Abstract
Aims
To evaluate whether comprehensive evaluation of coronary anatomy and delayed enhancement (DE) by multidetector-computed tomography (MDCT) would allow determination of etiology of left ventricular dysfunction (LVD) as compared with coronary angiography (CA) and DE-magnetic resonance (CMR).
Methods and results
Seventy-one consecutive patients (50 males, 59 ± 16 years) with LVD (ejection fraction: 26 ± 11%) of unknown etiology underwent MDCT, LGE (late Gd-DTPA-enhanced)-CMR and CA. Patients were classified into four groups according to coronary artery disease (CAD) by CA and LGE-CMR patterns. Patients (n = 24) with CAD and transmural or sub-endocardial DE by CMR were considered having definite ischaemic LVD (group 1). Patients (n = 36) without CAD by CA and with no/atypical LGE-CMR were considered non-ischaemic LVD (group 2). Further we identified four patients with transmural DE but no CAD (group 3) and seven patients with CAD but no DE (group 4). On per-patient basis, combined coronary and DE-MDCT had excellent agreement (κ = 0.89; P < 0.001) with CA/LGE-CMR to classify patients into the same four groups. Sensitivity, specificity and accuracy of MDCT were 97, 92 and 94%, respectively for detecting patients with definite (group 1) or likely (groups 3 and 4) ischaemic LVD.
Conclusion
Combined coronary and DE-MDCT can accurately differentiate ischaemic vs. non-ischaemic etiology of LVD.
Keywords: Computed tomography, Left ventricular dysfunction, Magnetic resonance imaging, Coronary angiography, Dilated cardiomyopathy, Infarct
Introduction
Recognizing the underlying etiology of left ventricular dysfunction (LVD) is essential for defining prognosis and selecting treatment of patients. Indeed coronary artery disease (CAD) is the most common cause of LVD,1 but may have worse outcome than some subtypes of non-ischaemic dilated cardiomyopathy (DCM).2 Conversely, coronary revascularization can improve clinical status and survival in patients with ischaemic LVD, especially if myocardial viability is present.3
Since non-invasive tests for detection of CAD perform poorly in patients with LVD, current guidelines4 recommend performing invasive coronary angiography (CA) to determine the underlying cause of LVD. Yet, this invasive approach is costly and carries a non-negligible risk of complications. More recently, late gadolinium diethylenetriamine penta-acetic acid (Gd-DTPA)-enhanced (LGE)5 cardiovascular magnetic resonance (CMR) has also been proposed to assess the etiology of LVD. Different patterns of LGE have been described in patients with LVD.6,7 Ischaemic LVD is typically associated with sub-endocardial or transmural LGE patterns. In contrast, patients with non-ischaemic DCM, usually display either no LGE, or in mid-ventricular or sub-epicardial LGE patterns,7 quite different from those seen in CAD patients.
Recently, it was demonstrated that also multidetector-computed tomography (MDCT) can detect myocardial necrosis and fibrosis on LGE images both in animals8–10 and humans.9,11–13 MDCT thus offers the unique possibility of combining myocardial tissue characterization with non-invasive coronary imaging in a single exam that lasts <15 min.
Accordingly, the aim of the present study was to evaluate the feasibility and diagnostic accuracy of using combined coronary and delayed enhancement (DE)-MDCT for determining the etiology of LVD. For this purpose, we compared coronary and DE-MDCT in 71 patients with LVD of unknown etiology to invasive CA and LGE-CMR.
Methods
Patient population
Consecutive patients with reduced left ventricular (LV) ejection fraction (<50%) referred to our institution for evaluation of etiology of LVD by CA and LGE-CMR were prospectively screened for inclusion into the study. Patients with an established diagnosis of LVD (definite history of infarction or prior CA) were not considered for inclusion. Presence of Q-waves on ECG was not considered a definite proof for ischaemic LVD and thus not an exclusion criterion. Other exclusion criteria were haemodynamic instability, atrial fibrillation, renal failure (serum creatinine >1.4 mg/dL), known allergy to iodinated contrast agents, or any contraindication to CMR imaging (cerebral aneurysm clips, pacemaker, or severe claustrophobia). The study protocol complied with the Declaration of Helsinki and was approved by the local Ethic Committee. All patients gave informed consent prior to inclusion into the study.
Study protocol
Patients underwent DE and coronary MDCT as well as clinically indicated CA and LGE-CMR in random order within 1 month. Because many patients had clinically symptomatic congestive heart failure, no systematic beta-blockers were given prior to MDCT. Also no sublingual nitroglycerin was given. Patients were not excluded based on high rate.
