Abstract
Purpose
Diabetes is a growing problem worldwide. Rates of diabetes are much higher in certain high-risk populations. Thus, a need exists for effective interventions that can be tailored to the needs and preferences of different populations. Diabetes is often a special concern for older African American women who have symptoms daily and must draw appropriate inferences from them. Symptoms are more than mere indicators of an underlying disease process. They indicate ways of knowing and understanding illness and can be effective guides or signals to implement appropriate self-care.
Conceptual Model
We developed a symptom-focused model for type 2 diabetes for older African American women based on the UCSF symptom management model. Key concepts in this model are symptom experience, symptom management, and health outcomes.
Methods
The Development of a conceptual model is described along with how it was used to guide the content and format of a community-based teaching and counseling intervention designed to improve self-care practices, perceptions of quality of life, and metabolic outcomes in older African American women with type 2 diabetes residing in rural areas of the Southeast in the United States.
Conclusions
The symptom-focused conceptual model is an innovative approach to tailoring care to a distinct population and to engaging participants in their own self-care.
Clinical Relevance: Diabetes is a major cause of morbidity and mortality in African Americans; and diabetes self-management is the cornerstone of care. To better meet the distinct needs of diverse populations and positively affect health outcomes, new tailored approaches should be developed that are culturally culturally sensitive and acceptable.
Keywords: symptoms, African American, diabetes mellitus, non-insulin-dependent
Diabetes is the fourth leading cause of disease-related death worldwide (International Diabetes Federation [IDF], 2007). World Health Organization (WHO) officials estimate that more than 180 million people have diabetes (WHO, 2006), and by 2025, this figure is expected to increase to 380 million (IDF). WHO officials further estimate that diabetes contributes to 2.9 million deaths each year. Additionally, the costs for treating and preventing diabetes and its complications in 2007 exceeded $232 billion USD (IDF). Diabetes rates are much higher in certain at-risk populations. Thus, a need exists for effective interventions that can be tailored to the needs and preferences of different populations
Diabetes is a special concern for African Americans. An estimated 2.7 million or 11.4% of all African Americans aged 20 years or older have diabetes (American Diabetes Association [ADA], 2005). After adjusting for population age differences, African Americans are 1.8 times as likely to have diabetes as are Caucasians (ADA). African Americans suffer disproportionately from the complications of diabetes including retinopathy, kidney failure, and amputations, adding to the burden (Harris et al., 1998; National Diabetes Information Clearinghouse [NDIC], 2002). Across ethnicities, women are more affected by diabetes than are men (NDIC).
The primary goal of diabetes management is to control the level of blood glucose. While randomized controlled studies have shown that careful blood glucose control can prevent or delay the onset of complications, achievement of this goal remains difficult for many (Diabetes Complications and Control Trial Research Group, 1993; Ohkubo et al., 1995; United Kingdom Prospective Diabetes Study [UKPDS], 1998). To achieve and maintain metabolic control, people with diabetes must follow a complex, time-consuming regimen of self care: adherence to a meal plan, daily foot care, regular program of physical activity, administration of insulin or oral medications, and monitoring of blood glucose. A person’s approach to self-care might vary because of differences in culture, resources, and functional abilities (Chipkin & deGroot, 1998). This is especially true for older African American women residing in the rural Southeast who often face other challenges in addition to managing their diabetes. Rural elders have more chronic disease and are in poorer health than are other elders and the counties where they live are more likely to have a shortage of healthcare providers (Williams, Lethbridge, & Chambers, 1997). African Americans living in rural areas also often have limited incomes. Many rural African American women have limited resources and multiple care-giving responsibilities that contribute to high levels of stress and might interfere with self care (Chipkin & deGroot, 1998; Samuel-Hodge et al., 2000).
Diabetes self-management education (DSME) is considered the cornerstone for achieving glycemic control and thereby, preventing or delaying the onset of complications (Mensing et al., 2006). DSME in the United States has been standardized through the National Standards for Diabetes Self-Management Education (Mensing et al.), which sponsors a curriculum for diabetes education that specifies content areas to be covered, structure of the classes, and outcomes to be evaluated. The intent of these standards is to ensure that programs provide comprehensive instruction. However, although program directors are advised to adapt the curriculum to the target population, the standards leave little room for meeting individual learning needs or addressing culture and age-related differences. Clearly, more individualized and culturally acceptable approaches to diabetes education should be developed and tested.
