Abstract
Embryo development after homologous intracytoplasmic sperm injection (ICSI) with sperm from testis tissue xenografts from pigs or any other farm animal species has not been evaluated critically. Here, we report development of porcine embryos in vitro following ICSI with sperm retrieved from xenografted neonatal pig testis. Small pieces of testis tissue from newborn piglets were grafted under the back skin of castrated immunodeficient mice (n = 4) and the xenografts were collected 8 months after grafting. Spermatozoa were recovered by mincing of the grafted tissue. For comparison, testicular, epididymal and ejaculated spermatozoa were also collected from mature boars. Oocytes injected with xenogeneic spermatozoa were either fixed to determine fertilisation processes (n = 89 in five replicates) or allowed to develop in vitro (n = 143 in four replicates). Xenogeneic porcine spermatozoa were fertilisation competent (24% v. 58%, 68%, 62% or 0% for xenogeneic v. control testicular, epididymal and ejaculated spermatozoa or no spermatozoa, respectively) and embryos developed to the blastocyst stage (8% v. 22%, 27%, 25% or 0%, respectively). These results demonstrate that porcine spermatozoa derived from immature testis tissue xenografted into mice are fertilisation competent, albeit at a lower rate than testicular, epididymal or ejaculated spermatozoa from control boars, and support embryo development after ICSI.
Introduction
Xenografting of testis tissue can serve as an in vivo culture system to mimic testis function from animals and humans ex situ. In this model, small fragments of immature testis tissue from a donor species are grafted into immunodeficient host mice, where they undergo testicular maturation and develop complete spermatogenesis (Honaramooz et al. 2002). This approach allows propagation and differentiation of male germ cells from a variety of donor species, including farm animals and primates (Honaramooz et al. 2002, 2004; Schlatt et al. 2002, 2003, 2006; Snedaker et al. 2004; Oatley et al. 2005; Rathi et al. 2005, 2006; Arregui et al. 2008). Xenografted newborn pig testis fragments showed a gradual progression from infantile seminiferous cords into mature seminiferous tubules mimicking testicular development in the donor species (Honaramooz et al. 2002). Spermatozoa recovered from these xenografts (xenogeneic spermatozoa) appear morphologically normal and, upon injection into mouse oocytes, have been shown capable of initiating the fertilisation process. We also reported blastocyst formation after intracytoplasmic sperm injection (ICSI) using xenogeneic rhesus spermatozoa into rhesus oocytes (Honaramooz et al. 2004) and progeny following ICSI with spermatozoa from mouse-to-mouse testis allografts and embryo transfer into surrogate mice (Schlatt et al. 2003). However, the fertilisation competence of xenogeneic spermatozoa from farm animals in a homologous system remains to be established.
Xenografting of neonatal porcine testis tissue into mice is a valuable tool in the study of testis development and a new window into the regulation of spermatogenesis. It can offer an accessible means for the experimental manipulation of the donor pig testis with the flexibility and convenience of working with a laboratory mouse in which the hormonal milieu can be more easily manipulated. The onset of spermatogenesis in porcine testicular xenografts appears slightly earlier compared with donor pigs (Honaramooz et al. 2002), although this is not the case with other farm species studied (Honaramooz et al. 2002; Oatley et al. 2005; Rathi et al. 2005, 2006), indicating that testicular maturation may be more readily accelerated in the pig. Furthermore, we recently demonstrated that individual cells obtained from neonatal pig testes after complete enzymatic dissociation can reorganise to form functional testicular tissue (Honaramooz et al. 2007), providing an excellent model to study cellular migration and organogenesis of the testis. Given the increasing importance of pigs in biomedical research, testis tissue xenografting may also prove to be of value in the genetic manipulation of pig gametes developing in host mice.
Therefore, to further establish this system for the study and manipulation of mammalian testis function, it was important to analyse the potential of xenogeneic pig spermatozoa originating from neonatal donors to direct embryonic development.
