Abstract
Transient asymmetric Nodal signaling in the left lateral plate mesoderm (L LPM) during tailbud/early somitogenesis stages is associated in all vertebrates examined with the development of stereotypical left-right (L-R) organ asymmetry. In Xenopus, asymmetric expression of Nodal-related 1 (Xnr1) begins in the posterior L LPM shortly after the initiation of bilateral perinotochordal expression in the posterior tailbud. The L LPM expression domain rapidly shifts forward to cover much of the flank of the embryo before being progressively downregulated, also in a posterior-to-anterior direction. The mechanisms underlying the initiation and propagation of Nodal/Xnr1 expression in the L LPM, and its transient nature, are not well understood. Removing the posterior tailbud domain prevents Xnr1 expression in the L LPM, consistent with the idea that normal embryos respond to a posteriorly derived asymmetrically acting positive inductive signal. The forward propagation of asymmetric Xnr1 expression occurs LPM-autonomously via planar tissue communication. The shifting is prevented by Nodal signaling inhibitors, implicating an underlying requirement for Xnr1-to-Xnr1 induction.
It is also unclear how asymmetric Nodal signals are modulated during L-R patterning. Small LPM grafts overexpressing Xnr1 placed into the R LPM of tailbud embryos induced the expression of the normally L-sided genes Xnr1, Xlefty, and XPitx2, and inverted body situs, demonstrating the late-stage plasticity of the LPM. Orthogonal Xnr1 signaling from the LPM strongly induced Xlefty expression in the midline, consistent with recent findings in the mouse and demonstrating for the first time in another species conservation in the mechanism that induces and maintains the midline barrier. Our findings suggest that there is long-range contralateral communication between L and R LPM, involving Xlefty in the midline, over a substantial period of tailbud embryogenesis, and therefore lend further insight into how, and for how long, the midline maintains a L versus R status in the LPM.
Keywords: Nodal, Xnr1, L-R asymmetry, lateral plate mesoderm, Xenopus laevis
Introduction
Vertebrates exhibit conserved anatomical left-right (L-R) asymmetry in, for example, the placement and anatomy of the cardiovascular system, visceral organs, and the number of lung lobes. In some species, such as zebrafish, L-R asymmetry is also apparent in the brain. The developmental mechanism by which L-R asymmetry is initiated, and the degree to which it is conserved between species, remains unknown (Burdine and Schier, 2000; Capdevila et al., 2000; Levin, 2005). Although a number of signaling molecules, including Shh, BMP, FGF, RA, and Notch, have been implicated in some vertebrates in the stage-setting phase of asymmetry establishment, there is currently no unifying “L-R specification model” that applies well to all species (Burdine and Schier, 2000; Capdevila et al., 2000; Whitman and Mercola, 2001). Despite the possible divergence in early mechanisms, however, they culminate in all species examined so far in the transient asymmetric LPM expression of a “L-side gene cassette”: Nodal, Lefty, and Pitx2. Such asymmetric expression may precede vertebrate evolution, as it is observed in ascidians and amphioxus (Boorman and Shimeld, 2002; Chea et al., 2005; Hudson and Yasuo, 2005; Morokuma et al., 2002; Yu et al., 2002), even if “L-sidedness” seems to be carried in a different germ layer. It is plausible that this gene cassette’s role in L-R asymmetry arose by redirecting a primitive role in specifying the oral-aboral axis, as in sea urchins (Chea et al., 2005; Duboc et al., 2005).
Gain-of-function experiments show that Nodal has asymmetry-instructive effects, and genetic loss-of-function experiments indicate its essential nature (Brennan et al., 2002; Capdevila et al., 2000; Cheng et al., 2000; Levin et al., 1997; Lowe et al., 2001; Saijoh et al., 2003; Sampath et al., 1997; Shiratori et al., 2001; Vincent et al., 2004). Asymmetric Nodal signaling directly induces Lefty and Pitx2 expression, which encode an antagonist and effector of L-R asymmetric signaling, respectively (Cheng et al., 2000; Juan and Hamada, 2001; Logan et al., 1998; Meno et al., 1999; Meno et al., 1998; Meno et al., 2001; Ryan et al., 1998; Yoshioka et al., 1998).
Probably because of its highly dynamic expression pattern, combined with the substantial tissue movement and rapid posteriorward node regression seen in some vertebrate embryos during early somitogenesis, there have been no concerted studies in mouse, chicken or zebrafish of the spatiotemporal pattern of Nodal expression in the LPM relative to anatomical landmarks, although a general anteriorward shifting of expression was reported in mice (e.g., Lowe et al., 1996). Fundamental gaps in our understanding therefore include how Nodal signaling in the L LPM is initiated and spatiotemporally regulated along the A-P axis as an instructor of asymmetric morphogenesis to the various organ primordia, and how the underlying molecular mechanisms are coordinated across the embryo to ensure an integrated morphogenetic process.
In all species, the mechanism initiating Nodal expression in the L LPM is poorly defined. In experiments in Xenopus, both the L and R LPM expressed Xnr1 when explanted away from axial midline tissues (Lohr et al., 1997). The addition of notochord to these explants suppressed expression (Lohr et al., 1998), which led to the proposal that LPM expresses Xnr1 by default, and that midline-derived tissues actively repress R-sided Xnr1 expression (Lohr et al., 1998; Lohr et al., 1997). However, Levin and Mercola (1998) showed that midline/node-type tissue could be formed in the “LPM-alone” explants, and suggested that positive-acting signals caused Xnr1 expression in both L and R LPM explants. The inference from these studies was that inductive signals are normally deployed specifically leftward in whole embryos, consistent with results from manipulating chicken embryos (Levin, 2005). In the mouse embryo, it has been suggested that Nodal itself is the node-derived positive inducing factor that travels to the L LPM to initiate Nodal expression (Yan et al., 1999; Lowe et al., 2001; Brennan et al., 2002; Saijoh et al., 2003). In support of this hypothesis, Nodal can exhibit long-range movement (Chen and Schier, 2001; Sakuma et al., 2002; Williams et al., 2004).
The ability of Nodal to autoinduce its expression in LPM may differ across species. In chicken embryos, Nodal-expressing cell pellets did not induce Nodal expression (Levin and Mercola, 1998). More recent studies, however, in early somitogenesis stage mouse embryos showed that Nodal-expressing tissue grafts, or electroporated expression vectors, did induce LPM expression of Nodal (Yamamoto et al., 2003). While the response to Xnr1 itself has not been demonstrated previously in Xenopus embryos, there is evidence that other TGFβ-related factors, which likely mimic Xnr1 to some degree, can induce Xnr1 expression when introduced into the LPM (Mogi et al., 2003; Toyoizumi et al., 2000; Toyoizumi et al., 2005). There is, however, no current evidence that these factors are normally expressed in or involved in L-R specification during tailbud stages; one issue addressed in the studies reported herein is the ability of Xnr1, specifically, to autoactivate its own expression within the LPM.
Embryological manipulations in Xenopus and mutant analyses in zebrafish and mouse have indicated that midline integrity is crucial for the development of proper L-R asymmetry (Danos and Yost, 1995; Danos and Yost, 1996; Izraeli et al., 1999; Lohr et al., 1997; Melloy et al., 1998; Rebagliati et al., 1998; Sampath et al., 1998). Analysis of mice deficient for Lefty1, a Nodal antagonist, indicated that left-sided Lefty1 expression in the prospective neural tube floor plate contributes to a midline barrier function that is proposed to prevent the wrong-sided diffusion of L-specifying signals (Meno et al., 1998). Observations of conjoined twins in human, frog, and chicken have led to the additional speculation that the midline may produce a R side-directed repressive signal that inhibits L-sided gene expression in the adjacent lateral regions of the twin, since asymmetry defects are only observed in the right-sided individual (Hyatt et al., 1996; Levin et al., 1997; Levin et al., 1996; Nascone and Mercola, 1997). While the midline seems to play a key role in the early establishment of asymmetry, it is uncertain if, and how, the midline plays a longstanding function in ensuring that L-R asymmetry is maintained in an integrated way across the entire embryo; we address this function during the phase of transient expression of the situs-instructive Nodal signal.
The mechanism underlying the establishment of the midline barrier during L-R patterning has remained vague. As stated above, Nodal can induce endogenous Nodal expression in the LPM of early mouse embryos, which also causes subsequent induction of Lefty1 expression in the midline. It has been proposed that, in the mouse, Nodal travels from the LPM to the midline to induce midline barrier function (Yamamoto et al., 2003).
In Xenopus, midline Xlefty expression occurs during two sequential phases of development. Xlefty expression is detected during gastrulation stages in the prospective dorsal midline tissues. This expression is maintained through early neurulation but becomes downregulated around the time of neural tube closure. Beginning at around stage 21, strong midline Xlefty expression, in a somewhat discontinuous pattern, is then re-established in the neural floorplate and hypochord (and transiently in notochord), in a P-to-A direction. At these stages, Xnr1 is already expressed in L LPM (Branford et al., 2000; Cheng et al., 2000). After completion of the mesendoderm inductive process, Organizer-derived Xnr signaling may be responsible for maintaining Xlefty expression in the prospective midline cells, and this early-phase midline expression may contribute midline barrier function during the period when asymmetric LPM gene expression begins to be instructed, as has been proposed by others (Danos and Yost, 1996; Lohr et al., 1997; Meno et al., 1998).
Here, we describe studies on the initiation of Xnr1 expression in the L LPM, its spatiotemporal expression pattern and the mechanism underlying its dynamic directional shift, and its subsequent inactivation. We show results suggesting that planar tissue communication, operating independent of axial tissues, underlies the rapid anteriorward expansion of Xnr1 expression, and that this process requires intercellular Xnr1 autoactivation. We demonstrate plasticity in L-R asymmetry at relatively late stages of embryogenesis and show conservation in the mechanism that initiates midline Xlefty expression. We present data strongly supporting the idea that orthogonal Xnr1 signaling from the LPM is responsible for the second-phase induction of midline Xlefty during late neurula/tailbud stages, in agreement with the recently published data in mouse (Yamamoto et al., 2003). Finally, we present evidence for tailbud-stage contralateral communication between the L and R LPM, via midline Xlefty, and discuss how this process may ensure that asymmetric morphogenesis occurs as a coordinated process between both sides of the embryo.
Materials and Methods
Embryo manipulations and microinjections
In vitro fertilized Xenopus embryos were microinjected in 1x Steinberg’s solution (SS; Kay, 1991) with 5% Ficoll, transferred at stage 9–9.5 to 0.1x SS; staging according to Nieuwkoop and Faber (1967). Xnr1, Xlefty, or Cerberus-short (Cer-S) (Piccolo et al., 1999) were placed in pCSKA plasmids to drive expression from early gastrulation (Condie et al., 1990). 8-cell embryos with differential dorsal/ventral pigmentation (Nieuwkoop, 1967) were injected into the right or left four blastomeres with CsCl-purified plasmids (in water) containing either β-galactosidase (150 pg total), Xnr1 (80 pg), Xlefty (150 pg), or Cer-S (150 pg). Injections were ~30° from the dorsal midline and ~20° above/below the equatorial cell boundary. Capped LacZ RNA (1.5 ng total; mMESSAGE mMACHINE kit; Ambion) was injected alone or together with plasmids for host-donor demarcation. pCSKA-β-galactosidase (pCSKA-497) encodes a nuclear-targeted form of β-galactosidase (gift from Richard Harland, UC Berkeley). pCSKA-Cer-S contained Cer-S protein-coding region in the pCSKA SmaI site, and pCSKA-Xnr1 and pCSKA-Xlefty were as published (Cheng et al., 2000; Sampath et al., 1997).