A symptom-focused approach offers an ideal method for tailoring self-management education to the needs and preferences of different populations and helping them to manage their disease over time. One of the goals of a symptom-focused approach is to help people with diabetes recognize and interpret symptoms so as to make appropriate decisions about self-care. For healthcare providers, understanding the reasoning that occurs in processing symptoms can help determine how patients assess and decide what to do about their symptoms and thereby improve self-care management (Teel, Meek, McNamara, & Watson, 1997).
In this paper we describe a symptom-focused conceptual model and how it was used to guide the content and format of a community-based teaching and counseling intervention designed to improve self-care practices, perceptions of quality of life, and metabolic outcomes in older African American women with type 2 diabetes residing in rural areas of the Southeast. Results of a pilot test of this intervention model are reported elsewhere (Skelly, Carlson, Leeman, Holditch-Davis, & Soward, 2005).
Symptom-Focused Self-Care
Symptoms are more than mere indicators of an underlying status. Symptoms indicate a way of understanding illness that can be independent of information from professionals (Peyrot, McMurray, & Hedges, 1987). A person’s experience of symptoms, or symptom identification, can become a signal to implement appropriate self-care practices to improve health outcomes. The experience and interpretation of symptoms will vary depending on the person’s context and is affected by ethnicity, gender, learning, and beliefs (Brown, 1988; Leventhal, 1986).
Perception of symptoms has been investigated in relationship to a variety of disease states including cancer (Brown, Carrieri, Jansen-Bjerklie, & Dodd, 1986; Giardino & Wolf, 1993; Hogan, 1997; Larson, Viele, Coleman, Dibble, & Cebulski, 1988; Munkres, Oberst, & Hughes, 1992), chronic respiratory symptoms (Janson-Bjerklie, Ferketich, Benner, & Becker, 1992; Janson-Bjerklie, Holzemer, & Henry, 1992), diabetes (Freund, Johnson, Rosenbloom, Alexander, & Hansen, 1986; Pennebaker et al., 1981; Van der Does et al., 1996); chronic pain (Faucett & Levine, 1991; Savedra, Tesler, Holzehemer, Wilkie, & Ward, 1990) and dizziness (Sloane, Coeytaux, Beck, & Dallara, 2001); and in different patient populations (Garcia, 2005). Investigators have studied the relationship between diabetes symptom burden and depression (Ludman et al., 2004), the relationship of depressive symptoms to symptom-reporting, self-care, and glucose control in diabetes (Ciechanowski, Katon, Russo, & Hirsch, 2003) and the relationships among depression, diabetes symptoms, and self-care adherence (McKellar, Humphreys, & Piette, 2004). While results of these studies varied, overall, the importance of the experience of symptoms as a mediator of self-care through the processes of perception and interpretation was supported.
Nurse researchers also have developed and tested a range of symptom-focused interventions. Through this research, nurses are building the evidence base for the value of a symptom focus at reducing symptom distress and improving lifestyle and self-care behaviors and health outcomes for a broad range of conditions and across diverse populations. For example, findings indicate that symptom-focused interventions might decrease fatigue in older women following postcoronary artery bypass surgery (Zimmerman et al., 2007), fatigue and sleep disturbance in patients with multiple sclerosis (Wassem & Dudley, 2003), and overall symptom severity in women with severe premenstrual syndrome (Taylor, 1999) and a subset of cancer patients receiving chemotherapy (Given et al., 2004). Symptom-focused interventions also improve behaviors and health outcomes. In a study with HIV/AIDs patients in Taipai, Chiou et al. (2005) reported that the experimental group had better medication adherence, lower CD4 counts and viral load, and higher QoL than did the control group.
Symptoms and Diabetes
People with diabetes experience symptoms (acutely and chronically) throughout the course of their disease, including the classic symptoms of polyuria, polydipsia, polyphagia, visual blurring, numbness and tingling in extremities, calf pain when walking, and impotence (Stover, Skelly, Holditch-Davis, & Dunn, 2001). Diabetes symptoms might be subtle and patients often do not recognize relationships between their symptoms and the disease. This is unlike asthma, where patients readily understand the relationship of shortness of breath to the underlying disease. Further, in diabetes, unlike asthma, short and long-term concerns are highly congruent; alleviation or control of acute symptoms can prevent or delay the onset of long-term complications (DCCT Research Group, 1993; Peyrot et al., 1987; UKPDS, 1998).
