Summary
Monkeypox is a disease that is endemic in Central and Western Africa. However, in 2003, there was an outbreak in the US, representing the first documented monkeypox cases in the Western hemisphere. Although monkeypox virus is less fatal and not as transmissible as variola virus, the causative agent of smallpox, there is concern that monkeypox virus could become a more efficient human pathogen. The reason for this may lie in the virus' genetic makeup, ecological changes, changes in host behavior, and the fact that with the eradication of variola virus, routine smallpox vaccination is no longer carried out. In this review, we focus on the viral proteins that are predicted to modulate the host immune response and compare the genome of monkeypox virus with the genomes of variola virus and the vaccinia virus, the orthopoxvirus that represented the smallpox vaccine. There are differences found in several of these immune-modulating genes including genes that express proteins that affect cytokines such as interleukin-1, tumor necrosis factor, and interferon. There are also differences in genes that code for virulence factors and host range proteins. Genetic differences likely also explain the differences in virulence between two strains of monkeypox virus found in two different regions of Africa. In the current setting of limited smallpox vaccination and little orthopoxvirus immunity in parts of the world, monkeypox could become a more efficient human pathogen under the right circumstances.
Keywords: monkeypox virus, immune evasion, immune modulatory
Introduction
Monkeypox virus is a member of the family Poxviridae, genus Orthopoxvirus. Additional members of this genus include variola virus, vaccinia virus, ectromelia (mousepox) virus, and cowpox virus. Variola virus caused smallpox and was ultimately eradicated as a disease through the combination of a successful containment strategy, the fact that there was no animal or environmental viral reservoirs, and the use of a very effective vaccine (1). The vaccine that helped eradicate smallpox was the closely related vaccinia virus. Vaccinia virus is commonly studied in laboratories as a model poxvirus. With the last naturally occurring case of smallpox in 1977 and the declaration of global eradication of smallpox by the World Health Organization (WHO) in 1980, routine smallpox vaccination around the world ended in the mid-1980s. Since routine smallpox vaccination ended decades ago, there is growing concern that monkeypox may become the next emerging poxvirus to plague humankind (2). The prospect of the global spread of monkeypox was recently seen with the first cases of monkeypox occurring in the Western Hemisphere due to the accidental importation of infected animals (3). Because the clinical features of monkeypox closely resemble smallpox, it makes it difficult to clinically distinguish one infection from the other unless specific diagnostic tests are performed. There are also concerns that in the current setting of limited smallpox vaccination, monkeypox could become a more efficient human pathogen. This review focuses on viral immune modulatory proteins that distinguish monkeypox virus from variola and vaccinia viruses. This review does not focus on the immune responses generated by live vaccinia virus vaccination (which provides cross protection to variola and monkeypox viruses), since this topic was recently reviewed in this journal (4).
Clinical features of monkeypox infection
The clinical features of monkeypox are very similar to those of smallpox. The first reported case of human monkeypox occurred in 1970 in a child from the Democratic Republic of Congo (DRC), formerly known as Zaire (5, 6). The main animal reservoir for monkeypox has not been conclusively determined, but rodents such as Gambian giant rats and rope squirrels are suspected to be a reservoir (7-10). The virus is thought to be transmitted to humans through close contact with infected animals. For example, infections are believed to occur through cuts in the skin while handling or eating infected animals (8). Some cases of human monkeypox that occurred in the US revealed that respiratory transmission from an infected animal to a human was possible (11). Similarly, some human-to-human transmissions are thought to occur through respiratory droplets (8). After exposure and infection, there is about a 10 to 14 day incubation period, which is then followed by a prodrome period of about two days. During the prodrome phase (a period prior to the development of a rash), an infected person can experience fever, chills, malaise, headache, backache, sore throat, shortness of breath, and swollen lymph nodes (12, 13). The finding of enlarged lymph nodes in the submandibular, cervical, or inguinal regions is interesting, because it potentially represents a distinguishing characteristic of human monkeypox from human smallpox infection (1). It is seen in about 90% of all human monkeypox infections (13).
After the prodrome period, a progressive maculopapular rash develops with lesion sizes that range from 0.2-1 cm (12, 13). This is also the time when the infected person is considered to be the most contagious (13). Lesions spread over the body in a centrifugal pattern starting on the face and trunk and spreading to the extremities with involvement of the palms and soles. Lesions progress through several stages during a two to four week period going from macules to papules, vesicles, pustules (Fig. 1), and finally a crusting phase that includes scabbing and desquamation (1). In some cases, lesions have resulted in dyspigmented scars. Extracutaneous manifestations such as secondary skin and/or soft-tissue infection, pneumonitis, ocular complications, and encephalitis have been seen in some cases (12).
Fig. 1. Monkeypox rash.
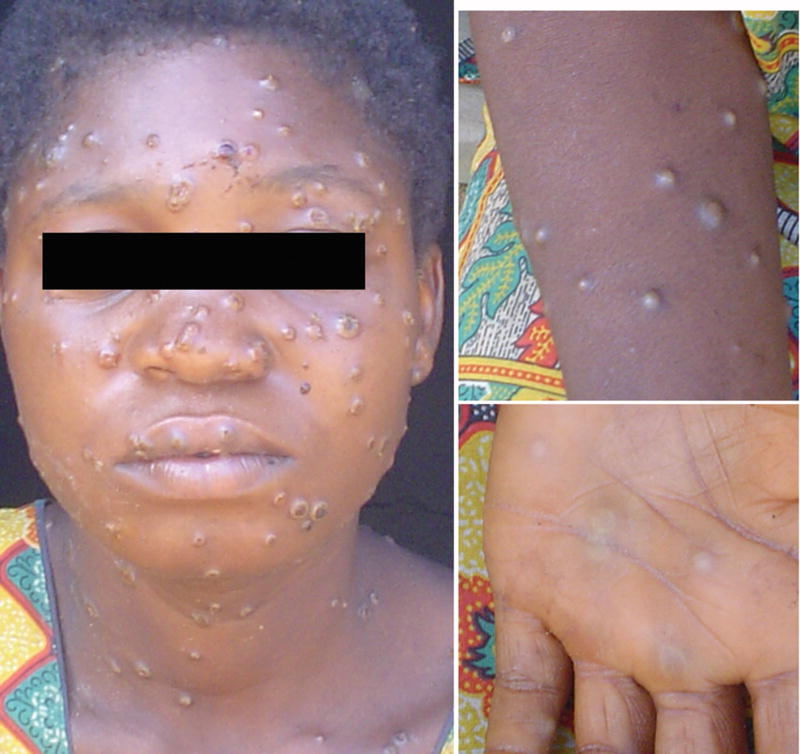
African woman with classical rash of monkeypox virus infection with deep seeded lesions on face, arms, and palms. Photos courtesy of the UCLA Monkeypox Project, Democratic Republic of Congo.
Laboratory diagnosis of monkeypox
Because the clinical features of smallpox and monkeypox are similar, laboratory diagnosis is of great importance, and newer methods that exploit differences in the host immune responses to orthopoxviruses are under development. There are several laboratory tests that can be performed at reference labs to differentiate between these viruses. Although the genomes of variola and monkeypox viruses are very similar, there are differences that allow the viruses to be distinguished by molecular techniques. Recently, real-time polymerase chain reaction (PCR) assays have been used to identify monkeypox virus that target two genes (14). In one assay that targets the DNA polymerase gene that is an ortholog to the vaccinia virus strain Copenhagen (COP) E9L gene, 13 different Eurasian orthopoxviruses can be detected. Another assay targets the vaccinia virus ortholog envelope protein COP-B5R gene. This assay takes advantage of single nucleotide polymorphisms within a small sequence of the monkeypox virus ortholog gene to COP-B5R that makes this assay sensitive and specific to only monkeypox virus (14). Such DNA testing requires collection of a specimen while the virus is still present and cannot be used to diagnose monkeypox after the infection has cleared; thus, other techniques are currently being developed that rely on the host immune response to the virus. The development of a standard antibody test for monkeypox is complicated by cross-reactive immune responses induced by prior smallpox vaccination. An immunoglobulin M (IgM) antibody assay has been used since anti-vaccinia IgM antibodies from distant past smallpox vaccination should not be present (15). Another approach under investigation that relies on subtle antigenic differences between the viruses is a whole-virus enzyme-linked immunosorbent assay (ELISA), where antibody titers to monkeypox and vaccinia viruses are measured and their ratio is determined (11). An example of such an epitope difference that might be used to diagnose monkeypox is a protein encoded by the monkeypox ortholog of the cowpox virus strain Brighton red (BR) 219 gene. This gene encodes a putative membrane-associated glycoprotein that is not present in vaccinia virus (16). An ELISA using a peptide from this protein was used to distinguish between prior smallpox vaccination and recent monkeypox infection (11). These prior assays rely on antibody responses, but another potential target of diagnostics may focus on cellular immune responses to specific pathogens. Such a technique under development is the measurement of a number of orthopoxvirus-specific T cells (11).
Two strains of monkeypox: Central African and West African
Before the 2003 outbreak of monkeypox in the United States (3), all previous cases of monkeypox had been only in Africa and mainly in Central and West African countries. The vast majority of cases have been in the Central African country DRC, where the virus is endemic (12). Depending on which part of Africa patients were from, epidemiologic studies revealed differences in the virulence of monkeypox infection. A milder form of disease with lower fatality and less human-to-human transmission was reported in strains from West Africa compared to disease occurring in Central Africa (5, 17). The case-fatality rate in Central Africa in the 1980s was about 10% in non-vaccinated individuals, while there were no fatalities in cases occurring in West Africa (18). These findings suggest that there are differences in the virulence of the West African and Central African strains of monkeypox.