Materials and methods
Experimental design
Small fragments of testis tissue from neonatal pigs were grafted under the back skin of castrated immunodeficient mice and the host mice were killed 8 months after grafting. Xenografts were minced, examined for the presence of spermatozoa and frozen until analysis. Spermatozoa were assessed for fertilisation competence using ICSI into porcine oocytes and the embryos were analysed for early developmental indicators. All experimental procedures were approved by the Institutional Animal Care and Use committees at the University of Pennsylvania and Chungbuk National University.
Preparation of donor testis tissue
One-week-old piglets at the University of Pennsylvania Swine Research Center served as testis donors for xenografting. The animals underwent routine aseptic castration and the testes, enclosed in the tunica albuginea, were maintained in Dulbecco's modified Eagle's medium (DMEM) on ice and transferred to the laboratory within 1 h. After removal of the capsule and overt connective tissue, donor testes were cut into small fragments (approximately 1 mm in diameter, weighing approximately 2 mg). Testis fragments were kept in DMEM on ice until grafting within 2 h. A few fragments were fixed in Bouin's solution as a reference for histology.
Recipient animals and procedures for xenografting
Six-week-old male immunodeficient (ICR-SCID) mice (Taconic, Germantown, NY, USA) were used as recipients (n = 4). Animals were anaesthetised using tribromoethanol (630 mg kg−1 bodyweight) and castrated through low abdominal incisions. Four skin incisions, approximately 5 mm each, were made on either side of the dorsal midline skin, spaced between the shoulder and rump. Eight grafts per recipient were affixed to the subcutaneous muscle layer using 6-0 braided silk sutures (Ethicon, Somerville, NY, USA). The incisions were closed with wound clips (Michel Clips, 7.5 mm; Miltex, York, PA, USA).
Analysis of xenografts
Eight months after grafting, recipient mice were killed by CO2 inhalation. A small portion of each testicular graft was fixed for histology and the remaining tissue was gently teased apart and dispersed in 1 mL Whittingham medium supplemented with bovine serum albumin (BSA; 3% w/v) under a dissecting microscope. The presence of spermatozoa among the mixture of dispersed cells was confirmed for each graft using an inverted microscope at ×400 magnification. The total number of spermatozoa in a graft was estimated using a haemocytometer and viability was determined using nigrosin-eosin staining. Samples were then diluted further in 10 mmL Whittingham medium containing 3% BSA, transferred into cryovials (0.5 mmL each) and snap frozen in liquid nitrogen. Vials were stored in liquid nitrogen until they were shipped, on dry ice, to Chungbuk National University for analysis.
Oocyte collection
Prepubertal porcine ovaries were collected at slaughter and transported to the laboratory at 25°C in Dulbecco's phosphate-buffered saline (DPBS) supplemented with 5.54 mm d-glucose, 0.33 mm sodium pyruvate, 75 μg mL−1 potassium penicillin G and 50 μg mL−1 streptomycin sulfate. Cumulus-oocyte complexes (COC) were aspirated from follicles with an 18-gauge needle into a disposable 10-mL syringe. Fifty porcine COC were matured in 500 μL of a defined protein-free medium consisting of tissue culture medium (TCM) 199 supplemented with 3.05 mm d-glucose, 0.91 mm sodium pyruvate, 10 ng mL−1 epidermal growth factor, 0.1% polyvinyl alcohol, 0.57 mm cysteine, 0.5 μg mL−1 luteinising hormone (LH) and 0.5 μg mL−1 follicle-stimulating hormone (FSH) under paraffin oil at 39°C for 40-44 h. After maturation, the cumulus cells were removed by vigorous pipetting of the COC in the presence of 0.3 mg mL−1 hyaluronidase.
Preparation of spermatozoa
In addition to xenogeneic spermatozoa, spermatozoa were also obtained from the testis, epididymis and ejaculates of mature boars for comparison. Spermatozoa were suspended in 1.5 mL HEPES-buffered Tyrode's medium for 30 min to induce capacitation. Some groups of spermatozoa were stained with the Mito-Tracker fluorochrome (Molecular Probes, Eugene, OR, USA) for 5 min at a final concentration of 10 μm and then washed in Tyrode's-lactate (TL)-HEPES buffered medium before injection.
