Summary
Schistosomiasis japonica is an endemic, zoonotic disease of major public health importance in China where water buffaloes account for approximately 75% of disease transmission. Interventions that reduce schistosome infection in water buffaloes will enhance their health simultaneously reducing disease transmission to humans. While chemotherapy has proved successful, it requires continued time consuming and expensive mass treatments. A more sustainable option would be development of vaccines that reduce transmission of S. japonicum from bovines to replace bovine chemotherapy. We performed two randomized double blind trials in water buffaloes to determine if DNA vaccines encoding triose phosphate isomerase (SjCTPI), or the tetraspanin 23 kDa integral membrane protein (SjC23), alone or fused to bovine heat shock protein 70 (Hsp70) could induce a level of immunity conducive to long-term sustainable control. Groups of water buffaloes (15/group) received 3 intramuscular injections, 4 weeks apart. Booster immunizations were co-administered with a plasmid DNA encoding IL-12. Four weeks after the last injection, water buffaloes were challenged with 1000 cercariae, and vaccine efficacy analyzed 8 weeks later. Water buffaloes vaccinated with SjCTPI-Hsp70 or SjCTPI plasmids had worm burdens reduced by 51.2% and 41.5%, respectively. Importantly, fecal miracidial-hatching was reduced by 52.1% and 33.2% respectively compared to control vaccinated water buffaloes. Vaccination with SjC23-Hsp70 and SjC23 plasmids reduced worm burdens by 50.9% and 45.5%, respectively, and fecal miracidial-hatching by 52.0% and 47.4%. A mathematical model of schistosome transmission predicts that schistosome vaccines capable of reducing water buffaloes’ fecal egg output by 45%, alone or in conjunction with praziquantel treatment, will lead to a significant reduction in transmission of schistosomiasis. Both DNA vaccines tested here exceed this hypothetical level. Indeed, mathematical modeling of SjCTPI-Hsp70 and SjC23-Hsp70 alone and in conjunction with human chemotherapy showed a significant reduction in transmission almost to the point of elimination.
Keywords: Schistosoma japonicum, DNA vaccine, Water buffaloes, SjCTPI-Hsp70, SjC23-Hsp70, IL-12
1. Introduction
Schistosomiasis is a parasitic disease affecting more than 200 million people worldwide [1]. Reassessment of schistosomiasis-related disability [2], combined with recent information on the global prevalence of schistosome infection [3] indicates that the true burden of schistosomiasis is substantially greater than previously appreciated. In Asia, particularly China, the causative agent is Schistosoma japonicum. Unlike the African species, S. mansoni and S. haematobium, S. japonicum is a zoonotic parasite, with bovines, particularly water buffaloes accounting for about 75% of schistosome transmission to humans in China [4–7].
Despite intensive control efforts, schistosomiasis remains endemic and a major public health problem in the lowland marsh and lakes regions of China, with approximately one million people and over 100,000 bovines infected [5,8]. These endemic foci have been largely refractory to the massive control programs that focus on annual mass chemotherapy and reduction in numbers of the snail intermediate host [8]. The inability to further reduce transmission of schistosomiasis has seriously restrained social/economic development and public health advances in these areas of China [9]. The seasonal transmission of schistosomiasis in these regions results in rapid re-infection and compounds the problem. This requires annual administration of praziquantel to residents, otherwise prevalence rates rapidly increase [8]. Unfortunately, the scenario for schistosomiasis in China is predicted to become more severe with the completion of the massive Three Gorges Dam [10,11]. New, currently unaffected areas, will become endemic with an exponential expansion of the habitats for the intermediate snail host Oncomelania hupensis [10]. The expansion of snail intermediate host habitats will increase the numbers of water buffaloes and humans at risk of infection [12].
Current control programs in many areas of China include simultaneous praziquantel (PZQ) treatment of humans and water buffaloes; while this has shown a reduction in the overall prevalence [7], it requires continued mass treatments that are both time consuming and expensive. A more sustainable option would be development of a vaccine which reduces transmission of S. japonicum from bovines to replace bovine chemotherapy. Indeed mathematical modeling [5] has demonstrated that reducing S. japonicum infection in bovine reservoirs using prophylactic vaccines with 45% efficacy alone or in combination with PZQ (parameters: 20% prevalence, 95% coverage), should over time reduce the equilibrium prevalence (Equilibrium prevalence is the predicted balance of all prevalences; the equilibrium prevalence approaches zero when the disease (parasite in this case) reproductive rate falls below one) and potentially lead to long-term sustainable control of schistosomiasis [11,13–15]. This two-pronged base intervention would significantly reduce transmission of schistosomiasis for the long term, increase bovine health and growth and would likely reduce overall morbidity in village populations [15].
A number of anti-S. japonicum vaccine candidates have been described; the majority are membrane proteins, muscle proteins or enzymes [11,13,16–23], but to date, no single recombinant protein vaccine candidate has induced the desired levels of protection required for potential long term sustainable control. We have focused on the development of plasmid DNA vaccines for schistosomiasis. A DNA-based vaccine strategy for induction of protective immunity is appealing as both humoral and cellular immune responses are recognized as being involved in eliminating schistosomes [11].
For the current study we focused on plasmid DNA vaccines encoding S. japonicum triose-phosphate isomerase (SjCTPI) or the tetraspanin 23 kDa integral membrane protein (SjC23). The 23 kDa surface membrane molecule was the first tetraspanin to be described from schistosomes [24], and is structurally similar to other recently identified and protective schistosome tetraspanins [20]. Both of these candidate vaccines have been shown to induce moderate to high levels of protection against challenge infection in extensive trials in mice and pigs [19,25–30]. Further, the protective effect of these vaccines was enhanced when co-administered with a plasmid expressing interleukin (IL)-12 [19,28]. In an attempt to enhance the immunogenicity of these two plasmid DNA vaccines we modified the SjCTPI and SjC23 plasmid DNA vaccines via the incorporation of the bovine Heat shock protein 70 (Hsp70) coding sequence. Hsp70 has been shown in numerous studies to target vaccine antigens to antigen presenting cells, including dendritic cells and macrophages, increasing vaccine immunogenicity [31–38]. The aim of this study was to determine if vaccination of water buffaloes with any of the four DNA vaccine constructs (SjCTPI, SjC23, SjCTPI-Hsp70 or SjC23-Hsp70) would induce 45% efficacy compared with control (pVAX DNA) vaccinated water buffaloes. Two randomized double-blinded trials were undertaken to test vaccine efficacy. Trial 1 evaluated efficacy of SjCTPI, SjCTPI-Hsp70 and pVAX; whereas Trial 2 evaluated efficacy of SjC23, SjC23-Hsp70 and pVAX. An English abstract of the SjCTPI vaccine efficacy (trial 1) was published [39]. Therefore, in this manuscript the complete SjCTPI trial results are included to compare their immunogenicity with that of the SjC23 vaccines as well as to incorporate them into the mathematical model. This paper reports the results of the two vaccine trials as well as predicted models of their influence on reducing transmission of schistosomiasis in China.