Multidetector row coronary computed tomography
MDCT was performed using a 40-slice (n = 35 patients) or a 64-slice (n = 36 patients) system (Brilliance 40 and 64, respectively, Philips Medical Systems, Cleveland, OH, USA) as previously described.14 The two systems had identical spatial and temporal resolution as well as rotation speed. They differed only in detector coverage length, which allowed 40% shorter scanning time for the 64-slice with respect to the 40-slice system. Coronary images were acquired immediately after intravenous injection of 120 mL of non-ionic contrast (Iomeron 400, Bracco, Milan, Italy) at a rate of 4 mL/min. Scan parameters were: tube voltage 120 kV and effective tube current 600 mA (for 40-slice MDCT) and 720 mA (for 64-slice MDCT). A second series of gated breath-hold images was acquired for DE imaging 10 min after contrast injection at the same location, but with lower tube current (400/600 mA) and voltage (80 kV) to increase signal-to-noise and reduce dose. Dose modulation was used only for patients with HR <70 b.p.m. Data sets were reconstructed at 75% of cardiac cycle with additional reconstructions at other phases if needed. Late images were reconstructed with a soft kernel (type CA), and resliced into serial 10 mm thick short-axis and two-, three- and four-chamber long-axis slices.9 Total estimated radiation dose for both scans calculated from dose length product was 17.8 ± 3.3 mSv (70% resulting from the coronary scan and 30% from the DE imaging).
LGE-cardiovascular magnetic resonance
LGE-CMR was performed on a 1.5 Tesla system (Philips Intera CV, Best, The Netherlands). Cine-breath-hold steady state free precession functional images were acquired in short-axis and two-, three- and four-chamber views. Two-dimensional (2D) and 3D short-axis and two-, three- and four-chamber LGE images were acquired 10–15 min after injection of 0.20 mmol/kg Gadodiamide (Omniscan, Nycomed) as previously described.9 Inversion recovery time was individually adapted (250–300 ms) based on a lock–locker sequence to null the signal of normal myocardium and maximize contrast between regions of LGE and normal myocardium.
LGE-CMR images were interpreted visually by one reader (A.C.P.) blinded to all clinical, CA and MDCT data. In each patient, presence and type of LGE pattern (typical ischaemic, i.e. sub-endocardial or transmural vs. typical non-ischaemic, i.e. mid-ventricular, patchy, or sub-epicardial) were recorded in a 17-segment AHA model.
Coronary angiography
Selective CA in multiple orthogonal projections was evaluated by different clinical physicians unaware of the MDCT results. The standard 16-segment American Heart Association classification system was used.15 All visually identified stenoses were measured quantitatively in two orthogonal views by a quantitative CA (QCA) with the CAAS II software (QCA, Cardiovascular Angiographic Analysis System II, Pie Medical Equipment, Switzerland), using catheter-based image calibration and automated vessel contour detection. Significant CAD was considered if diameter stenosis exceeded 50% in coronary segments >1.5 mm.
Data analysis
Coronary and LGE-MDCT images were transferred onto a dedicated workstation (Mxview, Philips Medical Systems). All patient-relevant information was removed and anonymous images were analysed offline by the visual consensual reading of two cardiologists (B.G. and J.B.L.P.D.W.) who were blinded to the patient’s clinical information, CA and LGE-CMR. Coronary images were interpreted on the data set containing the fewest motion artefacts. Using axial data sets, multiplanar reformations in various directions and maximum intensity projections, both readers first determined which segments were assessable and then visually categorized the severity of diameter stenosis as being <50% or ≥50%. Presence and type of MDCT-DE pattern were visually interpreted by the two reviewers on short-axis and two-, three- and four-chamber long-axis images in the same 17-segment model as CMR.
Patient classification
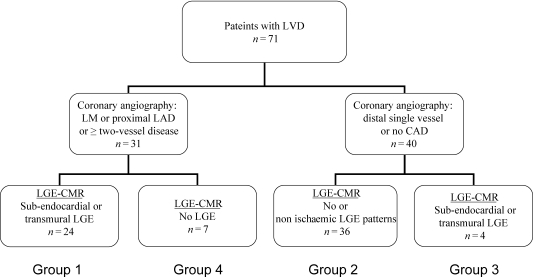
According to the results of CA and LGE-CMR, four groups of patients were identified. Group 1 consisted of patients who satisfied the criteria of Felker et al.16 for ischaemic LVD by CA, i.e. significant CAD stenosis in at least two coronary arteries, or in the proximal left anterior descending coronary artery (LAD) or left main coronary artery (LM); and who had also sub-endocardial or transmural LGE. Group two consisted of patients who had no significant (<50% stenosis) CAD, or CAD in only one small vessel, and who had either no LGE or a LGE pattern-described non-ischaemic LVD (mid-ventricular or sub-epicardial LGE).6,7 Group 3 consisted of patients without significant CAD, but with sub-endocardial or transmural LGE typical of ischaemic LVD. Finally, patients with CAD but without LGE or with non-ischaemic LGE patterns were classified in group 4. The same classification and criteria were used to classify patients using coronary and DE-MDCT.