Microdissections and LPM Transplantation
Embryo dissections used a Gastromaster® dissector with 400, 800, or 1500 μm size square loop tips, to cut square (i.e., box-shaped) or, by tilting, V-shaped explants. Dissections and culturing were in 0.75x normal amphibian medium (NAM; Sive, 2000). For LPM grafts, a square-shaped piece of donor LPM+ectoderm (~200 cells total area; ~12–15 cells wide) was excised; endoderm was carefully detached before transplantation. Explants were therefore somatopleure (LPM plus overlying ectoderm); they are referred to as “LPM” for simplicity because Xnr1 is only expressed within that tissue layer. In hosts, a shallow pocket of similar size, shape, and depth to the graft was prepared in the L or R flank. Engrafted embryos were healed (5 min) before transfer to fresh 0.75x NAM. Good engraftment was assured by quality of edge matching and rapid healing; only high quality embryos were maintained and analyzed. For midline extirpations, 400 μm square tips were tilted to remove a segment with a V-shaped cross-section, aiming to remove neural tube floorplate, notochord, and hypochord (e.g., see Fig. 7), as checked by serial histochemical analysis of post-fixed embryos (not shown).
In situ hybridization/Red-gal staining/histological analysis
Whole-mount in situ hybridization was as described (Harland, 1991) with DIG-labeled Xnr1, Xlefty, and XPitx2 probes (Cheng et al., 2000; Jones et al., 1995; Ryan et al., 1998). Red-gal (6-chloro-3-indolyl-βD-galactoside; Research Organics) staining was a modification of standard protocols: embryos were MEMFA-fixed (1 hr, room temperature), washed 3–4x in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and stained with 1.0 mg/ml Red-gal in PBS/0.1% Triton X-100, 5 mM potassium ferro/ferricyanide, 2 mM MgCl2 (1 hr, room temperature). Embryos were washed (3x, PBS), MEMFA post-fixed, and stored (−20ºC, 100% methanol) until in situ hybridization. After in situ analysis, embryos were re-fixed (1 hr, room temperature) in Bouin’s fixative (LabChem, Inc.), dehydrated and equilibrated to Histoclear:paraplast (National Diagnostics; 1:1 ratio), and paraplast-embedded. 8–10 μm microtome sections were counterstained with a 3:1 mixture of 95% ethanol:eosin (Sigma).
Immunohistochemistry
Stage 42–45 embryos were MEMFA-fixed (2 hr, room temperature) and washed 3–4x in PBS. Whole-mount immunohistochemistry (Harland, 1991) used a 1:5 dilution (in PBS, 2 mg/ml BSA, 0.1% Triton X-100) of MF20 monoclonal antibody against all sarcomeric myosin heavy chains (Bader et al., 1982). Secondary antibody was Alexa Fluor 594-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR), 1:200 dilution. Embryos were placed in PBS and immunofluorescent images recorded by an Olympus DP70 camera and Olympus BH2 microscope with appropriate filters. Images from any single experiment were post-processed identically.
Results
Anterior shifting and transient expression of Xnr1 and Xlefty in the left LPM
We previously described L-sided Xnr1 expression in Xenopus embryogenesis and noted a general anteriorward shift of expression during tailbud stages (stage 19/20–25; Lowe et al., 1996). We characterized this pattern at higher resolution and at more stages and compared it to Xlefty, which encodes a major feedback inhibitor of Nodal/Xnr1 signaling (Fig. 1A). After downregulation of gastrula stage Xnr1 expression (Jones et al., 1995; Lustig et al., 1996), expression appears posteriorly in small bilateral perinotochordal domains near the chordoneural hinge. Shortly thereafter, Xnr1 is first expressed in posterior L LPM relatively close to these domains. L LPM expression subsequently undergoes a large-scale, posterior-to-anterior (P-to-A) shift. A stage of broad expression encompassing much of the embryo’s flank, in both splanchnic and somatic mesoderm (Lowe et al., 1996), is followed by an anterior/ventralward shift, and progressive P-to-A shutdown of expression, which results in the Xnr1 signal becoming restricted to a small territory just posterior to the presumptive heart anlage. This signal disappears at stage 26–27. The Xlefty expression pattern follows the dynamic Xnr1 shift, consistent with the idea that Xlefty is a direct response gene of Nodal/Xnr1 signaling (Cheng et al., 2000; Tanegashima et al., 2000).
Figure 1. Anteriorwards shifting ofXnr1 expression in L LPM requires tissue communication.
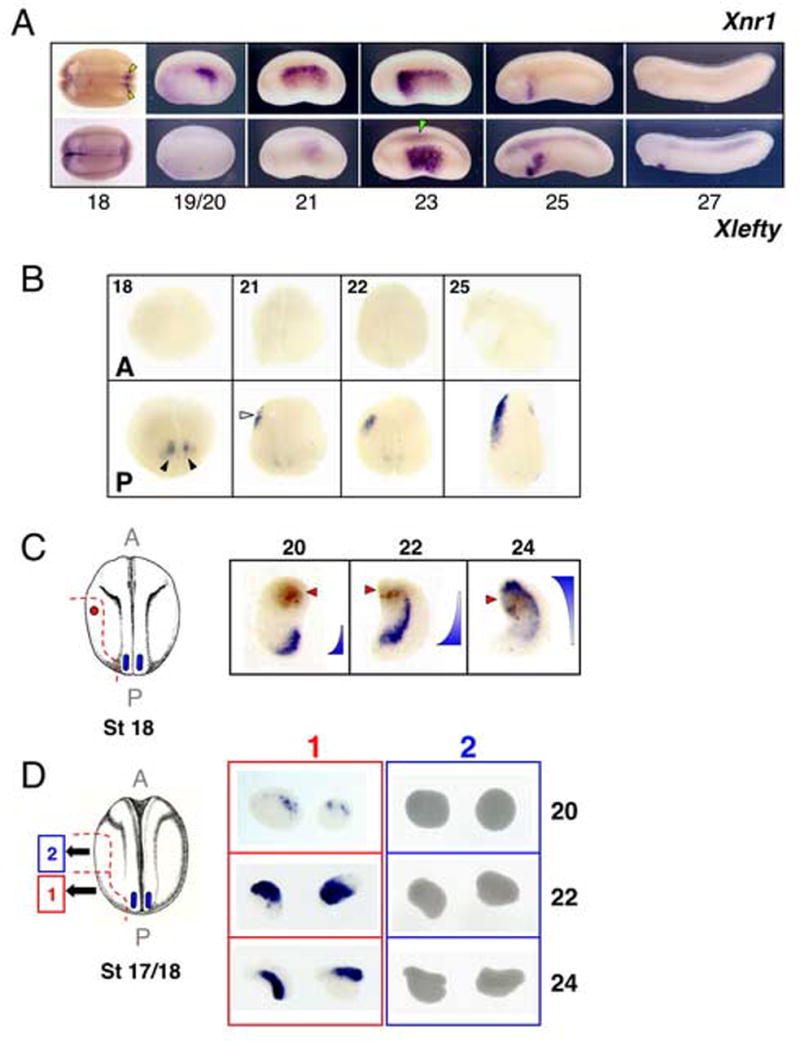
(A) Anterior shifting and transient expression of Xnr1 and Xlefty in L LPM. The dynamic expression pattern of Xnr1 in L LPM is mimicked with a temporal delay by Xlefty. The first Xnr1 expression at neurula/early tailbud is bilateral, flanking posterior notochord (yellow arrowheads). Asymmetric LPM expression begins at ~stage 19/20, shifts rapidly, and disappears by stage 27. Green arrowhead, axial Xlefty expression. Stage 18, dorsal view; other panels, lateral view. Anterior, left. (B) Transecting embryos (stage 18/19) prevents Xnr1 expression in anterior halves at all stages analyzed (stages indicated, panel top left). (Black arrowheads, perinotochordal Xnr1 expression; white arrowhead, L LPM Xnr1 expression). (C) LPM explants after neural tube closure, including tissue close to the bilateral posterior Xnr1 expression region, were marked anteriorly (Neutral Red; red arrowhead). Xnr1 expression shifted forward and showed graded expression as diagrammed. (D) Xnr1 expression in anterior explants (2) depends upon attached posterior tissue (1). Note perdurance of strong signal in posterior L explants at stage 24. A, anterior; P, posterior.
Requirement of posterior tissue for asymmetric activation of Xnr1 in LPM
We first readdressed the issue of whether asymmetric Xnr1 expression results from unilaterally directed positive signaling or R-side-specific inhibition, as proposed by Levin and Mercola (1998) and Lohr et al. 1997, respectively, as discussed in the Introduction. We found that two types of explants produced relevant information. The first explant type, from stage 15/16 embryos, was L or R mid-trunk LPM from the middle third of the embryo, with a dorsal limit ~20–25 cells below the intermediate mesoderm and a ventral limit approximately one-third from the embryo’s keel. Neither L nor R explants of this type developed Xnr1 expression at sibling stage 24 (Supplementary Fig. 1A). The second explant type included the same mid-trunk region, except that it was extended posteriorly to include a region approaching the tailbud and posterior-most axial tissue (Supplementary Fig. 1B). These explants therefore contained tissue encroaching upon the location where posterior bilateral Xnr1 expression develops at stage 17. In this case, both L and R explants expressed Xnr1, but we found L/R differences that varied with explantation stage. Stage 15/16 and stage 17 explants showed equivalently strong L and R expression when scored at sibling stage 24. Beginning at stage 18, R explants showed much less extensive and weaker Xnr1 expression than L explants, and this difference was more pronounced at stage 19 (Supplementary Fig. 1B). If explanted at stage 21/22, at a time when asymmetric Xnr1 expression in whole embryos is broad along the L LPM (Fig. 1A), R LPM explants did not develop Xnr1 expression (data not shown). Bisecting whole embryos along the axial midline at stage 15/16 led to L-sided Xnr1 expression only, despite the presence in both embryo-halves of the posterior perinotochordal Xnr1 expression domain (Supplementary Fig. 1C).
These data agree with the finding that removing the tailbud region encompassing the posterior bilateral Xnr1 expression from late neurula/early tailbud embryos (stage 17) led to the absence of asymmetric Xnr1, Xlefty, and (except as noted below) XPitx2 expression (Supplementary Fig. 2A). The lack of L-sided Xnr1 and Xlefty expression was associated with a lack of axial midline Xlefty expression. Control stage 20 extirpations, done just after asymmetric Xnr1 expression has initiated in posterior L LPM, developed robust expression of all three genes, including axial midline Xlefty (Supplementary Fig. 2B). In addition, in batches of embryos that lacked asymmetric Xnr1 expression, a substantial proportion (representative experiment, n=4/7) showed some L-sided XPitx2 expression that was much weaker than in sibling controls (Supplementary Fig. 2A). This result implies that non-Nodal-signaling mechanisms can induce L-sided Pitx2 expression, as reported in some genetic situations in the mouse (Constam and Robertson, 2000a; Constam and Robertson, 2000b; Meyers and Martin, 1999; Pennekamp et al., 2002).
The cardiac situs of posteriorly-cropped embryos was assessed at stage 43–45. In a population of embryos cropped at stage 17, heart looping was normally directed (22%), reversed (56%), or incomplete (22%) (Supplementary Fig. 2C). Our data are consistent with previous findings in the mouse, in which the absence of L LPM Nodal expression led to cardiac situs randomization (Brennan et al., 2002; Lowe et al., 2001; Saijoh et al., 2003). Cardiac situs remained normal in all control stage-20-cropped embryos (Supplementary Fig. 2C).
Preliminary data using pharmacological inhibitors of the Nodal signaling pathway support the idea that Xnr1 signaling from the posterior tailbud is required to initiate asymmetric Xnr1 expression in L LPM. Exposure of stage 17 embryos to SB-431542, a specific inhibitor of Type 1 Activin-Like Kinase receptors (ALK-4, -5, -7), prevented L LPM Xnr1 expression at later stages, without affecting the posterior bilateral Xnr1 expression (data not shown).
Overall, we conclude from these data that an inductive process involving Nodal/TGFβ signaling activates Xnr1 expression in L LPM, with the signal emerging from the region of the posterior tailbud that is the functional equivalent of the mouse and chicken embryonic node.