Experimental animal studies provided further evidence that the epidemiologic differences in mortality in the two regions of Africa are related to differences in the virus strains isolated from Central and West Africa (19). In a non-human primate study, cynomolgus monkeys were challenged with either a high dose or a low dose of either a Central African strain or a West African strain of monkeypox. All the monkeys died when challenged with the high dose of the Central African strain and displayed high morbidity with both doses. The monkeys challenged with the West African strain all survived and had little morbidity after challenge with both doses (19). The monkeypox outbreak that occurred in the US in 2003 provides additional support that the West African strain of monkeypox virus is less virulent (3). The outbreak in the U.S. resulted from the accidental importation of animals that were infected with the West African strain. The cases in the US resulted in no fatalities, and in general, they had less severe symptoms compared to the monkeypox cases being seen in the recent outbreaks in Central Africa.
Because there is a difference in virulence between the Central and West African strains of monkeypox, the genomes were compared (19, 20). In a comparison of a Central African strain (ZAI-96) with three West African strains (SL-V70, COP-58, and WRAIR-61) performed by Chen et al. revealed a 0.55-0.56% nucleotide difference between the Central African strains and the West African strains (19). Such genetic analysis revealed that the two strains of virus could be separated on a phylogenetic tree (Fig. 2). Further analysis by Chen et al. (19) of the Central and West African strains revealed that the Central African strain is predicted to have 173 functional unique genes, while the West African strain is predicted to have 171 unique genes. They share 170 orthologs and at the protein level are about 99.4% identical. There were no significant differences in the transcription regulatory sequences between the two genomes. Because there is a difference in virulence between the two strains, the authors examined the 56 virulence genes, 53 of which can be found in both strains (19). Within these 53 genes, there are 276 substitutions, which account for 61 conservative, 93 non-conservative, 121 silent amino acid changes. Sixteen proteins have changes in their predicted lengths mainly as extensions of the N- and C-termini. The most significant differences between the two strains are in the orthologs of BR-203, BR-209, and COP-C3L (19). Likos et al. (20) reported a similar set of genes as candidates that explain the difference in virulence between the two strains. We also used the Poxvirus Bioinformatics Resources Center Database (www.poxvirus.org) to compare the genomes of a Central African strain of monkeypox virus (strain Zaire-1979) and a West African strain of monkeypox (strain USA-2003-039) and tabulate major differences (Table 1). This website allows the user to compare two genomes in two ways: (i) the user is able to search for genes that are present in both genomes, and (ii) the user is able to search for genes that are present in one genome and absent in the other genome. Table 1 summarizes this type of analysis.
Fig. 2. Phylogenetic tree of different orthopoxvirus species, strain, or isolate based on nucleic acid sequence alignment.
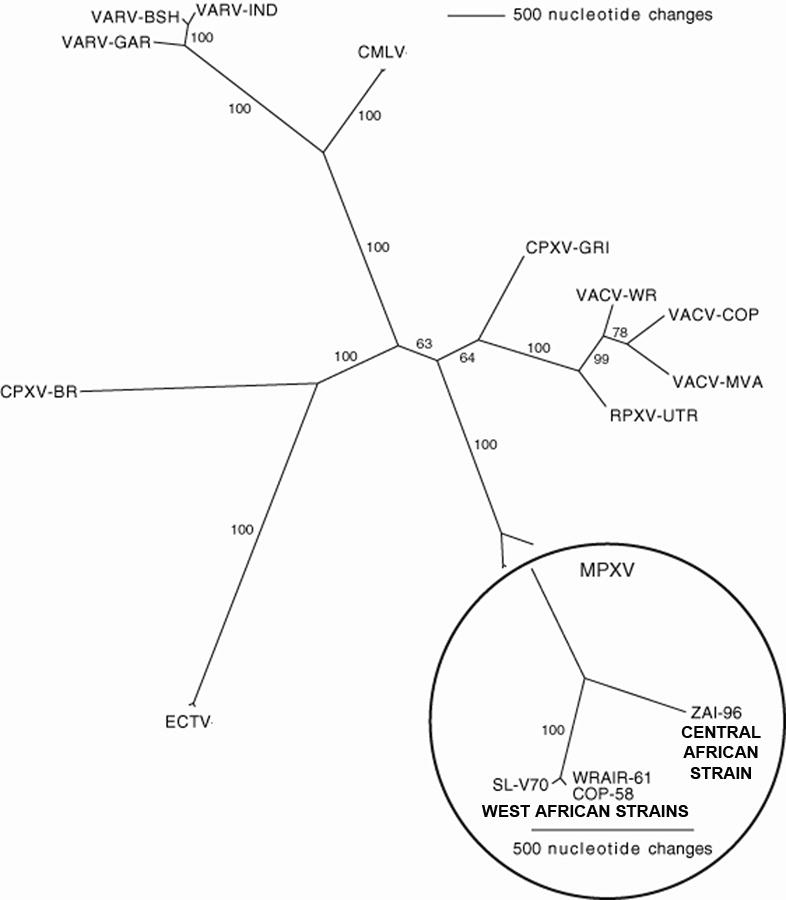
The circled portion of the tree represents the comparison of the genomes between three isolates of the West African strain and one isolate of the Central African strain of monkeypox virus (MPXV). The core regions of the genomes were used in each comparison with differences in nucleic acid sequence shown by varying branch length. CMLV: camelpox virus; CPXV: cowpox virus; ECTV: ectromelia virus; MPXV: monkeypox virus; RPXV: rabbitpox virus; VACV: vaccinia virus; VARV: variola virus. Adapted from (19) and reprinted with permission from Elsevier.
Table 1.
Comparison of the genomes of a central African strain of monkeypox virus (Zaire-1979) and a west African strain of monkeypox (USA-2003)
| Gene Ortholog Name | Predicted Protein Function | Present in MPV-ZAR_1979_005 | Present in MPV-USA_2003_039 |
|---|---|---|---|
| COP-A49R
162 aa |
Unknown | - | Fragment
69 aa: Central |
| COP-A52R
190 aa |
Bifunctional Toll-IL-1-receptor protein | - | Fragment
63 aa: N-term |
| COP-A55R
564 aa |
Kelch protein | Fragment
82 aa: C-term |
Fragment
112 aa: Central |
| COP-B17L
340 aa |
Unknown | Fragment
55 aa: Central 78 aa: C-term |
Fragment
55 aa: Central |
| COP-C2L
512 aa |
Kelch protein | Fragment
107 aa: N-term 176 aa: Central 105 aa: C-term |
Fragment
112 aa: N-term |
| COP-C3L
263 aa |
Inhibitor of complement enzymes | Truncated
216 aa: N-term/Central |
- |
| COP-E5R
331 aa |
Unknown | Fragment
67 aa: N-term 64 aa: C-term |
Fragment
62 aa: N-term 69 aa: N-term |
| BR-19
796 aa |
Unknown | Fragment
55 aa: N-term 56 aa: N-term 73 aa: C-term |
- |
| BR-20
170 aa |
Unknown | Fragment
64 aa: C-term |
- |
| BR-158
1284 aa |
CPV A-type inclusion protein | Fragment
696 aa: N-term/Central 54 aa: C-term 67 aa: C-term 75 aa: C-term |
Fragment
696 aa: N-term/Central 110 aa: Central 82 aa: C-term 100 aa: C-term |
| BR-203
225 aa |
Virulence protein | +
221 aa |
Fragment
51 aa: N-term |
| BR-209
326 aa |
IL-1β binding protein | Fragment
210 aa: N-term/Central 126 aa: C-term |
Fragment
163 aa: Central 132 aa: C-term |
Abbreviations: aa: amino acids; BR: cowpox virus strain Brighton Red; C-term: C-terminal fragment; COP: vaccinia virus, strain Copenhagen; CPV: cowpox virus; IL: Interleukin; MPV-Zar_1979_005: central African strain of monkeypox virus; MPV-USA_2003_039: west African strain of monkeypox virus; N-term: N-terminal fragment.
Gene nomenclature used in this review
The naming of genes in the orthopoxviruses has been evolving ever since whole viral genomes began to be routinely sequenced. To standardize the naming of genes discussed in this review, we identify gene orthologs by the nomenclature used when the first whole orthopoxvirus genome that was sequenced (21, 22). The first genome sequenced was vaccinia virus, strain COP. The naming of the genes was based on the position they held in HindIII restriction fragments of genomic DNA. The largest HindIII fragment carries the letter A, the next largest fragment is B, then C, and so on. Genes were then numbered in the order they appeared and then carried the letters R (right) and L (left) to indicate the direction of the coding frame. Since some genes in monkeypox and variola viruses are not present in this reference strain of vaccinia virus, for these genes we will use the name of the gene present in the cowpox virus, strain BR. The Poxvirus Bioinformatics Resources Center Database (www.poxvirus.org) is a useful resource to search for genes and actual gene names assigned to the genes of specific orthopoxviruses. In the tables, we list the COP genes and then BR genes.
