Abstract
BACKGROUND
Trazodone is commonly prescribed off-label for sleep disturbance in alcohol dependent patients, but its safety and efficacy for this indication is unknown.
METHODS
We conducted a randomized, double-blind, placebo-control trial of low dose trazodone (50−150 mg. at bedtime) for 12 weeks among 173 alcohol detoxification patients who reported current sleep disturbance on a validated measure of sleep quality or during prior periods of abstinence. Primary outcomes were the proportion of days abstinent and drinks per drinking day over six-months; sleep quality was also assessed.
RESULTS
Urn randomization balanced baseline features among the 88 subjects who received trazodone and 85 who received placebo. The trazodone group experienced less improvement in the proportion of days abstinent during administration of study medication (mean change between baseline and 3 months, −.12; 95% CI, −.15 to −.09), and an increase in the number of drinks per drinking day on cessation of the study medication (mean change between baseline and 6 months, 4.6; 95% CI, 2.1 to 7.1). Trazodone was associated with improved sleep quality during its administration (mean change on the Pittsburgh Sleep Quality Index between baseline and 3 months, −3.02; 95% CI, −3.38 to −2.67), but after it was stopped sleep quality equalized with placebo.
CONCLUSIONS
Trazodone, despite a short-term benefit on sleep quality, might impede improvements in alcohol consumption in the post-detoxification period and lead to increased drinking when stopped. Until further studies have established benefits and safety, routine initiation of trazodone for sleep disturbance cannot be recommended with confidence during the period after detoxification from alcoholism.
Keywords: Alcohol-Related Disorders, Sleep Disturbance, Insomnia, Trazodone
INTRODUCTION
Alcoholism has important public health impact (O'Connor & Schottenfeld, 1998), and sleep disturbance is common among alcohol-dependent persons (Stein & Friedmann, 2006). Their rate of sleep problems is highest (36% to 72%) during periods of abstinence (Brower, 2003). Sleep disturbance after alcohol detoxification might contribute to relapse through abnormalities in sleep architecture (i.e. effects on rapid eye movement or slow wave sleep) or in a general way such as through prolonged sleep latency, fatigue, depression or anxiety (Brower, 2003; Stein & Friedmann, 2006). Ingestion of alcohol quickly corrects many of these sleep disturbances (Wagman & Allen, 1975). In this manner, sleep problems during periods of abstinence contribute to the conditioned response to resume drinking in alcohol-dependent persons (Vitiello, 1997; Drummond et al., 1998).
Pharmacological management of sleep disturbance during early remission from alcohol dependence has been proposed to reduce relapse to alcohol (Brower, 2003). Off-label trazodone is among the most frequently prescribed agents for insomnia, even though the evidence supporting this indication is limited (Walsh & Schweitzer, 1999; Mendelson, 2005). It is the sleep agent most commonly prescribed by experts in addiction medicine for alcohol-dependent patients with sleep problems (Friedmann et al., 2003). Trazodone is commonly prescribed for alcohol-dependent patients with sleep problems because it is considered to be less habit-forming than benzodiazepine receptor agonists commonly prescribed for insomnia (Longo & Johnson, 2000). In the general population, trazodone decreases sleep latency (Ware & Pittard, 1990; Yamadera et al., 1999), improves sleep efficiency (Parrino et al., 1994; Haffmans & Vos, 1999), increases restorative slow wave sleep (Ware & Pittard, 1990), and exerts anxiolytic effects (Liebowitz & El-Mallakh, 1989). Trazodone is well tolerated (Feighner & Boyer, 1988), appears safe in combination with alcohol (except, perhaps, in massive overdose)(Goeringer et al., 2000), has low abuse potential (Rush et al., 1999), and induces sleep at bedtime dosing (Fabre, 1990).
Empirical evidence of trazodone's safety and effectiveness as a soporific agent in alcohol-dependent patients is limited. One small placebo-controlled trial suggested that trazodone improved sleep efficiency after alcohol detoxification (Le Bon et al., 2003). To examine whether trazodone improves drinking outcomes and sleep quality among sleep-disturbed, alcohol-dependent patients after detoxification, we performed a randomized, double-blind, placebo-controlled trial of short term, low dose trazodone (50−150 mg) at bedtime.
METHODS
Setting
The Institutional Review Boards of the Miriam Hospital and the Providence VA Medical Center, and the Clinical Trials Board of SSTAR of Rhode Island approved the Soporific Intervention to Enhance Sleep and Treatment of Alcoholism (SIESTA) study. Recruitment occurred at a short-term inpatient detoxification program for indigent persons. The standard 3 to 5 day protocol provides chlordiazepoxide on Days 1−3 as needed. After detoxification, patients are referred to residential or outpatient treatment; sleep management is not a typical feature of the treatment plan.
