites and echocardiographic indices of diastolic dysfunction points to a process related to heightened arginine methylation and limitation of global NO bioavailability rather than solely confined to endogenous NOS inhibition. This is further illustrated by the lack of relationship between diastolic function and methylation of non-arginine peptide, in this case methyl-lysine (which served as an ‘internal control’). The close relationship between the methylated arginine metabolites and NT-proBNP is confirmatory of previous reports,5 and also supported our hypothesis.
The second new finding is the clustering of more severe LV and RV systolic dysfunction with only elevated plasma ADMA levels and not with other metabolites of arginine or lysine methylation. There has been limited understanding of the role of SDMA in the pathophysiology of heart failure, and they are found at lower concentrations than ADMA levels in plasma. Only a small proportion of ADMA and MMA can be excreted in the urine, while SDMA is eliminated almost entirely by renal excretion. We confirmed that SDMA is a sensitive marker of renal dysfunction, and the differential relationships between ADMA and SDMA and myocardial function and outcomes may indicate compartmentalization related to its clearance mechanisms, especially if altered DDAH expression (which breaks down ADMA but not SDMA) is down-regulated with the progression of pump failure.
We found that plasma ADMA and MMA levels were significantly lower in those treated with beta-adrenergic blocker vs. those not on beta-blocker therapy. This difference was not as apparent with other methylated metabolites. Improved endothelial function has been observed following beta-adrenergic blocker use,26 and reports have linked the therapeutic benefit of selective beta-blockers to endogenous NO production.27 The precise mechanisms are yet to be defined, but there have been data supporting the role of beta-adrenergic blockers in preventing oxidative-stress induced upregulation of PRMT expression28 or inhibition of DDAH activity.29 The fact that SDMA is not metabolized by DDAH may in part account for the lack of an observed inverse association between its plasma levels and beta-blocker use. An alternative explanation can be the modulation of NOS expression (the target of ADMA antagonism) by beta-adrenergic blockade. Further studies are needed to better understand the clinical implications of therapeutic interventions targeting these cellular metabolic components such as PRMT, DDAH, or NOS in the setting of heart failure.
There are several limitations of this descriptive, cross-sectional study. The cross-sectional nature of our study population limits any direct demonstration of a cause-and-effect for different mechanistic pathways involved in the production and catabolism of methylated arginine derivatives. Furthermore, we recognize the limitations of a single time-point measurement of these metabolites that can present with significant biological variability, although it is generally accepted that tandem mass spectrometry techniques utilized in this study may provide greater precision than conventional HPLC or immunoassay analyses. In addition, there is no universally accepted quantification of diastolic dysfunction, and our analysis relied on accepted clinical criteria that have been established in the literature and in clinical practice. We also acknowledge the potential of inflated experiment-wise type I error due to multiple testing throughout the manuscript, but we chose not to adjust for a lower type I error rate (as it may lead to inflated type II error). Nevertheless, we hope our findings can stimulate interest for further investigations into how modulation of arginine metabolism can be clinically relevant.
Conclusions
Among patients with chronic systolic heart failure, accumulation of methylated arginine metabolites is associated with the presence of LV diastolic dysfunction. However, among the methylated derivatives of arginine, ADMA has the strongest association with disease progression and adverse long-term outcomes.
Funding
The original ADEPT study was funded by the American Society of Echocardiography, and received partial funding from GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc. This work was supported by National Institutes of Health grants P01 HL076491, P01 HL77107, P01 HL087018, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio, and the American Heart Association Ohio Valley Affiliates (0465266B).
Conflict of interest: none declared.