Directional expansion of Xnr1 expression is independent of the axial midline
One way of generating anteriorward-propagating Xnr1 expression in the L LPM could be that a developmental timing mechanism results in progressively anterior regions of the LPM activating Xnr1 expression slightly later than posterior neighboring tissue, with the activation not requiring contact with or signals from posterior tissue. In contrast, our results suggest a rolling-wave mechanism in which progressively anterior cell fields activate Xnr1 expression after induction from Xnr1 that is produced just-posteriorly. First, simple mid-trunk transection of stage 18/19 embryos, when Xnr1 expression has just begun in posterior L LPM, into anterior and posterior halves (which all developed well) prevented Xnr1 expression in anterior half-embryos (Fig. 1B). Stage 22 transections, performed when Xnr1 expression has just shifted into the anterior half, showed robust expression in both half-embryos. Subsequently (stages 23, 25, and 27 analyzed), there was expression domain shifting in the anterior half and downregulation in both the anterior and posterior half-embryos, similar to whole embryos (not shown). This result also shows that the development of anterior L LPM expression occurs via signaling from posterior LPM tissue, and does not require orthogonal induction from the trunk axial midline. The similar results obtained when L LPM explants alone were anterior-posterior transected (Fig. 1D) show that LPM integrity is required for anteriorward-propagating Xnr1 expression (Fig. 1C). We conclude that the directional P-to-A propagation of asymmetric Xnr1 expression requires planar tissue communication through the LPM and results from posteriorly-originated signals.
Autoregulation controls forward expansion of Xnr1 expression in the LPM
Evidence that an intercellular “rolling-wave” of Xnr1 autoinduction occurs in the L LPM came from the finding that small LPM transplants expressing Nodal-specific inhibitors could block the shift of Xnr1 expression. CerS, the short isoform of the secreted factor Cerberus, inhibits Nodal/Xnr signaling (Agius et al., 2000; Piccolo et al., 1999), and Xlefty was shown previously to block Xnr1 and XPitx2 expression in L LPM (Cheng et al., 2000). Each factor was encoded from pCSKA plasmids, to drive expression after gastrulation, and injected as a mixture with lacZ RNA lineage tracer. Control grafts were from embryos injected with lacZ RNA alone, or pCSKA-497 (encoding nuclear-targeted β-galactosidase). Based upon the fate maps, 8-cell-stage donor embryos were injected to enrich delivery to LPM (Methods). pCSKA-CerS or pCSKA-Xlefty LPM from stage 17 donor embryos was transplanted to a mid-trunk L-side location in stage 17 wild-type hosts (Fig. 2A). Engrafted embryos were cultured to stage 24/25 and analyzed for asymmetric Xnr1 expression.
Figure 2. Xnr1 inhibitors Xlefty and Cer-S suppress anteriorward shift of L-sided Xnr1 expression.
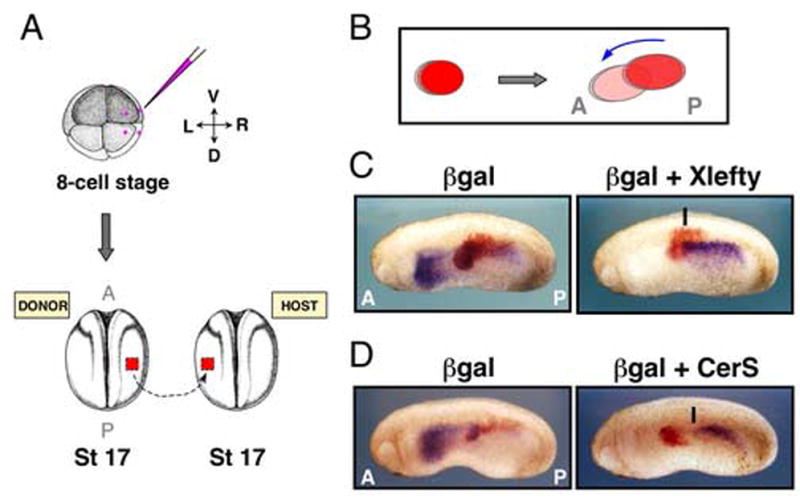
(A) 8-cell embryo injection with LacZ RNA +/− pCSKA-Xlefty or pCSKA-Cer-S enriched for LPM delivery (pink dots, injection points). R LPM was grafted mid-trunk into L LPM of host embryos. (B) Cartoon shows forward/ventral dislocation of graft LPM layer relative to overlying ectoderm that occurs after integration. (C,D) Stage 24/25 embryos showed shifting of Xnr1 expression through βgal-alone transplants, but suppressed or delayed shifting for Xlefty or Cer-S (black lines, anterior limit of Xnr1 expression). L, left; R, right; D, dorsal; V, ventral; A, anterior; P, posterior.
Grafts expressing either inhibitor blocked the forward expansion of Xnr1 expression (Fig. 2C,D; Table 1). The suppression of L-sided Xnr1 expression in host embryos receiving CerS- or Xlefty-expressing LPM grafts was of two types: complete absence of Xnr1 signal, or partial suppression in which Xnr1 expression shifted up to the posterior edge of the graft, and very seldom into its posterior margin (Fig. 2C,D; Table 1). In CerS-grafting experiments, ~75% of embryos showed complete or partial suppression. For Xlefty-expressing grafts, ~73% of embryos showed no signal or anteriorly halted expression (Fig. 2C,D; Table 1). The proportion of embryos (~20–30% overall) showing no effect on anteriorly shifting Xnr1 expression could reflect a dependence of the graft’s ability to block Xnr1 autoregulation upon the level of CerS and Xlefty produced from it, very likely including problems caused by the inevitable large-scale mosaic inheritance pattern of the non-chromosomally integrated factor-producing plasmids (e.g., see embryos in Fig. 3B). In embryos showing no L-sided expression, a high inhibitor level might have caused an early block to the beginning-stage rolling wave of Xnr1 expression within the L LPM (adding to the suppression caused by endogenous Xlefty expression). Alternatively, it may have completely prevented Xnr1 expression from initiating in the posterior L LPM. The control grafts did not perturb the Xnr1 expression pattern (Fig. 2C,D; Table 1). We conclude that once its asymmetric expression is initiated in L LPM, Nodal/Xnr1 signaling is required for the forward propagation of Xnr1 expression (Fig. 6).
Table 1.
Xnr1-specific inhibitors suppress anteriorward shifting of Xnr1 expression
| Donor graft | n embryos (#expts. pooled) | n (%) complete suppression | n (%) partial suppression | n (%) no suppression |
|---|---|---|---|---|
| LacZ alone | 49 (6) | - | - | 49 (100) |
| p497/LacZ | 9 (1) | - | - | 9 (100) |
| pCerS/LacZ | 28 (3) | 3 (11) | 18 (64) | 7 (25) |
| pXlefty/LacZ | 30 (3) | 5 (17) | 17 (57) | 8 (27) |
Donor grafts injected with LacZ RNA lineage tracer plus pCSKA/497, pCSKA/CerS, pCSKA/Xlefty; or LacZ alone were transplanted to the left side of host embryos at stage 17 and engrafted embryos were analyzed for Xnr1 expression at stage 24–25. All data refer to Figure 2.
Figure 3. Xnr1 induces Xnr1 expression in R LPM, which undergoes stereotypic P-to-A shifting.
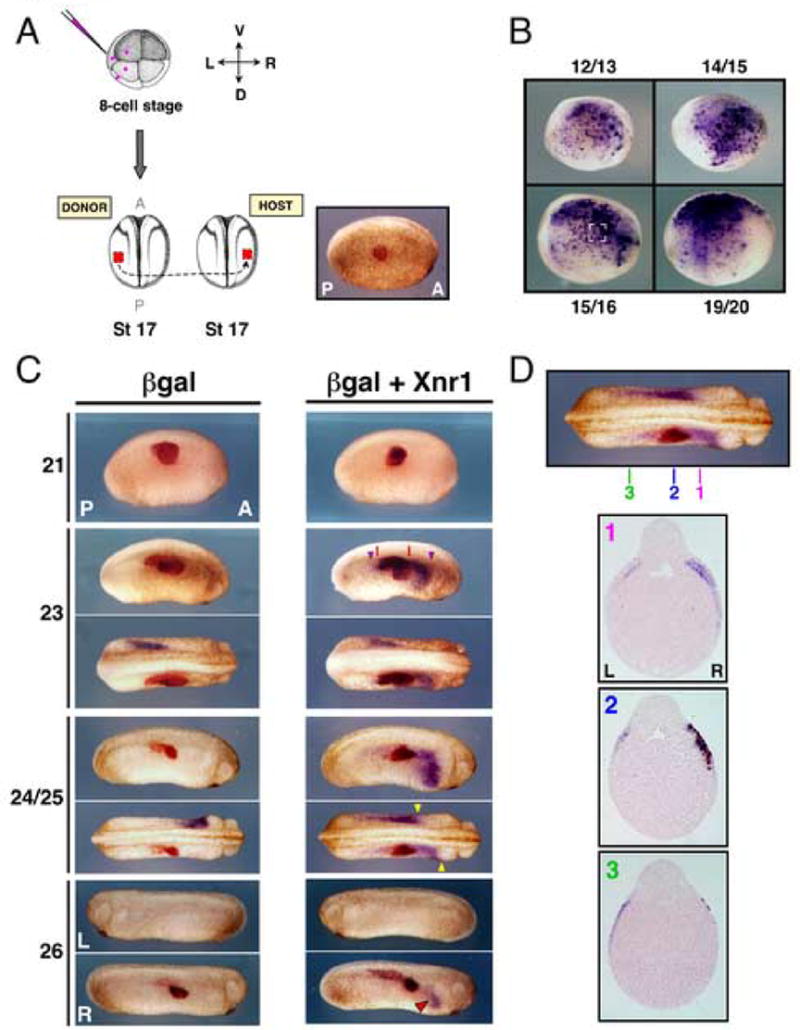
(A) 8-cell embryos injected as in Fig. 2 produced L LPM grafts that were placed into mid-trunk R LPM locations in stage 17 hosts. Right panel: engrafted embryo shortly after healing (red-gal stained), demonstrating medial placement. (B) Donor embryos injected with pCSKA-Xnr1 showed mosaic strong Xnr1 expression in L LPM from early neurula stage onward. Bracketed area (stage 15/16 panel) indicates explant size used in grafts. (C) βgal-alone engraftment did not induce R-sided Xnr1 expression at any stage (stages indicated left of panel). Xnr1-engrafted hosts showed extensive, anterior shifting, R LPM Xnr1 expression. At stage 23, R-sided Xnr1 expression had begun to shift significantly anterior-ward, with only limited posterior shifting. Red lines, A-P boundaries of graft. Purple arrowheads, A-P limits of induced Xnr1 expression. At stage 24/25, the anterior limit of R-sided Xnr1 expression was farther anterior than the endogenous L expression, as indicated by yellow arrowheads (dorsal view, embryo shown in panel above). At stage 26, R-side induced Xnr1 expression was prolonged compared to L side expression (red arrowhead). (D) Transverse sections, stage 24/25 Xnr1-engrafted embryo: grafts showed good laminar alignment with host tissues. Induced Xnr1 expression was restricted to R LPM. Sections as indicated demonstrate that R-sided induced Xnr1 expression progressed farther anterior than endogenous L-side expression, with minimal posterior shifting of Xnr1 expression. L, left; R, right; D, dorsal; V, ventral; A, anterior; P, posterior.
Figure 6. Model for asymmetric Nodal/Xnr1 signaling during L-R specification.