Participants
Research staff screened alcohol dependent patients. Inclusion criteria were: alcohol as the principal substance; DSM-IV criteria for current alcohol dependence; sleep disturbance during previous periods of abstinence or a global score of 5 or greater on the Pittsburgh Sleep Quality Index(Buysse et al., 1989); age between 18 and 65 years; adequate contraception if female; and ability to understand instructions. Exclusion criteria were: DSM-IV criteria for current dependence on drugs other than nicotine, or Axis I disorder (subjects with “substance-induced mood disorder,” or dysthymia were not excluded)(First et al., 1995); current suicidality (Miller et al., 1986); psychotropic, antidepressant, anxiolytic or antidipsogenic (naltrexone, disulfiram and acamprosate) medication; proerectile, herbal or sleep medication; pregnancy/lactation, ischemic heart disease, cardiac arrhythmias, priapism or hypotension; history of obstructive sleep apnea, emphysema, or poorly controlled diabetes mellitus with nocturia 2 or more times per night; life expectancy 6 months or less; inability to identify at least one contact person; or no address provided for the period after discharge.
Study Allocation
Eligible subjects completed informed consent and a baseline interview. Urn randomization software allocated subjects to bedtime trazodone or identical placebo while ensuring balance by gender, depressive symptoms (19 or below versus 20 or above on the Beck Depression Inventory-II) (Beck et al., 1996), and homelessness in the previous 30 days (Yes/No) (Stout et al., 1994). All subjects received a sleep hygiene pamphlet (American Sleep Disorders Association, 1997). Study medication was dispensed as scored 50 mg. tablets with a cap that recorded the date and time of each opening (MEMS, Aprex, Union City, Calif.). Subjects were instructed to begin with one tablet one hour before bedtime, and titrate dosage until they reached a balance between sleep response and morning lethargy, or up to 3 tablets maximum. Study medication ceased after 12 weeks.
Participants received $20, $30, $40 and $50 for four respective interviews (weeks 0, 4, 12, 24), and $5 for each daily sleep log returned weekly during the first four weeks (Monk et al., 1994). In addition to the outcomes described below, interviews assessed depressive symptoms (Beck et al., 1996), and treatment services received (McLellan et al., 1992). Checklists completed at the assessments and by telephone two-weeks after trazodone initiation monitored medical conditions, medications and side-effects.
Outcomes
Alcohol Consumption was assessed with a well-established calendar method (Sobell & Sobell, 1992). During the baseline interview, subjects recalled the number of standard drinks taken on each of ninety days prior to entry into the detoxification program. Each follow-up interview assessed alcohol consumption since the previous interview. From the calendar primary outcomes were the proportion reporting complete abstinence by the end of the study (6-month follow-up), the proportion of days abstinent (the number of non-drinking days reported during the period divided by the total number of days for which data were available); the number of drinks per drinking day (the total number of drinks reported during the period divided by the number of days on which alcohol consumption was reported), and the proportion of heavy drinking days (the number of days participants reported drinking at least 4 (women) or 5 (men) drinks during the period divided by the total number of days for which data were available). For the measure of complete abstinence, those who did not complete all follow-ups were assumed to have resumed drinking, and were therefore coded as not having achieved complete abstinence by the end of the study. Serum gamma-glutamyltransferase was tested at each assessment point. Global Sleep Quality during the past month was measured using the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989; Carpenter & Andrykowski, 1998). Health Status was assessed with the 12-item Medical Outcomes Study Short Form-12 (SF-12) (Ware et al., 1995).
Statistical Methods
We examined potential covariates by testing interactions of study condition by time by whether participants believed they were receiving active medication, baseline commitment to abstinence, and days of psychosocial treatment (inpatient treatment, medication, Alcoholics Anonymous, relapse prevention meetings, and/or individual or group discussion) in the first month. Logistic regression was used to test for treatment differences in the proportion who achieved complete abstinence by the end of the study, controlling for the proportion of days abstinent at baseline. Mixed linear regression analyses with full information maximum likelihood (FIML) estimation compared the other drinking outcome trajectories by treatment condition across baseline, 1-, 3-, and 6-month intervals. Each model assessed linear and quadratic change over time, treatment differences at baseline, and treatment by linear and quadratic time interactions. FIML allows available data from all participants to contribute to the estimation of intercepts and slopes. An alternative procedure, multiple imputation, replaced missing values in a total of 20 data sets based on gender, age, race, treatment group, homelessness, depressive symptoms, as well as baseline and available follow-up values for the primary and secondary outcomes; results (not shown) approximated those from the FIML estimation (Schafer, 1997).