Intracytoplasmic sperm injection
Spermatozoa were centrifuged (400g, 5 min) and resuspended in TL-HEPES: 10% polyvinylpyrrolidone solution (1 : 1). A microdrop (5 μL) of this suspension was placed on a slide on a Nikon Differential Interference Contrast inverted microscope (Nikon, Kanagawa, Japan) equipped with Narishige micromanipulators (Narishige, Tokyo, Japan). Oocytes with visible polar bodies and of excellent morphology were used for this experiment. Oocytes were centrifuged for 10 min in an Eppendorf centrifuge at 12 000g in 50 μL TL-HEPES medium in a 1.2-mL Eppendorf centrifuge tube. The injection of a spermatozoon into an oocyte's cytoplasm was performed using the method described by Kim et al. (1998). Briefly, the injection needle had inner and outer diameters of 6-7 and 8-9 μm, respectively. The polar body was at the 6 or 12 o'clock position, with the point of injection at 3 o'clock. An oocyte was penetrated by the injecting micropipette, a small amount of cytoplasm was drawn into the micropipette and then the cytoplasm, together with the spermatozoon and a small amount of medium, was expelled into the oocyte. Immediately after ooplasmic injection, the injecting micropipette was withdrawn quickly and the oocyte was released from the holding pipette to reduce intracytoplasmic pressure exerted on the oocyte. The same procedure was also performed for sham-injected oocytes, except that no spermatozoa were injected.
Assessment of fertilisation and early embryonic development
After injection, all oocytes were transferred to NCSU23 medium (Peters and Wells 1993) and cultured at 39°C under 5% CO2 in air. In order to determine fertilisation processes, some oocytes were fixed at 18 or 22 h following injection. Oocytes were permeabilised in an extraction medium containing 25% glycerol, 50 mm KCl, 0.5 mm MgCl2, 0.1 mm EDTA, 1 mm β-mercaptoethanol, 50 mm Imidazole, pH 6.7, 3% Triton X-100 and 25 mm phenylmethylsulfonyl fluoride for 10 min, fixed in methanol at −20°C for 10 min and stored in phosphate-buffered saline (PBS) containing 0.02% sodium azide and 0.1% BSA at 4°C. Fixed oocytes were incubated for 90 min at 39°C with 50 μg mL−1 propidium iodide (Sigma, St Louis, MO, USA) for 1 h in order to enable the fluorescent detection of DNA. Stained oocytes were then mounted under a coverslip with antifade mounting medium (Universal Mount; Fisher Scientific, Huntsville, AL, USA) to retard photobleaching. Slides were examined using a laser-scanning confocal microscope (MRC 1024; BioRad, Hercules, CA, USA) equipped with a kryptonargon ion laser for the simultaneous excitation of fluorescein in the tail of the spermatozoon (mitochondria) and propidium iodide in the DNA.
Statistical analysis
In vitro development of porcine embryos after ICSI with xenogeneic spermatozoa was compared with development rates after ICSI with ejaculated, epididymal and testicular mature boar spermatozoa or the development of sham-injected oocytes (parthenogeneic) by Chi-squared analysis. A P-value <0.05 was considered significant.
Results
One of the mice carrying the xenografts died and 20 of the 24 remaining grafts (83%) were recovered from the remaining mice. Spermatozoa were present in all dispersed grafts and complete spermatogenesis was also confirmed histologically in the grafts. The morphology of the seminiferous tubules in testis grafts appeared similar to tubular morphology in testes of mature boars (Fig. 1a). In representative fresh grafts, the concentration of spermatozoa was estimated to be approximately 15 × 106 in an average-sized graft (100 mg), at least 80% of which were viable. Isolated xenogeneic spermatozoa appeared morphologically normal (Fig. 1b), displaying typical characteristics of pig testicular spermatozoa; upon dilution in the buffer, approximately 10% displayed varying degrees of motility, ranging from very weak twitches to slow/moderate progressive movement.
Fig. 1.