People with diabetes must deal with symptoms on a regular and long-term basis and draw appropriate inferences from them. For people with diabetes, management of symptoms will influence the symptoms experienced (e.g., fatigue) as well as outcomes (e.g., blood glucose control; Lareau, 1997; Teel et al., 1997).
Conceptual Model
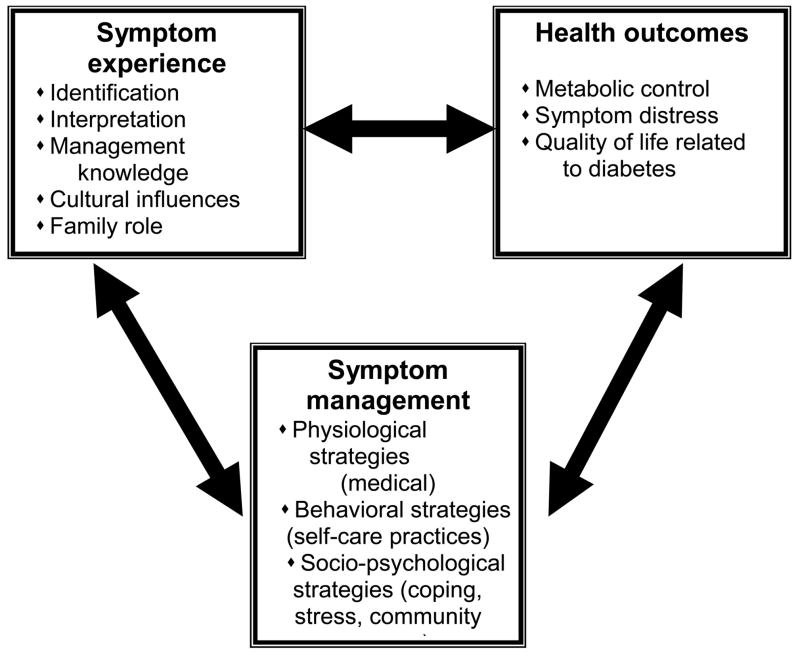
The symptom-focused intervention model developed by the research team is based on the UCSF symptom management model (Dodd et al., 2001; University of California at San Francisco Symptom Management Group, 1994) and a model developed by faculty at the University of North Carolina at Chapel Hill School of Nursing to manage symptoms of HIV infection in women (Miles et al., 2003). It was adapted for older African American women with type 2 diabetes residing in rural areas of the Southeast US based on findings from a series of small pilot studies that indicated key symptoms and how they affected the lives of women with diabetes, strategies women used to manage symptoms and their perceived effectiveness, and ways of learning about self-care that the women believed were most useful (Cagle, Appel, Skelly, & Carter-Edwards, 2002; Carter-Edwards, Skelly, Cagle, & Appel, 2004; Leeman, Skelly, Burns, Carlson, & Soward, in press; Skelly et al., 2005; Stover et al., 2001). The purpose of developing a conceptual model for the intervention was to be able to: (a) identify key concepts, (b) begin to describe how these concepts are interrelated, and (c) utilize the key concepts as the foundation for an intervention. The key concepts in this model are the symptom experience, symptom management, and health outcomes (Figure).
Figure.
Model: AH Skelly, Symptom Focused Diabetes Care for Older African American Women 2007.
Symptom experience refers to how a person identifies and interprets symptoms. This can be influenced by a variety of factors including a person’ knowledge and beliefs about heath and illness, culture and ethnicity, age and gender, and role in the family and community. Symptom management refers to the strategies people use to manage symptoms.
The model includes three classes of strategies: (a) physiologic (medical), (b) behavioral (self-care practices), and (c) sociopsychological (coping, stress reduction, social support). These classes of strategies are not mutually exclusive and a person might choose an action from several classes. This is the intention of tailoring.