Statistical analysis
Continuous values are reported as mean ± 1 standard deviation. Statistical analysis was made using SPSS 11.5 software. We estimated that prevalence of CAD in patients with LVD would be ∼50% and that MDCT would have 90% sensitivity and specificity to detect CAD on per-patient basis. Based on these assumptions we estimated that we need 35 with and 35 without disease to define sensitivity and specificity of MDCT with a 95% confidence interval (CI) of ±10%. Baseline characteristics of patients in the four groups were compared using ANOVA (continuous variables) and Kruskal–Wallis test (categorical variables). The diagnostic accuracy of coronary and DE-MDCT on segmental basis was evaluated using CA and LGE-CMR as reference standards. Diagnostic accuracy for detection of CAD and DE patterns was expressed as per cent with 95% CI. Agreement between the four groups of patients was assessed using κ-statistic. We computed predictive value of MDCT for the diagnosis of certain (group 1) and likely (groups 3 and 4) ischaemic aetiology of LVD. All tests were two-sided and P < 0.05 was considered indicative of statistical significance.
Results
Study population
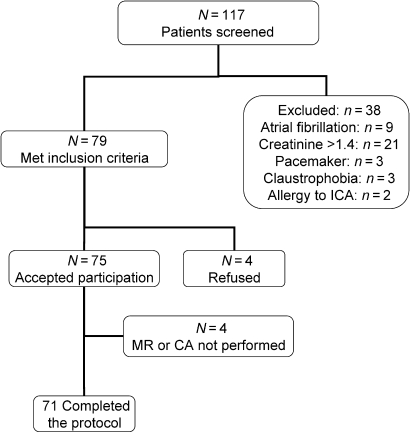
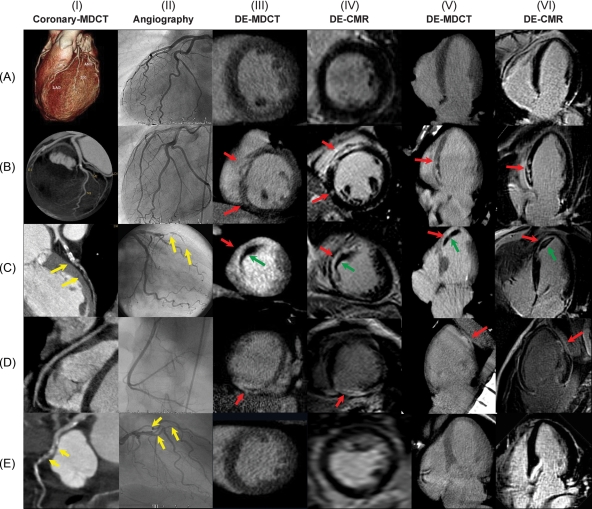
Figure 1 shows included and excluded patients. The 71 patients enrolled in this study successfully completed CA, MDCT, and CMR without complications. Examples of coronary and DE-MDCT, CA, and LGE-CMR images obtained in five representative patients are shown in Figure 2. Patient characteristics are summarized in Table 1. Most patients had new onset heart failure (<3 months duration of symptoms).
Figure 1.
Flow chart illustrating included and excluded patients.
Figure 2.
Examples of coronary (panel I) and delayed enhancement (DE)-multidetector-computed tomography (MDCT) (panels III and V) in five representative patients and their correlations to coronary angiography (panel II) and LGE-CMR (late Gd-DTPA-enhanced cardiovascular magnetic resonance; panels IV and VI). (A) No coronary artery disease (CAD) and absence of DE. (B) No CAD and mid-ventricular DE. (C) Occlusion of the proximal LAD (yellow arrow) with transmural DE in the apex, septum and antero-septal region (red arrows) and mural thrombus (green arrows). (D) Absence of CAD with transmural inferior necrosis. (E) Proximal and mid-LAD and first diagonal stenosis (yellow arrows) without DE.
Table 1.
