Abstract
Aims
To investigate whether genetic variants of the histidine-rich calcium (HRC)-binding protein are associated with idiopathic dilated cardiomyopathy (DCM) and its progression.
Methods and results
We screened 123 idiopathic DCM patients and 96 healthy individuals by single-strand conformation polymorphism analysis and direct sequencing for genetic variants in HRC. Six polymorphisms were detected: Leu35Leu (A/G), Ser43Asn (G/A), Ser96Ala (T/G), Glu202_Glu203insGlu (−/GAG), Asp261del (GAT/−), and an in-frame insertion of 51 amino acids at His321. The analysis of their frequencies did not reveal any significant correlation with DCM development. However, the Ser96Ala polymorphism exhibited a statistically significant correlation with the occurrence of life-threatening ventricular arrhythmias. During a follow-up of 4.02 ± 2.4 years, the risk for ventricular arrhythmias was higher (HR, 9.620; 95% CI, 2.183–42.394; P = 0.003) in the Ala/Ala patients, compared with Ser/Ser homozygous patients. On multivariable Cox regression analysis, the Ser96Ala polymorphism was the only significant genetic arrythmogenesis predictor in DCM patients (HR, 4.191; 95% CI, 0.838–20.967; P = 0.018).
Conclusion
The Ser96Ala genetic variant of HRC is associated with life-threatening ventricular arrhythmias in idiopathic DCM and may serve as an independent predictor of susceptibility to arrhythmogenesis in the setting of DCM.
Keywords: Calcium, Sarcoplasmic reticulum, Prognosis, Defibrillation
Introduction
Idiopathic dilated cardiomyopathy (DCM) represents the substrate for approximately 10% of sudden cardiac deaths (SCDs) in the adult population.1 Despite routine use of angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and spironolactone in patients with heart failure due to DCM, these patients still have a considerable annual mortality rate of 5–10%. Sudden unexpected death accounts for up to 50% of all deaths and is most often due to ventricular tachycardia (VT) or ventricular fibrillation (VF) and less often due to bradyarrhythmias or asystole.2
A consistent finding in failing myocardium is altered intracellular calcium (Ca) handling, manifested by a prolonged time course of intracellular Ca transients and changes in systolic and diastolic Ca levels.3 An increasing body of evidence indicates that abnormal intracellular Ca handling underlies contractile dysfunction,4,5 and contributes to ventricular arrhythmogenesis in failing myocardium.6,7
Recent studies have shown that histidine-rich calcium (HRC)-binding protein, a 165 kDa sarcoplasmic reticulum (SR) protein, may regulate SR Ca cycling during excitation–contraction coupling.8 Adenoviral-mediated HRC transfer in primary cultured rat ventricular myocytes was associated with an increase in SR Ca load, but decreased Ca release, resulting in depressed myocyte shortening and relengthening.9,10 Furthermore, isolated cardiomyocytes from transgenic mice with HRC overexpression revealed depressed SR Ca sequestration and delayed Ca decline, as well as relaxation rates, which triggered hypertrophy during the ageing process.11 Collectively, these findings suggest that HRC may play a regulatory role in both SR Ca release and uptake. Thus, it could be hypothesized that HRC mutations or polymorphisms may affect the SR Ca cycling and may be associated with depressed cardiac function and remodelling.
In the present study, we screened the HRC gene coding region in 96 healthy individuals and a well-characterized cohort of 123 non-ischaemic DCM patients, with a long follow-up period. Although the identified HRC genetic alterations occur in both DCM patients and controls with similar frequencies, we demonstrate that the genetic variant of Ser96Ala in HRC correlates with ventricular arrhythmogenesis and sudden death in DCM patients, suggesting that HRC may play a modifying role in the progression of this disease.
Methods
Patient selection
This study included patients with idiopathic DCM, who were referred to the Second Department of Cardiology, Onassis Cardiac Surgery Center Athens, and the Second Department of Cardiology, Attikon Hospital, University of Athens for diagnosis or treatment of heart failure, including implantable cardioverter-defibrillator (ICD) implantation. The patients were enrolled in the study between June 1999 and December 2005. The diagnosis of idiopathic DCM was based on the definition of the World Health Organization/International Society and Federation of Cardiology Task Force.12 The exclusion criteria included the presence of significant coronary artery stenosis on angiography and non-ischaemic DCM secondary to valvular heart disease, systemic hypertension, active myocarditis, and excessive alcohol abuse.
A total of 128 patients, who met the inclusion criteria (see Supplementary material online, Figure S1), underwent evaluation including NYHA classification, clinical examination, electrocardiogram (ECG), M-mode, and two-dimensional echocardiography. Ninety-seven of the enrolled patients had been referred for heart failure diagnosis and had complete diagnostic cardiac catheterization, which revealed DCM. Eighty-nine of them had also endomyocardial biopsy to rule out myocarditis prior to study entry. The remaining 31 of the enrolled patients were also referred for heart failure therapy. These patients had a long history of DCM, diagnosed after coronary angiography at least 9 months before inclusion in the study, without any indication of myocarditis. This group included 26 patients, who were referred for ICD implantation due to prior history of cardiac arrest (n = 3) or documented sustained VT episodes (n = 23). Five of the 128 patients initially enrolled did not have complete follow up data, and were excluded from the analysis.
The study was approved by the institutional review boards of the Onassis Cardiac Surgery Center and the Attikon Hospital of the University of Athens. All patients provided a written informed consent. An array of 96 Human Random Control DNA samples (panel 1 out of 5, Catalogue No.: #06041301), extracted from fresh, single donor blood samples of healthy Caucasian individuals (37.4 ± 9.7 years of age with 50% females), was obtained from the European Collection of Cell Cultures (ECACC, CAMR, Salisbury, Wiltshire, UK; distributed by Sigma-Aldrich Ltd, Poole, Dorset, UK). The samples were randomly selected without any constraints on age or gender. The DNA extraction, purification, and identification (determined by short tandem repeat DNA profiling) of these 96 control samples were performed by ECACC, and it is suitable for a wide range of genetic applications such as mutation analysis, single-nucleotide polymorphisms genotyping, and association studies.