References
- 1.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 2.Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2007;30:1834–1839. doi: 10.2337/dc07-0019. [DOI] [PubMed] [Google Scholar]
- 3.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Mallamaci F, Tripepi G. Asymmetric dimethylarginine (ADMA) as a cardiovascular risk factor in end-stage renal disease (ESRD) Eur J Clin Pharmacol. 2006;62(Suppl. 1):131–135. [Google Scholar]
- 5.Duckelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2037–2042. doi: 10.1161/ATVBAHA.107.147595. [DOI] [PubMed] [Google Scholar]
- 6.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 7.Saitoh M, Osanai T, Kamada T, Matsunaga T, Ishizaka H, Hanada H, Okumura K. High plasma level of asymmetric dimethylarginine in patients with acutely exacerbated congestive heart failure: role in reduction of plasma nitric oxide level. Heart Vessels. 2003;18:177–182. doi: 10.1007/s00380-003-0715-y. [DOI] [PubMed] [Google Scholar]
- 8.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–2430. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 9.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 10.Kaye DM, Ahlers BA, Autelitano DJ, Chin-Dusting JP. In vivo and in vitro evidence for impaired arginine transport in human heart failure. Circulation. 2000;102:2707–2712. doi: 10.1161/01.cir.102.22.2707. [DOI] [PubMed] [Google Scholar]
- 11.Paik WK, Paik DC, Kim S. Historical review: the field of protein methylation. Trends Biochem Sci. 2007;32:146–152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Li Y, Zhang P, Traverse JH, Hou M, Xu X, Kimoto M, Bache RJ. Dimethylarginine dimethylaminohydrolase and endothelial dysfunction in failing hearts. Am J Physiol Heart Circ Physiol. 2005;289:H2212–H2219. doi: 10.1152/ajpheart.00224.2005. [DOI] [PubMed] [Google Scholar]
- 13.Nicholls SJ, Wang Z, Koeth R, Levison B, Delfraino B, Dzavik V, Griffith OW, Hathaway D, Panza JA, Nissen SE, Hochman JS, Hazen SL. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–2324. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Park S, Li Y, Missov E, Hou M, Han X, Hall JL, Miller LW, Bache RJ. Alterations of gene expression in failing myocardium following left ventricular assist device support. Physiol Genomics. 2003;14:251–260. doi: 10.1152/physiolgenomics.00022.2003. [DOI] [PubMed] [Google Scholar]
- 16.Paulus WJ, Vantrimpont PJ, Shah AM. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994;89:2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- 17.Paulus WJ. Paracrine coronary endothelial modulation of diastolic left ventricular function in man: implications for diastolic heart failure. J Card Fail. 1996;2(Suppl. 4):S155–S164. doi: 10.1016/s1071-9164(96)80072-8. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, Bronzwaer JG. Nitric oxide's role in the heart: control of beating or breathing? Am J Physiol Heart Circ Physiol. 2004;287:H8–H13. doi: 10.1152/ajpheart.01147.2003. [DOI] [PubMed] [Google Scholar]
- 19.Bronzwaer JG, Heymes C, Visser CA, Paulus WJ. Myocardial fibrosis blunts nitric oxide synthase-related preload reserve in human dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003;284:H10–H16. doi: 10.1152/ajpheart.00401.2002. [DOI] [PubMed] [Google Scholar]
- 20.Heymes C, Vanderheyden M, Bronzwaer JG, Shah AM, Paulus WJ. Endomyocardial nitric oxide synthase and left ventricular preload reserve in dilated cardiomyopathy. Circulation. 1999;99:3009–3016. doi: 10.1161/01.cir.99.23.3009. [DOI] [PubMed] [Google Scholar]
- 21.Matter CM, Mandinov L, Kaufmann PA, Vassalli G, Jiang Z, Hess OM. Effect of NO donors on LV diastolic function in patients with severe pressure-overload hypertrophy. Circulation. 1999;99:2396–2401. doi: 10.1161/01.cir.99.18.2396. [DOI] [PubMed] [Google Scholar]
- 22.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 23.Fakhouri F, Placier S, Ardaillou R, Dussaule JC, Chatziantoniou C. Angiotensin II activates collagen type I gene in the renal cortex and aorta of transgenic mice through interaction with endothelin and TGF-beta. J Am Soc Nephrol. 2001;12:2701–2710. doi: 10.1681/ASN.V12122701. [DOI] [PubMed] [Google Scholar]
- 24.Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, Yamamoto H, Tamaki K, Shimokawa H, Takeshita A. Early induction of transforming growth factor-beta via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 1998;32:273–279. doi: 10.1161/01.hyp.32.2.273. [DOI] [PubMed] [Google Scholar]
- 25.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52:1593–1601. doi: 10.1038/ki.1997.490. [DOI] [PubMed] [Google Scholar]
- 26.Nishioka K, Nakagawa K, Umemura T, Jitsuiki D, Ueda K, Goto C, Chayama K, Yoshizumi M, Higashi Y. Carvedilol improves endothelium-dependent vasodilation in patients with dilated cardiomyopathy. Heart. 2007;93:247–248. doi: 10.1136/hrt.2006.087999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afonso RA, Patarrao RS, Macedo MP, Carmo MM. Carvedilol action is dependent on endogenous production of nitric oxide. Am J Hypertens. 2006;19:419–425. doi: 10.1016/j.amjhyper.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine- dependent methyltransferases. Circ Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.