Patient characteristics
| Group 1 (n = 24) | Group 2 (n = 36) | Group 3 (n = 4) | Group 4 (n = 7) | |
|---|---|---|---|---|
| Demographics | ||||
| Male/Female | 22/2 | 21/15 | 3/1 | 4/3 |
| Age (years) | 62 ± 10 | 56 ± 19 | 58 ± 22 | 63 ± 16 |
| Framingham risk (%) | 13 ± 7 | 13 ± 33 | 7 ± 8 | 15 ± 9 |
| NYHA class | ||||
| I | 3 (12%) | 3 (8%) | 0 (0%) | 1 (14%) |
| II | 7 (29%) | 11 (30%) | 2 (50%) | 1 (14%) |
| III | 13 (54%) | 20 (55%) | 1 (25%) | 3 (43%) |
| IV | 1 (4%) | 2 (6%) | 1 (25%) | 2 (29%) |
| ECG | ||||
| Q-waves | 10 (41%) | 4 (11%) | 0 (0%) | 0 0(%) |
| LBBB | 3 (12%) | 13 (36%) | 2 (50%) | 1 (14%) |
| CMR | ||||
| EF (%) | 25 ± 11 | 26 ± 12 | 29 ± 13 | 28 ± 7 |
| EDV (mL) | 280 ± 91 | 248 ± 111 | 233 ± 124 | 214 ± 45 |
| CAD by CA | ||||
| 0 vx | 0 (0%) | 35 (97%) | 4 (100%) | 0 (0%) |
| 1 vx | 1 (4%) | 1 (3%) | 0 (0%) | 1 (14%) |
| 2 vx | 6 (25%) | 0 (0%) | 0 (0%) | 2 (29%) |
| 3 vx | 17 (71%) | 0 (0%) | 0 (0%) | 4 (57%) |
CA, coronary angiography; CAD, coronary artery disease; EF, ejection fraction; EDV, end-diastolic volume; LBBB, left bundle branch block; NYHA, New York Heart Association class.
Coronary angiography and cardiovascular magnetic resonance
According to CA, 31 patients presented significant CAD, i.e. two patients had single-vessel disease (both had proximal LAD), nine had two-vessel disease and 20 presented with three-vessel disease. According to CMR, 24 patients had transmural LGE, four had sub-endocardial LGE, 11 had mid-ventricular or epicardial LGE and one patient had sub-epicardial and mid-ventricular LGE which extended transmurally in some regions.
Classification of patients by combining coronary angiography and late Gd-DTPA-enhanced cardiovascular magnetic resonance
Classification of patients by CA and LGE-CMR is shown in Figure 3. Among the 31 patients with CAD, 24 had transmural (n = 20) or sub-endocardial (n = 4) LGE (group 1). Among the 40 patients without significant CAD, 24 had no LGE on CMR, while 11 (15%) displayed mid-ventricular or sub-epicardial LGE and one patient presented with both a transmural and mid- and sub-epicardial LGE (the last patient was classified as a group 2 patient). Four patients without significant CAD, had transmural LGE on CMR (group 3). Finally seven patients with CAD (one with single proximal LAD stenosis, one with two-vessel disease involving the proximal LAD and distal left circumflex coronary artery (LCX), four patients with three-vessel disease and one patient with a single dominant right coronary artery (RCA) and no LM) had no LGE on CMR (group 4).
Figure 3.
Classification of patients according to coronary angiography and cardiovascular magnetic resonance.
Multidetector-computed tomography
Average heart rate during MDCT was 77 ± 14 (53–118 b.p.m.). No patient was excluded because of poor image quality. According to coronary MDCT, 33 patients exhibited significant CAD. Detection of CAD by MDCT on per-patient basis had very high agreement with CA (κ = 0.94; P < 0.001). MDCT was 100% (31/31) sensitive, 95% (95% CI: 88–100%) (38/40) specific, and 97% (95% CI: 89–100%) (69/71) accurate, for identifying CAD on per-patient basis. There were no false negative detections and only two false positive identifications of CAD by MDCT, both related to severe coronary calcifications that led to overestimation of non-significant stenoses.
On late images, MDCT identified 27 patients with transmural DE, three patients with sub-endocardial DE, 10 patients with mid-ventricular or epicardial DE and one patient who had mid-ventricular, sub-epicardial, and transmural DE. One patient with mid-ventricular LGE (non-ischaemic pattern) and one patient with transmural LGE by CMR were not identified by DE-MDCT. These false negatives were due to poor image quality and low signal-to-noise ratio. There were three false positive identifications of DE-MDCT (all of them considered ischaemic pattern). They were related to artefacts related to either adjacent bone structures (n = 2) or motion (n = 1). Overall DE-MDCT had very good agreement (κ = 0.88; P < 0.001) for detection of sub-endocardial or transmural DE using LGE-CMR as reference. The sensitivity for detection of sub-endocardial or transmural necrosis was 96% (28/29) (95% CI: 90–100%). Specificity was 92% (39/42) (95% CI: 85–100%). Diagnostic accuracy was 94% (67/71) (95% CI: 89–100%).