2. Materials and Methods
2.1. DNA vaccine constructs
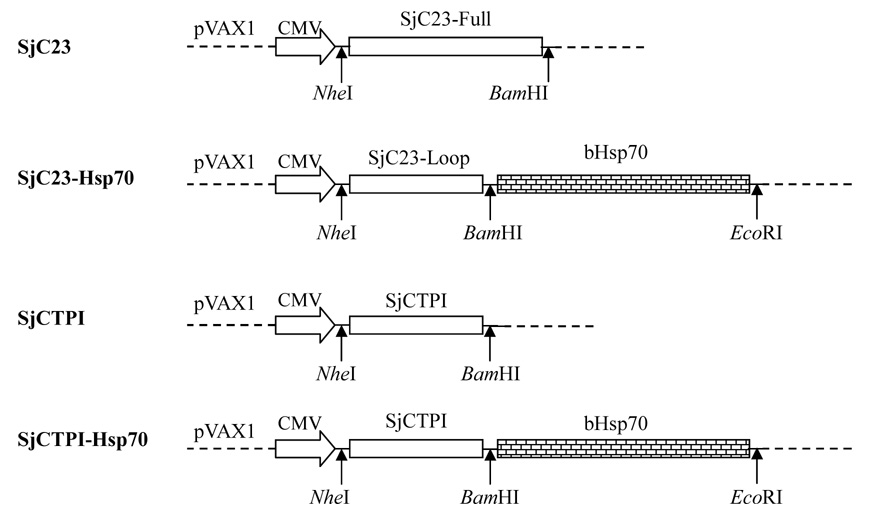
Four different plasmid DNA vaccine constructs were designed for this study (Fig. 1). cDNAs encoding the antigens were cloned in the pVAX1 plasmid (Invitrogen, Carlsbad, California USA) under the regulation of the cytomegalovirus (CMV) promoter. Using standard molecular biology techniques, the full length open reading frame (ORF) of SjC23 and SjCTPI were cloned in pVAX1 at the NheI (5’-end) and BamHI (3’-end) restriction sites. To generate heat shock protein 70 (Hsp70) fusion proteins, the inducible bovine Hsp70 coding region (kindly provided by Dr. V. Guerriero, University of Arizona), was used as a template for PCR. The Hsp70-fusion constructs were generated in a two step cloning procedure. First, the Hsp70 coding sequence was amplified by PCR and cloned in the pVAX1 plasmid at the BamHI (5’-end) and EcoRI (3’-end) restriction sites. Positive clones (Hsp70-pVAX) were identified and used as cloning vectors in the next step. In step 2, the immunodominant extracellular hydrophilic loop of SjC23 (amino acids 108–183) and the full length ORF of SjCTPI were amplified by PCR and cloned at the 5’-end of the Hsp70 coding region in Hsp70-pVAX plasmid at the NheI (5’-end) and BamHI (3’-end) restriction sites. The stop codons were eliminated from SjC23 and SjCTPI to allow the expression of the fusion proteins as a single polypeptide. The antigens were separated from Hsp70 by two amino acids (Gly-Ser), which were generated by the two codons introduced by BamHI restriction site used for joining the two sequences (Fig. 1). All clones were analyzed by restriction digests as well as by DNA sequencing. The plasmid DNA encoding the human IL-12 (pUMVC3-hIL12) was purchased from Aldevron (Fargo, ND USA). Endo-Free Mega plasmid preparation Kit (Qiagen, Valencia, CA. USA) was used for plasmid DNA preparation according to manufacturer’s instructions. Plasmids were resuspended in endotoxin-free saline solution (Sigma).
Figure 1.
Schematic representation of schistosome DNA vaccine constructs. DNA sequences encoding the antigens were cloned into the pVAX1 plasmid at the indicated restriction sites. In case of the Hsp70-fusion constructs, antigens were spaced from Hsp70 by two codons for the amino acids (Glycine-Serine) which were generated by the BamHI site used to join both sequences.
2.2. Vaccine trial site and study design
The study site selected for the vaccine trials was Xinan village, Gangkou township, Yueyang, at the lower reaches of the Shagang River, Hunan province. According to government records, there was no history of schistosomiasis transmission at this site.
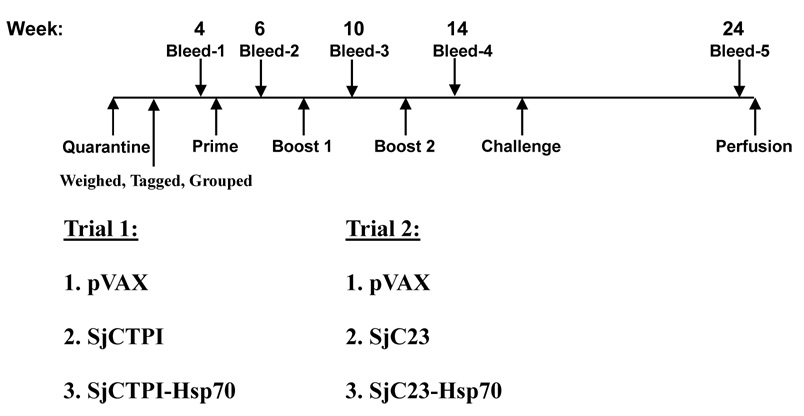
Two randomized double-blinded trials were set-up. Trial 1 evaluated SjCTPI and SjCTPI-Hsp70 plasmid DNA vaccines versus the pVAX (control). Trial 2 evaluated the SjC23 and SjC23-Hsp70 plasmid DNA vaccines versus the pVAX (control). Ninety water buffaloes (62 male and 28 female) aged 8–10 months with an average weight of 145.1±17.7 kg were purchased from Longhui county, Hunan province, an area with no history of schistosomiasis transmission. Female water buffaloes were distributed evenly between the different groups. All water buffaloes were initially quarantined at the site where they were confirmed as being schistosome-free by the miracidial hatching test (MHT), and by ELISA against schistosome antigens. The miracidial hatching test used 3 individual fecal samples from each water buffalo; each test was blinded (50 g feces/hatching) using a traditional Chinese sedimentation method [40]. All quarantined water buffaloes were treated with ivermectin (40mg/kg) to eliminate gastrointestinal nematodes before vaccination. Water buffaloes were then divided into two groups of 45 animals each for each of the two trials. Each water buffalo was assigned a unique identification number inscribed on the collar, the right ear, and into one of the horns. In each of the two trials, the water buffaloes were divided randomly into three groups (15 water buffaloes in each), each group receiving one of the vaccine constructs or the control. The research teams administering the vaccines or control plasmid were blinded until after the final data analysis. For the determination of vaccine efficacy, the primary endpoint of the trials was the reduction in the number of eggs per gram feces. This endpoint was used in the mathematical modeling because it is the key factor for disease transmission. Other endpoints were the reduction in liver eggs, miracidial hatching and worm burden.
2.3. Vaccination and challenge infection
Animals were immunized intramuscularly three times, 28 days apart, with the schistosome-DNA vaccines or the control vaccine (pVAX1 plasmid) (Fig. 2). All vaccinations were intramuscular into the shoulders, alternating sides with each boost. For the primary vaccination, water buffaloes received 300 µg of plasmid DNA in a final volume of 1 milliliter. For booster immunizations 300 µg schistosome vaccine or control plasmid DNA was administered along with 300 µg of human IL-12 plasmid as a molecular adjuvant. The final injection volume for the boosts (pVAX encoding antigen plus plasmid encoding IL-12) was maintained at 1 ml.
Figure 2.
Vaccination protocol of water buffaloes. Water buffaloes were obtained from schistosome-free area, treated with ivermectin, quarantined for 4 weeks, tagged and randomly divided into different groups (15 animals/group). Water buffaloes were injected intramuscularly 3 times, four weeks apart, challenged with 1000 cercariae, and perfused 8 weeks later. Blood samples were collected from animals at the indicated time points. The different groups in trials 1 and 2 are indicated.
Twenty-eight days after administration of the second boost, water buffaloes were challenged with cercariae. Oncomelanid snails infected with S. japonicum were kindly provided by Jiangsu Institute of Parasitic Diseases. Each water buffalo was challenged with 1000 cercariae, freshly shed from the infected snails. Cercariae were counted under a stereomicroscope and placed on glass slide cover slips, which were taped to the inner shaved thigh of the water buffalo for 30 minutes.
2.4. Examination of S. japonicum eggs in stool samples
Three fecal samples were collected from the rectum of each water buffalo two days and one day prior to perfusion and again on the day of perfusion. For each fecal sample, the number of eggs per gram of stool (EPG) was determined by microscopy and the number of hatched miracidia per gram stool was determined as previously described [40]. Briefly, a fresh stool specimen (approximately 200g) was collected and then a 50 g sub-sample was weighed and mixed with 100 ml water. The mixed sample was sieved sequentially through two nylon tissue bags/filters with different meshes (100 and 260 hole/inch). The sediment of the second sieve was removed to a clean conical flask. Water was added to the top of the flask and the flask was incubated at a constant temperature of 25°C. At 2, 4, 8, 12 and 24 hr post-incubation, hatched miracidia in the flask were monitored and collected on glass slides. A drop of iodine was added to kill and stain the miracidia. All miracidia collected from each sample were counted under a microscope. After a final check for hatched miracidia at 24 h post-incubation, the sediment at the bottom of flask was removed and all un-hatched eggs counted (6–9 slides) under the microscope. A routine method to validate the accuracy of the egg in the final sediment was to document infection intensity (eggs per gram, epg) for each water buffalo.