To characterize the magnitude of change over time, mean difference scores were calculated by subtracting the mean estimated change from baseline to each follow-up for the treatment group from the mean estimated change from baseline to follow-up for the placebo group. Predicted values from the mixed linear regression analyses generated these difference scores. We used a standard method to obtain Cohen's d treatment effect size by dividing the mean difference scores by the placebo group baseline standard deviation (Cohen, 1988). Cohen's d values of 0.20, 0.50, and 0.80 represent small, moderate and large effect sizes, respectively.
We calculated statistical power for a repeated measures regression model with two groups and attrition over time using the RMASS2 statistical power analysis program (Hedeker & Barlas, 1999). With a sample size of 173, we have power of .80 to detect a moderate effect size of .45.
RESULTS
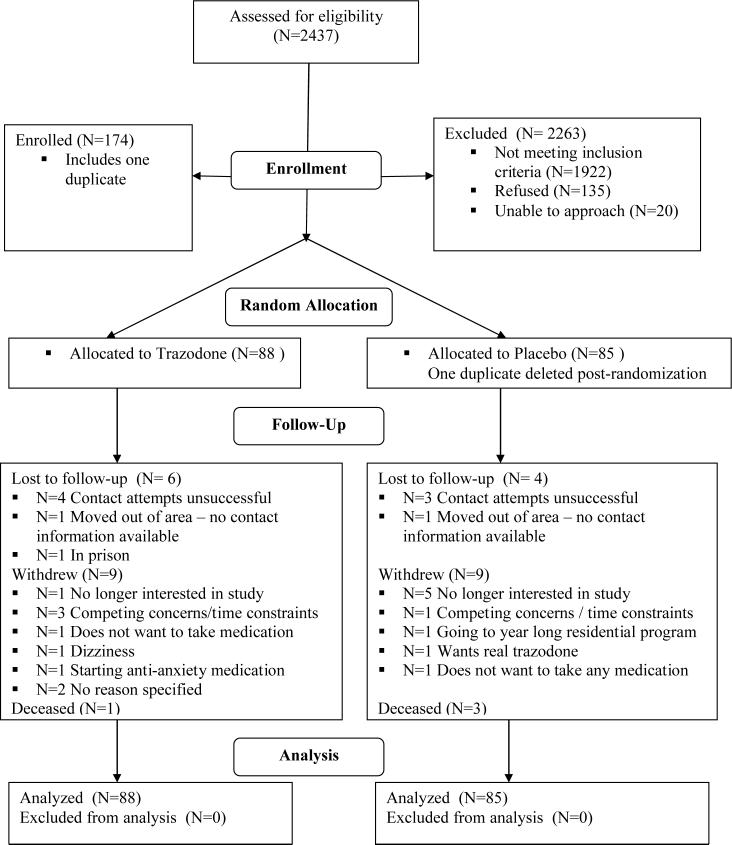
From June 2002 to January 2006, 2437 alcohol-dependent patients were screened (Figure 1) Among the 1922 ineligible individuals, 5.6% denied sleep problems; 45% were drug dependent and 28% received psychotropic medication. Research assistants were unable to approach 206 individuals and 135 refused. One duplicate placebo subject was deleted post-randomization. The trazodone (N=88) and placebo groups (N=85) did not differ at baseline on any measured characteristic (Table 1). Subjects were primarily male and Caucasian. Mean age was 41 years. Most (73%) reported 12 or more years of education and one-third was unemployed. Participants were heavy drinkers, having been abstinent only 21% of the 90 days prior to the baseline assessment, and consuming an average of 22 drinks per drinking day during that period.
Figure 1.
CONSORT Flowchart
Table 1.