Outcomes include metabolic control, symptom distress, and quality of life related to diabetes. The process by which these concepts are linked is bidirectional and dynamic with the symptom experience (including identification and assessment) being the signal to initiate appropriate symptom management strategies to improve health outcomes. Health outcomes can then influence both symptom management and experience. An important sidenote is that diabetes self-care practices, including home glucose monitoring (HGM), taking insulin/oral medications, diet, physical activity, and foot care, are not considered as outcomes, but as behavioral or self-care strategies (mediating variables) that have the potential to affect outcomes. The conceptual model was used to guide the content and scope of the intervention including the four intervention modules, choices of teaching methods, strategies and educational materials, decisions about the timing of the intervention and follow-up, and how cultural sensitivity is addressed.
Content and Scope of the Intervention
The purpose of the symptom-focused intervention is to provide older African American women who have type 2 diabetes with a culturally-sensitive, practical approach to identifying and interpreting their symptoms of diabetes, making appropriate inferences from them, and choosing appropriate management strategies to improve health outcomes. The content of the intervention was based on data obtained during our pilot work, augmented by a review of the literature.
In our pilot studies (Skelly et al., 2005; Stover et al., 2001), we identified symptoms in a sample of 75 women with type 2 diabetes mellitus attending a medical clinic. One of our major findings was that, in many cases, women did not recognize the relationship between their symptoms and diabetes. Therefore, we oriented the teaching modules around prevalent symptoms with a focus on identifying and assessing symptoms, how these symptoms relate to diabetes, and effective self-care strategies for these symptoms.
Based on the pilot data, modules were created to address the following: symptoms of hyperglycemia; symptoms of hypoglycemia; and numbness, tingling, or pain in the feet. A fourth module, prevention of cardiovascular disease, was created based on our experience in the pilot study in which 87.8% of the participants had hypertension, 41.5% had major cardiac diagnoses (e.g., MI, CHF), and 56.1% were being treated for lipid disorders (Skelly et al., 2005).
An intervention protocol guided nurses concerning what to include in each module. The approach used to implement the symptom intervention is teaching and counseling with a nurse as guide or facilitator. The intervention nurses engage in active listening during the visit and place only minimal emphasis on written materials (Randolph, 1996). Because an aim of the intervention is to increase awareness of the symptoms of diabetes and how to manage them, having older women become active participants is essential. This is accomplished through a variety of strategies such as providing opportunities for women to share their feelings and concerns, developing a trusting relationship with the women (and family members who are invited to “sit-in”), identifying and prioritizing the symptoms that are of most concern to women, using strategies that are culturally-meaningful, and using problem-solving strategies such as identifying barriers to self-care and adaptive coping.
Each learning module has a specific structure which includes: (a) a list of participant-centered objectives, (b) a symptom assessment guide, (c) management strategies, (d) key teaching points, (e) hints and tips, (f) educational materials, and (g) guidelines for ending the session that includes goal-setting and “homework” assignments for participants and intervention nurses. Based on an assessment of the woman’s symptoms, nurses adapted each module to one of three conditions—participants who are asymptomatic and show no clinical evidence (signs) of symptoms; participants who report no symptoms but show clinical evidence (signs) of the symptom (e.g., elevated blood glucose levels, decreased sensation in feet); and participants who are symptomatic, who in almost all cases also show clinical evidence (signs) of the symptoms. Thus, this model incorporates the use of clinical signs of diabetes to facilitate the identification and correct interpretation of symptoms.
Nurses introduce the content on management strategies with a brief overview of the symptom and how it relates to diabetes control. This includes identification and assessment of signs and symptoms, for example symptoms of neuropathy, and is tailored to the educational needs of individual patients and supplemented by relevant teaching materials that are always reviewed with participants. Tailoring is accomplished by giving participants choices in both strategies and actions. The management strategies available to participants are the physiologic strategies (medical management), behavioral strategies (self-care practices), and sociopsychological strategies (coping, stress, community resources). Examples of possible behavioral and sociopsychological self-care strategies are shown in Table 1. Nurses are guides and resources to participants as participants select among the strategies to address the symptom. This approximates a “real-world” situation where people with diabetes are required to assess their health status and take appropriate action.
Table 1.
Examples of Management Strategies and Actions for Symptoms of Hyperglycemia
| Strategy: Behavioral management strategies |
Actions: Tailored to individual
|
| Strategy: Sociopsychological strategies |
Actions: Tailored to individual
|
Participants proceed through all the modules as directed by their own symptoms or concerns. This approach differs from traditional diabetes education in that it is first focused on the specific symptoms each participant is experiencing (e.g., fatigue, urinary frequency) and then information and treatment relevant to those symptoms is offered. Thus, the intervention is individualized to each person’s experiences, rather than generalized.