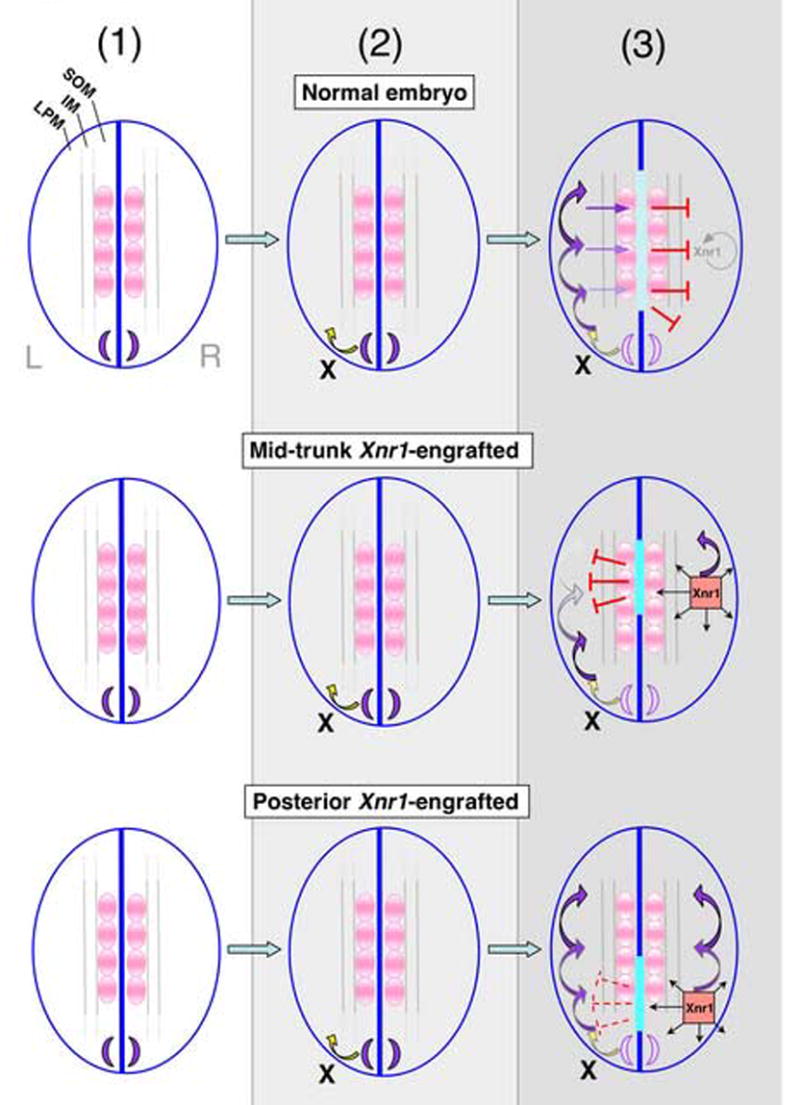
The transfer and propagation of L-R asymmetry is divided conceptually into three steps (arrows, direction of signal transfer. (1) Stage 17/18 normal embryos (top row), Xnr1 is first expressed symmetrically flanking posterior notochord (purple crescents). (2) At stage 19/20 an asymmetric inducing factor (X) initiates Xnr1 expression in posterior L LPM. (3) Between stages 21 and 25 a rolling wave autoactivation loop expands Xnr1 expression anteriorward. Orthogonal Xnr1 signaling from L LPM induces Xlefty expression in the midline (light blue bar) and rightward transfer of Xlefty prevents inappropriate activation of an Xnr1 autoregulatory loop in R LPM (SOM, somitic mesoderm; IM, intermediate mesoderm; LPM, lateral plate mesoderm). Middle row: effect of R-side mid-trunk Xnr1 grafts. Xnr1 induced in the R LPM causes orthogonal induction of robust ectopic midline Xlefty expression (turquoise bar); Xlefty travels contralaterally and suppresses the anterior shifting Xnr1 expression on the L. Accordingly, the R side becomes the dominant L side, and causes a concordant reversal of anatomical situs. Bottom row: with posterior Xnr1 grafts, orthogonal Xlefty induction does not precondition L LPM against the continued expansion of endogenous L-sided Xnr1 expression. The lack of a spatial advantage of R over the L (i.e., no “head-start”) leads to a competitive double-left situation; across the population, either side adopts “dominant L” status, causing randomization of situs that is concordant within each embryo.
Xnr1 induces Xnr1 in tailbud stage LPM
Although it has been demonstrated that various TGFβ-related factors (Activin, TGF-β5, and mouse Nodal), can induce robust Xnr1 expression in Xenopus embryos (Mogi et al., 2003; Toyoizumi et al., 2000; Toyoizumi et al., 2005), the response to Xnr1 itself has, however, not been demonstrated. As described below, our experiments go further than those previously published and, in some cases, have distinctly different findings.
Using the grafting method described above, we targeted pCSKA-Xnr1 to the L or R LPM of donor embryos, transplanted Xnr1-expressing grafts at stage 17 to a R-side mid-trunk location in wild-type hosts (Fig. 3A), and analyzed Xnr1 expression at various stages thereafter. Host Xnr1 expression was first detected both anteriorly and posteriorly of the graft at stage 22/23 (Fig. 3C; Table 2), slightly later than the time when endogenous Xnr1 expression is initiating in the posterior L LPM in normal embryos (Fig. 1A). Because the pCSKA-derived Xnr1 expression in donor embryos was strong at all stages between 12/13 and 19/20 (Fig. 3B), the stage 17 grafts were likely already producing significant amounts of Xnr1. We therefore attribute the inability to induce earlier R-sided endogenous Xnr1 expression to either an intrinsic competence window in the LPM to respond to Xnr1, or a requirement to reach a specific (unknown) threshold of graft-derived Xnr1.
Table 2.
Gene expression data, R side Xnr1-engrafted embryos
| A) Mid-trunk placement | |||||||
|---|---|---|---|---|---|---|---|
| Stage | Gene | n embryos (#expts. pooled) | n (%)L-sided | n (%)R-sided | n (%)bilateral | % Anterior truncation (%truncated plus suppressed) | Figure |
| St. 23 | Xnr1 | 12 (2) | 2 (17) | - | 10 (83) | - | Fig. 3 |
| St. 24/25 | Xnr1 | 21 (3) | - | - | 21 (100) | 90 (47) | Fig. 3 |
| St. 26 | Xnr1 | 9 (1) | - | 9 (100)* | - | - | Fig. 3 |
| St. 24/25 | Xlefty | 16 (3) | 2 (12) | - | 14 (88)† | 100 (0) | Fig. 4 |
| St. 28 | XPitx2 | 13 (2) | 2 (15) | 1 (8) | 10 (77) | 60 (0) | Fig. 4 |
| B) Posterior placement | |||||||
| Stage | Gene | n embryos (#expts. pooled) | n (%)L-sided | n (%)R-sided | n (%)bilateral* | Figure | |
|
| |||||||
| St. 24/25 | Xnr1 | 10 (1) | 2 (20) | - | 8 (80) | Fig. 5 | |
| St. 24/25 | Xlefty | 11 (2) | 1 (9) | 2 (18) | 8 (73) | Supp. Fig. 4 | |
| St. 28 | XPitx2 | 5 (1) | - | - | 5 (100) | Supp. Fig. 4 | |
| C) Mid-trunk placement plus midline extirpation | |||||||
| Stage | Gene | n embryos (#expts. pooled) | n (%)L-sided | n (%)R-sided | n (%)bilateral* | Figure | |
|
| |||||||
| St. 24/25 | Xnr1 | 6 (1) | - | - | 6 (100) | Fig. 5 | |
| St. 24/25 | Xlefty | 13 (2) | - | 1 (8) | 12 (92) | Supp. Fig. 6 | |
| St. 28 | XPitx2 | 6 (1) | - | - | 6 (100) | Supp. Fig. 6 | |
R-side βgal-engrafted controls showed normal L-side expression and anteriorward progression of Xnr1 (at St.24/25), Xlefty and XPitx2, respectively: n=14/14; n=12/12; n=9/9.
All R-side mid-trunk Xnr1-engrafted embryos at stage 26 showed perdurant Xnr1 expression in R LPM whereas endogenous L-sided expression had disappeared.
Ectopic midline and R side dorsal endoderm expression was also observed in 100% of engrafted embryos showing bilateral Xlefty expression (see text).
Posterior placement of R side Xnr1-graft resulted in mirror image L & R expression. Of embryos with bilateral expression, no suppression of anterior progression or intensity of L-sided expression was observed compared to the graft-induced R side expression.
Removal of midline tissues in mid-trunk R side Xnr1-engrafted embryos led to mirror image L & R expression.
By stage 24/25, the induced R-sided Xnr1 expression had shifted substantially anterior of the graft but minimally, if at all, posteriorly. Under these conditions, the induced R-sided Xnr1 expression extended farther forward than the endogenous L LPM expression, which itself had become stalled (extended less anteriorly) in comparison with unmanipulated or βgal control embryos (Fig. 3C; Table 2). For example, while R-sided expression abutted the presumptive heart anlage, L LPM expression had extended only mid-way through the LPM (Fig. 3C; Table 2). Another outcome occasionally observed (not shown) was that endogenous L-sided Xnr1 expression, in addition to anterior stalling, was overall significantly weaker than that induced on the R side, or seen in the L LPM of control embryos (Table 2). At stage 26/27, pCSKA-Xnr1/LPM engrafted embryos showed prolonged Xnr1 expression specifically in the anterior region of the R LPM, but Xnr1 expression was not detected around or within the graft, and there was no L-side signal (Fig. 3C; Table 2). It should be noted that L-sided Xnr1 expression disappears by this stage in normal embryos. LPM grafts from the L or R side of donor embryos induced R-sided host Xnr1 expression equivalently (not shown), suggesting that the meaningful signal is the ectopic Xnr1, with no requirement for additional L LPM tissue-derived signals.
Xnr1 activates L-sided gene expression program in R LPM and inverts situs
The graft-induced Xnr1 activated a robust L-side gene expression program in the R LPM as judged by the induced expression of Xlefty and XPitx2 at stage 25 and 28, respectively (Fig. 4A,B). Xlefty expression was strongly induced in the R LPM from the pCSKA-Xnr1 graft and, as for Xnr1, R-side LPM Xlefty expression in engrafted embryos extended more anteriorly than the endogenous L LPM expression, although we note that suppression of the level of L-sided Xlefty expression was not observed (Fig. 4A; Table 2). βgal controls showed the anterior-ventral localization of Xlefty expression within L LPM that is normally observed at stage 25 (Fig. 1A).
Figure 4. R-sided Xnr1 activates L-side gene expression program, midline Xlefty expression and inverts situs.
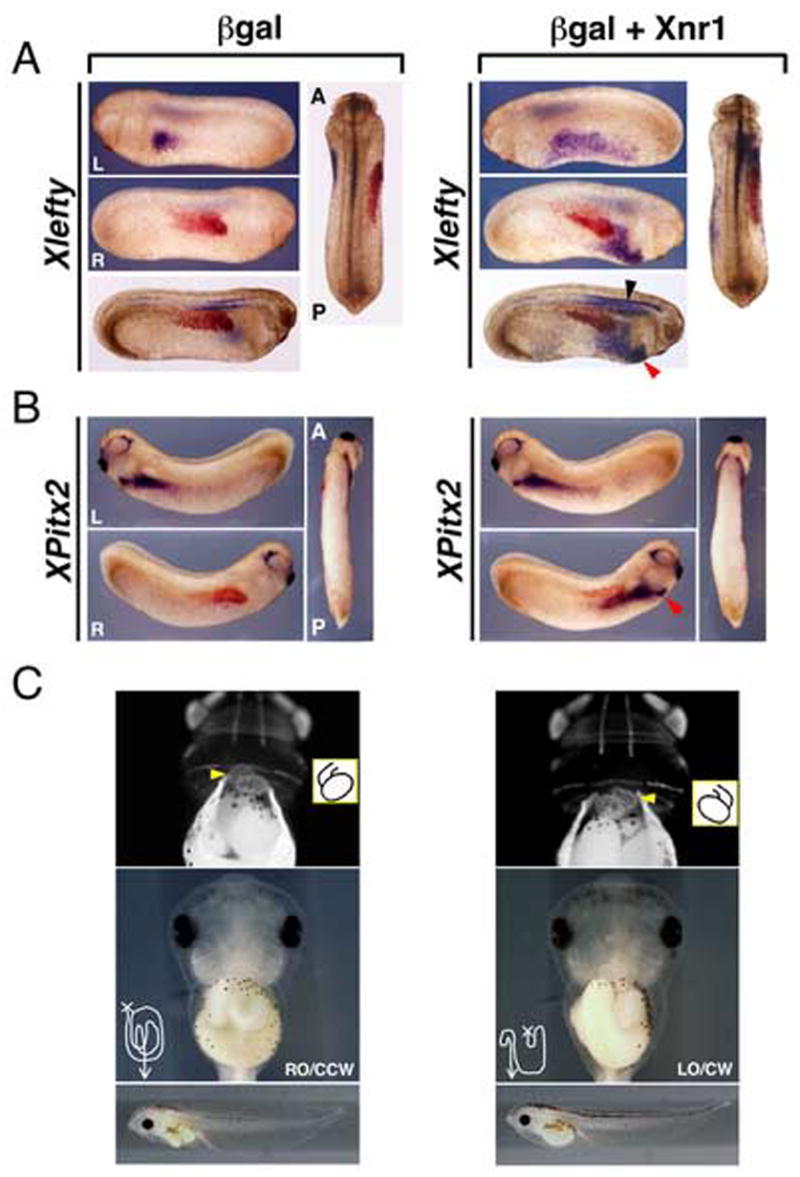
(A) While stage 25 βgal control-engrafted embryos (red-gal stained) showed no Xlefty expression, robust R-sided LPM expression (red arrowhead) was induced by Xnr1 grafts. Similar to Xnr1, R-sided Xlefty expression extended farther anterior than endogenous L-side expression. Strong induction of midline Xlefty expression orthogonal to the Xnr1-expressing grafts was detected (black arrowhead). (B) XPitx2 expression was induced in R LPM of stage 28 Xnr1-engrafted host embryos (red arrowhead), not by β-gal controls. (C) All embryos receiving βgal alone R-side grafts had normal cardiac and gut situs (stage 43–45: top panels, indirect immunofluorescence, MF20 antibody; middle panels, brightfield ventral views; bottom panels, lateral views, same embryos). All Xnr1-engrafted embryos showed concordant reversal of heart and gut looping, otherwise appearing normal (bottom panels). Careful gut uncoiling showed an overall reversed chirality, but with some disruption of architecture, although it did not resemble an inverted earlier-stage gut. Because grafts healed well, and control βgal engrafted embryos had normal gut coiling, this defect is likely not associated with the surgery per se, but because R-sided expression of Xnr1, and potentially its downstream targets, was prolonged compared to endogenous L-sided expression (see Fig. 3C). Yellow arrowheads, outflow tract; inset, diagram of heart looping; line drawings, gut tube coiling after partial unwinding. RO, right origin; LO, left origin; CCW, counter-clockwise; CW, clockwise. L, left; R, right; A, anterior; P, posterior.