2.5. Water buffaloes perfusion and analysis of worm burden
Fifty-six days after cercarial challenge, water buffaloes were weighed then euthanized. Adult worms were obtained from the portal vein of euthanized water buffaloes by perfusion of the descending thoracic aorta with physiological saline and any additional remaining worms in the intestinal mesentery and liver were collected. All adult worms recovered from each water buffalo were counted and recorded as total males, total females and total worm burdens.
2.6. Examination of eggs in liver
Two samples from the left lobe and 1 sample from the right lobe (5g each sample) were taken from the livers of each water buffalo and weighed. Each piece was placed in 20 ml of 5% (w/v) KOH for 2 days at 37°C. The suspension was then agitated to re-suspend the mixture, 1ml of the liver suspension was collected, centrifuged at 3000 rpm for 1 min, the pellet re-suspended in 200 µl of PBS and the total eggs present were counted under a microscope. Using the average of three separate samples, the number of eggs per gram of liver tissue was calculated for each animal.
2.7. Production of recombinant proteins and humoral immune responses
2.7.1. Production of recombinant SjC23 and SjCTPI
The large extracellular hydrophilic domain (amino acids 108–183) of SjC23 (SjC23-loop) was expressed in Escherichia coli using the prokaryotic expression plasmid pET32b (Novagen, Madison, WI, USA). Briefly, the sequence encoding the SjC23-loop was amplified with Pfu-DNA-polymerase (Stratagene) using sense (5’-CCGGATCCCATGTACAAGGATAAAATCGATGAC-3’) and antisense (5’-GGCTCGAGTTAGTTGCGTTTTAAGAATGCACT-3’) primers which contain BamHI and XhoI restriction sites (underlined), respectively. The PCR product was digested with the restriction enzymes BamHI and XhoI and subcloned in a BamHI/XhoI predigested pET32b. This system produces a recombinant thioredoxin fusion protein. Final clones were analyzed by sequencing and transformed into BL21(DE3)-Gold cells (Stratagene).
The pTrcHisB plasmid (Invitrogen) was used to produce recombinant SjCTPI. The full length ORF of SjCTPI was amplified with Pfu-DNA-polymerase (Stratagene) using sense (5’-CCGGATCCCATGTCTGGTTCTCGGAAATTTTTTG-3’, and antisense, 5’- GGCTCGAGTTATTGTCTAGCTTTACATATATC-3’ primers which contain BamHI and XhoI restriction sites (underlined), respectively. PCR products were digested with BamHI/XhoI restriction enzymes and subcloned in a predigested pTrcHisB plasmid. Resulting clones were analyzed by restriction digestion and sequencing, and those positive were transformed in TOP10 E. coli cells (Invitrogen).
To produce recombinant (r) SjC23 and SjCTPI proteins, a single colony from each clone was grown overnight at 37°C in LB-medium containing 75 µg/ml ampicillin. The overnight cultures were then diluted 100-fold in LB-medium-Amp and grown until an OD600 of 0.5 was reached. Protein expression was then induced by the addition of IPTG to 1.0 mM and the cells were grown for another 3 h. Cells were harvested by centrifugation and lysed by sonication. The recombinant proteins were then purified using His-Trap HP columns (GE Healthcare, Piscataway, NJ, USA). Purified recombinant proteins were analyzed by SDS-PAGE and Western Blotting.
2.7.2 Western blotting
Purified recombinant rSjC23 or rSjCTPI were separated onto a prep-SDS-PAGE, and transferred onto a Polyvinylidene Fluoride (PVDF) membrane. The membrane was cut into identical strips, pre-wetted using TBST (20 mM Tris-HCl, 150mM NaCl, pH 7.5, 0.05% (v/v) Tween 20), blocked with TBST containing 5% non-fat dry milk, under constant agitation at room temperature for one hour. Four serum samples from each group were diluted 1:50 and used to probe different strips. The serum samples were obtained from water buffaloes two weeks prior to challenge infection. Strips were incubated with the sera at room temperature for 2 hr with agitation. After rinsing (5X) with TBST, the strips were incubated for 1 h under agitation with secondary antibody (rabbit anti-bovine IgG-peroxidase conjugate, Sigma-Aldrich) diluted to 1:1000 in blocking solution. Blots were developed using H2O2 and 3, 3’-Diaminobenzidine (DAB) in PBS.
2.7.3 ELISA
Indirect ELISA was used to determine the levels of specific anti-SjC23 and anti-SjCTPI antibodies in buffalo sera. Blood samples were collected from all individual animals at five different time points during each trial; 2 days before primary vaccination, 15 days after each injection and at 56 days post-challenge infection (Fig. 2). Sera were prepared and stored at - 70°C until use. Flat-bottomed microplates (Maxi-Sorb; Nunc, Roskilde, Denmark) were coated with 10µg/ml of rSjCTPI or rSjC23 diluted in CB1 coating buffer (100µl/well) (Immunochemistry Technology, LCC, Bloomington, USA). Plates were incubated over night at room temperature, then washed 4 times with PBS-0.05%/Tween20 and blocked with 200µl of PBS-5% (v/v) normal pig serum albumin (PSA) and then washed 3 times with PBS-0.05%Tween20. Sera from all individual animals were diluted to 1:100 with PBS-PSA, and then 100µl of each diluted serum sample was added in duplicate to each well. Dilution buffer (PBS-PSA), pooled sera from the DNA plasmid control groups and pooled sera from the SjCTPI or SjC23 vaccinated groups just prior to perfusion were used as blank, negative control and positive control, respectively. The plates were incubated at room temperature for 2 h then washed 5 times with PBS-Tween20 followed by the addition of 100 µl of diluted secondary antibody (1:10000 rabbit anti-bovine IgG-peroxidase conjugate, Sigma-Aldrich, St Louis, USA) to each well. The plates were incubated at room temperature for 2 h, washed 6 times with PBS-Tween20, after which 100 ìl TMB substrate (Sigma-Aldrich, St Louis, USA), was added to each well. After 15 min incubation, the reactions were stopped by adding 50 µl of stop solution (5% (v/v) phosphoric acid) and the optical density (OD) was measured at 450nm. OD values were expressed as means ± SE of all individual animals within the same group.
2.8. Mathematical modeling
The mathematical model developed by Williams et al. [5] was used to determine if the resultant efficacies of the S. japonicum plasmid DNA vaccines evaluated here are sufficient to provide long term sustainable of S. japonicum in the marsh and lakes regions of Southern China. The best vaccine candidates were compared to the hypothetical vaccine providing 45% protective efficacy described in Williams et al. [5], and also incorporated with other control strategies and modeled as a realistic potential intervention program. The model used the same assumptions as those outlined in Williams et al. [5] with the resultant vaccine efficacies (based on the reduced fecal EPG) obtained in the two trials used to substitute for those in the original model. Basic parameters included endemic human, bovine and snail prevalences of 20%, 17% and 1% respectively, and vaccine coverage of 95% and no loss of vaccine efficacy over time.
2.9. Statistical analyses
Vaccine allocation was un-blinded after all testing was completed and all data entered. SPSS was used for data entry and preliminary data analysis. SAS was used for the final analysis. Negative binomial regression was used to analyze the total number of adult worms, number of eggs per gram of liver tissue, number of eggs per gram of feces and number of hatched miracidia per gram of feces, with vaccine group as the independent variable. Contrasts were used to compare each active vaccine with the control. Relative intensities of infection (vaccine:control) and a 95% confidence interval were derived from these effects after exponential transformation. Vaccine efficacy (VE) (95% confidence interval) was then calculated as (1-relative risk) × 100%.
3. Results
3.1 Worm burden reduction
The worm burden results of the two vaccine trials are summarized in Tables 1. In both trials a total of 4 out of 90 water buffaloes died of unknown natural causes, with symptoms including fever, diarrhea and lack of appetite. In trial 1, vaccination of water buffaloes with the SjCTPI construct or the SjCTPI-Hsp70 construct resulted in significant reductions of 41.5% (95% CI: 29.9–51.2%) and 51.2% (95% CI: 41.9–59.0%), respectively, in mean adult worm burdens when compared to immunization with the control plasmid DNA (pVAX) (Table 1). The inclusion of Hsp70 increased the efficacy of the vaccine construct (from 41.5% to 51.2%); statistically, the difference in efficacy with Hsp70 included was marginally insignificant (p=0.052). Similarly, vaccination of water buffaloes with SjC23 or SjC23-Hsp70 in trial 2 resulted in 45.5% (95% CI: 36.9–52.8%) and 50.9% (95% CI: 43.4–57.3%) reductions in mean adult worm burdens, respectively (Table 1). There was no significant difference in worm burdens from water buffaloes vaccinated with SjC23-Hsp70 when compared to SjC23 (p=0.15).