Patient follow-up
After initial evaluation, patients were scheduled for follow-up at 3 and 6 months. Subsequently, patients were evaluated at 6 month intervals or when device firing occurred for those carrying an ICD. During the follow up visits, the patients’ clinical status was evaluated in regard to heart failure symptoms and functional class changes. Echocardiography was performed in patients with clinical deterioration and 24 h ambulatory ECG was performed in patients who had arrhythmia symptoms. Device interrogation was performed in patients with ICD. Information regarding deceased patients was obtained from family members, their general practitioners, and the hospitals at which they had been admitted. Particular attention was given to the circumstances of each death. The endpoints during follow-up were: (i) life-threatening arrhythmic events, including SCD (defined as death occurring instantaneously within 60 min of a change in symptoms or unexpectedly during sleep), cardiac arrest due to VF (documented by the emergency service), and episodes of unstable VT (>180 bpm) or VF, which were terminated after ICD firing, as documented by the electrogram storage in patients with an ICD; (ii) cardiac death due to pump failure; and (iii) cardiac transplantation. The endpoints were determined by the clinicians involved in the study, who were blinded to the DNA data analysis. Cases were subject to censoring due to: (i) death from non-cardiac aetiology and (ii) study termination.
Genetic analysis
Total DNA was extracted from venous blood samples, using QIAamp DNA blood midi kit (Qiagen GmbH, Hilden, Germany). Using Platinum Taq DNA polymerase (Invitrogen Corp., Carlsbad, CA, USA), the HRC coding region, including −238 bp in the 5′ UTR, 20–50 bp of intronic sequences flanking each exon, and 137 bp downstream from the stop codon (3′ UTR), was amplified by polymerase chain reaction (PCR; see Supplementary material online, Table S1). The PCR products were denatured, rapidly cooled, analysed on MDE polyacrylamide gels (Cambrex Bio Science Rockland Inc., Rockland, ME, USA), and silver stained. For each electrophoresis pattern obtained, sequencing reactions were performed by Macrogen Inc. (Seoul, South Korea).
Statistics
Hardy–Weinberg equilibrium was assessed by χ2, likelihood ratio, and permutation exact test.13 One-way analysis of variance (ANOVA) for continuous and χ2 or Fisher’s exact test for categorical variables were used to compare differences in clinical parameters between the different genotypes. Multiple logistic regression analysis was used to estimate the relative risk (RR) of each genetic factor for the phenotype under investigation. The Mantel–Cox log-rank test was used to compare Kaplan–Meier survival curves. Univariable Cox proportional hazards model was performed to identify the significant variables. All the significant variables identified from univariable analysis, as well as known factors associated with the outcome, were entered in the multivariable Cox proportional hazards regression model. Data are presented as mean ± standard deviation. A P-value of <0.05 was considered statistically significant. Power calculations were performed, on the basis of frequencies of the rarer allele in the control group. The sample size in this study would allow detecting an RR (by allele) of 1.6 for any polymorphism with a frequency as rare as 1% in control population, and a power of 82.3% at the 0.05 significance level (two-sides). Statistical calculations were carried out by the SigmaStat 3.00 and SPSS 13.0 (SPSS Inc., USA) software.
Results
Patients and follow-up
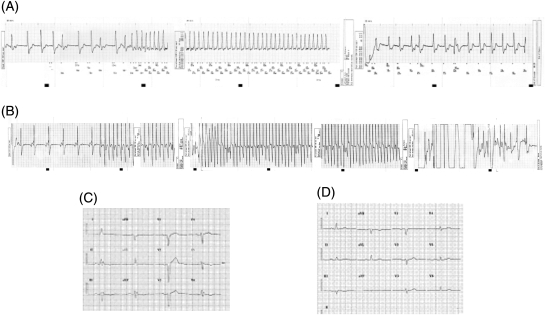
A total of 166 patients with non-ischaemic DCM were screened, and 123 of them with complete follow-up data, who met the inclusion criteria as described above, were enrolled in the study. The clinical characteristics at study entry are presented in Table 1. During follow-up, an ICD was implanted in another six patients, due to sustained VT episodes. The patients were followed up for a median period of 4.54 years (interquartile range, 4.92 years; from 1.33 to 6.25 years), and during this period, 28 of them (22.8%) presented one of the pre-specified life-threatening arrhythmic events. Specifically, six out of the 91 patients without ICD (6.6%) died suddenly and 22 out of the 32 patients with ICD (68.8%) presented haemodynamically unstable monomorphic VT (n = 20) or polymorphic VT/VF (n = 2), documented by the electrogram storage of the ICD (Figure 1). Interestingly, one of these patients who had presented an episode of unstable monomorphic VT died suddenly 3 months thereafter. In addition, seven other patients (5.6%) died due to pump failure and six (4.9%) underwent heart transplantation during the follow up period. Finally, two other patients (1.6%) died from a non-cardiac aetiology.
Table 1.