Patient classification by combining coronary angiography and delayed enhancement multidetector-computed tomography
By combining information on coronary anatomy and of DE by MDCT, we classified patients into the same four groups as by CA and CMR. This classification of patients by the combination of coronary and DE-MDCT had very good agreement with the classification of patients by the combination of CA and LGE-CMR (κ = 0.89; P < 0.001, Table 2). Only five patients were misclassified with respect to their suspected etiology of LVD. Among the two false positive detections of CAD, one was a true negative detection of DE-MDCT. The patient was thus misclassified as group 4 instead of group 2). The second false positive detection of CAD was also erroneously considered to have ischaemic pattern with DE-MDCT and thus misclassified as group 1 instead of group 2). There was a false positive detection of DE-MDCT in a patient correctly identified without CAD (misclassification of group 3 vs. group 2) and in a patient correctly identified to have CAD (misclassification as group 4 instead of group 1). In a fifth patient correctly identified not to have CAD, ischaemic type DE-MDCT was missed. Thus this patient was misclassified as group 2 instead of group 3. Finally a non-ischaemic (mid-ventricular DE pattern) was missed. Since this occurred in a patient who was correctly identified not to have CAD, the patient's group did not change (remained group 2).
Table 2.
Comparison of patient classification by multidetector-computed tomography vs. coronary angiography and cardiovascular magnetic resonance
| Coronary angiography and LGE-CMR |
|||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Total | |
| MDCT | |||||
| Group 1 | 24 | 1 | 0 | 1 | 26 |
| Group 2 | 0 | 33 | 1 | 0 | 34 |
| Group 3 | 0 | 1 | 3 | 0 | 4 |
| Group 4 | 0 | 1 | 0 | 6 | 7 |
| Total | 24 | 36 | 4 | 7 | 71 |
κ = 0.89; P < 0.001.
The combination of coronary and DE-MDCT was 97% (95% CI: 83–100%) (34/37) sensitive, 92% (33/36) (95% CI: 82–100%) specific, and 94% (95% CI: 89–100%) (67/71) accurate, for detecting definite (group 1) or probable (groups 3 and 4) ischaemic LVD as compared with CA and LGE-CMR. Since ‘group 3’ patients had typical ischaemic LGE, but ‘no’ CAD by CA, these probably represent embolic infarcts and/or transient occlusion followed by spontaneous recanalization. Thus, from a more clinical perspective, a second classification relative to the aetiology of the LVD was performed by including those patients as group 1. Using this second classification and considering only group 1 patients as definite ischaemic LVD, DE-MDCT was 96% (95% CI: 83–100%) (27/28) sensitive, 90% (40/43) (95% CI: 85–100%) specific, and 94% (95% CI: 89–100%) (67/71) accurate as compared with CA and LGE-CMR.
Discussion
Delineation of the etiology of left ventricular dysfunction
Precise delineation of the etiology of LVD is important for the risk stratification and treatment selection in patients with LVD. Unfortunately, clear definition of the etiology of LVD is not always straightforward. CA is currently considered the reference standard for detecting whether a patient has ischaemic LVD or non-ischaemic DCM. However, detection of epicardial stenosis by CA alone has its own limitations and different criteria for definition of ischaemic LVD have been proposed.16 Indeed, the detection of epicardial coronary artery stenosis by CA in a patient with LVD does not necessarily indicate that CAD is the underlying cause of LVD, as the fortuitous association of non-ischaemic DCM with CAD can be seen in older patients with coronary risk factors. On the other hand, a normal CA does not necessarily exclude CAD as initial cause of LVD, since patients with myocardial infarction may experience spontaneous recanalization of the culprit coronary artery.
Tissue imaging by LGE-CMR offers alternative and complementary information to elucidate the underlying mechanism of LVD. Indeed, it allows detecting myocardial fibrosis and necrosis with high spatial resolution and good histological correlation.5 Recent studies have shown that LGE-CMR allows differentiating ischaemic from non-ischaemic LVD by identifying different contrast enhancement patterns.6,7,17–19 Indeed, the majority of patients with ischaemic LVD exhibit at least one small region of either sub-endocardial or transmural LGE,6,7 although some may only be microscopically visible and occur at very late states of dysfunction.20 In contrast, patients with non-ischaemic LVD usually display no LGE or LGE regions whose spatial distribution differs from that seen in CAD, i.e. mid-ventricular or sub-epicardial DE zones.7 It was suggested that these ‘atypical’ LGE patterns may result from chronic or healed myocarditis.21
It is interesting to note that in the present study, classification of etiology by CA and CMR differed in a significant number of patients. Indeed, besides patients with both CAD and typical sub-endocardial or transmural LGE (group 1), and without both CAD and LGE (group 2), we came across several patients who definitely had CAD but did not show LGE or displayed ischaemic LGE pattern but had no significant CAD. We considered that the first group of patients without significant CAD, but with LGE (group 3) had likely undergone an embolic infarct or had reperfused their coronary artery after a transitory occlusion.6 Many of these patients had indeed CAD, but without significant (>50%) reduction of the lumen of the coronary arteries.