Table 1.
Worm burden (mean ± SE) in immunized and control water buffaloes in trials 1 and trial 2, and percent reduction in total worm burdens in immunized animals compared with their corresponding control group (pVAX) in each trial.
| Group |
Male Worms (Mean ± SE) |
Female Worms (Mean ± SE) |
Total Worms (Mean ± SE) |
Vaccine Efficacy (%) a (95% CI) |
|---|---|---|---|---|
| Trial 1 | ||||
| pVAX | 179.20 ± 10.3 | 115.73 ± 8.7 | 294.93 ± 17.3 | --- |
| (n = 15)b | ||||
| SjCTPI | 108.54 ± 6.8 | 64.00 ± 3.1 | 172.54 ± 9.1 | 41.5% |
| (n = 13) | (29.9%, 51.2%) | |||
| SjCTPI-Hsp70 | 84.73 ± 6.7 | 59.20 ± 5.2 | 143.93 ± 11.2 | 51.2% |
| (n = 15) | (41.9%, 59.0%) | |||
| Trial 2 | ||||
| pVAX | 217.00 ± 7.7 | 165.38 ± 9.8 | 382.38 ± 15.5 | --- |
| (n = 13) | ||||
| SjC23 | 122.62 ± 5.6 | 85.92 ± 5.3 | 208.54 ± 10.8 | 45.5% |
| (n = 15) | (36.9%, 52.8%) | |||
| SjC23-Hsp70 | 105.73 ± 7.3 | 82.13 ± 5.3 | 187.87 ± 11.5 | 50.9% |
| (n = 15) | (43.4%, 57.3%) |
Vaccine efficacy was calculated using total worms; differences were significant at p<0.0001; CI: 95% confidence intervals.
n represents the number of animals in each group at the time of perfusion.
3.2 Egg reduction in liver tissues
Pathology in schistosomiasis is due to the host immune response to parasite eggs trapped in host tissues. Therefore, we investigated the effect of the different vaccines on egg deposition in the livers of water buffaloes. The results of egg burdens of the two trials are summarized in Table 2. Vaccination of water buffaloes with SjCTPI resulted in a reduction (vaccine efficacy) of 42% (95% CI: 30.4–51.6%) in the egg burden in the liver (Table 2). This effect was significantly (p<0.000013) increased to 61.4% (95% CI: 54–67.7%) when the bovine Hsp70 sequence (SjCTPI-Hsp70) was included (Table 2). Similarly, vaccination with SjC23 or SjC23-Hsp70 resulted in liver egg reductions of 42.9% (95% CI: 32.3–51.8%) and 53.9% (95% CI: 45.6–60.9%), respectively, with the reduction rate significantly higher with the Hsp70 construct (p<0.01) (Table 2).
Table 2.
Egg burden (mean ± SE) in immunized and control water buffaloes in trials 1 and 2, and percent reduction in egg burden in immunized animals compared with their corresponding control group (pVAX) in each trial.
| Group | Eggs per gram liver |
Eggs per gram feces |
Hatched miracidia per gram feces |
|||
|---|---|---|---|---|---|---|
| Mean ± SE | Vaccine Efficacy (%)a, (95% CI) | Mean ± SE | Vaccine Efficacy (%)a, (95% CI) | Mean ± SE | Vaccine Efficacy (%)a, (95% CI) | |
| Trial 1 | ||||||
| pVAX | 459.0 ± 25.6 | --- | 23.8 ± 1.7 | --- | 18.4 ± 1.6 | --- |
| (n = 15) | ||||||
| SjCTPI | 266.4 ± 24.2 | 42.0% | 14.7 ± 1.3 | 38.3% | 12.3 ± 1.1 | 33.2% |
| (n = 13) | (30.4%, 51.6%) | (24.6%, 49.4%) | (16.2%, 46.7%)b | |||
| SjCTPI-Hsp70 | 177.0 ± 11.5 | 61.5% | 11.4 ± 0.7 | 52.1% | 8.8 ± 0.7 | 52.1% |
| (n = 15) | (54%, 67.7%) | (41.3%, 60.9%) | (39.4%, 62.2%) | |||
| Trial 2 | ||||||
| pVAX | 500.1 ± 26.5 | --- | 32.5 ± 2.1 | --- | 26.5 ± 1.0 | --- |
| (n = 13) | ||||||
| SjC23 | 285.7 ± 21.7 | 42.9% | 19.5 ± 0.9 | 40.2% | 13.9 ± 0.6 | 47.4% |
| (n = 15) | (32.3%, 51.8%) | (30.0%, 48.9%) | (37.1%, 56.0%) | |||
| SjC23-Hsp70 | 230.6 ± 13.6 | 53.9% | 16.9 ± 0.8 | 48.0% | 12.7 ± 0.7 | 52.0% |
| (n = 15) | (45.6%, 60.9%) | (39.1%, 55.5%) | (42.8%, 59.7%) | |||
Percent reduction in egg burdens were significant at p<0.0001;
significant at p< 0.001; CI: 95% confidence intervals. n represents the number of animals in each group at the time of perfusion.
3.3 Reduction in fecal eggs and hatched miracidia
Since the vaccine effect on the released eggs is a strong indicator of the ability of that vaccine to function as an anti-transmission vaccine, we assessed the effect of the four vaccine constructs on fecal egg counts as well as on the numbers of hatched miracidia (Table 2). Vaccination of water buffaloes with SjCTPI or SjCTPI-Hsp70 (trial 1) resulted in reductions in fecal egg outputs of 38.3% (95% CI: 24.6–49.4%) and 52.1% (95% CI: 41.3–60.9%) respectively, with the vaccine efficacy significantly higher in the SjCTPI-Hsp70 construct (p<0.028). Furthermore, the same constructs resulted in reductions of 33.2% (95% CI: 16.2–46.7%) and 52.1% (95% CI: 39.4–62.2%) respectively, in hatched miracidia per gram feces; the addition of Hsp70 significantly increased the vaccine efficacy (p<0.01) (Table 2). Compared with the pVAX, vaccination with SjC23 or SjC23-Hsp70 (trial 2) resulted in reductions of 40.2% (95% CI: 30–48.9%) and 48% (95% CI: 39.1–55.5%) respectively in fecal egg outputs; and 47.4% (95% CI: 37.1–56.0%) and 52% (95% CI: 42.8–59.7%) respectively in hatched miracidia per gram feces (Table 2). The reduction in fecal eggs and hatched miracidia was not statistically significant between the two constructs.