BR-203: virulence protein
The protein encoded by BR-203 is believed to have a role in avoiding apoptosis of infected lymphocytes (23, 24). The Central African strain of monkeypox virus ortholog to BR-203 gene encodes a full-length protein of 221 amino acids (aa). The West African strain is predicted to encode only an N-terminal fragment of about 51 aa. BR-203 is an ortholog to the myxoma virus M-T4 gene (23). The myxoma virus is a member of the poxvirus family that causes the disease known as myxomatosis in the European rabbit (23). Once a rabbit is infected with myxoma virus, there is viral replication at the site of infection, then viral infection of leukocytes, which results in spread throughout the host. The M-T4 protein is retained in the endoplasmic reticulum (ER) and is not secreted during viral infection in part due to a C-terminal–RDEL sequence, which helps anchor it in the ER (23). However, it was found that when the RDEL sequence was deleted, M-T4 protein was still retained in the ER, suggesting an additional mechanism for it staying in the ER (24). When this gene is deleted from myxoma virus, the virus is attenuated and infected lymphocytes undergo apoptosis, thus avoiding the primary mechanism of viral spread within the host (23). It was also found that the inflammatory response was heightened when M-T4 is deleted, suggesting a role in influencing the immune response to viral infection (24). The West African strain contains a two-base deletion, which causes a frameshift that results in expression of a short N-terminal fragment. Of note, this gene is not present in variola virus, strain Bangladesh-75 (BSH-75), indicating that while this gene may play a role in monkeypox virulence, it is not required for variola virus virulence.
BR-209: interleukin-1β binding protein
BR-209 encodes a 326 aa protein that functions as an interleukin-1β (IL-1β) binding protein that prevents IL-1β from binding to the IL-1 receptor (25, 26). IL-1 is a cytokine that affects the inflammatory response upon infection (27). IL-1 is found in three forms, IL-1α, IL-1β, and IL-1 receptor antagonist (28). In particular, IL-1β is secreted from cells and binds to IL-1 receptors at which point several signaling pathways are stimulated (28). These signaling pathways lead to the expression of tumor necrosis factor (TNF), IL-2, and certain cytokine receptors (27). IL-1 also helps in stabilizing mRNA levels for genes involved in inflammation (27). In vaccinia virus-infected cells, the IL-1β-binding protein was shown to inhibit the immune response by affecting the proliferation of murine B and T lymphocytes (26), a response is normally activated by IL-1 in vitro. However, there is conflicting data as to how the BR-209 gene contributes to vaccinia virus virulence in vivo. Mice that were injected intracranially with a vaccinia virus that had the BR-209 gene ortholog deleted were not as sick as mice infected intranasally with the deletion virus compared with wildtype virus (25, 26). Intranasal infection with the deletion virus results in more severe illness compared with wildtype virus. Thus, it appears that the route of infection influences the virulence of the virus when examining the role BR-209 plays in viral pathogenesis. The viral IL-1β-binding protein may also be responsible for controlling fever. In vaccinia viruses such as strain COP that do not express this protein, fever is not inhibited when compared to a recombinant virus that had the gene inserted (29). Chen et al. (19) reported the presence of a full-length 326 aa protein in the Central African strain they sequenced. The sequence of the monkeypox virus Central African strain Zaire_1979-005 contains open reading frames (ORFs) that are predicted to encode two fragments of the BR-209 gene: an N-terminal protein fragment of 210 aa and a C-terminal protein fragment of 126 aa. The West African strain of monkeypox contains a one-base insertion near the N-terminus and a four-base deletion resulting in two frameshifts that results in an N-terminal 163 aa fragment and a C-terminal 132 aa fragment. It is not known if any of the fragments function in a way similar to the full-length protein. Also, it is not known if the differences in the length of the N-terminal fragments of central versus West African strains of monkeypox contribute to the differences in virulence. Like BR-203, the BR-209 gene is not present in variola virus (strain BSH-75).
COP-C3L: complement control protein
The vaccinia virus COP-C3L gene encodes a 263 aa secreted protein that inhibits early steps of the host complement cascade and has been named the vaccinia virus complement control protein (VCP) (30-32). As opposed to the prior two genes discussed, this gene is present in variola virus. The ortholog to COP-C3L gene encoded by the Central African strain of monkeypox virus is a shorter protein of 216 aa and has been called monkeypox inhibitor of complement enzymes (MOPICE) (33). This gene is not present in the West African strain of monkeypox. The ortholog present in variola virus has been called the smallpox inhibitor of complement enzymes (SPICE) (34). These poxvirus proteins function is to inhibit the complement pathways by acting in a fashion similar to the mammalian regulators of complement activation (RCA) (35). Like the members of the RCA family, the poxviral inhibitors of complement are made of structural motifs known as short consensus repeats (SCRs) that are approximately 60 aa in length, have conserved disulfide-linked cysteines, and several highly conserved hydrophobic residues (36). The poxviral inhibitors are approximately 90% homologous to each other and about 35% similar to the human RCAs (19, 33).
Because multiple viruses have developed ways to inhibit the host complement attack, it is clear that complement is an important host defense against viruses. The complement cascade can be triggered by multiple activation signals, and the host has numerous proteins to regulate unwanted activation. Similar to members of the mammalian RCA family, the orthopoxvirus proteins can regulate complement activation by acting as a co-factor in the Factor I-mediated cleavage of complement components C3b and C4b (37-41). These proteins can also have decay-accelerating activity (DAA), which results in the more rapid breakdown of the C3 and C5 convertase protein complexes (36).
Many studies have been done that examine the activity of SPICE and VCP. Both SPICE and VCP act as cofactors for Factor I and inhibit both the classical and alternative complement pathway, with SPICE being 1000-fold more active than VCP at inhibiting the alternative pathway and 75-fold more active than VCP at inhibiting the classical pathway. However, SPICE is 100-fold more effective than VCP at cleaving human C3b and six-fold more effective at cleaving C4b compared to VCP (34, 42). SPICE and VCP differ by only 11 aa, with all the differences appearing to be on the surface of the proteins. The amino acid differences are found in SCR domains 2 (five amino acid differences), 3 (three amino acid differences), and 4 (three amino acid differences) (34). Through electrostatic modeling, it was postulated that SPICE interacts with C3b through long-range electrostatic attraction, with C3b being mostly negative and SPICE being mostly positive (42). SPICE binds C3b mainly through amino acids located in SCR 2 and SCR 3, while cofactor I activity of SPICE is due to amino acids located in SCR 2 and SCR 4 (42). Further studies revealed that four amino acids in SCR 2, Y98, Y103, K108, and K120, are responsible for the increased cofactor activity of SPICE compared to VCP (43).
SPICE and VCP have differences in the relative activity against complement from different species. SPICE is more active at inhibiting human and baboon complement, while VCP preferentially inhibits dog and guinea pig complement (34). The differences in activity against complement from different species have been speculated to be an example of a virulence factor that results in variola virus being such a dangerous human pathogen.
VCP has been characterized as the major secreted protein from vaccinia virus-infected cells. Work with recombinant forms of SPICE and VCP have revealed similar heparin-binding activity, which may allow the protein to bind to the cell surface (33). The heparin-binding ability is thought to be due to the presence of the heparin-binding consensus motif K/R-X-K/R (44). While VCP contains four of these motifs in SCR 1, 2, and 4, the heparin-binding activity has been mapped to ones in SCR 1 and 4 (44). SPICE is missing the fourth site due to mutations in SCR 4 (45). A net overall positive charge has also been suggested as being responsible for heparin-binding activity (44). Heparin binding of these proteins has been speculated to be a way that the protein may bind to cell surfaces and provide enhanced complement regulation. Recently, an additional and perhaps more biologically relevant way VCP is expressed on the surface of infected cells was discovered (46). VCP attaches to the cell surface through a direct interaction with another viral membrane protein, A56. Because A56 is highly conserved in variola and monkeypox viruses, it is possible that the interaction between VCP and A56 can occur in variola virus-infected cells and monkeypox virus-infected cells (46).
While VCP and SPICE have four SCR domains, MOPICE has only three full SCR domains due to a single base deletion that results in a stop codon that allows only 13 aa of the fourth SCR domain from being generated (33, 45). Because MOPICE has only three SCR domains, an obvious initial question asked was whether MOPICE had complement regulatory activity. In one study performed by Chen et al. (19), MOPICE was found to bind human C3b and C4b. It was also found to have cofactor activity to allow the Factor I-mediated cleavage of C3b and C4b. These findings were taken a step further in experiments by comparing the relative activities of MOPICE to VCP and SPICE (33). It was found that MOPICE was able to bind human C3b more efficiently than VCP but less efficiently than SPICE. MOPICE bound human C4b with efficiency equal to that of VCP but less efficiently compared to SPICE. MOPICE cleaved C3b into the same products as SPICE; however, it cleaved with the same efficiency as VCP but less efficiently compared to SPICE. MOPICE cleaved C4b just as efficiently as VCP but not as well as SPICE. MOPICE did not have DAA for C3 and C5 convertases of either the classical or alternative pathway. In contrast to MOPICE, both VCP and SPICE had DAA for C3 and C5 convertases of the classical pathway. The lack of DAA in MOPICE could be due to the need for a complete fourth SCR domain. An additional finding was that recombinant MOPICE binds heparin just as well as SPICE and VCP (33). As stated earlier, heparin binding has been mapped to SCRs 1 and 4; however, monkeypox virus lacks most of SCR 4 and therefore is missing one of the four heparin-binding sites.
Because of the ability of MOPICE to function in altering the complement pathway in ways similar to VCP and SPICE, the C3L gene might account for some of the differences in virulence between the Central and West African strains of monkeypox. Because of the absence of this gene in the less virulent West African strain the virus and virus-infected cells would be predicted to be susceptible to the host complement attack. The lack of this gene might result in better control of the infection and quicker clearing and/or alterations in the host immune response to the virus due to interplay of complement and the adaptive immune response (47, 48). Groups in multiple laboratories are seeking to understand the role of the complement control protein in monkeypox virus pathogenesis by generating monkeypox viruses with and without MOPICE and testing them in animal models.