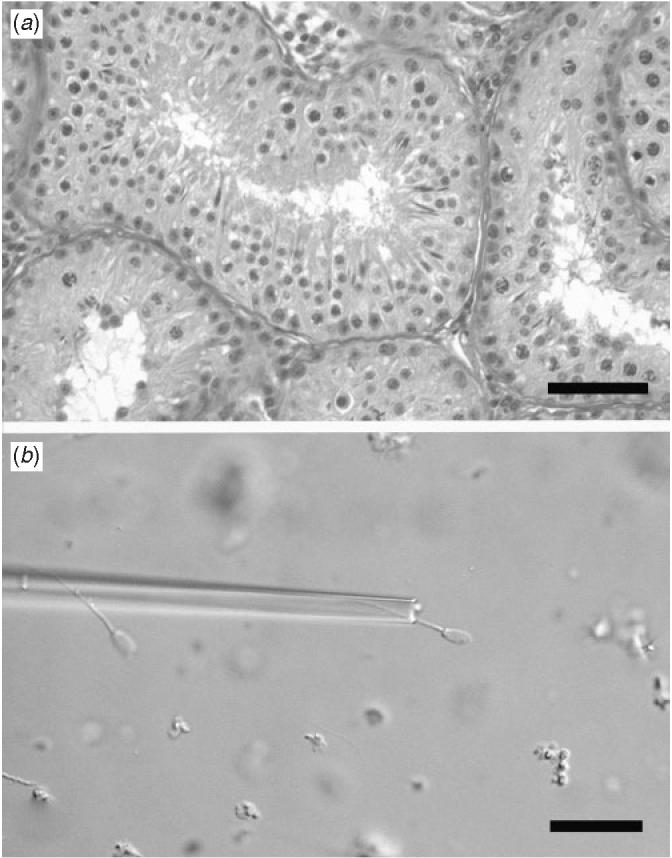
(a) Morphology of a testis tissue xenograft originating from a newborn pig grafted into an immunodeficient host mouse at 8 months after grafting. Scale bar = 50 μm. (b) Mature sperm in minced graft before use in intracytoplasmic sperm injection. Scale bar = 25 μm.
To determine whether the resultant spermatozoa were capable of supporting development, we used ICSI into in vitro-matured porcine oocytes. Fertilisation rates (at 18 or 22 h) and embryo development (at 6 or 7 days) after ICSI with spermatozoa of different origin are summarised in Table 1.
Table 1. In vitro development of porcine embryos after intracytoplasmic sperm injection.
Xenogeneic spermatozoa were collected from porcine testis tissue xenografts. All other spermatozoa were from mature boars. Xenogeneic and ejaculated spermatozoa were frozenthawed; testicular and epididymal spermatozoa were used fresh. Some oocytes were fixed and examined for the presence of male and female pronuclei as an indication of fertilisation. The remaining injected oocytes were allowed to develop in vitro and blastocysts were counted on Day 6 of culture. Means with different superscripts within a column are significantly different (P < 0.05)
| Source of spermatozoa | Replicates | No. fertilised/total oocytes (%) | No. blastocysts/total oocytes (%) |
|---|---|---|---|
| Xenogeneic | 5 | 21/89 (24%)a | 11/143 (8%)a |
| Testicular | 4 | 23/40 (58%)b | 18/82 (22%)b |
| Epididymal | 4 | 32/47 (68%)b | 24/89 (27%)b |
| Ejaculated | 4 | 26/42 (62%)b | 23/91 (25%)b |
| No sperm | 2 | 0/30 (0%)c | 0/30 (0%)c |
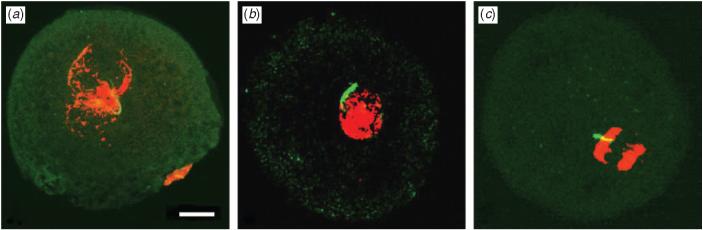
In fertilised oocytes, the female and male pronuclei formed at approximately 5 and 7-9 h, respectively, after sperm injection. Two large apposed pronuclei were observed as early as 10-12 h following injection. Oocytes fixed 22 h after injection were considered fertilised if the pronuclear membranes were broken, two small pronuclei had apposed (apposition) and mitotic prometaphase (synkaryogamy) or metaphase was observed. Conversely, if the two pronuclei had remained separate in the oocytes at 22 h following injection, the oocyte was considered to be unfertilised. Accordingly, xenogeneic spermatozoa recovered from ectopic xenografts of neonatal pig testis tissue in host mice were capable of fertilising porcine oocytes by ICSI (Fig. 2). We used Mito Tracker, which binds specifically to mitochondria and does not affect fertilisation ability or developmental competence, but allows tracking of the spermatozoon's tail within the oocytes.