Characteristics of patients (n = 123) upon study entry and healthy controls (n = 96)
| DCM | ECACC controls | |
|---|---|---|
| Age at study entry, years | 48.6 ± 13.9 | 37.4 ± 9.7 |
| Sex (female), n (%) | 28 (22.8) | 48 (50.0) |
| Clinical characteristics, n (%) | ||
| Familial DCM | 47 (38.2) | |
| NYHA | ||
| Class I | 56 (45.5) | |
| Class II | 51 (41.5) | |
| Class III | 16 (13.0) | |
| Atrial fibrillation (AF) | 21 (17.1) | |
| Left bundle branch block (LBBB) | 40 (32.5) | |
| LVEF, % | 29.3 ± 8.6 | |
| LVEDD, mm | 67.7 ± 9.3 | |
| LVESD, mm | 54.9 ± 11.0 | |
| ICD (history of sustained VT or VF), n (%) | 26 (21.1) | |
| History of unexplained syncope, n (%) | 8 (6.5) | |
| Medication at enrolment, n (%) | ||
| ACE inhibitor | 115 (93.5) | |
| Digitalis | 36 (29.3) | |
| Spirinolactone | 47 (38.2) | |
| Beta blockers | 93 (75.6) | |
| Amiodarone | 57 (46.3) | |
All values are mean ± standard deviation.
ECACC, European Collection of Cell Cultures; NYHA, New York Heart Association classification; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation; ACE, angiotensin-converting enzyme.
Figure 1.
Intracardiac electrograms showing recording of monomorphic (A) or polymorphic (B) ventricular tachycardia that terminated after implantable cardioverter-defibrillator firing, from two patients with Ala/Ala at codon 96. Their resting ECGs are also presented (C and D, respectively).
Genetic analysis for human histidine-rich calcium genetic variants
Six genetic alterations were identified in the human HRC coding region. Three of them were single-nucleotide substitutions. One was silent for A105G (CTG instead of CTA), which encodes leucine for codon 35. Two were non-synonymous changes, a G128A, which affects codon 43 and encodes for asparagine (AAC) instead of serine (AGC) (Ser43Asn), and a T286G, which affects codon 96 and encodes for alanine (GCC) instead of serine (TCC) (Ser96Ala).
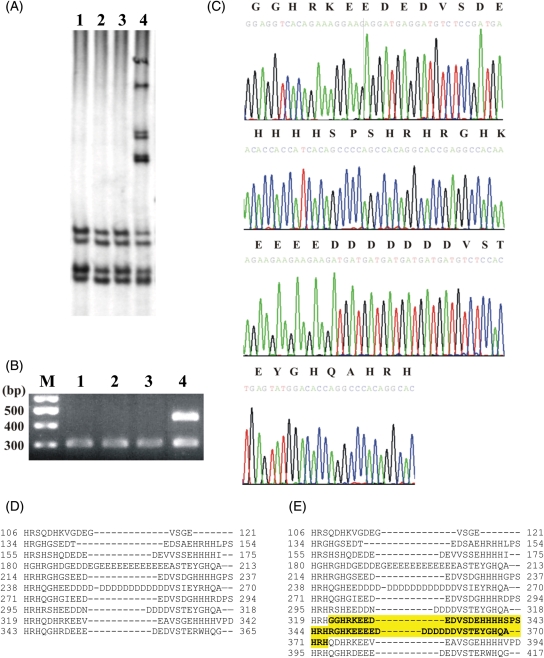
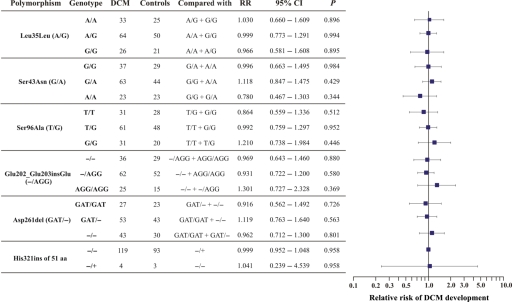
An insertion of a triplet 606_607insGAG was also detected in a minisatellite repeat of eight GAGs, encoding for a stretch of eight glutamic acid residues (Glu202_Glu203insGlu). In addition, a deletion of a triplet 781_783delGAT in a minisatellite repeat of 14 GATs, encoding for a stretch of 14 aspartic acid residues (Asp261del), was noted. Finally, an in-frame insertion of 153 bp was identified (Figure 2A and B) between nucleotides 963 and 964, which encodes 51 additional amino acid residues in HRC (His321ins51aa). The exact sequence of this insertion and its translation is depicted in Figure 2C. This polymorphism adds two histidine-rich acidic tandem repeats to the ten repeats already present in human HRC (Figure 2D and E). All the identified HRC polymorphisms were found to be consistent with the distribution predicted by the Hardy–Weinberg equilibrium in DCM patients and controls (see Supplementary material online, Table S2). The frequencies and the RR of DCM development, associated with the different HRC polymorphisms observed, did not show any statistical significance (Figure 3).
Figure 2.
Representative example of single strand conformational polymorphism analysis of HRC exon 1 with primer set 7, where the insertion of 51 amino acid residues was found. (A) Single strand conformational polymorphism analysis on 0.5× MDE polyacrylamide gels of three wild types (lanes 1–3), and one heterozygote for the insertion (lane 4). (B) Same set of DNA products (lanes 1–4) analysed in a 2% agarose gel, stained with ethidium bromide; M, 100 bp ladder; second to fourth lanes, wild-type samples; fifth lane, heterozygote for the insertion. (C) DNA sequences: top line, amino acid residues; bottom line, nucleotide sequence. (D) Alignment of the 10 histidine-rich and acidic tandem repeats of the wild-type histidine-rich calcium. The dashed lines indicate gaps for the best fit of the alignment. (E) Alignment of the histidine-rich and acidic tandem repeats of the mutant histidine-rich calcium with the 51 amino acids insertion (in bold, highlighted). The dashed lines indicate gaps for the best fit of the alignment. The in-frame insertion at position 321 adds two extra repeats to the protein.
Figure 3.
Group analysis of the relative risk of dilated cardiomyopathy development associated with each of the histidine-rich calcium genotypes detected in dilated cardiomyopathy patients and healthy controls. The number of dilated cardiomyopathy cases and controls with a specific genotype are presented. Each genotype was compared with the rest, as indicated. None of the differences between polymorphism frequencies was statistically significant.