In the Xnr1-engrafted embryos, a strikingly high level of Xlefty expression was detected in the midline perpendicularly closest to the graft, most notably enhanced in the notochord in cleared whole-mounts (Fig. 4A; Table 2). This effect was not observed in βgal controls; midline expression was restricted primarily to the neural tube floorplate and hypochord, comparable to the expression in unmanipulated sibling stage embryos (Fig. 4A; Table 2). These results strongly suggest that the mid-trunk region R-sided LPM Xnr1 expression signals orthogonally, and over a long range, to induce midline Xlefty expression. The result is also consistent with our finding that midline Xlefty expression was lost in posteriorly cropped embryos that lack L LPM Xnr1 expression. Engraftment of Xnr1-expressing LPM into posteriorly cropped embryos was able to induce robust and anteriorly shifting Xnr1 and Xlefty expression, which was associated with the restoration of axial Xlefty expression (Supplementary Fig. 3B).
Additionally, whereas βgal-engrafted control embryos all showed the L-sided dorsal anterior endoderm expression that is normally detected between stages 22–25 (Cheng et al., 2000), R-sided Xnr1-expressing grafts inverted this expression domain to the R anterior dorsal endoderm (Fig. 4A; Table 2).
XPitx2 was expressed at relatively equal intensities on both the L and R sides of Xnr1-engrafted embryos, although a substantial proportion showed induced R-sided XPitx2 expression that had progressed more anteriorly than on the left, as noted for Xnr1 and Xlefty (Fig. 4B; Table 2). Again, we infer that XPitx2 expression, induced in the R LPM by the robust and anteriorward-shifting Xnr1 expression, because of the mid-trunk graft placement, had a head-start in progressing anteriorly compared to the endogenous L LPM expression. In contrast, however, to the anterior truncation observed for L-sided Xnr1 and Xlefty expression in mid-trunk R side Xnr1-engrafted embryos, there was only an incremental difference in the anterior limits of the L versus R side XPitx2 expression domains.
Figure 4C shows that while embryos receiving control grafts exhibited normal cardiac and gut situs, there was a concordant reversal of heart and gut asymmetry in all pCSKA-Xnr1/LPM engrafted embryos (Table 3). This result is consistent with the idea that the induced R LPM Xnr1 expression, which is stronger, reaches more anteriorly, and is longer lasting than the endogenous L-sided expression, converts the R side to a dominant “L-sided specification state”, in accordance with the idea that Xnr1 is a true L-side instructive signal.
Table 3.
Morphological consequences of R side Xnr1-engraftment
| Donor graft | Graft position | n embryos (#of expts.) | n (%) Heart/ gut normal | n (%) Heart/ gut reversed* | Figure |
|---|---|---|---|---|---|
| LacZ alone | mid-trunk | 6 (1) | 6 (100) | - | Fig. 4 |
| pXnr1/LacZ | mid-trunk | 8 (1) | - | 8 (100) | Fig. 4 |
| LacZ alone | posterior | 6 (1) | 6 (100) | - | Supp. Fig. 5 |
| pXnr1/LacZ | posterior | 10 (1) | 5 (50) | 5 (50) | Supp. Fig. 5 |
Embryos engrafted at stage 17 in either a mid-trunk or posterior position of the R LPM were scored at stage 43–45 for heart and visceral orientation.
In cases where situs was inverted, both heart and gut situs were concordantly reversed (i.e., in one embryo both heart and gut were reversed).
Xnr1-mediated L-R switching depends upon the Xnr1-expressing graft location
The dominant L-R inversion caused by R-sided Xnr1 grafts depended upon their A-P location. Our working hypothesis was that the mid-trunk placement caused orthogonal midline induction of high levels of Xlefty, which by long-range leftward movement preconditioned the L LPM and interfered with the autoregulation-based anteriorward propagation of L-sided Xnr1 expression (Fig. 6). Our prediction was that more posterior engraftment would limit the “head-start” situation and allow the L-sided Xnr1 expression to escape contralateral blocking, and to undergo a more normal anteriorward shift. In this situation, a competitive “double-left” situation might develop with respect both to Xnr1 expression and L-R morphogenesis. Figure 5B and Supplementary Figure 4B show that more posterior grafts indeed led to mirror-image L- and R-sided Xnr1, Xlefty, and XPitx2 expression (Table 2). Randomization of heart and gut looping was observed in these embryos (50% normal: 50% reversed across the group, but concordant within each embryo; Supplementary Fig. 5C, Table 3). We conclude that a competitive double-left situation leads to a stochastic choice of one side or the other as the dominant left.
Figure 5. Posterior placement of R-side Xnr1 grafts or midline extirpation of mid-trunk engrafted embryos causes mirror-image expression of Xnr1.
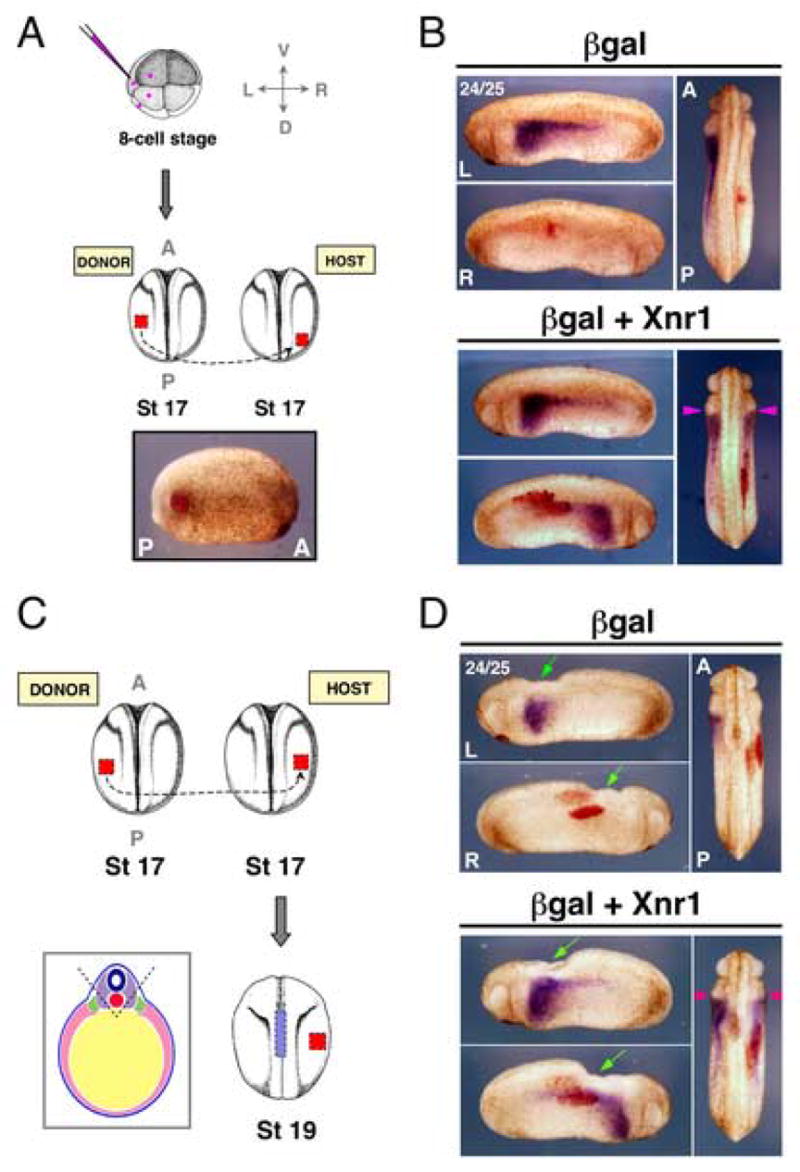
(A) Plasmids were targeted to the LPM as in Fig. 2. Compare posterior placement shown to medial location in Fig. 3 (engrafted embryo shortly after healing, red-gal stained). (B) βgal control-engrafted embryos (red-gal stained) showed endogenous L-sided expression of Xnr1 in LPM, and no R-sided expression. Host embryos carrying posterior R-side Xnr1 grafts developed mirror-image Xnr1 expression with equivalent anterior limits in L and R LPM (pink arrowheads). (C) Stage 17 Xnr1+βgal-expressing L LPM was mid-trunk grafted to the R LPM as in Fig. 3. At stage 19, midline orthogonal to the graft was removed (i.e., neural floorplate, notochord, and hypochord; A-P limits indicated by blue hatched bar). Left-hand panel, diagrammatic representation of tissue removed (neural tube, dark blue; notochord, red; paraxial mesoderm, purple; intermediate mesoderm, green; LPM, pink; endoderm, yellow; ectoderm, light blue). (D) βgal control-engrafted embryos showed normal L-specific Xnr1 expression. Midline extirpated embryos with mid-trunk placed R-side Xnr1-expressing grafts developed equivalent anterior limits of Xnr1 expression in both L and R LPM (pink arrowheads). Green arrows, midline area removed. Embryo stage indicated in top left of panel. L, left; R, right; D, dorsal; V, ventral; A, anterior; P, posterior.