3.4 Anti-SjCTPI/SjC23 IgG antibody response
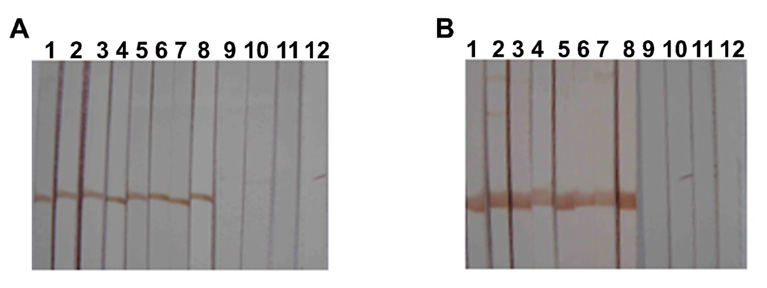
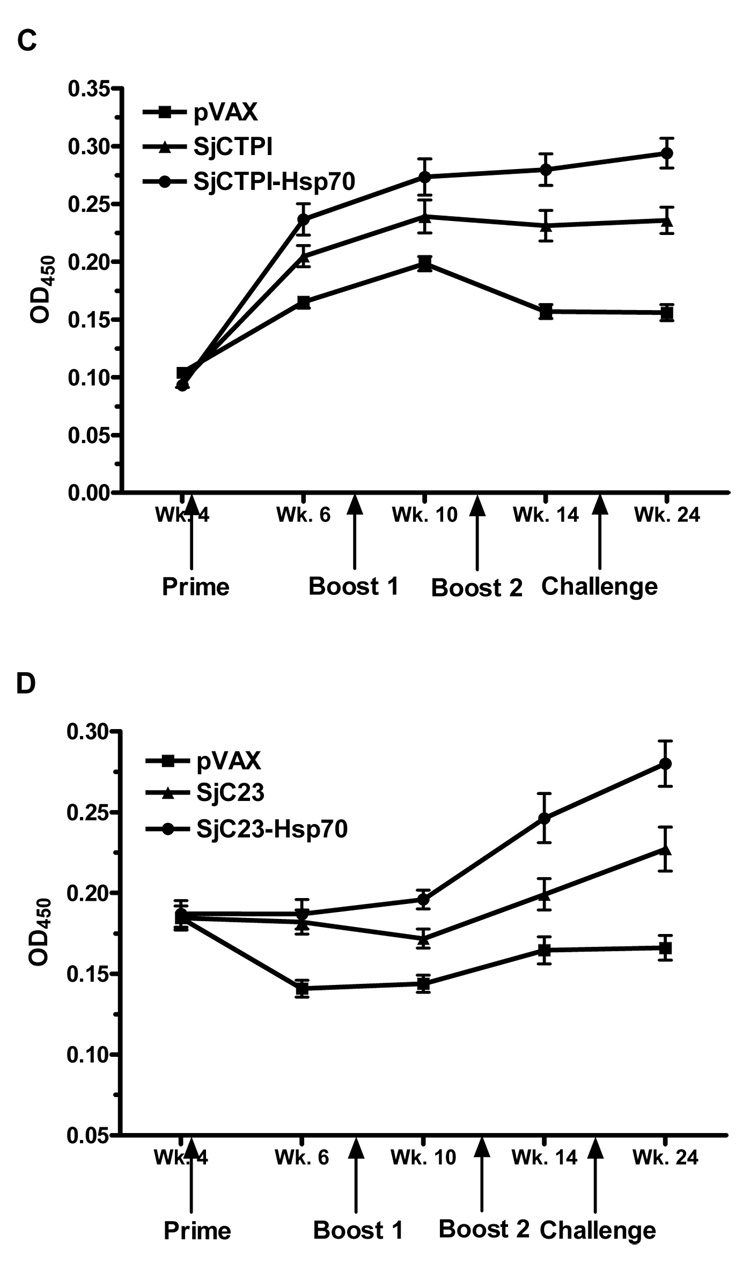
SjCTPI and the extracellular hydrophilic domain of SjC23 were expressed in E. coli as a poly-his fusion protein. They were purified using nickel chromatography, and the purified recombinant fusion proteins were analyzed by SDS-PAGE. Sera from control and immunized animals were examined for the presence of anti-SjC23 or SjCTPI specific antibodies for rSjC23 and rSjCTPI using Western Blots and ELISA. Figure 3 shows a Western Blot using four individual serum samples per group; sera were obtained from the different animals two weeks prior to challenge infection (Week 14). Animals immunized with SjC23 (Fig 3A, lanes 1–4), SjC23-Hsp70 (Fig. 3A, lanes 5–8), SjCTPI (Fig. 3B, lanes 1–4) and SjCTPI-Hsp70 (Fig. 3B, lanes 5–8) elicited high levels of specific antibodies. In contrast, sera from animals immunized with the control pVAX plasmid did not react with rSjC23 (Fig. 3A, lanes 9–12) or rSjCTPI (Fig. 3B, lanes 9–12). ELISA results using individual sera collected at different time points from animals in Trial 1 (SjCTPI) (Fig. 3C) or Trial 2 (SjC23) (Fig. 3D) show that antibody levels are slightly increased during the course of the experiment. Although the OD values in the vaccinated groups (SjCTPI and SjC23) were not very high when compared to control groups (pVAX), the differences between these values were statistically significant (P<0.01).
Figure 3.
Analysis of the humoral immune response. Western Blot analysis of four individual serum samples obtained from vaccinated animals from each group prior to challenge infection (A and B). Animals immunized with SjC23 (A, lanes 1–4) and SjC23-Hsp70 (A, lanes 5–8), SjCTPI (B, lanes 1–4) and SjCTPI-Hsp70 (B, lanes 5–8), elicited specific antibody response. Sera from animals immunized with the control pVAX plasmid did not react with rSjC23 (A, lanes 9–12) or rSjCTPI (B, lanes 9–12). Levels (OD values) of specific anti-SjCTPI (C) and anti-SjC23 (D) total IgG antibodies for all individual animals were analyzed by ELISA. Serum samples were obtained at 2 days prior to prime (Wk. 4), two weeks after the prime (Wk. 6), two weeks post the first boost (Wk. 10), two weeks post the second boost (Wk. 14), and two days prior to perfusion (Wk. 24). Arrows indicate the time of the different immunizations as well as the challenge infection. Values are expressed as means ± SE of the OD values of all animals within the same group.
3.5 Mathematical modeling
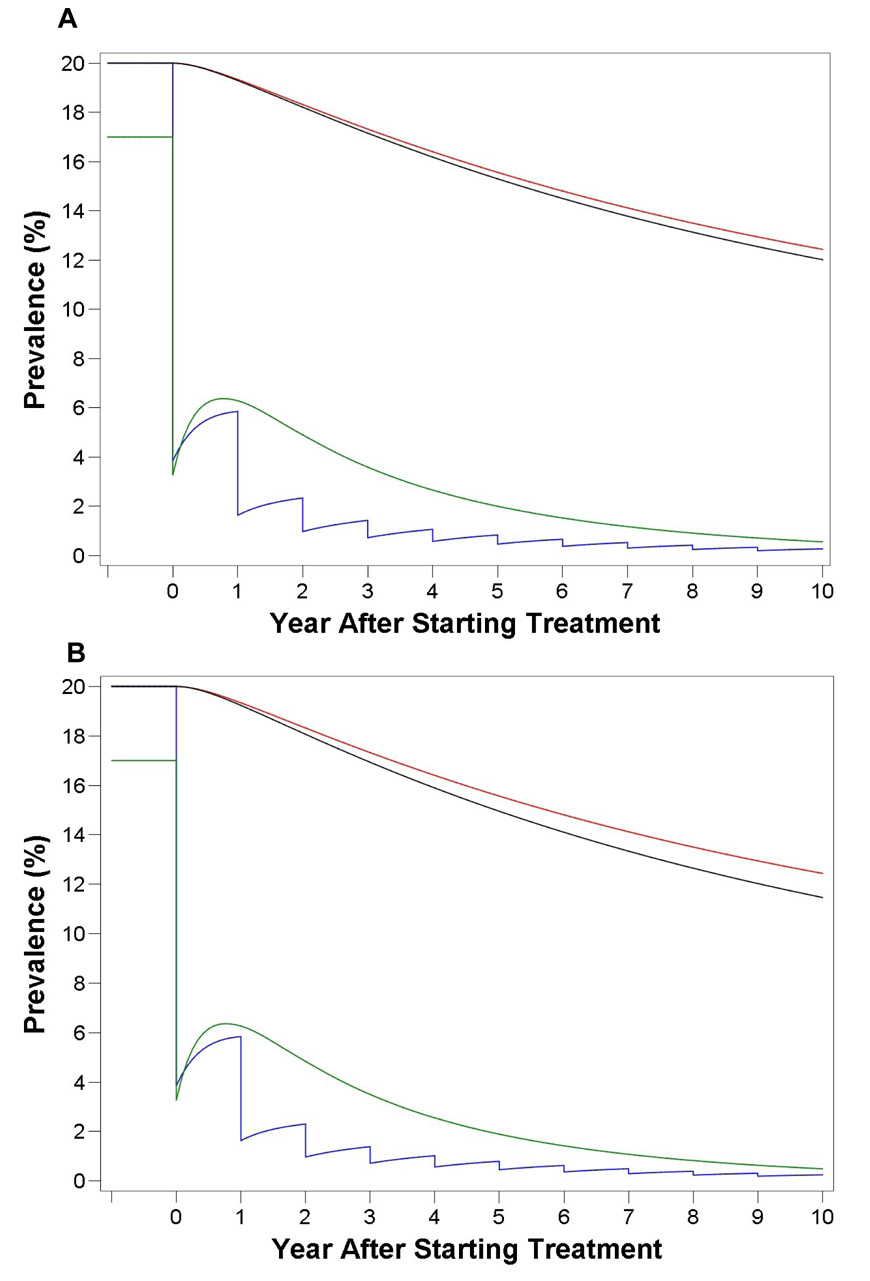
Mathematical modeling showed that of the 4 vaccine constructs tested in this study, only those incorporating Hsp70 had a marked effect on the reduction of prevalence over time. The SjC23-Hsp70 (Fig. 4A; 48% reduction in fecal egg output) and the SjCTPI-Hsp70 (Fig. 4B; 52% reduction in fecal egg output) constructs were modeled alone and as an intervention in conjunction with human annual mass treatment and an initial water buffaloes treatment; and were compared to the hypothetical vaccine providing 45% vaccine efficacy [5].