Analysis of histidine-rich calcium variants in dilated cardiomyopathy patients
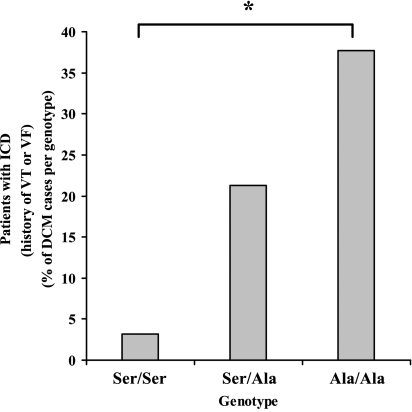
Comparison of the evaluated parameters at study entry, New York Heart Association (NYHA) classification, atrial fibrillation (AF), left bundle branch block (LBBB), left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension, left ventricular end-systolic dimension, ICD (history of sustained VT or VF prior to study entry), and unexplained syncope, revealed one statistically significant correlation against the identified HRC variants. Specifically, the G allele of the T to G polymorphism at position 286 (Ser96Ala) correlated well with the history of sustained VT or VF prior to study entry (patients treated ICD; χ2 = 11.710, df = 2, P = 0.003; see Supplementary material online, Table S3; Figure 4). The power of this comparison was 88.7% at the 0.050 significance level. The patients’ clinical characteristics in relation to Ser96Ala genotype are depicted in Table 2.
Figure 4.
The histidine-rich calcium polymorphism at position 96 is associated with the history of sustained ventricular tachycardia or ventricular fibrillation of dilated cardiomyopathy patients prior to study entry. Percentages of dilated cardiomyopathy patients with Ser/Ser, Ser/Ala, and Ala/Ala at codon 96 in need of implantable cardioverter-defibrillator implantation during study entry (*, χ2 = 11.710, df = 2, P = 0.003).
Table 2.
Clinical characteristics of patients upon study entry in relation to histidine-rich calcium genotype
| Ser/Ser (n = 31) | Ser/Ala (n = 61) | Ala/Ala (n = 31) | |
|---|---|---|---|
| Age at study entry, years | 42.5 ± 14.0 | 51.5 ± 11.8 | 48.9 ± 16.1 |
| Sex, male/female | 6 (19.4) | 13 (21.3) | 9 (29.0) |
| Clinical characteristics, n (%) | |||
| Familial DCM | 11 (35.5) | 24 (39.3) | 12 (38.7) |
| NYHA | |||
| Class I | 15 (48.4) | 27 (44.3) | 14 (45.2) |
| Class II | 13 (41.9) | 24 (39.3) | 14 (45.2) |
| Class III | 3 (9.7) | 10 (16.4) | 3 (9.7) |
| AF | 5 (16.1) | 9 (14.8) | 7 (22.6) |
| Left bundle branch block (LBBB) | 7 (22.6) | 23 (37.7) | 10 (32.3) |
| LVEF (%) | 29.6 ± 10.4 | 27.2 ± 9.6 | 30.2 ± 8.5 |
| LVEDD (mm) | 63.0 ± 15.3 | 65.6 ± 17.5 | 65.5 ± 14.8 |
| LVESD (mm) | 52.0 ± 13.9 | 52.1 ± 18.2 | 52.7 ± 13.6 |
| ICD (history of sustained VT or VF), n (%) | 1 (3.2) | 13 (21.3) | 12 (38.7) |
| History of unexplained syncope, n (%) | 4 (12.9) | 2 (3.3) | 2 (6.5) |
| Medication usage at enrolment, n (%) | |||
| ACE inhibitor | 27 (87.1) | 55 (90.2) | 25 (80.6) |
| Digitalis | 7 (22.6) | 19 (31.1) | 9 (29.0) |
| Spirinolactone | 15 (48.4) | 20 (32.8) | 12 (38.7) |
| Beta blockers | 23 (74.2) | 43 (70.5) | 27 (87.1) |
| Amiodarone | 15 (48.4) | 30 (49.2) | 12 (38.7) |
All values are mean ± standard deviation.
NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation; ACE, angiotensin-converting enzyme.
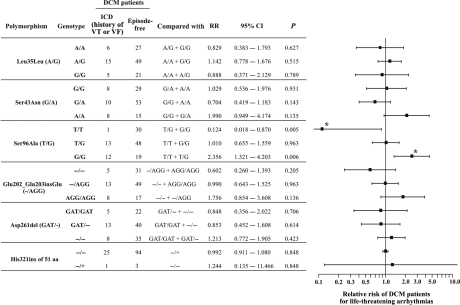
Importantly, the Ala96 (G) allele homozygous DCM patients were in need of ICD implantation (due to a history of sustained VT or VF prior to study entry) more frequently than the heterozygotes or the Ser96 (T) homozygotes. The RR for sustained VT or VF prior to study entry in DCM patients was evaluated in regard to each HRC polymorphism (Figure 5). Our results indicate that there was a protective trend for the Ser96Ser homozygous patients against these arrhythmias, with an RR of 0.124 (95% CI, 0.018–0.870; P = 0.005), compared with Ser96Ala and Ala96Ala patients. This value is eight times lower than the general risk for sustained VT or VF prior to study entry of a DCM patient. In contrast, the Ala/Ala homozygous patients exhibited an increased risk of sustained VT or VF (2.356; 95% CI, 1.321–4.203; P = 0.006) compared with Ser/Ser and Ser/Ala patients. The two DCM patient populations with and without ICD (history of sustained VT or VF) are in Hardy–Weinberg equilibrium (χ2 = 1.236, df = 2, P = 0.539 and χ2 = 0.0006, df = 2, P = 1.000, respectively). Therefore, the Ser96Ala mode of inheritance for the Ala allele was evaluated,14 with respect to the history of sustained VT or VF, as dominant (RR, 8.424; 95% CI, 1.190–59.619; P = 0.005), recessive (RR, 2.544; 95% CI, 1.322–4.896; P = 0.006), or multiplicative (RR for Ala/Ala, 6.086, and RR for Ala/Ser, 2.467 against Ser/Ser; 95% CI, 1.1936–14.0; χ2 = 11.80; df = 1; P < 0.001). Multiple logistic regression analysis revealed that the correlation between the Ala96Ala homozygosity and the need of ICD implantation (history of sustained VT or VF) in DCM patients is independent from other clinical characteristics (Table 3).