Differences in non-virulence genes
In addition to differences found in the virulence gene ortholog family, there were also differences found in genes involved in the viral life cycle that influence replication and transcription, which also could contribute to the difference in pathogenicity between the Central and West African strains. Likos et al. (20) compared sequences of five strains of monkeypox virus: three African strains (ZAI-1979-005, RCG-2003-358, and LIB-1970-184) and two American strains (USA-2003-039 and USA-2003-044). The strains ZAI-1979-005 and RCG-2003-358 are part of the Central African family, while the LIB-1970-184 and the two American strains are considered to members of the West African family. The two American strains differ by only one nucleotide. They reported on proteins that differed by greater than or equal to five amino acid changes to have the potential to significantly alter protein function and therefore influence the difference between the two strains of monkeypox virus. A total of four genes were found that fit this criterion: COP-H5R (late transcription factor), COP-A9L (morphogenesis factor), COP-A50R (DNA ligase), and COP-A36R (role in actin tail formation). It is unclear exactly how these amino acid differences affect the two strains of monkeypox virus.
Genomic differences between monkeypox and variola viruses
To help understand the potential differences in the disease resulting from infection with monkeypox versus variola viruses as well as the potential of monkeypox virus to become a more efficient human pathogen, it is useful to understand major genetic differences between monkeypox and variola viruses. Monkeypox and smallpox share many similar symptoms, yet monkeypox has a lower fatality rate and lower person-to-person transmission rate and results in more lymphadenopathy. The genomes of monkeypox and variola viruses are about 96% similar in the central regions but differ in the terminal regions, the place where most of the virulence and host-range genes are located (49). Phylogenetic analysis has shown that the variola and monkeypox viruses have a common ancestor but have not evolved one from the other (19, 49) (Fig. 2). Shchelkunov et al. (49) compared the genomes of a Central African strain of monkeypox virus [strain Zaire-96 (MPXV ZAI-96)] and two variola virus strains [strain India-1967 (VARV IND) and strain BSH-75]. When comparing amino acid sequence similarity between MPXV ZAI-96 and VARV IND, the terminal regions were found to be 83.5-93.6% identical. Chen et al. (19) also sequenced and compared a Central African strain of monkeypox virus (strain ZAI-96) and a strain of variola virus (strain BSH-75) specifically examining the virulence family orthologs. We used the website poxvirus.org to compare the genomes of a Central African strain of monkeypox virus (strain Zaire-1979) and variola virus (strain Bangladesh-1975maj) looking for genes present in one and absent in the other. Most differences between monkeypox and variola viruses are in ORFs of unknown function (Table 2). There are several differences in virulence genes and other genes with various known functions. The ortholog to COP-A44L (hydroxysteriod dehydrogenase) is a full-length protein in monkeypox virus but variola virus encodes a protein that is predicted to be about 140 aa shorter. The orthologs to COP-B7R (virulence protein) and BR-203 (virulence protein) are present in monkeypox virus but missing in variola virus. BR-209 (IL-1β binding protein) is full length in some Central African strains and fragmented in other Central African strains; however, the ortholog to this gene is not present in variola virus. The orthologs to COP-C3L (complement control protein), COP-C10L (IL-1β antagonist protein), COP-E3L [interferon (IFN)-resistance protein], and COP-K3L (eIF-2α homolog protein), are absent/fragmented proteins in monkeypox virus but are full-length proteins in variola virus. The orthologs to BR-203, BR-209, and COP-C3L were already discussed and are not discussed again in this section.
Table 2. Comparison of monkeypox virus and variola virus genomes.
A. Genes with known function present in monkeypox virus and absent/fragmented in variola virus
| Gene Ortholog Name | Predicted Function | Present in MPV | Present in VARV |
|---|---|---|---|
| COP-A35R
176 aa |
Unknown | +
176 aa |
- |
| COP-A44L
346 aa |
Hydroxysteriod dehydrogenase | +
346 aa |
Fragment
210 aa: Central/C-term |
| COP-B7R
182 aa |
Virulence, ER resident | +
182 aa |
- |
| COP-B11R
88 aa |
Unknown | +
113 aa |
- |
| COP-B12R
283 aa |
Ser/Thr kinase protein | +
282 aa |
Fragment
104 aa: C-term |
| COP-E7R
166 aa |
Soluble myristylated protein | +
166 aa |
- |
| COP-K1L
284 aa |
Host range protein/Ankyrin protein | +
284 aa |
Fragment
66 aa: N-term 70 aa: C-term |
| COP-K4L
424 aa |
Phospholipase-D protein | +
424 aa |
- |
| BR-18
171 aa |
Unknown | +
176 aa |
- |
| BR-25
668 aa |
Chinese Hamster Ovary Host Range protein | +
660 aa |
Truncated
452 aa: N-term/Central |
| BR-26
63 aa |
Unknown | +
64 aa |
- |
| BR-45
276 aa |
Putative monoglyceride lipase | +
276 aa |
- |
| BR-181
81 aa |
Unknown | +
74 aa |
- |
| BR-197
505 aa |
Schlafen protein | +
503 aa |
- |
| BR-203
225 aa |
Virulence protein | +
221 aa |
- |
| BR-218
193 aa |
Unknown | +
190 aa |
- |
| B. Poxvirus genes with known function absent/fragmented in monkeypox and variola viruses | |||
| Gene Ortholog Name | Predicted Function | Present in MPV | Present in VARV |
| COP-C2L
512 aa |
Kelch protein | Fragment
107 aa: N-term 176 aa: Central 105 aa: C-term |
Fragment
79 aa: Central 221 aa: C-term |
| COP-B_ORF-C
95 aa |
Unknown | - | Fragment
65 aa: C-term |
| BR-08/BR-223
672 aa |
Ankyrin protein | - | Fragment
91 aa: N-term |
| BR-19
796 aa |
Unknown | Fragment
55 aa: N-term 56 aa: N-term 73 aa: C-term |
- |
| BR-158
1284 aa |
CPV A-type inclusion protein | Fragment
696 aa: N-term/Central 54 aa: C-term 67 aa: C-term 75 aa: C-term |
Fragment
702 aa: N-term/Central 65 aa: C-term 96 aa: C-term 192 aa: C-term |
| BR-176
409 aa |
Semaphorin | - | Fragment
122 aa: Central 139 aa: C-term |
| BR-195
197 aa |
Guanylate kinase | - | Fragment
151 aa: Central/C-term |
| BR-209
326 aa |
IL-1β binding protein | Fragment
210 aa: N-term/Central 126 aa: C-term |
- |
| BR-215
557 aa |
Kelch protein | Fragment
70 aa: N-term |
Fragment
70 aa: N-term 88 aa: Central |
| C. Genes with known function present in variola virus but absent/fragmented in monkeypox virus | |||
| Gene Ortholog Name | Predicted Function | Present in MPV | Present in VARV |
| COP-C3L
263 aa |
Inhibitor of complement enzymes | Truncated
216 aa: N-term/Central |
+
263 aa |
| COP-C10L
331 aa |
IL-1β antagonist protein | Fragment
83 aa: N-term |
+
330 aa |
| COP-E3L
190 aa |
IFN-resistance protein | Fragment
153 aa: Central/C-term |
+
192 aa |
| COP-A30.5L
42 aa |
Unknown | - | +
42 aa |
| COP-A49R
162 aa |
Phosphotransferase/Anion transport | - | +
162 aa |
| COP-B_ORF-B
66 aa |
Unknown | - | +
66 aa |
| COP-E_ORF-C
70 aa |
Unknown | - | +
76 aa |
| COP-F14.5L
49 aa |
Unknown | - | +
49 aa |
| COP-K3L
88 aa |
eIF-2α homolog protein | - | +
87 aa |
Abbreviations: aa: amino acids; BR: Cowpox virus strain Brighton Red; C-term: C-terminal fragment; COP: vaccinia virus, strain Copenhagen; CPV: cowpox virus; eIF: eukaryotic initiation factor; ER: endoplasmic reticulum; IFN: interferon; IL-1: Interleukin-1; MPV: central African strain of monkeypox virus Zaire_1979_005; N-term: N-terminal fragment; ORF: open reading frame; Ser/Thr: serine/threonine; VARV: variola virus strain Bangladesh75maj.
Genes with known function present in monkeypox virus and absent/fragmented in variola virus (Table 2A)
COP-A44L: hydroxysteriod dehydrogenase
Monkeypox virus contains an ortholog to COP-A44L that is predicted to encode a protein of 346 aa. In the variola virus, a 210 aa fragment of the ortholog is found that is missing the N-terminal domain and thus is likely to be inactive in variola virus (50, 51). COP-A44L is predicted to encode a 3-β-hydroxysteriod dehydrogenase that would convert pregnenalone to progesterone and dehydroepiandrosterone to androstendione (52). This reaction is necessary for making all types of steroid hormones, including glucocorticoids (GCs). GCs, which are immunosuppressive and anti-inflammatory, can affect the antiviral immune response of the host. Although A44L was not needed for viral replication, vaccinia A44L-deleted viruses were attenuated in vivo. Mice injected intranasally with the vaccinia A44L-deleted virus mounted a strong pulmonary inflammatory response that included but was not limited to an early and fast recruitment of lymphocytes and an increase in IFN-γ production (53). They also recovered more quickly from infection and had less weight loss than mice infected with wildtype vaccinia virus (53). It is thought that A44L influences virulence by increasing steroid production, leading to suppression of the immune system and therefore affecting the immune response (28).
COP-B7R: virulence, ER-resident protein
Monkeypox virus contains a 182 aa ortholog to COP-B7R, which is not present in variola virus. COP-B7R is a protein that resides in the ER; however, it is unclear how this protein is retained, since it does not contain a known retention signal (54). Viral replication is not affected when this gene is deleted. However, B7R-deleted vaccinia virus is less virulent when tested in vivo in a murine intradermal model. The mechanism of how B7R affects virulence is unknown. It is thought that B7R can either affect apoptosis similar to the way the myxoma virus M-T4 gene does or it interacts and retains in the ER a normally secreted or cell surface-expressed protein that is important in the immune response (54).