Participant Characteristics at Enrollment
| Trazodone N=88 |
Placebo N=85 |
P-value | |
|---|---|---|---|
| Age (SD) | 41 (6.8) | 41 (7.7) | 0.80 |
| % Female | 9.8 | 7.5 | 0.36 |
| % Caucasian | 87.1 | 85.2 | 0.73 |
| % 12+ years of school | 75.6 | 72.6 | 0.63 |
| % Unemployed | 35.8 | 33.0 | 0.25 |
| % Homeless | 20.2 | 21.4 | 0.62 |
| % Depressed | 31.2 | 28.9 | 0.73 |
| Past Three Months Drinking | |||
| Mean proportion of days abstinent (SD) | 0.23 (0.29) | 0.18 (0.24) | 0.22 |
| Mean drinks per drinking day (SD) | 22.2 (13.8) | 21.5 (11.4) | 0.70 |
| Mean proportion of heavy drinking days | 71.6 | 78.5 | 0.10 |
| % Using other medication | 94.3 | 94.1 | 0.97 |
| Serum gamma glutamyltransferase (GGT) | 116.9 (170.9) | 144.3 (162.0) | 0.35 |
| Sleep Quality (SD) | 12.2 (3.6) | 11.6 (3.3) | 0.28 |
| SF-12 Mental Health Composite Score (SD) | 33.8 (13.0) | 35.1 (11.0) | 0.54 |
| SF-12 Physical Health Composite Score (SD) | 46.4 (8.1) | 45.3 (8.4) | 0.42 |
Of the 32 participants (18.5%) lost to follow-up, 20 (11.6%) were lost after the baseline, 9 (5.2%) after the 1-month and 3 (1.7%) after the 3-month assessment. Attrition rates did not differ by treatment group at any point. Participants who were lost to follow up also did not differ from retained subjects on age, race, gender, education level, employment status, homelessness, depressive symptoms, or baseline measures of proportion of days abstinent, number of drinks per drinking day, sleep quality, and health status. However, participants lost to follow up who had completed the 4-week sleep log reported taking the study medication on 62.8% (N=14) of days compared to 85.5% (N=106) of days for retained participants (P=0.002). Participants lost to follow up who completed a 2-week telephone check-in (N=20) did not differ on the number of reported side effects, nor were they more or less likely to believe they were taking trazodone compared with retained participants.
The study conditions did not differ in their rated baseline commitment to abstinence (mean ± standard deviation, 5.54 ± 1.23 and 5.58 ± 0.92 for the trazodone and placebo groups, respectively), nor did they differ in self-reported use of other medications (either prescribed or over the counter) for help with sleep, anxiety, emotional problems, alcohol detoxification, drinking prevention, drug use stabilization, and/or to block the effects of other drugs either at baseline or follow-up. At the 1-month follow-up (McLellan et al., 1992), 72.7% reported having received informal intervention (attending at least one Alcoholics Anonymous meeting), 25.3% reported formal treatment, and 2% reported no intervention at all after leaving the detoxification program. Compared to the placebo group, the trazodone group tended to be more likely to attend Alcoholics Anonymous (Odd Ratio [OR], 1.91; 95% CI, 0.91, 4.03; P=0.09), but the two groups did not differ in the mean number of days of psychosocial intervention at the 1-month follow up (5.55 ± 6.23 and 5.55 ± 4.35 for the trazodone and placebo groups, respectively). No significant moderator effect over time was detected for any of these covariates; hence, they were excluded from the mixed linear regression models for drinking and secondary outcome variables.
Drinking Outcomes
Mixed linear regression results indicated a linear increase in the proportion of days abstinent over time and a quadratic effect (Table 2), which indicates a leveling off in the rate of change over time. A linear decrease in the number of drinks per drinking days and the proportion of heavy drinking days also leveled off over time.
Table 2.
Mixed Linear Regression Results for Drinking Outcomes and Secondary Outcomes of Sleep Quality and Quality of Life†
| Predictor Variables |
Unstandardized Parameter Estimate (95% CI) |
|||||
|---|---|---|---|---|---|---|
| |
Drinking Outcomes |
Secondary Outcomes |
||||
| Proportion of Days Abstinent | Mean Drinks per Drinking Day | Proportion of Heavy Drinking Days | Sleep Quality | SF-12 Mental Health Composite | SF-12 Physical Health Composite | |
| Linear Slope | 0.28*** (0.22, 0.35) | −5.18** (−8.33, −2.02) | −0.27*** (−0.36, −0.21) | 3.43*** (−4.18, −2.68) | 7.39*** (4.93, 9.84) | 0.01 (−1.50, 1.52) |
| Quadratic Slope | −0.04*** (−0.05, −0.03) | 0.79** (0.28, 1.30) | 0.04*** (0.03, 0.05) | 0.47*** (0.35, 0.59) | −0.97*** (−1.36, −0.59) | −0.01 (−0.25, 0.23) |
| Baseline Intervention Difference | −0.03 (−0.12, 0.05) | −0.32 (−3.85, 3.20) | 0.06 (−0.03, 0.14) | −0.12 (−1.15, 0.91) | −0.06 (−3.53, 3.42) | −1.10 (−3.24, 1.04) |
| Intervention Difference in Linear Slope | 0.06 (−0.03, 0.15) | 0.68 (−3.84, 5.20) | −0.07 (−0.17, 0.02) | 1.94*** (0.87, 3.01) | −1.16 (−4.65, 2.32) | 0.81 (−1.34, 2.96) |
| Intervention Difference in Quadratic Slope | −0.01 (−0.02, 0.01) | −0.24 (−0.98, 0.49) | 0.01 (−0.01, 0.02) | −0.31*** (−0.48, −0.14) | 0.29 (−0.26, 0.84) | −0.10 (−0.44, 0.24) |
Mixed linear model including linear and quadratic change in the outcomes over time (measured at baseline 1-, 3-, and 6-month intervals), intervention difference at baseline, and intervention by linear and quadratic time interactions.