Figure 5.
Relative risk of dilated cardiomyopathy patients for implantable cardioverter-defibrillator implantation (history of sustained ventricular tachycardia or ventricular fibrillation prior to study entry) with respect to histidine-rich calcium polymorphisms. The numbers of dilated cardiomyopathy patients with and without an implantable cardioverter-defibrillator, respectively, with a specific genotype are presented. Each genotype was compared with the rest as indicated. Asterisks indicate statistically significant difference. Homozygosity for alanine of the histidine-rich calcium Ser96Ala polymorphism was associated with an increased risk for malignant ventricular arrhythmias in dilated cardiomyopathy patients prior to study entry, while homozygosity for serine was protective.
Table 3.
Multiple logistic regression analysis of dilated cardiomyopathy patients clinical characteristics and genotype to implantable cardioverter-defibrillator (history of ventricular tachycardia or ventricular fibrillation) prior to study entry (χ2 = 25.098; df = 10; P = 0.005)
| Variable | ICD (history of VT or VF) |
||
|---|---|---|---|
| OR | 95% CI | P-value | |
| Genetic variant | |||
| Ser96Ala (T→G) | |||
| T/G compared with T/T | 6.166 | 0.714–53.228 | 0.098 |
| G/G compared with T/T | 18.213 | 1.974–168.073 | 0.010 |
| Clinical characteristics | |||
| Age | 1.061 | 1.014–1.110 | 0.010 |
| Sex | 1.504 | 0.422–5.359 | 0.529 |
| Familial DCM | 0.394 | 0.133–1.166 | 0.093 |
| NYHA | |||
| Class I compare with III | 1.946 | 0.335–11.299 | 0.458 |
| Class II compare with III | 1.530 | 0.298–7.863 | 0.610 |
| AF | 0.630 | 0.164–2.421 | 0.501 |
| LBBB | 0.439 | 0.136–1.415 | 0.168 |
| LVEF | 0.963 | 0.890–1.041 | 0.338 |
ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; AF, atrial fibrillation; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; OR, odds ratio; CI, confidence interval; df, degrees of freedom; with bold statistical significant associations.
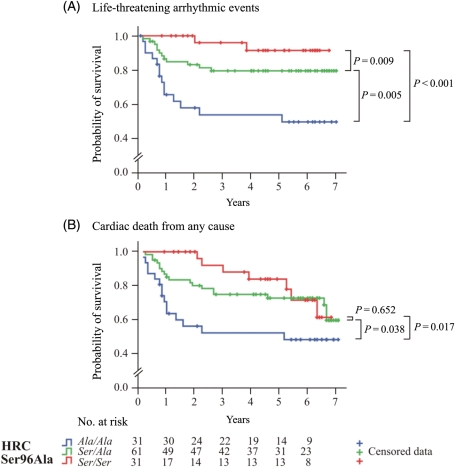
During follow-up, with the exception of a history of sustained VT or VF prior to study entry, none of the other clinical characteristics correlated with a life-threatening arrhythmic event, based on Kaplan–Meier event-free plots (see Supplementary material online, Figure S2). However, the Kaplan–Meier survival curves for life-threatening arrhythmic events (Figure 6A) and for cardiac death from any cause, including life-threatening arrhythmic events, pump failure, and transplantation (Figure 6B), showed that the Ala/Ala patients were more susceptible to malignant ventricular arrhythmias than the Ser/Ala or Ser/Ser patients at HRC codon 96 (Madel–Cox log-rank test, P = 0.005 and <0.001, for life-threatening arrhythmic events; and P = 0.038 and 0.017, for cardiac death from any cause, respectively). All the genetic variants identified in this study (Ser43Asn, Ser96Ala, Glu202_Glu203insGlu, and Asp261del) and patients’ characteristics were included in the univariable Cox proportional hazards models, with the exception of Leu35Leu, which results in a synonymous codon, and His321ins51aa, that has a low frequency (Table 4). The unadjusted hazard ratio (HR) for life-threatening ventricular arrhythmias was 9.620 (95% CI, 2.183–42.394; P = 0.003), and for cardiac death from any cause was 2.719 (95% CI, 1.103–6.704; P = 0.030; Table 4) for the Ala/Ala, compared with Ser/Ser genotypes. Furthermore, the Ala/Ala genotype was identified as an independent factor for prediction of life-threatening ventricular arrhythmias, with an HR of 4.191 (95% CI, 0.838–20.967; P = 0.018; Table 5), compared with Ser/Ser, using the multivariable Cox proportional hazards regression model and controlling for history of sustained VT or VF prior to study entry and known predictors of the outcome, namely patient’s age, sex, NYHA classification, LBBB, and LVEF, during the study entry.
Figure 6.
Kaplan–Meier plots for the probability of survival from: (A) life-threatening ventricular arrhythmic events including sudden cardiac death and episodes of unstable VT (>180 b.p.m.) or ventricular fibrillation, which were recorded by an implantable cardioverter-defibrillator device and (B) cardiac death from any cause, including pump failure, transplantation, sudden cardiac death, and episodes of unstable VT (>180 b.p.m.) or ventricular fibrillation, which were recorded by an implantable cardioverter-defibrillator device. Each event is depicted as a step down. Each censored case [due to (A) death from other causes except sudden cardiac death, heart transplantation, and study termination and (B) death from non-cardiac aetiology and study termination] is marked with a cross. The table at the bottom of the plots indicates the number of dilated cardiomyopathy patients in risk for each year of the follow-up study. The Ala/Ala homozygotes for the Ser96Ala polymorphism were statistically more susceptible to ventricular arrhythmic events, compared with Ser/Ala heterozygotes and Ser/Ser homozygotes.