Genes with known function present in variola virus but absent/fragmented in monkeypox virus (Table 2C)
COP-C10L: IL-1β antagonist
The ortholog to COP-C10L, an IL-1β antagonist, is present in variola virus but is fragmented in monkeypox virus. The full-length ortholog in variola virus is 330 aa, while monkeypox is predicted to encode only an 83 aa fragment homologous to the N-terminal domain. It is thought that COP-C10L influences virulence by blocking IL-1 receptors by binding to them and therefore allowing the virus to evade the effects of IL-1 activities (55). C10L was found to structurally resemble IL-1 receptor antagonist, a protein whose function is to inhibit IL-1 by binding to its receptors. Interestingly, in a yeast two-hybrid assay, C10L was found to interact with K1L, a host range protein that is full-length in monkeypox virus but fragmented in variola virus, suggesting the importance of both proteins in interactions between the host and virus (56).
COP-E3L: IFN-resistance protein
A 192 aa ortholog to COP-E3L is expressed in variola virus, while the ortholog expressed in the Central African strain of monkeypox virus is only a 153 aa fragment lacking the N-terminus. COP-E3L encodes a protein that has a C-terminal domain that binds double-stranded (DS) RNA and an N-terminal domain that binds Z-DNA (57, 58). Both monkeypox and variola virus are predicted to express this C-terminal, dsRNA binding domain. This domain has activity that affects IFN, a cytokine with antiviral activity. There are two classes of IFN, type I IFN and type II IFN, and each type binds to its own type of receptor (28). Type I IFN includes IFN α and β, while type II includes IFN γ. E3L inhibits both types of IFN (28, 59-61). There are several ways in which IFN is activated including viral infection and exposure to dsRNA. Once IFN is activated, it will bind to its designated receptor and stimulate certain signaling pathways that lead to gene expression and activation of several antiviral proteins including protein kinase R (PKR) and 2′-5′ oligoadenylate synthetase (2′-5′ A), two proteins that bind dsRNA. The function of PKR is to inhibit the step of initiation in protein translation by phosphorylating eIF-2α (57). The second antiviral protein 2′-5′A inhibits protein synthesis by activating RNase L, which then acts to degrade rRNA and most likely mRNA (62). Because the virus inhibits PKR and 2′-5′A, protein synthesis can continue. E3L gene has two start codons resulting in two different size proteins (63). The longer sized protein contains both the N-terminal domain and the C-terminal domain, while the shorter size protein contains only the C-terminal domain (57).
The N-terminal domain (that is missing in monkeypox) binds Z-DNA and therefore is thought to either activate or repress gene expression involved in a wide array of cellular processes including the immune response and apoptosis (64, 65). Based on microarray data that used cells infected with a vaccinia virus that had the N-terminal domain deleted, the expression of certain genes involved in the inflammatory response increased; therefore, it was concluded that the binding of the N-terminal to Z-DNA represses the expression of these immune response genes (65). However, studies using a plasmid that expressed E3 in uninfected cells, N-terminal domain binding to Z-DNA activated some genes that influence the immune response (64). Interestingly, only the C-terminal domain is needed for IFN- resistance in vitro; however, the full-length protein is needed for IFN-resistance in mice (58). The monkeypox virus only codes the short E3L protein (the C-terminal domain that binds double-stranded RNA) due to mutations in the gene (49). Thus it is unclear what activity the protein encoded by monkeypox has.
COP-K3L: IFN-resistance protein
COP-K3L is a homolog to the eukaryotic translation initiation factor 2α (eIF-2α). This 88 aa protein ortholog is present in variola virus but absent in most strains of monkeypox virus; however, one monkeypox strain is predicted to expresses a protein that is fragmented and about 43 aa long. Once the IFN pathway is activated, there is an enzyme called dsRNA-activated (DAI) protein kinase that phosphorylates the α subunit of eIF-2α and therefore inhibits initiation in the translation pathway (66). Because K3L is a mimic of eIF-2α, it is believed to bind the eIF-2α site on DAI and prevent DAI from autophosphorylating itself (66). If initiation is not inhibited, protein synthesis can continue. Importantly, when the K3L gene is deleted, the virus becomes IFN-sensitive (66, 67). The two genes, COP-E3L and COP-K3L, affect IFN resistance and therefore influence virulence. Taken together, COP-C10L, COP-E3L, and COP-K3L, which are present in variola virus but absent or fragmented in monkeypox virus, may contribute to the differences in morbidity, mortality, and transmissibility seen between variola and monkeypox viruses.
Additional genomic differences
There are additional genomic differences between monkeypox and variola viruses. As shown in Table 2, there are genes that have various functions that are present in monkeypox virus and absent or fragmented in variola virus. Some of these genes have been characterized, while some have not. Monkeypox virus contains orthologs to COP-E7R and COP-K4L that are not present in variola virus. The orthologs to COP-B12R and COP-K1L are present in monkeypox virus but are fragmented in variola virus. The function of E7R, which is predicted to be a soluble myristylated protein, remains unclear; however, it was found within infectious mature virions, which could indicate that it plays a role in viral replication (68). COP-K4L, a phospholipase-D protein, is not needed for viral replication and did not influence virulence (69). This protein functions as a nicking-joining enzyme by cutting and rejoining DNA. It was found to be the only protein required for this activity. COP-B12R, a ser/thr kinase protein, was found not to have an effect on virulence or viral replication in vitro or in vivo (70). COP-K1L is a host range/ankyrin protein, which is important in viral infection because ankyrin proteins act as scaffolds in protein-protein interactions (71). This protein is thought to be needed for viral replication in some human cells and rabbit cells but is fragmented in variola virus (72, 73). Although K1L is only present in a few orthopoxviruses, a closely related host range protein COP-C7L is present in most orthopoxviruses including variola virus. It appears that orthopoxviruses that lack K1L can still replicate in certain cell lines due to the compensatory effect of C7L (73). The orthologs to BR-25 and BR-45 are present in monkeypox virus but fragmented or absent in variola virus. The ortholog to BR-25, the Chinese hamster ovary (CHO) host range protein, allows cowpox virus to replicate in CHO cells, which vaccinia viruses cannot do (74). When recombinant vaccinia virus is made expressing this protein, productive infection can occur in CHO cells before the cells die (75). The ortholog to BR-45 is a putative monoglyceride lipase protein, and it is unclear how this protein influences virulence. The ortholog to BR-197 is a Schlafen protein. These proteins are believed to influence cell growth and T-cell maturation (76). It is unclear how these proteins regulate these processes, and therefore, the effect this protein has on virulence is unknown.
Prior smallpox vaccination affects severity of monkeypox infection
Similar to the protection from smallpox conferred by vaccination, vaccinia vaccination protects against monkeypox. Doctors caring for patients during monkeypox outbreaks noticed that patients with less severe illness had been vaccinated with the smallpox vaccine (77). Vaccinated patients had a less intense rash, and about half had evidence of lymphadenopathy. There were also no fatalities in vaccinated patients. It is estimated that smallpox vaccination gives 85% protection against monkeypox (78). Patients' vaccination status was based on having a vaccination scar. In one study that examined patients infected with a West African strain of monkeypox in the 2003 US outbreak, it was found that three patients who had been vaccinated with the smallpox vaccine three to 48 years previously had evidence of potential recent monkeypox virus exposure but had no evidence of disease (11). These vaccinated patients were believed to be infected with monkeypox virus based on potential exposure to infected animals and a serological ELISA that compared the ratio of antiviral antibody responses to monkeypox virus versus the antibody response to vaccinia virus. This ratio was high compared to uninfected vaccinated contacts, which the authors of this paper believed to represent a recent orthopoxvirus infection. As further evidence of asymptomatic monkeypox infection in these vaccinated individuals, antibody titers declined 60% from levels measured at an early time point (two to four months after potential exposure to infected animals) when compared to a late time point (one year). While this study identified some vaccinated individuals that appeared to be fully protected from monkeypox, there were five other vaccinated individuals that were infected with monkeypox and displayed mild to moderate symptoms (3, 11, 79). Thus distant vaccination is likely only to be partially protective.
Genomic differences between monkeypox and vaccinia viruses
While smallpox vaccination confers immunity from monkeypox disease, it is useful to think about the differences between monkeypox and vaccinia viruses. Proteins that are expressed in monkeypox that are missing from vaccinia virus may represent specific targets for diagnostics as well as for future subunit-based vaccines that might be developed against monkeypox. It is also interesting to think about these genes, because they encode proteins to which smallpox vaccination does not generate a monkeypox-specific immune response. Using poxvirus.org and two published papers (16, 80), a list of genes that differed between the Central African strain of monkeypox (ZAI-1979) to one human vaccine strain (ACAM2000) was compiled (Table 3). Vaccinia virus strain ACAM2000 is a clone isolated from the Dryvax® vaccine that was plaque purified and grown in Vero cells under serum-free conditions (81). ACAM2000 has been FDA approved and has now replaced Dryvax as the smallpox vaccine (82). Some of the genes that differ between monkeypox and variola viruses also differ in the vaccinia strain. There are additional proteins that are present in monkeypox virus but are fragmented in the vaccinia strain including COP-B19R (IFN-α/β binding protein), BR-05/BR-226 (TNF binding protein), and BR-207 (serpine-2/apoptosis protein).
Table 3. Comparison of monkeypox virus and vaccinia virus genomes.