Fig. 2.
Demonstration of fertilisation after injection of a xenogeneic pig spermatozoon into a porcine oocyte. Spermatozoa were stained with Mito Tracker (Molecular Probes, Eugene, OR, USA) and oocytes were stained with propidium iodide. Following intracytoplasmic sperm injection, the resumption of meiosis, polar body extrusion and pronuclear formation were observed (a), followed by synkaryogamy (b) and anaphase (c). Scale bar = 20 μm.
Embryos resulting from ICSI with xenogeneic pig spermatozoa could develop in vitro at least to the blastocyst stage (Fig. 3). None of the sham-injected oocytes developed, indicating that blastocyst formation was not due to parthenogenic activation of oocytes during manipulation. Sham-injected oocytes had remained as one cell or become fragmented by Day 2 after injection.
Fig. 3.
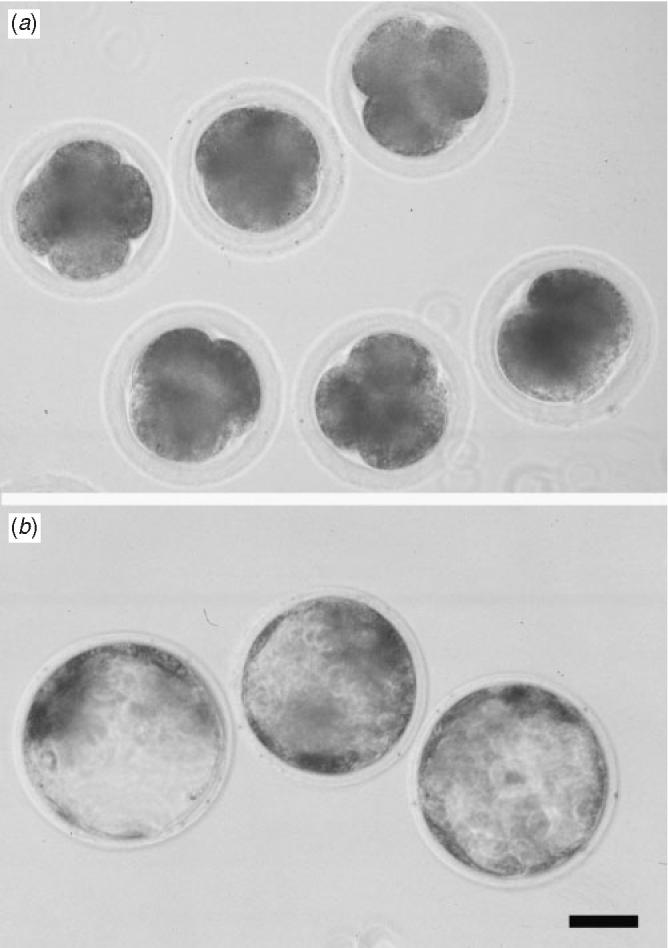
(a) Four-cell- and (b) blastocyst-stage embryos obtained by intracytoplasmic sperm injection of testicular spermatozoa isolated from a neonatal pig-to-mouse testis graft. Scale bar = 60 μm.
Discussion
As reported previously (Honaramooz et al. 2002), xenografting of testis tissue from neonatal donor pigs resulted in the complete development of porcine spermatogenesis in the host mice. Morphologically mature pig spermatozoa could be retrieved from the majority of grafts. Successful fertilisation and preimplantation development of porcine oocytes showed that xenogeneic spermatozoa can be used for assisted fertilisation. Although we had indirect evidence for the fertilisation competence of xenogeneic pig spermatozoa in a heterologous system (pig spermatozoa fertilising mouse oocytes; Honaramooz et al. 2002), the present report demonstrates homologous fertilisation and early embryonic development by xenogeneic spermatozoa for this farm animal species.