Extirpation experiments showed that the axial midline was required for the contralateral suppressive effect on L-sided Xnr1 expression by the mid-trunk R-sided grafts (Fig. 5D, Supplementary Fig. 6B). Xnr1-expressing grafts were placed in the mid-trunk R LPM at stage 17 and the embryos developed until stage 19/20, when approximately half of each group underwent localized midline extirpation. Xnr1 expression was then compared later (stage 24/25), when its expression has shifted relatively far forward (Fig. 1A), between extirpated and non-extirpated Xnr1/βgal or βgal-alone-engrafted embryos, and to unmanipulated siblings. The posterior limit of the removed midline region was set approximately to the posterior graft margin. The anterior limit was just anterior of the graft’s anterior margin, to take into account the forward dislocation of the graft LPM relative to the ectoderm (e.g., Fig. 2B). Embryos with midline integrity reproduced the contralateral block on the anterior shift of L-sided Xnr1 expression (as in Fig. 3C,D). In contrast, midline removal prevented communication between the L and R sides; extirpated embryos showed bilateral Xnr1 expression of equivalent intensity and anterior progression (Fig. 5D, Supplementary Fig. 6B; Table 2). Extirpations from R-side βgal control-engrafted embryos did not affect L-sided Xnr1 expression compared to non-extirpated βgal control or unmanipulated embryos (this latter result agrees with findings that extirpating midline tissues after neural plate closure (stages 20–28) does not significantly alter cardiac situs (Danos and Yost, 1996). The result with Xlefty was similar: anteriorward shifting was blocked on the L side without midline extirpation, but became bilaterally equivalent with extirpation (Supplementary Fig. 6B; Table 2). The finding that the ability of the R-sided graft to suppress the forward propagation of Xnr1 expression within the L LPM is prevented by the local removal of a strip of midline tissue orthogonally closest to the Xnr1-expressing graft supports the idea that the relevant event is the induced high level of midline Xlefty expression (Fig. 6).
Discussion
Our finding that posterior inductive signals from the tailbud region of Xenopus embryos induce L-sided Xnr1 expression supports the idea that a conserved asymmetrically directed inducer emanates from the node or its functional equivalent in all vertebrate species. While studies on various Nodal and Cryptic mutants in the mouse suggest that Nodal signaling from the node is involved in initiating Nodal expression in the L LPM, there has, however, been little to no study in any vertebrate of the mechanism underlying the spreading of Nodal expression within the LPM after its initiation. Our inhibitor results suggest that Xnr1 autoregulation is a required component of the mechanism for the rapid and unidirectional anteriorward propagation of its expression domain. We also provided evidence that embryonic L-R asymmetry, determined by Xnr1 signaling activity from the LPM, remains plastic until stages that are close to the actual onset of asymmetric morphogenesis. In this latter respect, it is possible that there is no specification of definitive L or R fates, but that the earlier L-R biases only becomes fixed by the structurally irreversible process of asymmetric morphogenesis.
In addition to the role that the midline plays in preventing the incorrect transfer to the R side of leftness-inducing signals that are active in the left LPM, our results support the idea that, in normal embryos, Xlefty, induced orthogonally by L LPM-derived Xnr1, diffuses from the midline into the R LPM and helps to prevent the spurious ectopic expression of “L-specifying” genes. Such a process may be particularly important in suppressing the R-sided activation of genes whose expression is subject to self-amplification, such as Xnr1 (Fig. 6).
Induction of asymmetric Xnr1 expression during Xenopus embryogenesis
Lohr et al. 1997 proposed that L or R LPM expresses Xnr1 by default, and that R-side specific inhibition causes the L-specific expression seen in normal embryos, while Levin and Mercola (1998) suggested that asymmetric L-specific positive induction is involved. Our results are largely consistent with, but extend the findings of, Levin and Mercola (1998). We showed that Xnr1 expression does not develop simply as a default condition within LPM removed from the repressive influence of the midline, but that an inductive signal is asymmetrically deployed from the tailbud region, the area of nascent mesoderm formation and bilateral Xnr1 expression. These conclusions were generated from both posterior cropping and LPM explantation experiments (Supplementary Figs. 1, 2).
Studies in the mouse embryo suggest that Nodal signals originating from the node are involved in initiating Nodal expression in the L LPM. Embryos lacking node Nodal expression because of a deletion of specific cis-regulatory regions show an absence of L LPM Nodal expression (Brennan et al., 2002; Saijoh et al., 2003). Consistent with these findings, we showed that disruption of Xnr1 signaling from the posterior tailbud, either by extirpation or pharamacological approaches, abolished L LPM Xnr1 expression. These results imply that the posterior bilateral perinotochordal Xnr1 expression domains are functionally equivalent to the bilateral Nodal expression in the 0-7 somite-stage mouse node (Lowe et al., 1996). In contrast to the spreading of Xnr1 expression through the L LPM, Xnr1 autoregulation is apparently not involved in maintaining the posterior bilateral tailbud expression, because it was unaffected by the Nodal receptor inhibitor SB-431542.
Dynamics of asymmetric Xnr1 expression and LPM plasticity during L-R specification
Yamamoto et al. 2003 showed that Nodal-expressing LPM grafts or Nodal expression vectors could induce Nodal expression in the LPM of early somite-stage mouse embryos. Intriguingly, the electroporation of Nodal expression vectors into the R LPM caused an extensive spreading of Nodal expression along the A-P axis, although the authors did not speculate on the underlying mechanism. Based on their report, one cannot conclude that the locally electroporated vectors induced bidirectionally shifting Nodal expression, or the degree to which the expression in the LPM became expanded by the rearward movement of Nodal-expressing cells in association with the movements driving node regression. Future time-course studies of the expansion of Nodal expression with respect to the position of the node might gain insight in this respect.
We demonstrated that Xnr1-expressing grafts caused R-sided induction of the Xnr1, Xlefty, and Pitx2, which underwent the dynamic directional shift that occurs in the L side of normal embryos (we note here the substantially prolonged Xnr1 expression observed at the end phase; Fig. 3C). Our results disagree with those of Toyoizumi et al. 2005, who use hypodermic injection to deliver bacterially expressed and refolded mouse Nodal to the R LPM of neurula/tailbud stage Xenopus embryos. Toyoizumi et al. detected the induced expression of Xnr1 and XPitx2, but not of Xlefty, a surprising finding as Xlefty is a direct downstream target of Xnr1 signaling (Cheng et al., 2000; Tanegashima et al., 2000). Toyoizumi et al. 2005 also concluded that mouse Nodal could not activate the autoregulatory Xnr1 expression loop in the R LPM. While their hypodermic delivery method allows easier control over the time of ligand presentation than our plasmid expression/grafting methods, our method may be advantageous in misexpressing Xnr1 itself from its normal source tissue, thereby presumably presenting this intercellular signal in a state much closer to that encountered in normal embryos.
While mid-trunk Xnr1-expressing grafts did initially induce host Xnr1 expression both posterior and anterior of the graft, the subsequently induced host Xnr1 expression shifted only anteriorly, revealing an inherent directionality within the LPM in the ability to propagate an Xnr1 autoregulated expression wave. Further work will be needed to determine if a specific repressive influence works to oppose a posteriorward Xnr1 expression wave. We speculate that anterior cues may somehow be given by the anterior movement of the graft’s LPM layer relative to the overlying epidermis (e.g. Fig. 2B), a movement of the interior germ layer that could be considered similar to that undergone by the endoderm relative to the rest of the tailbud-stage embryo (Chalmers and Slack (2000). It is, however, not known how this displacement might orient the Xnr1 autoregulatory wave. Another possibility is that Xnr1 is somehow moved vectorially within the plane of the LPM through the anteriorly-disposed cell surfaces in association with some form of planar cell polarity.
A potential limitation of the rolling-wave Xnr1-to-Xnr1 model comes from considering the observed speed of Xnr1 expression shifting in the LPM as compared to the time required for transcription, translation, propeptide processing and secretion, ligand diffusion/transport, receptor binding and intracellular signal transduction. The time from posterior initiation of Xnr1 expression to the maximal forward progression of expression in the L LPM can be estimated at ~6–8 hours. Studies on TGF-β signaling have shown that peak levels of phosphorylated Smad2 are detected as soon as 0.5–1 hour after ligand addition (Di Guglielmo et al., 2003; Lo and Massague, 1999). The 8-hour expression-shift-period could be sufficient if the underlying mechanism was not a long series of individual cell-to-cell signaling events along the entire LPM, but as a lower number of “block steps” between broad fields of cells. While our data strongly support the idea that Xnr1 autoregulation is a required part of the anteriorward-shifting process, there may be an additional and faster tissue communication process, acting synergistically with Xnr1 autoactivation, which contributes to the rapid field-propagation.
Following the P-to-A expression shift, Xnr1 expression is progressively downregulated in the same direction. The inactivation wave may be directly connected with the induced expression of Xlefty, which mimics Xnr1 with a spatiotemporal delay, as expected for a direct target of Xnr signaling. The model arising is that Xlefty inhibits the Xnr1 autoregulatory loop and shuts off Xnr1 expression, thereby ensuring transient Nodal signaling. While delayed expression of Lefty2 with respect to Nodal was noted during gastrulation stages in the mouse embryo (Juan and Hamada, 2001), more precise comparative studies will be needed to determine if this relationship holds true during during early somitogenesis stages.
Our results further confirm the view that unilateral Xnr1 expression is the asymmetry-instructive event that the preceding L-R biases converge towards. We have found conditions in which altering the relative level of Xnr1 expression, or the timing of its production from LPM to the organ primordia, can dominantly invert L-R anatomy. Mogi and Toyoizumi (2000, 2003) showed a rapid decline at stages 26–28 in the ability of Activin or TGFβ5 to reverse embryonic situs. Their observations support the model that it is the transient Xnr1 wave in the LPM that is the main determinant of asymmetry, because at this stage the asymmetric Xnr1 expression wave would be becoming extinguished, and asymmetric expression of downstream effectors, such as XPitx2, would be beginning in the organ primordia to drive the chiral morphogenetic program. The inability of inducers placed on the R-side to invert situs at even later stages might reflect the closing of a window of competence for LPM responsiveness. But, it is also possible, even if older R LPM were still competent to activate Xnr1 expression, that the earlier passage of a L-sided Xnr1 expression wave would have already initiated the asymmetric morphogenetic program, which would maintain a temporal advantage over any effects induced in the R LPM.
Orthogonal induction of midline Xlefty and contralateral communication in Xenopus
Yamamoto et al. 2003 demonstrated in mouse embryos that Nodal produced in the LPM could induce midline Lefty1 expression. Using similar experimental approaches, we have recapitulated these results for the first time in another species, showing conservation in the mechanism that induces the molecular midline barrier. First, embryos without L LPM expression of Xnr1 lack midline Xlefty expression, which is restored by placing Xnr1-expressing grafts into the LPM. Second, R side Xnr1-engrafted embryos displayed a strong orthogonal induction of Xlefty expression in the axial midline, most noticeably in notochord that, although in general proximity to the graft, was relatively extensive along the A-P axis. The abnormally high midline expression of Xlefty induced by grafts placed in a mid-trunk location, which is proposed by contralateral suppression to give the R-side-induced Xnr1 expression a significant head-start compared to the endogenous L-side, was associated with a dominant and concordant reversal of cardiac and gut situs (Fig. 4C). To our knowledge, this is the most dramatic demonstration of the induction of downstream gene expression, contralateral gene expression responses, and anatomical consequences, of long-range orthogonal Nodal signaling, which was shown by extirpation experiments to require the axial midline. For unknown reasons, the Nodal/Xnr1 loss- and gain-of-function manipulations of Toyoizumi et al. 2005 did not affect the midline expression of Xlefty, and we do not know how to explain this discrepancy by differences in our technical and/or experimental approach.
The observation that the R-sided Xnr1 grafts caused either an anterior truncation of L-sided Xnr1 and Xlefty expression, or caused Xnr1 expression to be both anteriorly truncated and substantially suppressed, could be related to variability in the precise A-P location of the R-side graft, or how rapidly and efficiently the Xnr1 signal was registered by the host tissue. Both variables may be hard to control with the current experimental technique. On the other hand, the expression of XPitx2 showed only an incremental anterior truncation in embryos carrying R-sided Xnr1 grafts. Analyzing stages in addition to those shown here could reveal that XPitx2 expression does shift forward faster on the R side. In addition, we have not yet determined if the L-sided Xnr1 expression, even when reduced and/or delayed compared to the R side, can still shift anteriorly to induce the anterior domain of L-sided XPitx2 expression. Another possibility is that the forward diffusion of Xnr1 along the L LPM from a completely stalled L-sided expression wave could induce the anterior XPitx2 expression.