A. Genes with known function present in monkeypox virus and absent/fragmented in vaccinia virus (ACAM2000)
| Gene Ortholog Name | Predicated
Function |
Present in MPV | Present in ACAM2000 |
|---|---|---|---|
| COP-B19R
353 aa |
IFN-α/β binding protein | +
352 aa |
Truncated
265 aa: N-term/Central |
| BR-05/226
355 aa |
TNF binding protein | +
348 aa |
Fragment
122 aa: N-term 146 aa: Central |
| BR-18
171 aa |
Unknown | +
176 aa |
- |
| BR-45
276 aa |
Putative monoglyceride lipase | +
276 aa |
Fragment
81 aa: N-term 136 aa: Central |
| BR-158
1284 aa |
CPV A-type inclusion protein | Fragment
696 aa: N-term/Central 54 aa: C-term 67 aa: C-term 75 aa: C-term |
Fragment
721 aa: N-term/Central 227 aa: C-term 65 aa: C-term 154 aa: C-term |
| BR-181
81 aa |
Unknown | +
74 aa |
- |
| BR-197
505 aa |
Unknown | +
503 aa |
Fragment
219 aa: N-term 124 aa: Central |
| BR-203
225 aa |
Virulence protein | +
221 aa |
Fragment
77 aa: N-term |
| BR-207
341 aa |
Serpine-2/apoptosis protein | +
344 aa |
Fragment
126 aa: N-term 222 aa: Central/C-term |
| BR-219
1919 aa |
Surface glycoprotein | +
1879 aa |
Fragment
51 aa: N-term |
| B. Poxvirus genes with known function absent/fragmented in monkeypox and vaccinia viruses | |||
| Gene Ortholog Name | Predicted Protein Function | Present in MPV | Present in ACAM2000 |
| BR-19
796 aa |
Unknown | Fragment
55 aa: N-term 56 aa: N-term 73 aa: C-term |
- |
| BR-20
170 aa |
Unknown | Fragment
64 aa: C-term |
- |
| BR-176
409 aa |
Semaphorin protein | - | Fragment
132 aa: N-term 142 aa: C-term |
| BR-215
557 aa |
Kelch protein | Fragment
70 aa: N-term |
- |
| C. Genes with known function present in vaccinia virus (ACAM2000) and absent/fragmented in monkeypox virus | |||
| Gene Ortholog Name | Predicted Protein Function | Present in MPV | Present in ACAM2000 |
| COP-C3L
263 aa |
Inhibitor of complement enzymes | Truncated
216 aa: N-term/Central |
+
263 aa |
| COP-C10L
331 aa |
IL-1β antagonist | Fragment
83 aa: N-term |
+
331 aa |
| COP-E3L
190 aa |
IFN-resistance protein | Fragment
153 aa: Central/C-term |
+
190 aa |
| COP-A40R
168 aa |
C-type lectin type II membrane protein | - | +
168 aa |
| COP-A49R
162 aa |
Phosphotransferase/Anion transport protein | - | +
162 aa |
| COP-A52R
190 aa |
Bifunctional Toll / IL-1-receptor protein | - | +
190 aa |
| COP-A57R
151 aa |
Guanylate kinase | - | +
151 aa |
| COP-B10R
166 aa |
Unknown | - | +
166 aa |
| COP-C8L
184 aa |
Unknown | - | +
177 aa |
| COP-K3L
88 aa |
eIF-2α homolog | - | +
88 aa |
| BR-174
63 aa |
Unknown | - | +
62 aa |
| BR-209
326 aa |
IL-1β binding protein | Fragment
210 aa: N-term/Central 126 aa: C-term |
+
326 aa |
Abbreviations: aa: amino acids; ACAM2000: vaccine strain of vaccinia virus; BR: cowpox virus strain Brighton Red; C-term: C-terminal fragment; COP: vaccinia virus, strain, Copenhagen; CPV: cowpox virus; eIF: eukaryotic initiation factor; IFN: interferon; IL: interleukin; MPV: central African strain of monkeypox virus Zaire_1979_005; N-term: N-terminal fragment; ORF: open reading frame; TNF: tumor necrosis factor.
COP-B19R: IFN-α/β-binding protein
The ortholog to COP-B19R is present in monkeypox virus and is 352 aa; however, the vaccine strain of vaccinia virus contains a truncated protein. ACAM2000 expresses a 265 aa protein that is missing the C-terminal domain. The vaccinia virus strain Western Reserve ortholog to COP-B19R encodes a 351 aa protein that binds type I IFN-α/β and prevents it from binding to its cellular receptors, thereby inhibiting certain signaling pathways (83, 84). This protein is also secreted from infected cells and can bind both infected and uninfected cells due to its three Ig domains (85). Although it is unclear which of three domains is responsible for cell surface binding or IFN binding, it is thought that the N-terminal domain is involved in binding to cells, while the C-terminal domain is responsible for binding IFN. While deletion of this gene from vaccinia virus only minimally affected virulence, it was recently published that this type I IFN binding protein was critical to ectromelia virus (ECTV) virulence based on the severe attenuation of the virus when the ECTV gene was deleted (86). In addition to influencing ECTV virulence, the protein encoded by the ECTV B19R ortholog was also shown to be targeted by the antibody immune response. In fact, Xu et al. (86) showed that immunizing mice with the purified ECTV protein could protect mice from lethal ECTV challenge. It is not known if the immune response to the fragment expressed by vaccinia virus results in a similar protective response.
BR-05/BR-226: TNF-α receptor
Monkeypox virus contains the ortholog to BR-05/BR-226 and is 348 aa. This gene is found within the inverted terminal repeats of the viral genome; thus, there are two copies of this gene in the monkeypox virus genome. The ortholog to this gene in ACAM2000 is fragmented into two pieces, a 122 aa piece containing the N-terminal domain and a 146 aa piece containing the central region. The gene codes for TNF-α receptors and has been named cytokine response modifier (Crm) B in poxviruses. TNF-α is a cytokine secreted from T cells and macrophages that protects cells from viral infection and can kill cells that have been infected with virus. CrmB is a viral protein that is secreted and binds TNF-α and TNF-β (87). CrmB has been shown to bind TNF-α but not lymphotoxin-α, a cytokine similar to TNF-α, and therefore prevents the binding of TNF-α to its cellular receptor (88). Similar to the fragmented COP-B19R protein that might be expressed by ACAM2000, it is not known if antibody responses to the fragments of BR-05/BR-226 expressed by ACAM2000 induce antibodies that block the viral TNF-binding protein activity.
BR-207: IL-1 convertase
Monkeypox virus contains an IL-1 convertase that is 344 aa and is found in some strains of vaccinia virus but is fragmented in the vaccine strain ACAM2000. ACAM2000 is predicted to encode two fragments, a 126 aa fragment homologous to the N-terminal domain and a 222 aa fragment homologous to the C-terminal domain. The monkeypox protein is an ortholog to BR-207, which is also known as serine protease inhibitor-2 (SPI-2) (28). To secrete active IL-1, intracellular pro-IL-1 must be cleaved by the enzyme caspase 1 into mature IL-1 and then released from the cell. The viral IL-1 convertase inhibitor inhibits caspase 1 and therefore prevents mature IL-1 from being formed. However, the virus can only inhibit the production of mature IL-1 from cells it has infected. Since this is not an extracellular protein, it is unlikely that an antibody response to the protein would alter monkeypox pathogenesis.
Person-to-person transmission of monkeypox and models for an epidemic
Although monkeypox and variola viruses generate similar clinical diseases, they differ in their resulting mortality and their transmissibility from person-to-person. Historically, monkeypox virus has a low person-to-person transmission rate. This transmissibility is in contrast to variola virus, which has a high transmission rate, and this finding helps explain the epidemic potential of one virus and not the other. The low person-to-person transmissibility of monkeypox is supported by early epidemiologic studies. For example, between 1981-1986 in the DRC, there were 338 cases of identified monkeypox (9). Of these 338 cases, 67% were confirmed by virus culture. Secondary transmission rates in households that had unvaccinated members were about 10%. Twenty-eight percent of infected patients reported having contact with another infected patient during the incubation period. However, it was rare to have an extended transmission chain beyond secondary cases (89, 90). Such data are from an era when smallpox vaccination was still wide spread. During 1996 and 1997, the largest recorded outbreak of monkeypox virus in the Kasai Oriental region in the DRC raised concern that the epidemiology may be different in the post-smallpox vaccination era. The WHO launched an investigation, which was complicated by military unrest in the region. First in February 1997, they reported 92 cases, and then in October of that year an additional 419 cases were reported (91, 92). Initially, there was raised concern because there were a much higher proportion of cases due to secondary transmissions (78%) compared to previous surveillance times in 1981-1985 (28%). However, the fatality rate (1.5%) was much lower than the outbreaks reported previously (10%) (12, 93). The reported high rate of person-to-person transmission and low mortality is likely explained by a concurrent varicella zoster (chickenpox) outbreak that resulted in the misdiagnosis of monkeypox cases (9). It is estimated that 88 of the 419 cases were chickenpox (12). While there is still a question about the increased rate of person-to-person transmission during this outbreak, these studies do suggest that as the number of vaccinia vaccinated individuals decrease, the population becomes more susceptible to monkeypox virus.