Figure 4.
Mathematical modeling of the schistosome DNA vaccine constructs. The DNA vaccine constructs SjC23-Hsp70 (A) and SjTPI-Hsp70 (B) were modeled alone and as an intervention (in conjunction with human annual mass treatment and an initial water buffaloes treatment) and compared to a hypothetical vaccine providing 45% efficacy. The red lines represents the effect of a hypothetical vaccine providing 45% efficacy alone on human prevalence; the black lines represents the effect of SjC23-Hsp70 vaccine (A) and SjCTPI-Hsp70 vaccine (B) providing 48% and 52% efficacy respectively on human prevalence; the blue lines represents the effect of the of SjC23-Hsp70 vaccine (A) or SjCTPI-Hsp70 (B) in combination with an initial bovine mass treatment and annual mass treatment of humans for 10 years on human prevalence; and the green lines represents the effect of SjC23-Hsp70 vaccine (A) and SjCTPI-Hsp70 vaccine (B) in combination with an initial bovine mass treatment and annual mass treatment of humans for 10 years on water buffaloes prevalence.
Both vaccine constructs alone showed greater reductions in human prevalence compared to the 45% hypothetical vaccine over the 10-year period (Fig. 4), with human prevalence reduced from 20% to 12.4% for the 45% vaccine, 12% for SjC23-Hsp70 and 11.5% for SjCTPI-Hsp70. Additionally, over a 25-year period these were further reduced to 8.3% for the 45% hypothetical vaccine, 7.7% for SjC23-Hsp70 and 6.9% for SjCTPI-Hsp70. The equilibrium prevalence (the predicted balance of all prevalences; it approaches zero when the disease reproductive rate falls below one) for all 3 vaccines over a 25-year period remained constant at 4.6% for the 45% hypothetical vaccine, 3.4% for SjC23-Hsp 70 and 1.6% for SjCTPI-Hsp70.
When the SjC23-Hsp70 and SjCTPI-Hsp70 vaccines were combined with annual mass human treatment and an initial bovine mass treatment and modeled as an intervention program, human prevalence was reduced considerably compared to that of the vaccine alone. Over the 10-year period, human prevalence was reduced to 0.03% for SjC23-Hsp70 and 0.02% for SjCTPI-Hsp70, however over a 25-year period they slightly increased from the 10-year time point to 0.04% for SjC23-Hsp 70 and 0.03% for SjCTPI-Hsp70. The equilibrium prevalence was reduced to zero for both vaccines after 5 years, but these returned to the levels seen when the vaccine was modeled alone at the 25-year mark.
4. Discussion
Schistosomiasis japonica is a zoonotic disease affecting human as well as several vertebrate animals including water buffaloes which account for about 75% of disease transmission to humans [4–7]. The aim of this study was to evaluate the efficacy of two schistosome vaccine candidates, SjCTPI and SjC23, as plasmid DNA vaccines against S. japonicum in water buffaloes. Two randomized double blind control vaccine trials were performed to determine the efficacy levels of the SjC23 and SjCTPI vaccines on their own, or when fused together with the dendritic cell targeting molecule, heat shock protein 70 (Hsp70) (SjCTPI-Hsp70 and SjC23-Hsp70) [38]. Each trial utilized three groups of 15 water buffaloes, and in both trials, all booster vaccines, including controls, were co-administered with an interleukin-12 (IL-12) expressing plasmid.
All DNA vaccine constructs elicited an IgG immune response specific for SjCTPI or SjC23 as determined by Western Blot and ELISA. The DNA vaccine constructs significantly reduced the worm burden (41.5–51.2%, Table 1) and egg burden (33.2–61.5%, Table 2) in vaccinated/challenged water buffaloes. Constructs incorporating Hsp70 generated a greater immune response and increased vaccine efficacy. The most successful vaccine was the SjCTPI-Hsp70 construct, which produced a 51.2% reduction in worm burden, 61.5% liver egg reduction, 52.1% fecal egg reduction and 52.1% reduction in fecal miracidia.
We focused on DNA vaccines for these trials based on earlier success in vaccinating mice and pigs with similar plasmid DNA vaccines encoding these same two antigens [26–30,41]. Additionally, preparation and production of DNA vaccines is relatively easy and cost effective, and for use in the field, can even be used without a cold chain. Another advantage of applying DNA vaccines when compared to other approaches is the possibility of targeting the in vivo expressed recombinant antigen to different cell compartments [42–45]. In this study, we chose the heat shock protein 70 sequence to target antigen presenting cells to increase vaccine immunogenicity/efficacy. Furthermore, using DNA vaccines, molecular adjuvants such as plasmid DNAs encoding cytokines can be co-administered with the vaccine antigen. Based on our earlier studies and those of others, we chose an IL-12 encoding plasmid as adjuvant [19,28,46,47].
We selected SjCTPI ad SjC23 as our candidate vaccine antigens based on more than a decade of research on these two schistosome molecules, initially as recombinant antigens, then as multiple antigen synthetic peptides and finally as plasmid DNA constructs [16,19,25–30,48,49]. In addition to our studies, these two antigens have been evaluated as vaccines in mice and livestock in numerous studies by others, always showing reasonable levels of efficacy [29,30]. Triose-phosphate isomerase (TPI) is a dimeric enzyme which converts glyceraldehyde-3-phosphate to dihydroxyacetone phosphate, an important step in the glycolytic pathway. SjCTPI is located at the surface membrane of newly transformed schistosomula and in all cells of schistosomes [50]. The 23 kDa surface membrane molecule was the first tetraspanin to be described from helminth parasites [24,51,52]. It is a member of the trans-membrane-4 superfamily (TM4SF), and it is an integral membrane protein consisting of four hydrophobic trans-membrane domains and large and small hydrophilic domains that are thought to be extracellular. The 23 kDa protein has been detected in all stages of the parasite in vertebrates, and in virtually all cells, thus making it is an excellent vaccine target [52]. When administered to sheep with Freund’s adjuvant, rSj23 was reported to be highly immunogenic with a worm burden reduction of 59% [53]. Immunization of pigs with SjCTPI and SjCTPI plus IL-12 DNA vaccine [28], or with SjC23 and SjC23 plus IL-12 DNA vaccine [19] showed that these vaccines were able to induce strong immune protection. Interleukin-12 has the potential to activate natural killer (NK) cells and promote cytolytic T cell proliferation. IL-12 is an important cytokine that when used as an adjuvant promotes Th1-type and cellular immune responses [16,54,55]. These studies confirm earlier observations that both S. mansoni and S. japonicum 23 kDa protein and TPI vaccines function optimally as Th1-type vaccines [16,19,28,41,49,56].
Several efforts have been made to enhance the immunogenicity and the efficacy of DNA vaccines; targeting dendritic cells is one of the most promising methods. We performed several studies in mice to enhance the immunogenicity and the efficacy of the S. mansoni plasmid DNA vaccines. Some of these studies showed that the incorporation of the coding region of the dendritic cell targeting molecule, Hsp70, was able to enhanced immunogenicity of these vaccines (unpublished data). Our results were similar to those reporting demonstrated increased immunogenicity of mycobacterial vaccines when the coding region of Hsp70 was incorporated into the vaccine [57]. Hsp70 is a member of a family of molecular chaperones that have important cellular roles in protein synthesis and modifying processes [58–60]. Hsp70 can combine with many kinds of antigenic peptides to form antigen peptide-Hsp compounds. As discussed in Triantafilou et al. [61] Hsp70 and other Hsps are usually found in dendritic cell signalosomes following PAMP stimulation/activation. Taken together, there was significant background literature to support the inclusion of Hsp70 into our two vaccines as a mean of enhancing immunogenicity and efficacy. As evident from the results of the two vaccine trials presented here, the vaccines incorporating Hsp70 are more immunogenic and efficacious, as determined by its effect on egg burden, than the homologous constructs without Hsp70.