There is substantial evidence that the L-R symmetry of the Xenopus embryo begins to be broken long before gastrulation and this L-R bias eventually becomes converted into the qualitatively different L and R gene expression programs seen during tailbud stages (Bunney et al., 2003; Levin and Mercola, 1998; Levin and Mercola, 1999; Levin et al., 2002) (Hyatt et al., 1996; Hyatt and Yost, 1998; Kramer et al., 2002; Kramer and Yost, 2002). The facility with which the normally L-sided expression of Xnr1 can be activated within R LPM, by our manipulations or by others, means that there must be a mechanism(s) that ensure L-R compartmentalization. The importance of suppressing the spurious R-sided activation of the L-sided program during tailbud stages is shown by the fact that the R-sided activation of an Xnr1 expression wave has a highly significant effect on L-R asymmetry (Fig. 4C).
With respect to this issue, previous extirpation studies in Xenopus suggested that the midline functions as a regulator of laterality only up until neurula/early tailbud stages. Extirpations were done between stages 15–28, but no effect was noted after stage 20, a time just around the onset of asymmetric gene expression in the LPM (Danos and Yost, 1996; Lohr et al., 1997). We now provide evidence that the midline barrier may serve a compartmentalization function during the tailbud-stage period of asymmetric gene expression (stages 20–25), with diffusion of Xlefty from the midline conditioning R LPM against the activation of Xnr1 expression (Fig. 6).
Future hurdles will be to understand how Xnr1, and any other asymmetrically produced factors, generate inducer gradients that change with time, and how the level of intracellular effectors (e.g., Pitx2) established from these activity gradients work to regulate asymmetric morphogenesis. In addition to the active conditioning of the R LPM against initiating the expression of L-sided genes, the long-range regulation of the level of Nodal signaling by Xlefty distributed within the tissues is an integral determinant of the activity gradient. Further challenges will be to understand how such activity gradients in some cases dictate the emergence of chiral anatomy from tissue sheets or tubes, but in others cause asymmetric regression of specific tissues, as occurs, for example, for the cardiovascular system primordia.
Supplementary Material
Figure S1. Explantation of different regions of LPM affects ability to express Xnr1. (A) Approximately the middle one-third of L and R LPM was explanted at either (a) stage 15/16 or (d) 19/20, and cultured to sibling stage 24. (b,c) L or R LPM explanted at stage 15/16 failed to develop Xnr1 expression. (d,e,f) Only L LPM explanted at stage 19/20 developed Xnr1 expression. The Xnr1 expression territory observed at stage 19/20 is schematized in blue. (B) (b,c,e,f,h,i) Explanting L or R LPM that extended more posteriorly, approaching the bilateral Xnr1 expression domain, resulted in L- and R-sided Xnr1 expression when explanted at (a,d,g) stages 17, 18, or 19/20 and developed to stage 24. Note stronger intensity of Xnr1 signal in L LPM explanted at stages 18 and 19/20. (C) (a) Bisections down the midline from stage 15/16 embryos that were then cultured to stage 24 (b,c) led to only L-specific Xnr1 expression, despite presence in L and R embryo-halves of the respective posterior perinotochordal Xnr1 expression domain (c, black arrowheads). Red dotted lines, region of explanted LPM. L, left; R, right.
Figure S2. Posterior tailbud induces asymmetric Xnr1 expression. Tissue encompassing the posterior bilateral Xnr1 expression area was removed at (A) stage 17 or (B) stage 20, and posteriorly-cropped embryos analyzed for Xnr1, Xlefty, and XPitx2. In (A), asymmetric gene expression was absent, except for occasional weak XPitx2 signal (stage 34 shown, black arrowhead). Note absence of midline Xlefty expression (red arrowheads, compare to Fig. 2B). (B) Unilateral induction occurs by stage 20, and midline Xlefty expression develops. (Anterior, left; stages analyzed indicated) The removed posterior tissue from both stages showed the bilateral Xnr1 expression. (C) L-R anatomy after cropping (MF20 immunofluorescence analysis for heart, brightfield analysis for gut). Stage 17 cropping produced (from left to right) normal, reversed, and indeterminate heart looping at percentages indicated (n=18); stage 20 cropping (rightmost panel) all had normal looping (n=21). Yellow arrowheads, cardiac outflow tract; inset, diagram of heart looping. Stage 17 posterior cuts damaged posterior endoderm more than at stage 20, the gut is more normal in the latter.
Figure S3. Xnr1-expressing grafts restore LPM Xnr1, Xlefty expression and midline Xlefty in posterior cropped embryos. (A) Posterior tailbud tissues encompassing the posterior bilateral Xnr1 expression area were removed at stage 17. Xnr1+βgal-expressing L LPM was then grafted to L side of posterior cropped embryos, which were analyzed for Xnr1 and Xlefty expression at stage 24/25. (B) Xnr1 and Xlefty expression was absent in LPM of βgal control-engrafted embryos that also lacked axial Xlefty expression, except for occasional small fleck of expression observed in posterior midline tissues, most likely due to incomplete posterior tailbud cropping (black arrowhead). Robust L-sided Xnr1 and Xlefty expression was observed in Xnr1-engrafted hosts (red arrowheads), as well as restoration of midline Xlefty (green arrowhead). Xlefty expressing embryos were cleared. A, anterior; P, posterior.
Figure S4. Posterior placement of R-side Xnr1 grafts causes mirror-image expression of L-side genes. (A) Plasmids were targeted to the LPM as in Fig. 2. Compare posterior placement shown to medial location in Fig. 3 (engrafted embryo shortly after healing, red-gal stained). (B) βgal control-engrafted embryos (red-gal stained) showed endogenous L-sided expression of Xnr1, Xlefty, and XPitx2 in LPM, and no R-sided expression. Host embryos carrying posterior R-side Xnr1 grafts developed mirror-image Xnr1, Xlefty, and XPitx2 expression with equivalent anterior limits in L and R LPM (pink arrowheads). Same embryos shown have been cleared in bottom panels and dorsal views of Xlefty expressing embryos to show axial expression. Black arrowheads, endogenous Xlefty expression in anterior regions of the neural floorplate and hypochord with weak expression in the notochord, as normally observed at stage 25. Embryo stages indicated. L, left; R, right; D, dorsal; V, ventral; A, anterior; P, posterior.
Figure S5. Loss of Xnr1-engrafted R-to-L dominance with posterior engraftment. (A) All unmanipulated sibling embryos and (B) βgal-alone-engrafted embryos had normal heart and gut looping. (C) Posterior R-sided Xnr1 grafts caused randomization of heart and gut situs across the population, but situs concordance within each embryo was noted (stage 43–45 embryos analyzed, MF20 immunofluorescence shown; gut analysis not shown; yellow arrowheads, cardiac outflow tract; insets, schematic representation of heart looping).
Figure S6. Midline extirpation blocks the spatial advantage of mid-trunk grafts in dominantly converting R to L. (A) Stage 17 Xnr1+βgal-expressing L LPM was mid-trunk grafted to the R LPM as in Fig. 3. At stage 19, midline orthogonal to the graft was removed (i.e., neural floorplate, notochord, and hypochord; A-P limits indicated by blue hatched bar). Right-hand panel, diagrammatic representation of tissue removed (neural tube, dark blue; notochord, red; paraxial mesoderm, purple; intermediate mesoderm, green; LPM, pink; endoderm, yellow; ectoderm, light blue). Top right panel in (B), transverse section (eosin stained) at plane indicated (white lines) demonstrating extirpation of midline tissues. (B) βgal control-engrafted embryos showed normal L-specific gene expression. Midline extirpated embryos with mid-trunk placed R-side Xnr1-expressing grafts developed equivalent anterior limits of expression of Xnr1, Xlefty, and XPitx2 in both L and R LPM (pink arrowheads). Same embryos shown have been cleared in bottom panels and dorsal views of Xlefty expressing embryos to show axial expression. Black arrowheads, relatively broad Xlefty expression in dorsal endoderm that is anterior of extirpated region. Green arrows, midline area removed. Note increased curvature of stage 28 embryos related to midline extirpation (compare to Fig. S4). Stages indicated in top left of panels. L, left; R, right; A, anterior; P, posterior.
Acknowledgments
We thank Richard Harland for the pCSKA and pCSKA-497 plasmids, David Bader for the MF20 antibody used in this study, and Wright lab members for thoughtful comments on the experiments and manuscript. We also thank Guoqiang Gu and Anna Means for critical feedback. This work was supported by the NIH grant GM56238-08.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–83. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–70. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman CJ, Shimeld SM. The evolution of left-right asymmetry in chordates. Bioessays. 2002;24:1004–11. doi: 10.1002/bies.10171. [DOI] [PubMed] [Google Scholar]
- Branford WW, Essner JJ, Yost HJ. Regulation of gut and heart left-right asymmetry by context-dependent interactions between xenopus lefty and BMP4 signaling. Dev Biol. 2000;223:291–306. doi: 10.1006/dbio.2000.9739. [DOI] [PubMed] [Google Scholar]
- Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–44. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development. 2003;130:4847–58. doi: 10.1242/dev.00698. [DOI] [PubMed] [Google Scholar]
- Burdine RD, Schier AF. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 2000;14:763–76. [PubMed] [Google Scholar]
- Capdevila J, Vogan KJ, Tabin CJ, Izpisua Belmonte JC. Mechanisms of left-right determination in vertebrates. Cell. 2000;101:9–21. doi: 10.1016/S0092-8674(00)80619-4. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Slack JM. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development. 2000;127:381–92. doi: 10.1242/dev.127.2.381. [DOI] [PubMed] [Google Scholar]
- Chea HK, Wright CV, Swalla BJ. Nodal signaling and the evolution of deuterostome gastrulation. Dev Dyn. 2005;234:269–78. doi: 10.1002/dvdy.20549. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–10. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV. The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in xenopus. Development. 2000;127:1049–61. doi: 10.1242/dev.127.5.1049. [DOI] [PubMed] [Google Scholar]
- Condie BG, Brivanlou AH, Harland RM. Most of the homeobox-containing Xhox 36 transcripts in early Xenopus embryos cannot encode a homeodomain protein. Mol Cell Biol. 1990;10:3376–85. doi: 10.1128/mcb.10.7.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constam DB, Robertson EJ. SPC4/PACE4 regulates a TGFbeta signaling network during axis formation. Genes Dev. 2000a;14:1146–55. [PMC free article] [PubMed] [Google Scholar]
- Constam DB, Robertson EJ. Tissue-specific requirements for the proprotein convertase furin/SPC1 during embryonic turning and heart looping. Development. 2000b;127:245–54. doi: 10.1242/dev.127.2.245. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Linkage of cardiac left-right asymmetry and dorsal-anterior development in Xenopus. Development. 1995;121:1467–74. doi: 10.1242/dev.121.5.1467. [DOI] [PubMed] [Google Scholar]
- Danos MC, Yost HJ. Role of notochord in specification of cardiac left-right orientation in zebrafish and Xenopus. Dev Biol. 1996;177:96–103. doi: 10.1006/dbio.1996.0148. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Lapraz F, Besnardeau L, Lepage T. Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev Cell. 2005;9:147–58. doi: 10.1016/j.devcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods in Cell Biology. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hudson C, Yasuo H. Patterning across the ascidian neural plate by lateral Nodal signalling sources. Development. 2005;132:1199–210. doi: 10.1242/dev.01688. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Lohr JL, Yost HJ. Initiation of vertebrate left-right axis formation by maternal Vg1. Nature. 1996;384:62–5. doi: 10.1038/384062a0. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell. 1998;93:37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Izraeli S, Lowe LA, Bertness VL, Good DJ, Dorward DW, Kirsch IR, Kuehn MR. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature. 1999;399:691–4. doi: 10.1038/21429. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CV. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Juan H, Hamada H. Roles of nodal-lefty regulatory loops in embryonic patterning of vertebrates. Genes Cells. 2001;6:923–30. doi: 10.1046/j.1365-2443.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- Kay BK, Peng HB. Xenopus laevis: Practical uses in cell and molecular biology. Academic Press; San Diego: 1991. [PubMed] [Google Scholar]