As a positive control for comparing fertilisation competence, we used mature boar spermatozoa of different origin (i.e. testicular, epididymal and ejaculated). Although our results clearly showed that xenogeneic spermatozoa are fertile and can direct embryonic development, their fertilisation efficiency was lower than that of spermatozoa from mature boars. Similarly, a lower developmental potential of oocytes recovered from mouse ovarian allografts compared with control oocytes recovered from the oviducts of superovulated mice has been reported (Yang et al. 2006). Conversely, spermatozoa obtained from neonatal mouse testes ectopically grafted into immunodeficient mice showed a normal fertilisation rate after ICSI into mouse oocytes (80% and 50% at the two- and four-cell stage, respectively, v. 88% and 65% for control epididymal sperm at the two- and four-cell stage, respectively; Schlatt et al. 2003). Xenogeneic spermatozoa derived from immature rhesus testis tissue grafted in mice showed a fertilisation rate after ICSI into rhesus oocyte (Honaramooz et al. 2004) that was similar to that reported for rhesus testicular sperm (Hewitson et al. 2002).
The lower rates of fertilisation and development seen with xenograft-derived spermatozoa compared with testicular, epididymal or ejaculated spermatozoa from control boars could be due to both technical and biological reasons. Xenogeneic spermatozoa were frozen in a generic medium (snap frozen in Whittingham and 3% BSA), not one designed specifically to maintain the viability of porcine spermatozoa, whereas ejaculated spermatozoa were frozen in a cryopreservation medium for porcine semen and testicular and epididymal spermatozoa were used fresh. Therefore, in a follow-up experiment (data not shown), we performed ICSI with testicular, epididymal and ejaculated boar spermatozoa frozen with the same medium and freezing protocol as that used for xenogeneic spermatozoa. However, the results were similar (40-60% fertilisation for testicular, epididymal or ejaculated boar spermatozoa), indicating that the reason for the reduced fertility of xenogeneic spermatozoa may be biological. Graft-derived spermatozoa do not undergo epididymal maturation, making them comparable to testicular spermatozoa in their maturation status. However, in situ, spermatozoa are continuously being moved out of the seminiferous tubules in testes; there is no excurrent duct system for the removal of spermatozoa in grafted tissue. Therefore, spermatozoa formed in xenografts may accumulate, undergo aging and lose their fertilisation ability over time. However, previously, we observed comparable fertilisation rates after ICSI with graft-derived spermatozoa and spermatozoa from intact male mice or rhesus monkeys (Schlatt et al. 2003; Honaramooz et al. 2004). In these experiments, mouse and rhesus monkey spermatozoa were recovered within 1 month after first observing complete spermatogenesis. In the present study, sperm were retrieved approximately 4 months after spermatozoa were first produced. The percentage of grafts with spermatozoa and the concentration of spermatozoa per graft increase over time (data not shown), but the fertilisation competence of the spermatozoa in older grafts may decrease owing to senescence of the spermatozoa. Therefore, collection of spermatozoa from older grafts may yield a mixture of recently produced fertile spermatozoa and subfertile older spermatozoa, resulting in a decreased fertilisation rate.
As a negative control, we used sham injection of medium into oocytes without spermatozoa; none of the sham-injected oocytes developed, showing an absence of parthenogenesis. This, along with the observed fertilisation progress, provided further evidence that the blastocysts obtained had indeed resulted from fertilisation and not parthenogenesis. There are differences in the rate of parthenogenesis among different laboratories working with pig oocytes but, in our system, without artificial activation, we do not usually see parthenogenic development. In an earlier study, we conducted parentage analysis of rhesus blastocysts resulting from ICSI with xenogeneic rhesus spermatozoa and ascertained that the embryos indeed originated from the xenogeneic donor spermatozoa because DNA types obtained for the embryo were compatible with those of the putative parents (Honaramooz et al. 2004). We also have shown previously that mouse embryos generated by ICSI of mouse spermatozoa from ectopic grafts can develop normally and give rise to healthy off-spring (Schlatt et al. 2003). Therefore, these results collectively indicate that the porcine embryos in the present study are normal and will likely develop further if transferred to a surrogate female.