*P <0.05
P < 0.01
P <0.001
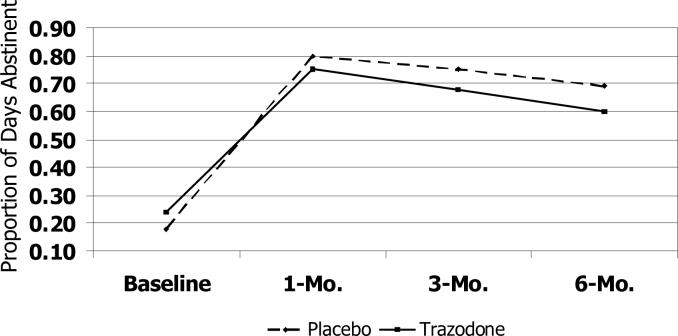
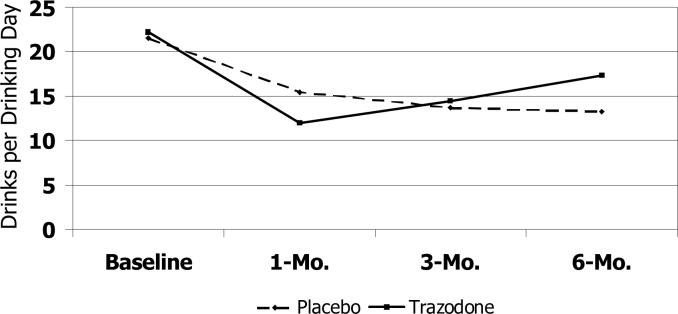
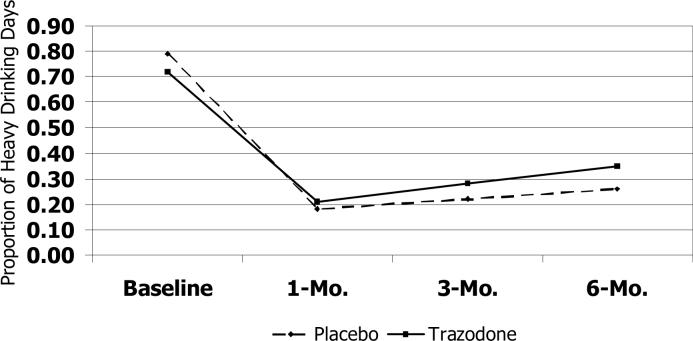
The trazodone group showed a smaller increase in the proportion of days abstinent at all time points relative to the placebo group (Figure 2a); effect sizes for the estimated mean difference between groups in the change in the proportion of days abstinent were moderate from baseline to 1-month (Cohen's d, −0.22), 3-month (Cohen's d, −0.50) and 6-month (Cohen's d, −0.51) follow-up assessments. No clinically significant differences between the treatment groups were detected in the number of drinks per drinking day from baseline to the 1- and 3-month assessments. However, by the 6-month assessment, the trazodone group had a greater increase relative to the placebo group in the number of drinks per drinking day (Cohen's d, 0.41), such that the estimated number of drinks per drinking day exceeded that of the placebo group (Figure 2b). Finally, compared to placebo, the trazodone group showed a smaller decrease in the proportion of heavy drinking days through the 3-month follow-up (Cohen's d, −0.53) compared to the placebo group, followed by a smaller increase from 3−6 months (Figure 2c).
Figure 2a.
Trajectory of Abstinence Days
Figure 2b.
Trajectory of Drinks per Drinking Days
Figure 2c.
Trajectory of Heavy Drinking Days
After controlling for the proportion of days abstinent at baseline, logistic regression analyses indicated no differences in the probability of complete abstinence by study condition: only 9.1% of the trazodone group and 14.1% of the placebo group achieved complete abstinence at the 6-month follow-up (adjusted OR, 0.60; 95% CI, 0.23, 1.56; P=0.30).
Biological Verification of Drinking
Serum gamma-glutamyltransferase (GGT) drawn at baseline (N=133), 1 month (N=99), 3 months (N=86), and 6 months (N=95) verified self-reports of abstinence. Participants reporting abstinence over the prior two weeks had a 32% decrease in GGT level from baseline, compared with a 2% decrease in GGT those who reported drinking. Participants reporting abstinence in the prior two weeks had a mean GGT of 56.0 ± 71.0 compared with 129.0 ± 171.6 for those reporting any drinking (P=0.07).