In regards to the impact of either of these two vaccines on transmission of schistosomiasis in China, both the SjC23-Hsp70 and SjCTPI-Hsp70 alone were predicted by the mathematical model to be effective at achieving long term sustainable control of schistosomiasis. Either vaccine reduces the equilibrium prevalence to a level below that of the hypothetical 45% vaccine modeled by Williams et al. [5]. The purpose of the model is to show the application of this vaccine in an integrated control program and predict such program’s outcome. This is particularly important for policy making decisions in public health. While the vaccine alone impacts considerably on the equilibrium prevalence, it is very slow acting on the actual human prevalence due to the indirect mechanism of action by the vaccine upon human infection rates. As a result human prevalence slowly decreases over time. However, when the efficacy of the SjC23-Hsp70 or SjCTPI-Hsp70 vaccines were incorporated into an intervention program coincident with annual human mass treatment (praziquantel) for 10 years and an initial bovine mass treatment (praziquantel) (Fig. 4), the equilibrium prevalence was predicted to be reduced to zero after 5 years and a major initial reduction was predicted in both the human and bovine prevalence due to the treatment regimen. After the cessation of human treatment at the 10-year mark the equilibrium prevalence returned to that seen when the vaccine was administered alone however the human prevalence was maintained at low levels due to the action of the vaccine. While the model predicts that either vaccine alone is effective at long term control, the combination of treatment and vaccination is predicted to be effective at both the short and long term control of schistosomiasis in the lake and marsh region of China.
The results of this study are of direct public health relevance as a recently concluded bovine drug intervention trial in schistosome-endemic communities indicated that water buffaloes are responsible for 75–80% of schistosomiasis transmission in the marshland areas of China [7]. Control programs in China employing mass human treatment with praziquantel (PZQ) have failed to reduce transmission to desired levels. We propose a novel control program for schistosomiasis in China, involving, in addition to treatment with PZQ, would be vaccination of water buffaloes with partially protective vaccines such SjC23-Hsp70 and SjCTPI-Hsp70 as a means to reduce numbers of egg-laying parasites in livestock, leading to measurable declines in prevalence, intensity and transmission of S. japonicum. Currently, a field trial to test this hypothesis using the SjC23-Hsp70 vaccine is underway in China.
Given the levels of vaccine efficacy shown in this study, and based on the mathematical modeling [5] undertaken, the employment in tandem of human PZQ treatment and bovine vaccination would significantly reduce transmission of schistosomiasis in the lake and marshland areas of southern China, even to the point of elimination. Such a novel control program for schistosomiasis in China would impact positively on social and economic conditions for local inhabitants by reducing morbidity due to schistosomiasis as well as improved physical maturation and cognition in children [9,62,63]. Similarly it has been shown that non-schistosome infected animals grow and gain weight faster and are overall healthier than schistosome infected animals. Thus a control program that reduces prevalence, intensity and transmission of schistosomiasis will directly benefit the health of animals in endemic sites.
5. Acknowledgements
We thank Drs. He Young Kang and other colleagues from Hunan Institute of Parasitic Diseases and Norman Lautsch at Harvard School of Public Health for their invaluable assistance. We also thank Dr. Vince Guerriero at the University of Arizona, USA for providing the bovine Hsp70 plasmid. This project was supported by grants to D.H. from the Wellcome Trust (GR 075816) and the National Institute of Health (NIH) (R01AI068109). The mathematical modeling work was developed as part of an ICRGS Award supported by the Wellcome Trust and the NHMRC (Australia)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chitsulo L, Loverde P, Engels D. Schistosomiasis-TDR/WHO. Nat Rev Microbiol. 2004;2(1):12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- 2.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 3.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86(2–3):125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 4.Xianyi C, Liying W, Jiming C, Xiaonong Z, Jiang Z, Jiagang G, et al. Schistosomiasis control in China: the impact of a 10-year World Bank Loan Project (1992–2001) Bull World Health Organ. 2005;83(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 5.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, et al. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People's Republic of China. Acta Trop. 2002;82(2):253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Zhang S, Wu W, Zhang G, Lu D, Ornbjerg N, et al. Treatment and reinfection of water buffaloes and cattle infected with Schistosoma japonicum in Yangtze River Valley, Anhui province, China. J Parasitol. 2006;92(5):1088–1091. doi: 10.1645/GE-806R.1. [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Li Y, Gray D, Ning A, Hu G, Chen H, et al. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People's Republic of China. Am J Trop Med Hyg. 2006;74(2):335–341. [PubMed] [Google Scholar]
- 8.Li YS, Sleigh AC, Li Y, Tanner M, Dessein A, Williams GM, et al. Five-year impact of repeated praziquantel treatment on subclinical morbidity due to Schistosoma japonicum in China. Trans R Soc Trop Med Hyg. 2002;96(4):438–443. doi: 10.1016/s0035-9203(02)90386-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang YX, Manderson L. The social and economic context and determinants of schistosomiasis japonica. Acta Trop. 2005;96(2–3):223–231. doi: 10.1016/j.actatropica.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J, Gu XG, Xu YL, Ge JH, Yang XX, He CH, et al. Relationship between the transmission of schistosomiasis japonica and the construction of the Three Gorge Reservoir. Acta Trop. 2002;82(2):147–156. doi: 10.1016/s0001-706x(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 11.McManus DP. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 2005;27(7–8):297–308. doi: 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang GJ, Vounatsou P, Zhou XN, Utzinger J, Tanner M. A review of geographic information system and remote sensing with applications to the epidemiology and control of schistosomiasis in China. Acta Trop. 2005;96(2–3):117–129. doi: 10.1016/j.actatropica.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;133 Suppl:S43–S61. doi: 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- 14.Wu ZD, Lu ZY, Yu XB. Development of a vaccine against Schistosoma japonicum in China: a review. Acta Trop. 2005;96(2–3):106–116. doi: 10.1016/j.actatropica.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21(3):112–117. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Da'dara AA, Skelly PJ, Wang MM, Harn DA. Immunization with plasmid DNA encoding the integral membrane protein, Sm23, elicits a protective immune response against schistosome infection in mice. Vaccine. 2001;20(3–4):359–369. doi: 10.1016/s0264-410x(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 17.Auriault C, Gras-Masse H, Pierce RJ, Butterworth AE, Wolowczuk I, Capron M, et al. Antibody response of Schistosoma mansoni-infected human subjects to the recombinant P28 glutathione-S-transferase and to synthetic peptides. J Clin Microbiol. 1990;28(9):1918–1924. doi: 10.1128/jcm.28.9.1918-1924.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Auliff A, Jones MK, Yi X, McManus DP. Immunogenicity and immunolocalization of the 22.6 kDa antigen of Schistosoma japonicum. Parasite Immunol. 2000;22(8):415–424. doi: 10.1046/j.1365-3024.2000.00319.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Ren J, Da'dara A, Harn D, Xu M, Si J, et al. The protective effect of a Schistosoma japonicum Chinese strain 23 kDa plasmid DNA vaccine in pigs is enhanced with IL-12. Vaccine. 2004;23(1):78–83. doi: 10.1016/j.vaccine.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006 doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 21.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37(3–4):257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Niles EG, El-Sayed N, Berriman M, LoVerde PT. Schistosoma mansoni (Platyhelminthes, Trematoda) nuclear receptors: sixteen new members and a novel subfamily. Gene. 2006;366(2):303–315. doi: 10.1016/j.gene.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui AA, Pinkston JR, Quinlin ML, Saeed Q, White GL, Shearer MH, et al. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005;23(12):1451–1456. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Wright MD, Henkle KJ, Mitchell GF. An immunogenic Mr 23,000 integral membrane protein of Schistosoma mansoni worms that closely resembles a human tumor-associated antigen. J Immunol. 1990;144(8):3195–3200. [PubMed] [Google Scholar]