The classification of the last group of patients with CAD, but without typical LGE (group 4) is somewhat more problematic. These patients might have a pure form of collateral dependent non-infarcted ‘hibernating myocardium’.22,23 They could also have non-ischaemic DCM with secondary presence of CAD. Since we did not perform endocardial biopsies in our patients, the precise etiology of LVD in these patients remained uncertain. These intricacies illustrate clearly, that a correct classification of the etiology of LVD is difficult and requires not only depiction of coronary anatomy, but also identification of myocardial tissue damage by LGE-CMR or MDCT.
Use of multidetector-computed tomography to define the etiology of left ventricular dysfunction
So far, only few prior studies have employed MDCT to characterize patients with LVD. Budoff et al.24,25 reported that the calcium score alone had 97–99% sensitivity and 68–82% specificity for identification of ischaemic vs. non-ischaemic LVD and that it also was more accurate than thallium stress testing for delineating the etiology of LVD. More recently, Andreini et al.26 and Cornily et al.27 evaluated the feasibility of using 16-slice MDCT for detecting coronary stenosis in LVD patients and reported good diagnostic accuracy of detecting CAD on segmental basis. Although detection of DE by MDCT was reported in infarction,9,11–13 myocarditis,21–23 sarcoidosis,28 and hypertrophic cardiomyopathy,29 DE-MDCT has not yet been proposed for detection of etiology of LVD.
The present study is thus the first to evaluate the combination of coronary and DE-MDCT to assess the etiology of LVD in a large number of patients with LVD of unknown origin. Our results indicate that MDCT has a high accuracy for detection of significant CAD in comparison with CA in this population and that it is capable of detecting similar DE patterns as LGE-CMR, with an equally good accuracy. Accordingly, in our study, MDCT allowed to accurately define the origin of LVD in comparison with CA and CMR. The major advantage of using MDCT in this setting is that it allows for a comprehensive assessment in a single non-invasive test. An additional advantage of MDCT is its more widespread availability, compared with CA and CMR and that it can be performed in patients with implantable devices, such as resynchronization pacemakers or AICD. One should nonetheless recognize that the use of MDCT in patients with LVD also carries significant limitations. Since injection of iodinated contrast can aggravate pre-existing renal failure, it is contraindicated in patients with renal failure. This condition is however common in patients with LVD due to low cardiac output, coexisting renal disease and treatment with diuretics or inhibitors of the renin–angiotensin–aldosterone system. Injection of large volumes of iodinated contrast agents at high injection rates might also cause haemodynamic complications, such as acute pulmonary oedema. Patients should therefore be well monitored. No such complication was however observed in our study. Another limitation of current retrospectively gated DE-MDCT technology is the high radiation dose with the potential of radiation-induced cancer.30 As opposed to using invasive angiography as a first test, which may allow the possibility of coronary revascularization in the same setting; using MDCT as a screening test for CAD might potentially cause double radiation exposure if invasive angiography will be needed later for revascularization purposes. The late-enhanced study adds 30% additional dose (>5 mSv) to the dose of CA-MDCT. Such considerations of radiation-induced cancer are certainly important in young asymptomatic patients with long life expectancy, where the benefits of the test must be weighted against the theoretical risk of inducing cancer due to radiation exposure. However in the present population of older patients with severe LVD, the potential harm from radiation is likely negligible as opposed to disease-related prognosis.31 Also in the future, dose exposure of MDCT will likely be reduced significantly by prospective ECG gating.32
A final important limitation is that image quality of DE-MDCT is currently less than that of LGE-CMR. It may not allow detection of very small areas of necrosis. Yet, in the present study, most patients presented with large areas of DE that could easily be picked up by MDCT, and agreement of DE-MDCT and LGE-CMR was high.
Other value of delayed enhancement multidetector-computed tomography in patients with left ventricular dysfunction
In patients with CAD, the presence of LGE by CMR indicates irreversible myocardial necrosis, and thus non-viable myocardium and a low likelihood of functional recovery after revascularization.33 Detection of LGE is thus useful for deciding whether patients with ischaemic LVD should undergo revascularization or not. The ability to predict myocardial viability was also recently demonstrated for DE-MDCT.13,34 LGE as detected by CMR also carries prognostic information in patients with LVD. Kwong et al.35 demonstrated that the presence of unrecognized LGE in CAD patients unfavourably influenced outcome. Similarly in patients with non-ischaemic DCM, Assomull et al.36 reported that the presence of LGE was associated with higher risk of death. Given the high degree of concordance between DE-MDCT and LGE-CMR patterns, one might anticipate that DE-MDCT would carry also a similar prognostic value as CMR in LVD patients. This was however not verified in the present work.