Secondary Outcomes
Sleep quality did not differ by treatment group at baseline, but linear and quadratic treatment effects emerged (Table 2). Sleep quality improved more for the trazodone group than the placebo group at the 1- and 3-month follow-ups, with a moderate effect size from baseline to the 1-month assessment (Cohen's d, −0.50) and a large effect from baseline to the 3-month follow-up (Cohen's d, −0.93). However, sleep quality degraded for the trazodone group between the 3- and 6-month assessments (Figure 2d); such that sleep quality was equal for both groups at the 6-month assessment. Trazodone similarly improved mental health status modestly from baseline through three months (Cohen's d, −0.18) then leveled off. Physical health status did not improve over time or by treatment.
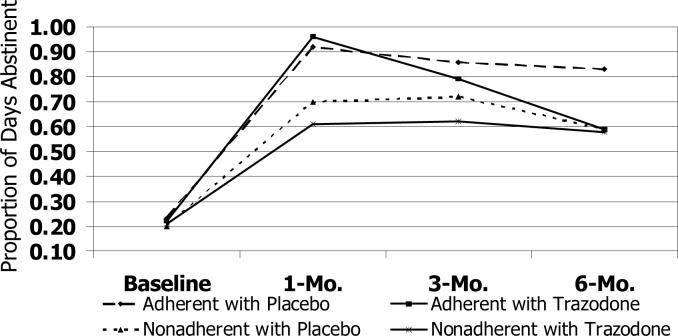
Figure 2d.
Trajectory of Abstinence Days by Treatment Adherence
Adherence and Side-Effects
Reported adherence with the medication was high during the first month (Table 3). MEMS data correlated with self-report (ρ, 0.34, P=0.003; N=76) but suggested lower adherence. Either way adherence did not differ between groups. Participants in both groups reported taking an average of two pills per day over the first 4 weeks. In a mixed linear regression model the number of pills per day increased similarly in both groups (unstandardized β, 0.01; 95% CI, −0.01 to 0.03; P=0.27). The trazodone group tended to report more dry mouth, but other side effects were not reported more often than in the placebo group (Table 3). The two groups did not differ in the number of reported side effects, though those in the treatment group more often believed they were probably or definitely taking trazodone.
Table 3.
Comparison of Study Medication Adherence and Side Effects, by Study Condition
| Trazodone | Placebo | P-value | |
|---|---|---|---|
| % Days took medication | |||
| By self-report (N)* | 82.4 (63) | 83.3 (57) | .87 |
| From MEMSCAP data (N) | 43.4 (51) | 37.7 (46) | .38 |
| Mean ± SD number of pills per day (N)* | 1.98±0.7 (63) | 1.98±0.6 (57) | .97 |
| Side effects reported, % (N)† | 3.06±3.3 (64) | 3.67±3.5 (64) | .27 |
| Dry mouth | 37.5 (24) | 23.9 (16) | .13 |
| Morning drowsiness | 31.3 (20) | 48.5 (32) | .05 |
| Weight gain | 23.8 (15) | 28.4 (19) | .69 |
| Increased thirst | 23.8 (15) | 31.3 (21) | .43 |
| Daytime tiredness | 21.9 (14) | 36.9 (24) | .08 |
| Nervousness | 18.8 (12) | 19.4 (13) | 1.0 |
| Decreased interest in sex | 14.8 (9) | 10.6 (7) | .60 |
| Headache | 14.1 (9) | 23.9 (16) | .19 |
| Fast heartbeat | 12.5 (8) | 9.0 (6) | .58 |
| Muscle aches | 12.5 (8) | 14.9 (10) | .80 |
| Decreased appetite | 11.1 (7) | 10.5 (7) | 1.0 |
| Diarrhea | 10.9 (7) | 7.5 (5) | .35 |
| Dizzy on standing | 9.4 (6) | 6.0 (4) | .52 |
| Runny nose | 7.8 (5) | 22.7 (15) | .03 |
| Confusion | 7.8 (5) | 7.5 (5) | 1.0 |
| Difficulty concentrating | 6.3 (4) | 14.9 (10) | .16 |
| Uncontrolled hand movements | 6.3 (4) | 9.0 (6) | .74 |
| Rash | 6.3 (4) | 3.0 (2) | .33 |
| Decreased urine flow | 6.3 (4) | 3.0 (2) | .43 |
| Painful/prolonged erections | 5.8 (3) | 0.0 (0) | .24 |
| Nausea/vomiting | 4.7 (3) | 9.0 (6) | .49 |
| Breathing trouble | 4.7 (3) | 1.5 (1) | .36 |
| Blurred vision | 3.2 (2) | 10.5 (7) | .17 |
| Constipation | 3.1 (2) | 9.0 (6) | .27 |
| Swelling of hands/feet | 1.6 (1) | 6.0 (4) | .37 |
| Mean ± SD number of side effects reported (N)†‡ | 3.06±3.3 (64) | 3.67±3.5 (64) | .27 |
| % Believed they were taking trazodone (N)† | 79.0 (49) | 48.5 (32) | .0003 |
From daily sleep log data over the first 4 weeks (total N=121)
From 2-week follow-up data (total N=128)
Log-transformed variable