In our pilot work, we found timing visits at 2-week intervals and lasting from 1 to 1 1/2 hours to be most effective. This allows sufficient time for participants to try the new strategy and see its effect while still remaining engaged. A mid-week telephone call is made to reinforce goals and discuss any new problems.
An intervention is culturally sensitive to the degree that it is meaningful and relevant to an individual’s cultural identity. Based on our pilot work and the work of Miles and Holditch-Davis (1996), many procedures were used to increase cultural sensitivity of the intervention. Before beginning the actual intervention, nurses did a semi-structured, in-depth interview with participants to learn participants’ perspectives on their symptoms and managing their diabetes. This information was used to tailor the intervention to each participant’s situation. For example, while one participant might prefer relying on family members for support, another might feel more comfortable “not bothering” her family and prefer professional support. Some participants were active in their church family while others preferred reading scripture at home and meditating privately.
These preferences were used to make the intervention’s strategies and actions more acceptable and useful to participants. Other approaches included acknowledging the multicaregiver role of older African American women in their families and communities, recognizing the importance of religiosity/spirituality in the women’s lives, recognizing their unique value as individuals and caretakers of the family (“family first”) and recognizing the importance of the extended family in African American culture (Samuel-Hodge et al., 2000). Throughout the intervention, the nurses emphasized active-listening rather than written materials (Randolph, 1996). Storytelling and the use of personal anecdotes (from nurses and participants) were effective and enjoyable for participants.
In intervention research, the choice of which processes and outcomes to evaluate is strategic (Campbell et al., 2000; Mannheim, 1999). Evaluation should include not only outcomes relevant to a specific condition or disease but also encompass measures of wider relevance (Fitzpatrick, Davey, Buxton, & Jones, 1998). The conceptual model directed our choice of outcome variables in that our expectation of the intervention was that it would increase participants’ knowledge of symptoms and how to effectively manage them (self-care), which would presumably decrease symptom distress, improve metabolic control, and increase participants’ perceptions of overall quality of life as well as quality of life related to their diabetes (Dalton et al., 1999). The outcome measures of symptoms distress, metabolic control, and quality of life were pilot tested during the exploratory phase of the research. In the larger field trial, we expanded our outcomes to include measures of cost to the healthcare system and society. Diabetes self-care practices and symptom knowledge are measured as mediating variables (or intermediate outcomes).
Conclusions
Living with diabetes is a proactive process closely intertwined with the larger contexts of an individual’s life. As part of their personal construction of illness, individuals organize and shape the nature of a disorder such as diabetes and how it is managed and treated (Peyrot et al., 1987).
People with diabetes must assume the major responsibility for managing their diabetes in order to achieve and maintain good glycemic control and prevent complications. This self-management requires a commitment of time, physical and mental energy, resources, and an immediate and intimate knowledge of the disease based on personal experience. This personal knowledge of diabetes is conceptually and empirically distinct from professional knowledge and reflects the experience of having diabetes. In a real-world situation, both of these types of knowledge may provide alternatives, competing perspectives on the individual’s condition (Peyrot et al., 1987). For example, when diabetes is first diagnosed, professional knowledge is more important because people generally have little information about the disease and seek to reduce their uncertainty about their condition. However, as these people develop more experience living with diabetes, they build a personal store of knowledge about their own conditions which they call upon to make management decisions. Therefore, an intervention that can be focused on a person’s own personal experience of diabetes (e.g., symptoms) should be effective in helping them to make more informed decisions about self-care and ultimately improve diabetes-related outcomes. This, we believe, is the major contribution of a conceptual model that is focused on symptoms. To date, our pilot work has supported the effectiveness of this approach (Skelly et al., 2005).
Walker and Avant (2005) suggest evaluating conceptual models for their usefulness, generalizability, parsimony, and testability. The usefulness of a model can be determined by the amount of research it generates, its relevance to the clinical setting, and its potential to influence practice, education, administration, and research. Research in numerous disease conditions has been guided by a symptom-focused model.