Study limitations
The present work was performed using MDCT scanners with, respectively, 40 and 64 detector rows and 420 ms rotation speed. The diagnostic performance of more recent dual-source MDCT scanners in patients with LVD and high heart rates might be even higher.37,38 Another limitation of this present study is that we used CA and LGE-CMR as gold standard for classification of etiology of LVD. Histological verification by endomyocardial biopsy was not performed in our study.
Conclusion
The present study demonstrates that combined coronary and DE-MDCT can identify the underlying etiology of patients with LVD in a manner much similar to CMR and CA. Our results also raised questions about the use of a single standard of reference for the diagnosis of LVD etiology since significant discrepancies between LGE-CMR and CA were observed in several patients. In this setting, combination of coronary and DE-MDCT may be thus extremely useful for better understanding of the etiology of LVD. DE-MDCT being more widely available, it could thus allow for a faster and cheaper characterization of the etiology of LVD.
Funding
Grant support by the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRSM 3.4557.02). J.B.L.P.D.W. was supported by a fellowship of the Damman foundation.
Funding to pay the Open Access publication charges for this article was provided by a grant support by the Fondation Nationale de la Recherche Scientifique of the Belgian Government (FRSM 3.4557.02).
Conflict of interest: the authors have no conflicts of interest relative to the findings of this study.
References
- 1.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Ay T, Pasquet A, Robert A, D’Hondt AM, Noirhomme P, Goenen M, Melin JA, Vanoverschelde JL. Usefulness of low-dose dobutamine echocardiography for selection of patients with severely depressed left ventricular function for coronary bypass grafting. Am J Cardiol. 2002;90:319–323. doi: 10.1016/s0002-9149(02)02473-6. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC, Jr. ACC/AHA guidelines for the evaluation management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim RJ, Fieno D, Parrish RB, Harris K, Chen EL, Simonetti O, Bundy J, Finn P, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 6.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 7.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 8.Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S, Halperin HR, Wu KC, Hare JM, Lima JA. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394–404. doi: 10.1161/CIRCULATIONAHA.105.521450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 10.Baks T, Cademartiri F, Moelker AD, Weustink AC, van Geuns RJ, Mollet NR, Krestin GP, Duncker DJ, de Feyter PJ. Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J Am Coll Cardiol. 2006;48:144–152. doi: 10.1016/j.jacc.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 11.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Gunther RW, Kuhl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042–2047. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Sanz J, Weeks D, Nikolaou K, Sirol M, Rius T, Rajagopalan S, Dellegrottaglie S, Strobeck J, Fuster V, Poon M. Detection of healed myocardial infarction with multidetector-row computed tomography and comparison with cardiac magnetic resonance delayed hyperenhancement. Am J Cardiol. 2006;98:149–155. doi: 10.1016/j.amjcard.2006.01.093. [DOI] [PubMed] [Google Scholar]
- 13.Habis M, Capderou A, Ghostine S, Daoud B, Caussin C, Riou J-Y, Brenot P, Angel CY, Lancelin B, Paul JF. Acute myocardial infarction early viability assessment by 64-slice computed tomography immediately after coronary angiography. Comparison with low-dose dobutamine echocardiography. J Am Coll Cardiol. 2007;49:1178–1185. doi: 10.1016/j.jacc.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Pouleur AC, le Polain de Waroux JB, Kefer J, Pasquet A, Coche E, Vanoverschelde JL, Gerber BL. Usefulness of 40-slice multidetector row computed tomography to detect coronary disease in patients prior to cardiac valve surgery. Eur Radiol. 2007;17:3199–3207. doi: 10.1007/s00330-007-0676-0. [DOI] [PubMed] [Google Scholar]