Participants who reported less than full adherence with study medication in the first month had had fewer days abstinent at baseline than those who reported 100% adherence (unstandardized β; −0.12; 95% CI, −0.22 to −0.02; P=0.02) Mixed linear regression analyses examined interactions between self-reported adherence and treatment condition on the trajectories of the outcome variables. Significant interactions emerged for proportion of days abstinent (unstandardized β, 0.03; 95% CI, 0.01 to 0.04; P=0.001; N=120) and sleep quality (unstandardized β, −0.20; 95% CI, −0.39 to −0.02; P=0.029; N=120). Fully adherent subjects in both groups and partially adherent trazodone subjects experienced the greatest increases in the proportion of days abstinent during the first three months, which declined in the 3- to 6-month interval (Figure 2c). Among subjects who were partially adherent with placebo, abstinence days reached a plateau after the first month. Trazodone subjects and fully adherent placebo subjects experienced the greatest improvements in sleep quality during the first three months, which dissipated in the 3- to 6-month interval. In the partially adherent placebo group, sleep quality did not improve further after 1 month.
DISCUSSION
In this randomized trial of sleep-disturbed, alcohol-dependent patients, compared with placebo controls the trazodone group experienced less improvement in abstinence days and an increase in the number of drinks per drinking day with cessation of the study medication. Trazodone was associated with improved sleep quality during its administration, but after it was stopped sleep quality equalized with placebo.
Few studies have evaluated trazodone as a sleep aid among newly-detoxified alcohol-dependent patients. Le Bon and colleagues (2003) performed a double-blind, placebo-controlled clinical trial among 18 insomniac, alcohol-dependent patients who returned after a 2-week washout period after alcohol detoxification. Over 4 weeks of follow-up, polysomnographic studies revealed that trazodone reduced awakenings and enhanced sleep maintenance. Trazodone also produced subjective improvements on the Clinical Global Impression Scale. These improvements in sleep maintenance and global impression are consistent with the observation in the current study that trazodone increased subjective sleep quality.
Although the scope of the current study limited the ability to perform polysomnography on all participants, the trade-off was the ability to examine trazodone's effects on alcohol-related outcomes. Despite short-term effects on sleep quality, the current study dampens enthusiasm for the widespread practice of prescribing trazodone for sleep-disturbance after alcohol detoxification. We cannot discern the reason for the decrement in abstinence days in the trazodone group, but pre-clinical research has suggested that trazodone's metabolite mchlorophenylpiperazine (m-CPP) exerts ethanol-like effects and induces alcohol craving in recently detoxified alcoholics (Krystal et al., 1994). Similarly obscure is the reason for the exacerbation of drinking severity upon stoppage of trazodone but not placebo. One prior study suggested that trazodone can alleviate symptoms of alcohol withdrawal (Roccatagliata et al., 1980) Although causal direction is uncertain, one can speculate that post-acute alcohol withdrawal symptoms including sleep disturbance, reemerged with cessation of trazodone and triggered alcohol consumption. Concern about the cessation of trazodone leading to more drinking is salient because research and clinical experience suggest that medication adherence is problematic in alcohol-dependent populations (Swift, 1999). Rebound effects on sleep disturbance, anxiety and depressive symptoms with abrupt cessation of trazodone, and whether a slow taper might prevent them, merit further study.
The study's strengths include a randomization procedure that balanced possible baseline confounders; a double-blind, placebo-controlled design, a reasonable rate of follow-up in a socially disconnected sample; and sophisticated analyses that examined several methods for handling missing data. The study's major weaknesses are its relatively small sample size, single site and heavy drinking population; its findings generalize best to alcohol-dependent populations requiring inpatient detoxification. In addition, little is known about the types or intensity of subsequent alcohol treatment, although days of treatment appear balanced between study conditions.