- Kramer KL, Barnette JE, Yost HJ. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell. 2002;111:981–90. doi: 10.1016/s0092-8674(02)01200-x. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–24. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Evolutionary conservation of mechanisms upstream of asymmetric Nodal expression: reconciling chick and Xenopus. Dev Genet. 1998;23:185–93. doi: 10.1002/(SICI)1520-6408(1998)23:3<185::AID-DVG4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development. 1999;126:4703–14. doi: 10.1242/dev.126.21.4703. [DOI] [PubMed] [Google Scholar]
- Levin M, Pagan S, Roberts DJ, Cooke J, Kuehn MR, Tabin CJ. Left/right patterning signals and the independent regulation of different aspects of situs in the chick embryo. Dev Biol. 1997;189:57–67. doi: 10.1006/dbio.1997.8662. [DOI] [PubMed] [Google Scholar]
- Levin M, Roberts DJ, Holmes LB, Tabin C. Laterality defects in conjoined twins. Nature. 1996;384:321. doi: 10.1038/384321a0. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat Cell Biol. 1999;1:472–8. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94:307–17. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Danos MC, Groth TW, Yost HJ. Maintenance of asymmetric nodal expression in Xenopus laevis. Dev Genet. 1998;23:194–202. doi: 10.1002/(SICI)1520-6408(1998)23:3<194::AID-DVG5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lohr JL, Danos MC, Yost HJ. Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–72. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–61. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–43. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll K, Sun E, Ramos R, Elmendorf H, Kirschner MW. A Xenopus nodal-related gene that acts in synergy with noggin to induce complete secondary axis and notochord formation. Development. 1996;122:3275–82. doi: 10.1242/dev.122.10.3275. [DOI] [PubMed] [Google Scholar]
- Melloy PG, Ewart JL, Cohen MF, Desmond ME, Kuehn MR, Lo CW. No turning, a mouse mutation causing left-right and axial patterning defects. Dev Biol. 1998;193:77–89. doi: 10.1006/dbio.1997.8787. [DOI] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, Schier AF, Hamada H. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–98. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–97. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Meno C, Takeuchi J, Sakuma R, Koshiba-Takeuchi K, Ohishi S, Saijoh Y, Miyazaki J, ten Dijke P, Ogura T, Hamada H. Diffusion of nodal signaling activity in the absence of the feedback inhibitor Lefty2. Dev Cell. 2001;1:127–38. doi: 10.1016/s1534-5807(01)00006-5. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science. 1999;285:403–6. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- Mogi K, Goto M, Ohno E, Azumi Y, Takeuchi S, Toyoizumi R. Xenopus neurula left-right asymmetry is respeficied by microinjecting TGF-beta5 protein. Int J Dev Biol. 2003;47:15–29. doi: 10.1387/13. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Ueno M, Kawanishi H, Saiga H, Nishida H. HrNodal, the ascidian nodal-related gene, is expressed in the left side of the epidermis, and lies upstream of HrPitx. Dev Genes Evol. 2002;212:439–46. doi: 10.1007/s00427-002-0242-3. [DOI] [PubMed] [Google Scholar]
- Nascone N, Mercola M. Organizer induction determines left-right asymmetry in Xenopus. Dev Biol. 1997;189:68–78. doi: 10.1006/dbio.1997.8635. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. North-Holland Publishing Company; Amsterdam: 1967. [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–43. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–10. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB. Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev Biol. 1998;199:261–72. doi: 10.1006/dbio.1998.8935. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, Norris DP, Robertson EJ, Evans RM, Rosenfeld MG, Izpisua Belmonte JC. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394:545–51. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Oki S, Ohishi S, Hamada H. Left-right patterning of the mouse lateral plate requires nodal produced in the node. Dev Biol. 2003;256:160–72. doi: 10.1016/s0012-1606(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H. Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells. 2002;7:401–12. doi: 10.1046/j.1365-2443.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development. 1997;124:3293–302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–9. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishino J, Saijoh Y, Whitman M, Hamada H. Two-step regulation of left-right asymmetric expression of Pitx2: initiation by nodal signaling and maintenance by Nkx2. Mol Cell. 2001;7:137–49. doi: 10.1016/s1097-2765(01)00162-9. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Tanegashima K, Yokota C, Takahashi S, Asashima M. Expression cloning of Xantivin, a Xenopus lefty/antivin-related gene, involved in the regulation of activin signaling during mesoderm induction. Mech Dev. 2000;99:3–14. doi: 10.1016/s0925-4773(00)00465-2. [DOI] [PubMed] [Google Scholar]
- Toyoizumi R, Mogi K, Takeuchi S. More than 95% reversal of left-right axis induced by right-sided hypodermic microinjection of activin into Xenopus neurula embryos. Dev Biol. 2000;221:321–36. doi: 10.1006/dbio.2000.9666. [DOI] [PubMed] [Google Scholar]
- Toyoizumi R, Ogasawara T, Takeuchi S, Mogi K. Xenopus nodal related-1 is indispensable only for left-right axis determination. Int J Dev Biol. 2005;49:923–38. doi: 10.1387/ijdb.052008rt. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Norris DP, Le Good JA, Constam DB, Robertson EJ. Asymmetric Nodal expression in the mouse is governed by the combinatorial activities of two distinct regulatory elements. Mech Dev. 2004;121:1403–15. doi: 10.1016/j.mod.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Whitman M, Mercola M. TGF-beta superfamily signaling and left-right asymmetry. Sci STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.64.re1. [DOI] [PubMed] [Google Scholar]
- Williams PH, Hagemann A, Gonzalez-Gaitan M, Smith JC. Visualizing long-range movement of the morphogen Xnr2 in the Xenopus embryo. Curr Biol. 2004;14:1916–23. doi: 10.1016/j.cub.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Mine N, Mochida K, Sakai Y, Saijoh Y, Meno C, Hamada H. Nodal signaling induces the midline barrier by activating Nodal expression in the lateral plate. Development. 2003;130:1795–804. doi: 10.1242/dev.00408. [DOI] [PubMed] [Google Scholar]
- Yan YT, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 1999;13:2527–37. doi: 10.1101/gad.13.19.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, Hamada H, Noji S. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- Yu JK, Holland LZ, Holland ND. An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol Dev. 2002;4:418–25. doi: 10.1046/j.1525-142x.2002.02030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Explantation of different regions of LPM affects ability to express Xnr1. (A) Approximately the middle one-third of L and R LPM was explanted at either (a) stage 15/16 or (d) 19/20, and cultured to sibling stage 24. (b,c) L or R LPM explanted at stage 15/16 failed to develop Xnr1 expression. (d,e,f) Only L LPM explanted at stage 19/20 developed Xnr1 expression. The Xnr1 expression territory observed at stage 19/20 is schematized in blue. (B) (b,c,e,f,h,i) Explanting L or R LPM that extended more posteriorly, approaching the bilateral Xnr1 expression domain, resulted in L- and R-sided Xnr1 expression when explanted at (a,d,g) stages 17, 18, or 19/20 and developed to stage 24. Note stronger intensity of Xnr1 signal in L LPM explanted at stages 18 and 19/20. (C) (a) Bisections down the midline from stage 15/16 embryos that were then cultured to stage 24 (b,c) led to only L-specific Xnr1 expression, despite presence in L and R embryo-halves of the respective posterior perinotochordal Xnr1 expression domain (c, black arrowheads). Red dotted lines, region of explanted LPM. L, left; R, right.
Figure S2. Posterior tailbud induces asymmetric Xnr1 expression. Tissue encompassing the posterior bilateral Xnr1 expression area was removed at (A) stage 17 or (B) stage 20, and posteriorly-cropped embryos analyzed for Xnr1, Xlefty, and XPitx2. In (A), asymmetric gene expression was absent, except for occasional weak XPitx2 signal (stage 34 shown, black arrowhead). Note absence of midline Xlefty expression (red arrowheads, compare to Fig. 2B). (B) Unilateral induction occurs by stage 20, and midline Xlefty expression develops. (Anterior, left; stages analyzed indicated) The removed posterior tissue from both stages showed the bilateral Xnr1 expression. (C) L-R anatomy after cropping (MF20 immunofluorescence analysis for heart, brightfield analysis for gut). Stage 17 cropping produced (from left to right) normal, reversed, and indeterminate heart looping at percentages indicated (n=18); stage 20 cropping (rightmost panel) all had normal looping (n=21). Yellow arrowheads, cardiac outflow tract; inset, diagram of heart looping. Stage 17 posterior cuts damaged posterior endoderm more than at stage 20, the gut is more normal in the latter.
Figure S3. Xnr1-expressing grafts restore LPM Xnr1, Xlefty expression and midline Xlefty in posterior cropped embryos. (A) Posterior tailbud tissues encompassing the posterior bilateral Xnr1 expression area were removed at stage 17. Xnr1+βgal-expressing L LPM was then grafted to L side of posterior cropped embryos, which were analyzed for Xnr1 and Xlefty expression at stage 24/25. (B) Xnr1 and Xlefty expression was absent in LPM of βgal control-engrafted embryos that also lacked axial Xlefty expression, except for occasional small fleck of expression observed in posterior midline tissues, most likely due to incomplete posterior tailbud cropping (black arrowhead). Robust L-sided Xnr1 and Xlefty expression was observed in Xnr1-engrafted hosts (red arrowheads), as well as restoration of midline Xlefty (green arrowhead). Xlefty expressing embryos were cleared. A, anterior; P, posterior.
Figure S4. Posterior placement of R-side Xnr1 grafts causes mirror-image expression of L-side genes. (A) Plasmids were targeted to the LPM as in Fig. 2. Compare posterior placement shown to medial location in Fig. 3 (engrafted embryo shortly after healing, red-gal stained). (B) βgal control-engrafted embryos (red-gal stained) showed endogenous L-sided expression of Xnr1, Xlefty, and XPitx2 in LPM, and no R-sided expression. Host embryos carrying posterior R-side Xnr1 grafts developed mirror-image Xnr1, Xlefty, and XPitx2 expression with equivalent anterior limits in L and R LPM (pink arrowheads). Same embryos shown have been cleared in bottom panels and dorsal views of Xlefty expressing embryos to show axial expression. Black arrowheads, endogenous Xlefty expression in anterior regions of the neural floorplate and hypochord with weak expression in the notochord, as normally observed at stage 25. Embryo stages indicated. L, left; R, right; D, dorsal; V, ventral; A, anterior; P, posterior.
Figure S5. Loss of Xnr1-engrafted R-to-L dominance with posterior engraftment. (A) All unmanipulated sibling embryos and (B) βgal-alone-engrafted embryos had normal heart and gut looping. (C) Posterior R-sided Xnr1 grafts caused randomization of heart and gut situs across the population, but situs concordance within each embryo was noted (stage 43–45 embryos analyzed, MF20 immunofluorescence shown; gut analysis not shown; yellow arrowheads, cardiac outflow tract; insets, schematic representation of heart looping).
Figure S6. Midline extirpation blocks the spatial advantage of mid-trunk grafts in dominantly converting R to L. (A) Stage 17 Xnr1+βgal-expressing L LPM was mid-trunk grafted to the R LPM as in Fig. 3. At stage 19, midline orthogonal to the graft was removed (i.e., neural floorplate, notochord, and hypochord; A-P limits indicated by blue hatched bar). Right-hand panel, diagrammatic representation of tissue removed (neural tube, dark blue; notochord, red; paraxial mesoderm, purple; intermediate mesoderm, green; LPM, pink; endoderm, yellow; ectoderm, light blue). Top right panel in (B), transverse section (eosin stained) at plane indicated (white lines) demonstrating extirpation of midline tissues. (B) βgal control-engrafted embryos showed normal L-specific gene expression. Midline extirpated embryos with mid-trunk placed R-side Xnr1-expressing grafts developed equivalent anterior limits of expression of Xnr1, Xlefty, and XPitx2 in both L and R LPM (pink arrowheads). Same embryos shown have been cleared in bottom panels and dorsal views of Xlefty expressing embryos to show axial expression. Black arrowheads, relatively broad Xlefty expression in dorsal endoderm that is anterior of extirpated region. Green arrows, midline area removed. Note increased curvature of stage 28 embryos related to midline extirpation (compare to Fig. S4). Stages indicated in top left of panels. L, left; R, right; A, anterior; P, posterior.