In addition to the concerns this large outbreak raised, a report of up to six sequential person-to-person transmissions in a hospital community in the Republic of the Congo in 2003 raises concerns about increased transmissibility of the virus that could result in better adaptations to humans (94). This is the first time such a long uninterrupted chain of seven generations of person-to-person transmission has been documented. Since such a chain of transmission is so unusual, several points can be made that support serial transmission. First, the length of time between case onsets is consistent with the expected incubation period after a transmission and thus less likely to be a result of multiple individual random reintroductions of monkeypox virus from an animal source. Second, cases occurred sequentially within families. This shows that family members who were either nursing the infected individual or in very close proximity to the infected individual became infected. Interestingly, this is not the first time person-to-person transmission has occurred within a hospital setting. There were three sequential passages or four generations of transmission reported previously (89). The worry about such extended chains of infection is that this process may allow the selection of a virus that is more efficient at person-to-person transmission.
Researchers have examined what type of conditions would be right for monkeypox to become endemic in the population. Based on current data, it is believed that only repeated reintroduction of the virus from the wild could sustain monkeypox in humans and that person-to-person transmission would not be sufficient to do this (9, 78). An alternative epidemiologic model suggested by Antia et al. (95) about the role ecological changes, changes in host behavior, and viral evolution plays in the transmissibility of monkeypox into a population. These factors can lead to increased introduction of the virus into the population, which in turn increases the number of primary cases. This can give rise to longer transmission chains which gives the virus a chance to mutate and spread more efficiently from person-to-person eventually becoming an epidemic (95).
Orthopoxvirus seroprevalence and its relation to monkeypox virus infections
It is speculated that most individuals become infected with monkeypox through the hunting, skinning, and eating of infected animals (8). In endemic regions, it is unclear if every infection leads to disease or if asymptomatic infections occur. Furthermore, it is not known if exposure to other naturally occurring orthopoxviruses in Africa leads to immunity to monkeypox virus infection. A seroprevalence study was conducted in the Likouala region of the Republic of Congo to determine if villagers in this region who were being exposed to monkeypox were developing immunity to the virus without showing signs of illness (96). Researchers used ELISAs to measure levels for IgM and IgG antibodies to orthopoxviruses. While such antibody testing has a high sensitivity and a high specificity to prior orthopoxvirus infections, it cannot differentiate between antibodies made in response to particular orthopoxvirus. It was found that residents in this area had an overall anti-orthopoxvirus IgG seroprevalence of 57%. This high seroprevalence was different from the seroprevalence surveys done decades earlier. For example, in one previous survey, using a hemagglutinin inhibition assay (HIA) on serum from residents of Kole, DRC, they detected an overall seroprevalence of only 19% for antibodies made in response to orthopoxviruses (96, 97). Another survey that used serum from residents of Pool and Sangha provinces of Republic of Congo found a seroprevalence of 16% for orthopoxvirus antibodies. There are several points that could explain why there is such a large difference in seroprevalences between the recent and earlier studies. One explanation may be the age of the residents from each survey and the relation to prior smallpox vaccination. In the earlier studies in Kole or Pool and Sangha provinces, 90% or more of the participants were less than 30 or 15 years old, respectively (96). Thus, the majority of these individuals were young enough that they may not have ever been vaccinated with the smallpox vaccine. However, in the recent study from the Likouala region, about 50% of the participants were old enough to have been previously vaccinated. Another point that may explain the differences in seroprevalence was the use of the HIA assay in the earlier studies. The ELISA could be a more sensitive assay than HIA in detecting anti-orthopoxvirus antibodies. Finally, the higher seroprevalence may represent exposure to other orthopoxviruses due to either changes in ecology that allowed more interactions between humans and animals.
Because ELISA was used to determine seroprevalence, IgG and IgM antibodies can be distinguished. It was found that older participants had higher IgG and IgM anti-orthopoxvirus antibodies compared to younger individuals with the higher IgG levels, perhaps being due to smallpox vaccination and/or exposure to other orthopoxviruses (96). However, the presence of high IgM antibody levels suggests a recent exposure to an orthopoxvirus. The older individuals would be exposed to orthopoxviruses due to their adult duties of hunting and cooking. These are the activities that have the highest risk of exposure, since they involve working with potentially infected animals that may carry the virus. The authors of this study suggest that there may be a higher exposure rate to monkeypox virus than researchers are aware of and thus a higher level of asymptomatic infections than previously thought. An alternative explanation is that the high seroprevalence is due to antibody responses from exposure to some other non-pathogenic orthopoxviruses that may actually provide some protection from monkeypox in Africa. Since there are no common naturally occurring orthopoxvirus infections in places like the US, one can speculate that introduction of a virulent strain of monkeypox could result in even more disease than what is seen in Central Africa because of this lack of any pre-existing anti-orthopoxvirus immunity.
Summary and conclusions
Because of the continuous outbreaks of human monkeypox in Africa and the recent accidental importation of monkeypox virus resulting in cases in the US in 2003, it is of great importance that this orthopoxvirus remains at the forefront of potential emerging infectious diseases. Although smallpox has been eradicated from the human population since 1980, there is the potential for monkeypox to fill this void. An extended chain of person-to-person transmissions of monkeypox in 2003 in the Republic of Congo reveals the potential of further adaptation of the virus to become a more successful human pathogen (94). Also, since the human population is constantly changing due to such things as ecological disturbances and development of new infections such as human immunodeficiency virus, this may provide monkeypox virus the opportunity it needs to spread more efficiently. Based on the genome comparisons between the Central and West African strains of monkeypox virus, it is interesting to speculate that the ortholog to COP-C3L, a gene coding for an innate immune response protein (a complement control protein), may contribute to the difference in virulence between the more virulent and less virulent strains of monkeypox virus. The ortholog to COP-C3L in the Central African strain is truncated by one SCR domain, which may affect its function, in particular, its lack of decay accelerating activity (33). The Central African strain of monkeypox also contains truncated orthologs of COP-E3L and COP-K3L, two proteins that function in IFN resistance, that are full-length in variola and vaccinia viruses. In contrast to COP-E3L and COP-K3L, the ortholog to BR-203 (a protein that prevents apoptosis of infected lymphocytes) is full length in monkeypox virus and absent in variola virus and fragmented in vaccinia virus. These differences in proteins that affect the host immune system could explain the difference in virulence between these three orthopoxviruses. Further research needs to be done to determine each gene's contribution to virulence and if genes that are predicted to encode fragments of proteins with known functions retain some function. Such an understanding may help determine what made variola virus such a successful human pathogen as well as help in making safer vaccines and better therapeutics.
Monkeypox infection is an important emerging pathogen that, based on serologic studies in Africa, may result in more infections than originally believed. If a virulent strain of monkeypox were introduced in a setting where individuals have no immunity to orthopoxviruses, this may provide the virus with the opportunity to exploit this naive population, which could lead to an epidemic.
Acknowledgments
S.N.I. is supported by NIH NIAID Middle Atlantic Regional Center of Excellence (U54 AI057168). We would also like to thank Dr, Anne Rimoin for providing photographs of a person with monkeypox virus infection.
References
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. First. Geneva: World Health Organization; 1988. [Google Scholar]
- 2.Breman JG, Henderson DA. Poxvirus dilemmas--monkeypox, smallpox. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 3.Reed KD, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 4.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 5.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 6.Marennikova SS, Seluhina EM, Mal'ceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 7.Khodakevich L, et al. The role of squirrels in sustaining monkeypox virus transmission. Trop Geogr Med. 1987;39:115–122. [PubMed] [Google Scholar]
- 8.Khodakevich L, et al. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Trop Geogr Med. 1987;39:56–63. [PubMed] [Google Scholar]
- 9.Hutin YJ, et al. Outbreak of human monkeypox, democratic republic of congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutson CL, et al. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- 11.Hammarlund E, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 12.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41:1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Olson VA, Laue T, Laker MT, Damon IK. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karem KL, et al. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shchelkunov SN, et al. Analysis of the monkeypox virus genome. Virology. 2002;297:172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 18.Jezek Z, Fenner F. Human monkeypox. Monographs Virol. 1988;17:1–140. [Google Scholar]
- 19.Chen N, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Likos AM, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 21.Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. 517–563. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 22.Johnson GP, Goebel SJ, Paoletti E. An update on the vaccinia virus genome. Virology. 1993;196:381–401. doi: 10.1006/viro.1993.1494. [DOI] [PubMed] [Google Scholar]
- 23.Barry M, Hnatiuk S, Mossman K, Lee SF, Boshkov L, McFadden G. The myxoma virus M-T4 gene encodes a novel RDEL-containing protein that is retained within the endoplasmic reticulum and is important for the productive infection of lymphocytes. Virology. 1997;239:360–377. doi: 10.1006/viro.1997.8894. [DOI] [PubMed] [Google Scholar]
- 24.Hnatiuk S, et al. Role of the C-terminal RDEL motif of the myxoma virus M-T4 protein in terms of apoptosis regulation and viral pathogenesis. Virology. 1999;263:290–306. doi: 10.1006/viro.1999.9946. [DOI] [PubMed] [Google Scholar]