Table 4.
Univariable Cox proportional hazards model of time to life-threatening ventricular arrhythmic events or cardiac death from any causea
| Variable | Life-threatening arrhythmic events |
Cardiac death from any cause |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Genetic variant | ||||||
| Ser96Ala (T→G) | ||||||
| T/G compared with T/T | 3.281 | 0.734–14.660 | 0.120 | 1.319 | 0.551–3.155 | 0.534 |
| G/G compared with T/T | 9.620 | 2.183–42.394 | 0.003 | 2.719 | 1.103–6.704 | 0.030 |
| Ser43Asn (G→A) | ||||||
| G/A compared with G/G | 0.412 | 0.159–1.069 | 0.068 | 0.382 | 0.167–0.871 | 0.052 |
| A/A compared with G/G | 1.230 | 0.474–3.191 | 0.671 | 0.966 | 0.394–2.370 | 0.940 |
| Glu202_Glu203insGlu (−→GAG) | ||||||
| −/GAG compared with −/− | 7.192 | 1.553–33.317 | 0.062 | 3.637 | 1.119–11.824 | 0.078 |
| GAG/GAG compared with −/− | 4.098 | 0.931–18.035 | 0.076 | 2.589 | 0.875–7.656 | 0.086 |
| Asp261del (GAT→−) | ||||||
| GAT/− compared with GAT/GAT | 1.183 | 0.346–4.043 | 0.788 | 0.606 | 0.234–1.571 | 0.302 |
| −/− compared with GAT/GAT | 1.949 | 0.642–5.923 | 0.239 | 0.888 | 0.384–2.054 | 0.782 |
| Clinical characteristics | ||||||
| Age | 1.058 | 1.023–1.094 | 0.001 | 1.026 | 1.001–1.051 | 0.041 |
| Sex | 1.104 | 0.469–2.597 | 0.821 | 1.209 | 0.606–2.414 | 0.590 |
| Familial DCM | 0.683 | 0.301–1.552 | 0.363 | 0.732 | 0.373–1.436 | 0.364 |
| NYHA | ||||||
| Class I compare with III | 0.388 | 0.130–1.163 | 0.091 | 0.192 | 0.084–0.442 | <0.001 |
| Class II compare with III | 0.730 | 0.263–2.031 | 0.547 | 0.389 | 0.183–0.829 | 0.014 |
| AF | 1.030 | 0.357–2.970 | 0.957 | 1.311 | 0.580–2.961 | 0.515 |
| LBBB | 1.298 | 0.608–2.771 | 0.500 | 1.263 | 0.674–2.367 | 0.465 |
| LVEF | 0.973 | 0.929–1.018 | 0.230 | 0.962 | 0.925–1.000 | 0.051 |
| ICD (history of sustained VT or VF) | 12.266 | 5.496–27.373 | <0.001 | 5.558 | 2.986–10.346 | <0.001 |
aCardiac death from any cause, including death due to pump failure, transplantation, sudden cardiac death, and episodes of unstable VT (>180 b.p.m.) or VF, which were recorded by an ICD device.
AF, atrial fibrillation; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter-defibrillator; HR, hazard ratio; CI, confidence interval; df, degrees of freedom; with bold statistical significant associations.
Table 5.
Multivariable Cox proportional hazards model of time to life-threatening ventricular arrhythmic events or cardiac death from any causea
| Overall model fit | Life-threatening arrhythmic events |
Cardiac death from any cause |
||||
|---|---|---|---|---|---|---|
| (χ2 = 69.621; df = 9; P < 0.001) |
(χ2 = 61.420; df = 9; P < 0.001) |
|||||
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Genetic variant | ||||||
| Ser96Ala (T→G) | ||||||
| T/G compared with T/T | 1.293 | 0.264–6.344 | 0.751 | 0.856 | 0.314–2.334 | 0.761 |
| G/G compared with T/T | 4.191 | 0.838–20.967 | 0.018 | 2.041 | 0.688–6.054 | 0.199 |
| Clinical characteristics | ||||||
| Age | 1.027 | 0.989–1.067 | 0.170 | 1.004 | 0.972–1.036 | 0.819 |
| Sex | 0.854 | 0.296–2.466 | 0.770 | 0.862 | 0.366–2.026 | 0.733 |
| NYHA | ||||||
| Class I compare with III | 0.743 | 0.208–2.659 | 0.648 | 0.213 | 0.077–0.590 | 0.003 |
| Class II compare with III | 0.840 | 0.284–2.483 | 0.7536 | 0.354 | 0.159–0.789 | 0.011 |
| LBBB | 0.762 | 0.323–1.797 | 0.535 | 0.734 | 0.353–1.527 | 0.734 |
| LVEF | 0.955 | 0.894–1.020 | 0.174 | 0.965 | 0.914–1.018 | 0.194 |
| ICD (history of sustained VT or VF) | 7.770 | 3.201–18.856 | <0.001 | 5.364 | 2.533–11.358 | <0.001 |
aCardiac death from any cause, includes death due to pump failure, transplantation, sudden cardiac death, and episodes of unstable VT (>180 b.p.m.) or VF, which were recorded by an ICD device.
AF, atrial fibrillation; LBBB, left bundle branch block; ICD, implantable cardioverter-defibrillator; HR, hazard ratio; CI, confidence interval; df, degrees of freedom; with bold statistical significant associations.