- 25.Gan XX, Shen LY, Wang Y, Ding JZ, Shen HY, Zeng XP, et al. Recombinant tegumental protein Shistosoma japonicum very lowdensity lipoprotein binding protein as a vaccine candidate against Schistosoma japonicum. Mem Inst Oswaldo Cruz. 2006;101(1):9–13. doi: 10.1590/s0074-02762006000100003. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Ren J, Harn DA, Si J, Yu C, Ming X, et al. Protective immunity induced with 23 kDa membrane protein dna vaccine of Schistosoma japonicum Chinese strain in infected C57BL/6 mice. Southeast Asian J Trop Med Public Health. 2003;34(4):697–701. [PubMed] [Google Scholar]
- 27.Zhu Y, Si J, Ham DA, Yu C, He W, Hua W, et al. The protective immunity produced in infected C57BL/6 mice of a DNA vaccine encoding Schistosoma japonicum Chinese strain triose-phosphate isomerase. Southeast Asian J Trop Med Public Health. 2002;33(2):207–213. [PubMed] [Google Scholar]
- 28.Zhu Y, Si J, Harn DA, Xu M, Ren J, Yu C, et al. Schistosoma japonicum triose-phosphate isomerase plasmid DNA vaccine protects pigs against challenge infection. Parasitology. 2006;132(Pt 1):67–71. doi: 10.1017/S0031182005008644. [DOI] [PubMed] [Google Scholar]
- 29.Shi F, Zhang Y, Lin J, Zuo X, Shen W, Cai Y, et al. Field testing of Schistosoma japonicum DNA vaccines in cattle in China. Vaccine. 2002;20(31–32):3629–3631. doi: 10.1016/s0264-410x(02)00398-5. [DOI] [PubMed] [Google Scholar]
- 30.Shi F, Zhang Y, Ye P, Lin J, Cai Y, Shen W, et al. Laboratory and field evaluation of Schistosoma japonicum DNA vaccines in sheep and water buffalo in China. Vaccine. 2001;20(3–4):462–467. doi: 10.1016/s0264-410x(01)00340-1. [DOI] [PubMed] [Google Scholar]
- 31.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1(2):151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 32.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14(3):303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 33.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in Dendritic Cell-Mediated Antigen Cross-Presentation. Immunity. 2002;17(3):353. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 34.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 35.Binder RJ, Karimeddini D, Srivastava PK. Adjuvanticity of alpha 2-macroglobulin, an independent ligand for the heat shock protein receptor CD91. J Immunol. 2001;166(8):4968–4972. doi: 10.4049/jimmunol.166.8.4968. [DOI] [PubMed] [Google Scholar]
- 36.Singh-Jasuja H, Hilf N, Arnold-Schild D, Schild H. The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem. 2001;382(4):629–636. doi: 10.1515/BC.2001.074. [DOI] [PubMed] [Google Scholar]
- 37.Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, et al. Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood. 2004;103(5):1747–1754. doi: 10.1182/blood-2003-08-2828. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, et al. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169(5):2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 39.Yu XL, He YK, Xiong T, Zhao YQ, Shi MZ, Zhou J, et al. [Protective effects of coimmunization with SjCTPI-Hsp70 and interleukin-12 DNA vaccines against Schistosoma japonicum challenge infection in water buffalo] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24(6):433–436. [PubMed] [Google Scholar]
- 40.Guo JG, Ross AG, Lin DD, Williams GM, Chen HG, Li Y, et al. A baseline study on the importance of bovines for human Schistosoma japonicum infection around Poyang Lake, China. Am J Trop Med Hyg. 2001;65(4):272–278. doi: 10.4269/ajtmh.2001.65.272. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Si J, Harn DA, Yu C, Liang Y, Ren J, et al. The protective immunity of a DNA vaccine encoding Schistosoma japonicum Chinese strain triose-phosphate isomerase in infected BALB/C mice. Southeast Asian J Trop Med Public Health. 2004;35(3):518–522. [PubMed] [Google Scholar]
- 42.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27(12):573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Raviprakash K, Marques E, Ewing D, Lu Y, Phillips I, Porter KR, et al. Synergistic neutralizing antibody response to a dengue virus type 2 DNA vaccine by incorporation of lysosome-associated membrane protein sequences and use of plasmid expressing GM-CSF. Virology. 2001;290(1):74–82. doi: 10.1006/viro.2001.1136. [DOI] [PubMed] [Google Scholar]
- 44.Delogu G, Li A, Repique C, Collins F, Morris SL. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect Immun. 2002;70(1):292–302. doi: 10.1128/IAI.70.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005;175(2):633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, et al. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003;21(21–22):2882–2889. doi: 10.1016/s0264-410x(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca CT, Pacifico LG, Barsante MM, Rassi T, Cassali GD, Oliveira SC. Co-administration of plasmid expressing IL-12 with 14-kDa Schistosoma mansoni fatty acid-binding protein cDNA alters immune response profiles and fails to enhance protection induced by Sm14 DNA vaccine alone. Microbes Infect. 2006;8(9–10):2509–2516. doi: 10.1016/j.micinf.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Harn DA, Reynolds SR, Chikunguwo S, Furlong S, Dahl C. Synthetic peptide vaccines for schistosomiasis. Pharm Biotechnol. 1995;6:891–905. doi: 10.1007/978-1-4615-1823-5_40. [DOI] [PubMed] [Google Scholar]
- 49.Da'dara AA, Skelly PJ, Fatakdawala M, Visovatti S, Eriksson E, Harn DA. Comparative efficacy of the Schistosoma mansoni nucleic acid vaccine, Sm23, following microseeding or gene gun delivery. Parasite Immunol. 2002;24(4):179–187. doi: 10.1046/j.1365-3024.2002.00453.x. [DOI] [PubMed] [Google Scholar]
- 50.Harn DA, Gu W, Oligino LD, Mitsuyama M, Gebremichael A, Richter D. A protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triosephosphate isomerase. J Immunol. 1992;148(2):562–567. [PubMed] [Google Scholar]
- 51.Gaugitsch HW, Hofer E, Huber NE, Schnabl E, Baumruker T. A new superfamily of lymphoid and melanoma cell proteins with extensive homology to Schistosoma mansoni antigen Sm23. Eur J Immunol. 1991;21(2):377–383. doi: 10.1002/eji.1830210219. [DOI] [PubMed] [Google Scholar]
- 52.Harn DA, Mitsuyama M, Huguenel ED, David JR. Schistosoma mansoni: detection by monoclonal antibody of a 22,000-dalton surface membrane antigen which may be blocked by host molecules on lung stage parasites. J Immunol. 1985;135(3):2115–2120. [PubMed] [Google Scholar]
- 53.Taylor MG, Huggins MC, Shi F, Lin J, Tian E, Ye P, et al. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine. 1998;16(13):1290–1298. doi: 10.1016/s0264-410x(98)00055-3. [DOI] [PubMed] [Google Scholar]
- 54.Scott P, Trinchieri G. IL-12 as an adjuvant for cell-mediated immunity. Semin Immunol. 1997;9(5):285–291. doi: 10.1006/smim.1997.0084. [DOI] [PubMed] [Google Scholar]
- 55.Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158(2):816–826. [PubMed] [Google Scholar]
- 56.Da'Dara AA, Skelly PJ, Walker CM, Harn DA. A DNA-prime/protein-boost vaccination regimen enhances Th2 immune responses but not protection following Schistosoma mansoni infection. Parasite Immunol. 2003;25(8–9):429–437. doi: 10.1111/j.1365-3024.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 57.Harmala LA, Ingulli EG, Curtsinger JM, Lucido MM, Schmidt CS, Weigel BJ, et al. The adjuvant effects of Mycobacterium tuberculosis heat shock protein 70 result from the rapid and prolonged activation of antigen-specific CD8(+) T cells in vivo. J Immunol. 2002;169(10):5622–5629. doi: 10.4049/jimmunol.169.10.5622. [DOI] [PubMed] [Google Scholar]
- 58.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava PK, Amato RJ. Heat shock proteins: the 'Swiss Army Knife' vaccines against cancers and infectious agents. Vaccine. 2001;19(17–19):2590–2597. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 60.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80(2):183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 61.Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32(Pt 4):636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- 62.McGarvey ST, Zhou XN, Willingham AL, 3rd, Feng Z, Olveda R. The epidemiology and host-parasite relationships of Schistosoma japonicum in definitive hosts. Parasitol Today. 1999;15(6):214–215. doi: 10.1016/s0169-4758(99)01409-x. [DOI] [PubMed] [Google Scholar]
- 63.Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, et al. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60(4):556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]