- 25.Alcami A, Smith GL. A soluble receptor for interleukin-1β encoded by vaccinia virus - a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 26.Spriggs MK, et al. Vaccinia and cowpox viruses encode a novel secreted Interleukin-1-Binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 28.Smith GL, Symons JA, Khanna A, Vanderplasschen A, Alcami A. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 29.Alcami A, Smith GL. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci USA. 1996;93:11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotwal GJ, Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988;335:176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- 31.Kotwal GJ, Isaacs SN, McKenzie R, Frank MM, Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990;250:827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs SN, Kotwal GJ, Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci USA. 1992;89:628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liszewski MK, et al. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol. 2006;176:3725–3734. doi: 10.4049/jimmunol.176.6.3725. [DOI] [PubMed] [Google Scholar]
- 34.Rosengard AM, Liu Y, Nie Z, Jimenez R. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc Natl Acad Sci USA. 2002;99:8808–8813. doi: 10.1073/pnas.112220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blue CE, Spiller OB, Blackbourn DJ. The relevance of complement to virus biology. Virology. 2004;319:176–184. doi: 10.1016/j.virol.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 37.McKenzie R, Kotwal GJ, Moss B, Hammer CH, Frank MM. Regulation of complement activity by vaccinia virus complement-control protein. J Infect Dis. 1992;166:1245–1250. doi: 10.1093/infdis/166.6.1245. [DOI] [PubMed] [Google Scholar]
- 38.Sahu A, Isaacs SN, Soulika AM, Lambris JD. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J Immunol. 1998;160:5596–5604. [PubMed] [Google Scholar]
- 39.Rosengard AM, et al. Functional characterization of soluble and membrane-bound forms of vaccinia virus complement control protein (VCP) Mol Immunol. 1999;36:685–697. doi: 10.1016/s0161-5890(99)00081-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, et al. Mapping of regions within the vaccinia virus complement control protein involved in dose-dependent binding to key complement components and heparin using surface plasmon resonance. Biochim Biophys Acta. 2003;1650:30–39. doi: 10.1016/s1570-9639(03)00189-4. [DOI] [PubMed] [Google Scholar]
- 41.Bernet J, Mullick J, Panse Y, Parab PB, Sahu A. Kinetic analysis of the interactions between vaccinia virus complement control protein and human complement proteins C3b and C4b. J Virol. 2004;78:9446–9457. doi: 10.1128/JVI.78.17.9446-9457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sfyroera G, Katragadda M, Morikis D, Isaacs SN, Lambris JD. Electrostatic modeling predicts the activities of orthopoxvirus complement control proteins. J Immunol. 2005;174:2143–2151. doi: 10.4049/jimmunol.174.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav VN, Pyaram K, Mullick J, Sahu A. Identification of hot spots in the variola virus complement inhibitor (SPICE) for human complement regulation. J Virol. 2008;82:3283–3294. doi: 10.1128/JVI.01935-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA, et al. Conserved surface-exposed K/R-X-K/R motifs and net positive charge on poxvirus complement control proteins serve as putative heparin-binding sites and contribute to inhibition of molecular interactions with human endothelial cells: a novel mechanism for evasion of host defense. J Virol. 2000;74:5659–5666. doi: 10.1128/jvi.74.12.5659-5666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uvarova EA, Shchelkunov SN. Species-specific differences in the structure of orthopoxvirus complement-binding protein. Virus Res. 2001;81:39–45. doi: 10.1016/S0168-1702(01)00332-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girgis NM, Dehaven BC, Fan X, Viner KM, Shamim M, Isaacs SN. Cell surface expression of the vaccinia virus complement control protein is mediated by interaction with the viral a56 protein and protects infected cells from complement attack. J Virol. 2008;82:4205–4214. doi: 10.1128/JVI.02426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 48.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 49.Shchelkunov SN, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massung RF, et al. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994;201:215–240. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- 51.Shchelkunov SN, Massung RF, Esposito JJ. Comparison of the genome DNA sequences of Bangladesh-1975 and India-1967 variola viruses. Virus Research. 1995;36:107–118. doi: 10.1016/0168-1702(94)00113-q. [DOI] [PubMed] [Google Scholar]
- 52.Moore JB, Smith GL. Steroid hormone synthesis by a vaccinia enzyme - a new type of virus virulence factor. EMBO J. 1992;11:1973–1980. doi: 10.1002/j.1460-2075.1992.tb05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reading PC, Moore JB, Smith GL. Steroid hormone synthesis by vaccinia virus suppresses the inflammatory response to infection. J Exp Med. 2003;197:1269–1278. doi: 10.1084/jem.20022201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price N, Tscharke DC, Hollinshead M, Smith GL. Vaccinia virus gene B7R encodes an 18-kDa protein that is resident in the endoplasmic reticulum and affects virus virulence. Virology. 2000;267:65–79. doi: 10.1006/viro.1999.0116. [DOI] [PubMed] [Google Scholar]
- 55.Kluczyk A, Siemion IZ, Szewczuk Z, Wieczorek Z. The immunosuppressive activity of peptide fragments of vaccinia virus C10L protein and a hypothesis on the role of this protein in the viral invasion. Peptides. 2002;23:823–834. doi: 10.1016/s0196-9781(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 56.McCraith S, Holtzman T, Moss B, Fields S. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:4879–4884. doi: 10.1073/pnas.080078197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang HW, Jacobs BL. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993;194:537–547. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- 58.Brandt TA, Jacobs BL. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001;75:850–856. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beattie E, Paoletti E, Tartaglia J. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L- and E3L- mutant viruses. Virology. 1995;210:254–263. doi: 10.1006/viro.1995.1342. [DOI] [PubMed] [Google Scholar]
- 61.Alcami A, Smith GL. Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus. Trends Microbiol. 1996;4:321–326. doi: 10.1016/0966-842x(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 62.Rivas C, Gil J, Melkova Z, Esteban M, Diaz-Guerra M. Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2-5A synthetase enzyme. Virology. 1998;243:406–414. doi: 10.1006/viro.1998.9072. [DOI] [PubMed] [Google Scholar]
- 63.Yuwen H, Cox JH, Yewdell JW, Bennink JR, Moss B. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology. 1993;195:732–744. doi: 10.1006/viro.1993.1424. [DOI] [PubMed] [Google Scholar]
- 64.Kwon JA, Rich A. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc Natl Acad Sci USA. 2005;102:12759–12764. doi: 10.1073/pnas.0506011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langland JO, Kash JC, Carter V, Thomas MJ, Katze MG, Jacobs BL. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J Virol. 2006;80:10083–10095. doi: 10.1128/JVI.00607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies MV, Elroy-Stein O, Jagus R, Moss B, Kaufman RJ. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992;66:1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beattie E, Tartaglia J, Paoletti E. Vaccinia virus encoded eIF-2a homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- 68.Martin KH, Grosenbach DW, Franke CA, Hruby DE. Identification and analysis of three myristylated vaccinia virus late proteins. J Virol. 1997;71:5218–5226. doi: 10.1128/jvi.71.7.5218-5226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eckert D, Williams O, Meseda CA, Merchlinsky M. Vaccinia virus nicking-joining enzyme is encoded by K4L (VACWR035) J Virol. 2005;79:15084–15090. doi: 10.1128/JVI.79.24.15084-15090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banham AH, Smith GL. Characterization of vaccinia virus gene B12R. J Gen Virol. 1993;74:2807–2812. doi: 10.1099/0022-1317-74-12-2807. [DOI] [PubMed] [Google Scholar]
- 71.Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- 72.Oguiura N, Spehner D, Drilien R. Detection of a protein encoded by the vaccinia virus C7L open reading frame and study of its effect on virus multiplication in different cell lines. J Gen Virol. 1993;74:1409–1413. doi: 10.1099/0022-1317-74-7-1409. [DOI] [PubMed] [Google Scholar]
- 73.Meng X, Chao J, Xiang Y. Identification from diverse mammalian poxviruses of host-range regulatory genes functioning equivalently to vaccinia virus C7L. Virology. 2008;372:372–383. doi: 10.1016/j.virol.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spehner D, Gillard S, Drillien R, Kirn A. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J Virol. 1988;62:1297–1304. doi: 10.1128/jvi.62.4.1297-1304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ink BS, Gilbert CS, Evan GI. Delay of vaccinia virus-induced apoptosis in nonpermissive Chinese hamster ovary cells by the cowpox virus CHOhr and adenovirus E1B 19K genes. J Virol. 1995;69:661–668. doi: 10.1128/jvi.69.2.661-668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 77.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 78.Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 79.Karem KL, Reynolds M, Olson V, Li Y, Damon IK. Monkeypox outbreak diagnostics and implications for vaccine protective effect. Nat Med. 2006;12:495–496. 496–497. doi: 10.1038/nm0506-495. author reply. [DOI] [PubMed] [Google Scholar]
- 80.Manes NP, et al. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J Proteome Res. 2008;7:960–968. doi: 10.1021/pr070432+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monath TP, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) - a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8(Suppl):31–44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.CDC. Notice to readers: newly licensed smallpox vaccine to replace old smallpox vaccine. MMWR Morb Mortal Wkly Rep. 2008;57:207–208. [Google Scholar]
- 83.Pelletier J, Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985;40:515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- 84.Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- 85.Alcami A, Symons JA, Smith GL. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J Virol. 2000;74:11230–11239. doi: 10.1128/jvi.74.23.11230-11239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu RH, et al. The orthopoxvirus type I IFN binding protein is essential for virulence and an effective target for vaccination. J Exp Med. 2008;205:981–992. doi: 10.1084/jem.20071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu FQ, Smith CA, Pickup DJ. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 88.Alcami A, Khanna A, Paul NL, Smith GL. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80:949–959. doi: 10.1099/0022-1317-80-4-949. [DOI] [PubMed] [Google Scholar]
- 89.Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 90.Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 91.CDC. Human monkeypox -- Kasai Oriental, Democratic Republic of Congo. MMWR Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 92.CDC. Human monkeypox -- Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 93.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 94.Learned LA, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–434. [PubMed] [Google Scholar]
- 95.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lederman ER, et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: evidence for monkeypox virus exposure. Am J Trop Med Hyg. 2007;77:1150–1156. [PubMed] [Google Scholar]
- 97.Jezek Z, Nakano JH, Arita I, Mutombo M, Szczeniowski M, Dunn C. Serological survey for human monkeypox infections in a selected population in Zaire. J Trop Med Hyg. 1987;90:31–38. [PubMed] [Google Scholar]