Discussion
We identified herein the first genetic variant of an SR Ca-cycling gene associated with malignant arrhythmias in DCM. The variant encodes for the Ser96Ala polymorphism in the human HRC and appears to be a powerful predictor for the occurrence of ventricular arrhythmias and sudden death in DCM patients. Although new therapeutic advances have improved survival, the occurrence of sudden arrhythmogenesis continues to constitute one of the leading causes of death in DCM. Various approaches for predicting the risk of SCD have been suggested, but they all yielded limited insights into the identification of positive predictor factors in fatal arrhythmias.15 To date, left ventricular dysfunction (LVEF, <30%) serves as the only independent predictor for major arrhythmic events, as revealed by multivariable analysis in one of the best controlled studies for arrhythmia risk stratification and primary prevention of sudden death in DCM.16 However, depressed left ventricular dysfunction does not appear to be an effective predictor, as it has failed to identify or has presented low accuracy in the identification of patients, that would benefit from implanted cardioverter defibrillation (ICD) therapy.17,18 Thus, efforts to identify new predictive factors for mortality and especially sudden death in DCM are very important, since these may hold promise for effective therapy in individual patients.
Recently, abnormalities in intracellular calcium handling have been implicated in ventricular arrhythmogenesis of the failing myocardium.6,7 The impaired calcium handling has been found to predispose to delayed afterdepolarizations and triggered activity of arrhythmogenesis, which are considered as main mechanisms, underlying ventricular arrhythmias in DCM patients. Genetic studies have also begun to reveal the role of defective myocardial calcium handling in the pathogenesis of various inherited arrhythmic syndromes. Support for this notion has been provided by the identification of human mutations in the ryanodine receptor 219–21 and calsequestrin (CSQ)22 genes, which implicated them as the SR Ca-handling candidates underlying catecholaminergic/polymorphic VT and arrhythmogenetic right ventricular cardiomyopathy.
Another SR Ca-handling protein, which has been recently shown to play a role in myocardial calcium handling, is HRC.8 Increases in HRC levels are associated with impaired SR Ca uptake and depressed heart contractility, leading to cardiac remodelling upon ageing.9–11 To assess whether genetic variants in HRC may be associated with human DCM, we sequenced the HRC coding region in a well-characterized DCM group. Five out of six of the identified HRC variants, Leu35Leu, Ser43Asn, Ser96Ala, Glu202_Glu203insGlu, and Asp261del, were described previously,23,24 while the 51 amino acid insertion at codon 321 is reported for the first time. Each genetic variant exhibited similar frequency in controls and DCM patients. However, the Ser96Ala HRC polymorphism appeared to significantly correlate with malignant ventricular arrhythmias or SCDs only in DCM patients. Specifically, the Ala/Ala variant was associated with a four-fold increased risk compared with Ser/Ser variant. Although the functional significance of the Ser96Ala variant is not known, bioinformatical analysis of the HRC amino acid sequence, using the NetPhos 2.025 and NetPhosK 1.025,26 servers, revealed that Ser96 may be phosphorylated by casein kinase II (prediction score, 0.972). Indeed, previous studies showed that HRC could be phosphorylated by casein kinase II in cardiac SR,27,28 similar to CSQ.29 Interestingly, dephosphorylation of CSQ enhances the RyR channel opening probability and thus, SR Ca release.30 Considering the similarities between HRC and CSQ,8 it is possible that the lack of the HRC phosphorylation site (Ser96Ala) may also impair the regulatory effects of HRC on RyR or SERCA2 function10,31 and initiate delayed afterdepolarization and ventricular arrhythmogenesis. Furthermore, since the interaction of HRC to triadin has been shown to be modulated by Ca,8,31 reduced SR Ca levels in DCM would be expected to diminish this complex. Thus, triadin may be free to interact and modulate RyR activity. The increased triadin/RyR interaction, which may be further enhanced by the Ser96Ala HRC variant, coupled with destabilization of RyR, may exacerbate spontaneous Ca release and delayed afterdepolarization initiation leading to lethal arrhythmias in DCM. The delayed afterdepolarization as a possible mechanism for ventricular arrhythmia is also supported from stored electrogram data, which revealed that nine out of 12 (85%) Ala/Ala homozygous patients with ventricular arrhythmias exhibited the first beat of tachycardia late in the cardiac cycle.32
Nevertheless, the current study is subject to several potential limitations, such as (i) the small sample size, which may limit identification of rare mutations; (ii) the calculation of correlations of genotypes with clinical parameters, which may not reflect the sole risk estimate for disease development and progression; other parameters, such as patients’ environment, diet, life-style, and geography may also be involved; (iii) the sensitivity of the single-strand conformational polymorphism–PCR (SSCP–PCR) method for mutation screening may limit the identification of certain human mutations;33 and (iv) the inclusion of DCM patients with a history of life-threatening ventricular arrhythmias in the follow up study.
In conclusion, we report here six genetic variants of the human HRC gene. Importantly, the homozygosity for Ala at amino acid position 96 was associated with sustained VT in DCM patients. This is the first report of a human HRC polymorphism, which correlates with life-threatening ventricular arrhythmic episodes in non-ischaemic DCM patients. Future studies may elucidate the functional significance of the Ser96 Ala genetic variant in DCM.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: none declared.
Funding
This study was supported by research funds from the Biomedical Research Foundation, Academy of Athens, the John F. Kostopoulos Foundation, the Hellenic Cardiological Society, NIH HL26057, HL64018 and HL77101, and the Leducq Foundation Trans-Atlantic alliance. E.V. and D.S. are supported by the European Union 6th Framework Program for Research and Technological Development, ‘Life sciences, genomics and biotechnology for health’, VALAPODYN, contract #LSHG-CT-2006-037277. Funding to pay the Open Access publication charges for this article was provided by Evangelia G. Kranias, University of Cincinnati College of Medicine.