In summary, the present study demonstrated, for the first time, that porcine oocytes injected with xenogeneic porcine spermatozoa are capable of developing at least to the blastocyst stage.
The results reported here add to the value of testis tissue xenografting for the cross-species production of spermatozoa, even from neonatal donors. This system not only can be used to study and manipulate testis function in farm animals, but it can also salvage genetic material from superior individuals that are sexually immature or cannot be maintained for sperm production.
Acknowledgments
The authors thank Janet Turpin and Terry Jordan for animal care. This study was supported by National Research Initiative Competitive Grant no. 2003-35203-13486 (to I.D.) from the USDA Cooperative State Research, Education, and Extension Service and by grant no. 2R01 RR17359-06 (to I.D.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. This study was also supported by a grant from the BioGreen 21 program, RDA, Republic of Korea (to N.H.K.).
References
- Arregui L, Rathi R, Zeng W, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I. Xenografting of adult mammalian testis tissue. Anim. Reprod. Sci. 2008;106:65–76. doi: 10.1016/j.anireprosci.2007.03.026. doi:10.1016/J.ANIREPROSCI.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson L, Martinovich C, Simerly C, Takahashi D, Schatten G. Rhesus offspring produced by intracytoplasmic injection of testicular sperm and elongated spermatids. Fertil. Steril. 2002;77:794–801. doi: 10.1016/s0015-0282(01)03281-2. doi:10.1016/S0015-0282(01)03281-2. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. doi:10.1038/NATURE00918. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo CT, Meyers SA, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. doi:10.1095/BIOLREPROD.103.025536. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol. Reprod. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. doi:10.1095/BIOLREPROD.106.054999. [DOI] [PubMed] [Google Scholar]
- Kim N-H, Lee JW, Jun SH, Lee HT, Chung KS. Fertilization of porcine oocytes following intracytoplasmic spermatozoon or isolated sperm head injection. Mol. Reprod. Dev. 1998;51:436–444. doi: 10.1002/(SICI)1098-2795(199812)51:4<436::AID-MRD11>3.0.CO;2-Q. doi:10.1002/(SICI)1098-2795(199812)51:4<436::AID-MRD11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Reeves JJ, McLean DJ. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol. Reprod. 2005;72:358–364. doi: 10.1095/biolreprod.104.030783. doi:10.1095/BIOLREPROD.104.030783. [DOI] [PubMed] [Google Scholar]
- Peters RM, Wells KD. Culture of pig embryos. J. Reprod. Fertil. 1993;48(Suppl):61–73. [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Schlatt S, Dobrinski I. Germ cell fate in bovine testis tissue xenografts. Reproduction. 2005;130:923–929. doi: 10.1530/rep.1.00912. doi:10.1530/REP.1.00912. [DOI] [PubMed] [Google Scholar]
- Rathi R, Honaramooz A, Zeng W, Turner R, Dobrinski I. Germ cell development in equine testis tissue xenografted into mice. Reproduction. 2006;131:1091–1098. doi: 10.1530/rep.1.01101. doi:10.1530/REP.1.01101. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim SS, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124:339–346. doi: 10.1530/rep.0.1240339. doi:10.1530/REP.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Schöler HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol. Reprod. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. doi:10.1095/BIOLREPROD.102.014894. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rübben H, Dhir R, Dobrinski I, Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006;21:384–389. doi: 10.1093/humrep/dei352. doi:10.1093/HUMREP/DEI352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedaker A, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J. Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Yang HY, Cox S-L, Jenkin G, Findlay J, Trounson A, Shaw J. Graft site and gonadotrophin stimulation infulences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction. 2006;131:851–859. doi: 10.1530/rep.1.00916. doi:10.1530/REP.1.00916. [DOI] [PubMed] [Google Scholar]