- 15.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A, Jr, Russell RO, Jr, Ryan TJ, Smith SC, Jr. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 17.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–748. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 18.Soriano CJ, Ridocci F, Estornell J, Perez-Bosca JL, Pomar F, Trigo A, Planas A, Nadal M, Jacas V, Martinez V, Paya R. Late gadolinium-enhanced cardiovascular magnetic resonance identifies patients with standardized definition of ischemic cardiomyopathy: a single centre experience. Int J Cardiol. 2007;116:167–173. doi: 10.1016/j.ijcard.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Casolo G, Minneci S, Manta R, Sulla A, Del Meglio J, Rega L, Gensini G. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am Heart J. 2006;151:101–108. doi: 10.1016/j.ahj.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz ER, Schaper J, vom Dahl J, Altehoefer C, Grohmann B, Schoendube F, Sheehan FH, Uebis R, Buell U, Messmer BJ, Schaper W, Hanrath P. Myocyte degeneration and cell death in hibernating human myocardium. J Am Coll Cardiol. 1996;27:1577–1585. doi: 10.1016/0735-1097(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 21.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 22.Vanoverschelde JL, Wijns W, Depre C, Essamri B, Heyndrickx GR, Borgers M, Bol A, Melin JA. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513–1523. doi: 10.1161/01.cir.87.5.1513. [DOI] [PubMed] [Google Scholar]
- 23.Depre C, Vanoverschelde JL, Melin JA, Borgers M, Bol A, Ausma J, Dion R, Wijns W. Structural and metabolic correlates of the reversibility of chronic left ventricular ischemic dysfunction in humans. Am J Physiol. 1995;268:H1265–H1275. doi: 10.1152/ajpheart.1995.268.3.H1265. [DOI] [PubMed] [Google Scholar]
- 24.Budoff MJ, Shavelle DM, Lamont DH, Kim HT, Akinwale P, Kennedy JM, Brundage BH. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. J Am Coll Cardiol. 1998;32:1173–1178. doi: 10.1016/s0735-1097(98)00387-8. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Jacob B, Rasouli ML, Yu D, Chang RS, Shavelle DM. Comparison of electron beam computed tomography and technetium stress testing in differentiating cause of dilated versus ischemic cardiomyopathy. J Comput Assist Tomogr. 2005;29:699–703. doi: 10.1097/01.rct.0000175503.87578.0d. [DOI] [PubMed] [Google Scholar]
- 26.Andreini D, Pontone G, Pepi M, Ballerini G, Bartorelli AL, Magini A, Quaglia C, Nobili E, Agostoni P. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:2044–2050. doi: 10.1016/j.jacc.2007.01.086. [DOI] [PubMed] [Google Scholar]
- 27.Cornily JC, Gilard M, Gal GL, Pennec PY, Vinsonneau U, Blanc JJ, Mansourati J, Boschat J. Accuracy of 16-detector multislice spiral computed tomography in the initial evaluation of dilated cardiomyopathy. Eur J Radiol. 2007;61:84–90. doi: 10.1016/j.ejrad.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Kanao S, Tadamura E, Yamamuro M, Kubo S, Kimura T, Kita T, Togashi K. Demonstration of cardiac involvement of sarcoidosis by contrast-enhanced multislice computed tomography and delayed-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2005;29:745–748. doi: 10.1097/01.rct.0000177519.25045.01. [DOI] [PubMed] [Google Scholar]
- 29.Shiozaki AA, Santos TS, Artega E, Rochitte CE. Images in cardiovascular medicine. Myocardial delayed enhancement by computed tomography in hypertrophic cardiomyopathy. Circulation. 2007;115:e430–e431. doi: 10.1161/CIRCULATIONAHA.106.674911. [DOI] [PubMed] [Google Scholar]
- 30.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 31.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 32.Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, Veit-Haibach P, Tatsugami F, von Schulthess GK, Kaufmann PA. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–197. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]
- 33.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 34.Lessick J, Ghersin E, Dragu R, Litmanovich D, Mutlak D, Rispler S, Agmon Y, Engel A, Beyar R. Diagnostic accuracy of myocardial hypoenhancement on multidetector computed tomography in identifying myocardial infarction in patients admitted with acute chest pain syndrome. J Comput Assist Tomogr. 2007;31:780–788. doi: 10.1097/rct.0b013e318033d6fc. [DOI] [PubMed] [Google Scholar]
- 35.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 36.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Weustink AC, Meijboom WB, Mollet NR, Otsuka M, Pugliese F, van Mieghem C, Malago R, Van Pelt N, Dijkshoorn ML, Cademartiri F, Krestin GP, de Feyter PJ. Reliable high-speed coronary computed tomography in symptomatic patients. J Am Coll Cardiol. 2007;50:786–794. doi: 10.1016/j.jacc.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 38.Leber AW, Johnson T, Becker A, von Ziegler F, Tittus J, Nikolaou K, Reiser M, Steinbeck G, Becker CR, Knez A. Diagnostic accuracy of dual-source multislice CT-coronary angiography in patients with an intermediate pretest likelihood for coronary artery disease. Eur Heart J. 2007;28:2354–2360. doi: 10.1093/eurheartj/ehm294. [DOI] [PubMed] [Google Scholar]
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.