Supplementary Material
References
- 1.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 2.Grimm W, Maisch B. Sudden cardiac death in dilated cardiomyopathy—therapeutic options. Herz. 2002;27:750–759. doi: 10.1007/s00059-002-2425-0. [DOI] [PubMed] [Google Scholar]
- 3.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 4.Haghighi K, Schmidt AG, Hoit BD, Brittsan AG, Yatani A, Lester JW, Zhai J, Kimura Y, Dorn GW, II, MacLennan DH, Kranias EG. Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem. 2001;276:24145–24152. doi: 10.1074/jbc.M102403200. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AG, Zhai J, Carr AN, Gerst MJ, Lorenz JN, Pollesello P, Annila A, Hoit BD, Kranias EG. Structural and functional implications of the phospholamban hinge domain: impaired SR Ca2+ uptake as a primary cause of heart failure. Cardiovasc Res. 2002;56:248–259. doi: 10.1016/s0008-6363(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 6.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Lee HG, Kang H, Kim DH, Park WJ. Interaction of HRC (histidine-rich Ca(2+)-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J Biol Chem. 2001;276:39533–39538. doi: 10.1074/jbc.M010664200. [DOI] [PubMed] [Google Scholar]
- 9.Kim E, Shin DW, Hong CS, Jeong D, Kim do H, Park WJ. Increased Ca2+ storage capacity in the sarcoplasmic reticulum by overexpression of HRC (histidine-rich Ca2+ binding protein) Biochem Biophys Res Commun. 2003;300:192–196. doi: 10.1016/s0006-291x(02)02829-2. [DOI] [PubMed] [Google Scholar]
- 10.Fan GC, Gregory KN, Zhao W, Park WJ, Kranias EG. Regulation of myocardial function by histidine-rich, calcium-binding protein. Am J Physiol Heart Circ Physiol. 2004;287:H1705–H1711. doi: 10.1152/ajpheart.01211.2003. [DOI] [PubMed] [Google Scholar]
- 11.Gregory KN, Ginsburg KS, Bodi I, Hahn H, Marreez YM, Song Q, Padmanabhan PA, Mitton BA, Waggoner JR, Del Monte F, Park WJ, Ii GW, Bers DM, Kranias EG. Histidine-rich Ca binding protein: a regulator of sarcoplasmic reticulum calcium sequestration and cardiac function. J Mol Cell Cardiol. 2006;40:653–665. doi: 10.1016/j.yjmcc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 13.Guo SW, Thompson EA. Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- 14.Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002;3:146–153. doi: 10.1093/bib/3.2.146. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib SM, Sanders GD, Bigger JT, Buxton AE, Califf RM, Carlson M, Curtis A, Curtis J, Fain E, Gersh BJ, Gold MR, Haghighi-Mood A, Hammill SC, Healey J, Hlatky M, Hohnloser S, Kim RJ, Lee K, Mark D, Mianulli M, Mitchell B, Prystowsky EN, Smith J, Steinhaus D, Zareba W. Preventing tomorrow’s sudden cardiac death today: part I: current data on risk stratification for sudden cardiac death. Am Heart J. 2007;153:941–950. doi: 10.1016/j.ahj.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–2891. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 17.Bansch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, Block M, Gietzen F, Berger J, Kuck KH. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–1458. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 18.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 19.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 21.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 22.Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med. 2003;13:148–151. doi: 10.1016/s1050-1738(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 23.Haga H, Yamada R, Ohnishi Y, Nakamura Y, Tanaka T. Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet. 2002;47:605–610. doi: 10.1007/s100380200092. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann SL, Topham M, Hsieh CL, Francke U. cDNA and genomic cloning of HRC, a human sarcoplasmic reticulum protein, and localization of the gene to human chromosome 19 and mouse chromosome 7. Genomics. 1991;9:656–669. doi: 10.1016/0888-7543(91)90359-m. [DOI] [PubMed] [Google Scholar]
- 25.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 26.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- 27.Shoshan-Barmatz V, Orr I, Weil S, Meyer H, Varsanyi M, Heilmeyer LM. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim Biophys Acta. 1996;1283:89–100. doi: 10.1016/0005-2736(96)00079-x. [DOI] [PubMed] [Google Scholar]
- 28.Hadad N, Meyer HE, Varsanyi M, Fleischer S, Shoshan-Barmatz V. Cardiac sarcalumenin: phosphorylation, comparison with the skeletal muscle sarcalumenin and modulation of ryanodine receptor. J Membr Biol. 1999;170:39–49. doi: 10.1007/s002329900536. [DOI] [PubMed] [Google Scholar]
- 29.Cala SE, Jones LR. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J Biol Chem. 1991;266:391–398. [PubMed] [Google Scholar]
- 30.Szegedi C, Sarkozi S, Herzog A, Jona I, Varsanyi M. Calsequestrin: more than ‘only’ a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem J. 1999;337:19–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, Kontrogianni-Konstantopoulos A, Sanoudou D, Kranias EG. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol. 2007;293:H1581–H1589. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- 32.Rosen MR, Fisch C, Hoffman BF, Danilo P, Jr, Lovelace DE, Knoebel SB. Can accelerated atrioventricular junctional escape rhythms be explained by delayed afterdepolarizations? Am J Cardiol. 1980;45:1272–1284. doi: 10.1016/0002-9149(80)90489-0. [DOI] [PubMed] [Google Scholar]
- 33.Nataraj AJ, Olivos-Glander I, Kusukawa N, Highsmith WE., Jr Single-strand conformation polymorphism and heteroduplex analysis for gel-based mutation detection. Electrophoresis. 1999;20:1177–1185. doi: 10.1002/(SICI)1522-2683(19990101)20:6<1177::AID-ELPS1177>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.