The symptom-focused diabetes care model is relevant to the clinical setting because it engages the individual with diabetes by directly linking information about self-care to a person’s own experiences. Generalizability is concerned with how widely the framework or model can be applied and parsimony relates to how simply the theory can be stated. Our study participants are older African American women with type 2 diabetes living in rural areas and the choice of symptom-management strategies is tailored to this population. However, this is an approach that can be easily adapted to a variety of cultures, settings, and populations. The symptom-focused model is parsimonious in the number of concepts and well-defined boundaries. Testability is defined by Walker and Avant (2005) as the degree to which a theory or model can be supported by empirical evidence. If a theory can generate hypotheses it is testable. The symptom-focused model is testable. At present, we are analyzing data from a larger, community-based, randomized, controlled trial to test the effectiveness of the symptom-focused diabetes care intervention. Data from this study will provide additional information on the effectiveness of this approach in a sample of older African American women as well as valuable data on cultural acceptability and appropriateness.
Clinical Resources
American Diabetes Association. (2008). Standards of medical care in diabetes. Diabetes Care, 31(1), S12–S54.
Funnell, M., Brown, T., Childs, B., Hass, L. Hosey, G., et al. (2008). National standards for diabetes self-management education. Diabetes Care, 31(1), S97–S104.
Table 2.
Baseline Structured Interview
|
References
- American Diabetes Association. Diabetes 4-1-1 Facts, figures & statistics at a glance: Who’s getting diagnosed, why and what it means for society. Canada: Author; 2005. [Google Scholar]
- Brown S. Diabetes interventions for minority populations. Diabetes Spectrum. 1988;11:145–149. [Google Scholar]
- Brown M, Carrieri V, Janson-Bjerklie S, Dodd MJ. Lung cancer and dyspnea: The patient’s perspective. Oncology Nursing Society. 1986;13 (5):19–24. [PubMed] [Google Scholar]
- Cagle C, Appel S, Skelly A, Carter-Edwards L. Mid-life African American women with type 2 diabetes: Influence on work and the multicaregiver role. Ethnicity and Disease. 2002;12(4):555–566. [PubMed] [Google Scholar]
- Campbell M, Fitzpatrick R, Haines A, Kinmonth A, Sandercock P, Spiegelholter D, et al. Framework for design and evaluation of complex interventions to improve health. British Medical Journal. 2000;321:694–696. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Edwards L, Skelly A, Cagle C, Appel S. “They care but they don’t understand:” Social support of African American women with type 2 diabetes. The Diabetes Educator. 2004;30(4):1–21. doi: 10.1177/014572170403000321. [DOI] [PubMed] [Google Scholar]
- Chipkin S, deGroot M. Contextual variables influencing outcome measures in minority populations with diabetes. Diabetes Spectrum. 1998;11:149–160. [Google Scholar]
- Chiou PY, Kuo BI, Lee MB, Chen YM, Chuang P, Lin LC. A programme of symptom management for improving quality of life and drug adherence in AIDS/HIV patients. Journal of Advanced Nursing. 2006;55 (2):169–179. doi: 10.1111/j.1365-2648.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. General Hospital Psychiatry. 2003;25(4):246–52. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- Dalton J, Blau W, Lindley C, Carlson J, Youngblood R, Greer S. Changing acute pain management to improve patient outcomes: An educational approach. Journal of Pain and Symptom Management. 1999;17(4):277–287. doi: 10.1016/s0885-3924(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Diabetes Complications and Control Trial Research Group. The effect of intensive therapy for diabetes on the development and progression of long-term complications. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Faucett JA, Levine JD. The contributions of interpersonal conflict to chronic pain in the presence or absence of organic pathology. Pain. 1991;44:35–43. doi: 10.1016/0304-3959(91)90144-M. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient based outcome measures for use in clinical trials. Health Technology Assessment. 1998;2(14):1–74. [PubMed] [Google Scholar]
- Freund A, Johnson S, Rosenbloom A, Alexander B, Hansen C. Subjective symptoms, blood glucose estimation, and blood glucose concentrations in adolescents with diabetes. Diabetes Care. 1986;9(3):236–243. doi: 10.2337/diacare.9.3.236. [DOI] [PubMed] [Google Scholar]
- Garcia A. Symptom prevalence and treatments among Mexican Americans with type 2 diabetes. The Diabetes Educator. 2005;31(4):543–554. doi: 10.1177/0145721705278801. [DOI] [PubMed] [Google Scholar]
- Giardino E, Wolf Z. Symptoms: Evidence and experience. Holistic Nursing Practice. 1993;7(2):1–12. [PubMed] [Google Scholar]
- Given C, Given B, Rahbar M, Jeon S, McCorkle R, Cimprich B, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. Journal of Clinical Oncology. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: The Third National Health and Nutrition Examination Survey, 1998–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Hogan C. Cancer nursing: The art of symptom management. Oncology Nurse Forum. 1997;24(8):1335–1340. [PubMed] [Google Scholar]
- International Diabetes Federation. Diabetes atlas. (3) 2007 Retrieved January 12, 2008, from http://www.eatlas.idf.org/
- Janson-Bjerklie S, Holzhemer W, Henry S. Patients’ perceptions of pulmonary problems and nursing interventions during hospitalization for pneumocystis carinii pneumonia. American Journal of Critical Care Nursing. 1992;1(1):114–121. [PubMed] [Google Scholar]
- Janson-Bjerklie S, Ferketich S, Benner P, Becker G. Clinical markers of asthma severity and risk: Importance of subjective as well as objective factors. Heart & Lung. 1992;21(3):265–272. [PubMed] [Google Scholar]
- Larson PJ, Viele C, Coleman S, Dibble SL, Cebulski C. Comparison of perceived symptoms of patients undergoing bone marrow transplant and the nurses caring for them. Oncology Nurses Forum. 1988;20(1):81–87. 87–88. [PubMed] [Google Scholar]
- Lareau S. Clinical sidebar. Image: Journal of Nursing Scholarship. 1997;29(2):180. [Google Scholar]
- Leeman J, Skelly A, Burns D, Carlson J, Soward A. Tailoring a diabetes self-care intervention for use with older, rural African-American women. The Diabetes Educator. doi: 10.1177/0145721708316623. in press. [DOI] [PubMed] [Google Scholar]
- Leventhal H. Symptom reporting: A focus on processing. In: McHugh S, Vallis T, editors. Illness behavior: A multidisciplinary model. New York: Plenum Press; 1986. pp. 219–237. [Google Scholar]
- Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, et al. Depression and diabetes symptom burden. General Hospital Psychiatry. 1994;26:430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Mannheim I. Health services research clinical trials: Issues in the evaluation of economic costs and benefits. Controlled Clinical Trials. 1999;19:149–158. doi: 10.1016/s0197-2456(97)00146-3. [DOI] [PubMed] [Google Scholar]
- McKellar JD, Humphreys K, Piette JD. Depression increases diabetes symptoms by complicating patients’ self-care adherence. Diabetes Educator. 2004;30(3):485–92. doi: 10.1177/014572170403000320. [DOI] [PubMed] [Google Scholar]
- Mensing C, Boucher J, Cypress M, Weinger K, Mulcahy K, Barta P, et al. National standards for diabetes self-management education. Diabetes Care. 2006;29(Suppl 1):S78–S85. [PubMed] [Google Scholar]
- Miles M, Holditch-Davis D. HIV symptom management with African American women. Funded National Institutes of Health, National Institute of Nursing Research; 1996. [Google Scholar]
- Miles MS, Holditch-Davis D, Eron J, Black BP, Pedersen C, Harris DA. An HIV self-care symptom management intervention for African American mothers. Nursing Research. 2003;52(6):350–360. doi: 10.1097/00006199-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Munkres A, Oberst M, Hughes S. Appraisal of illness, symptom distress, self-care burden, and mood states in patients receiving chemotherapy for initial and recurrent cancer. Oncology Nurses Forum. 1992;19 (8):1201–1209. [PubMed] [Google Scholar]
- National Diabetes Information Clearinghouse. Diabetes in African Americans. 2002. NIH Publication No. 02–3266. [Google Scholar]
- Ohkubo Y, Kishikawa H, Araki E, Isami S, Motoyoshi S, Kojima Y, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Research and Clinical Practice. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- Pennebaker J, Cox D, Gonder-Frederick L, Wunsch M, Evans S, Pohl S. Physical symptoms related to blood glucose in insulin-dependent diabetics. Psychosomatic Medicine. 1981;43(6):489–500. doi: 10.1097/00006842-198112000-00005. [DOI] [PubMed] [Google Scholar]
- Peyrot M, McMurry J, Jr, Hedges R. Living with diabetes: The role of personal and professional knowledge in symptom and regimen management. In: Brooks N, Matson R, editors. Research in the sociology of health care. Vol. 6. Greenwich, CT: JAI Press; 1987. pp. 107–146. [Google Scholar]
- Randolph S. Cultural Considerations in Research with African American Mothers. Paper presented at the International Infancy Conference, Providence; Rhode Island. 1996. [Google Scholar]
- Samuel-Hodge CD, Headen SW, Skelly AH, Ingram AF, Keyserling TC, Jackson EJ, et al. Influences on day-to-day self-management of type 2 diabetes among African American women: Spirituality, the multi-caregiver role, and other social context factors. Diabetes Care. 2000;23:928–33. doi: 10.2337/diacare.23.7.928. [DOI] [PubMed] [Google Scholar]
- Savedra M, Tesler M, Holzhemer WL, Wilkie DJ, Ward JA. Testing a tool to assess postoperative pediatric and adolescent pain. In: Tyler DC, Kraus EJ, editors. Advances in pain research and therapy (15), pediatric pain. New York: Raven Press; 1990. pp. 850–93. [Google Scholar]
- Skelly A, Carlson J, Leeman J, Holditch-Davis D, Soward A. Symptom-focused management for African American women with type 2 diabetes: A pilot study. Applied Nursing Research. 2005;18:213–220. doi: 10.1016/j.apnr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sloane P, Coeytaux R, Beck R, Dallara J. Dizziness: State of the science. Annals of Internal Medicine. 2001;134(9, Part 2):823–832. doi: 10.7326/0003-4819-134-9_part_2-200105011-00005. [DOI] [PubMed] [Google Scholar]
- Stover J, Skelly A, Holditch-Davis D, Dunn P. Perceptions of health and their relationship to symptoms in African American women with type 2 diabetes. Applied Nursing Research. 2001;14(2):72–80. doi: 10.1053/apnr.2001.22372. [DOI] [PubMed] [Google Scholar]
- Taylor D. Effectiveness of professional-peer group treatment: Symptom management for women with PMS. Research in Nursing & Health. 1999;22:496–511. doi: 10.1002/(sici)1098-240x(199912)22:6<496::aid-nur7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Teel C, Meek P, McNamara A, Watson L. Perspectives unifying symptom interpretation. Image: Journal of Nursing Scholarship. 1997;29 (2):175–181. doi: 10.1111/j.1547-5069.1997.tb01553.x. [DOI] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study. UKDS demonstrates benefit of intensive glucose control in type 2 diabetes. Issues in Type 2 Diabetes, 2 (3) Seacaucus, NJ: Professional Postgraduate Services; 1998. [Google Scholar]
- University of California–San Francisco Nursing Symptom Management Faculty Group. A model for symptom management. Image: Journal of Nursing Scholarship. 1994;26:272–276. [PubMed] [Google Scholar]
- Van der Does F, De Neeling J, Snoek F, Kostense P, Grootenhuis P, Bouter L, et al. Symptoms and well-being in relation to glycemic control in type II diabetes. Diabetes Care. 1996;19(3):204–210. doi: 10.2337/diacare.19.3.204. [DOI] [PubMed] [Google Scholar]
- Walker LO, Avant KC. Strategies for theory construction in nursing. 4. Upper Saddle River, NJ: Pearson Education; 2005. [Google Scholar]
- Wassem R, Dudley W. Symptom management and adjustment of patients with multiple sclerosis. Clinical Nursing Research. 2003;12:102–117. doi: 10.1177/1054773803238743. [DOI] [PubMed] [Google Scholar]
- Williams R, Lethbridge D, Chambers W. Development of a health promotion inventory for poor rural women. Family and Community Health. 1997;20:13–23. [Google Scholar]
- World Health Organization. Fact sheet N312. 2006 Retrieved January 12, 2008, from http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- Zimmerman L, Barnason S, Schulz P, Nieveen J, Miller C, Hertzog M, et al. The effects of a symptom management intervention on symptom evaluation, physical functioning, and physical activity for women after coronary artery bypass surgery. Journal of Cardiovascular Nursing. 2007;22:493–500. doi: 10.1097/01.JCN.0000297379.06379.b6. [DOI] [PubMed] [Google Scholar]