Other limitations are typical for studies of alcohol-dependent populations. Alcohol consumption outcomes derive from self-report, which provide useful estimates but might reflect underreporting (Sobell & Sobell, 1990; Del Boca & Noll, 2000); that said, GGT generally confirmed reported alcohol use. Participants' self-reports may overstate the severity of sleep problems (Currie et al., 2004), although a recent report suggests that subjective sleep measures predict recurrent drinking better than does polysomnography in this population (Conroy et al., 2006). Standardized research assessments and procedures partly mitigate measurement bias (Sobell & Sobell, 1990; Del Boca & Noll, 2000). Many subjects guessed their study allocation, but uncertainty about the accuracy of their guesses during the course of the study should limit the effect of expectation bias on their reports. Study attrition could have introduced follow-up bias, but follow-up rates did not differ by baseline characteristics and were typical for similar studies (Hansten et al., 2000). Adherence with study medication was fair; thus, the results likely reflect real-world effect rather than efficacy among alcohol dependent populations. The extensive exclusions for medical and psychiatric comorbidities and other drug dependence further suggest that these findings generalize best to “pure” alcohol dependent patients who are increasingly rare in medical and addiction treatment settings.
It remains possible that the combination of trazodone with antidipsogenic medications (such as naltrexone or acamprosate) or psychosocial interventions (such as relapse prevention) might improve outcomes compared to a similarly treatment-receiving placebo group; future research should examine this possibility. Nonetheless, based on current findings, we conclude that trazodone, despite a short-term effect on sleep quality, cannot be recommended with confidence for the management of sleep disturbance during the period after detoxification from alcohol dependence. Although this study is not definitive, its findings increase the probability that trazodone use or cessation might impede improvements in alcohol consumption during the early post-detoxification period. This finding contradicts clinical “common sense” that has assumed that since insomnia might be related to relapse, hypnotic treatment would decrease recurrent drinking. However, it is consistent with the traditional recommendation that alcohol-dependent patients should avoid all sedative-hypnotic medications during recovery, because of their potential to trigger relapse. In this regard, some treatment centers and recovery groups have been known to prohibit even over-the-counter remedies such as diphenhydramine. That said, very little clinical information exists on the use of any hypnotic agent or its cessation in the alcohol dependent population (Brower, 2003) Until further studies have established benefits and safety, the pharmacological treatment of sleep disturbance should be initiated with caution among patients in early recovery from alcohol dependence.
ACKNOWLEDGMENTS
The National Institute on Alcohol Abuse and Alcoholism (R01AA13243) supported this study (ClinicalTrials.gov Identifier, NCT00027053). Dr. Friedmann directs the Program to Integrate Psychosocial & Health Services in Chronic Disease & Disability, a Targeted Research Enhancement Program (TRP 04−179) supported by the Department of Veterans Affairs Health Services Research & Development Service, at the Providence Veterans Affairs Medical Center (Rhode Island). The authors thank the patients and staff of SSTAR of Rhode Island; G. Linda Bigden; Anthony Charuvastra; Jumi Hayaki; Debra Herman; Susan Ramsey; Kristina Richards; M. Andrea Monckeberg; and Hossein Zia. The views expressed in this article are the authors' and not necessarily those of the National Institute on Alcohol Abuse and Alcoholism or Department of Veterans Affairs.
Sponsored by National Institute on Alcohol Abuse and Alcoholism (NIAAA) (R01AA13243), ClinicalTrials.gov Identifier, NCT00027053.
REFERENCES
- American Sleep Disorders Association . Behaviors that help promote sound sleep. American Sleep Disorders Association; Rochester, MN: 1997. Sleep Hygiene. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosomatic Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Conroy DA, Todd AJ, Brower KJ, Strobbe S, Consens F, Hoffmann R, Armitage R. Perception of sleep in recovering alcohol-dependent patients with insomnia: relationship with future drinking. Alcohol Clin Exp Res. 2006;30(12):1992–1999. doi: 10.1111/j.1530-0277.2006.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Malhotra S, Clark S. Agreement among subjective, objective, and collateral measures of insomnia in postwithdrawal recovering alcoholics. Behav.Sleep Med. 2004;2:148–161. doi: 10.1207/s15402010bsm0203_4. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95(Suppl 3):S347–S360. doi: 10.1080/09652140020004278. [DOI] [PubMed] [Google Scholar]
- Drummond S, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22(8):1796–1802. [PubMed] [Google Scholar]
- Fabre LF. Trazodone dosing regimen: experience with single daily administration. J Clin Psychiatry. 1990;51:23–26. [PubMed] [Google Scholar]
- Feighner JP, Boyer WF. Overview of USA controlled trials of trazodone in clinical depression. Psychopharmacology. 1988;95:S50–S53. doi: 10.1007/BF00172631. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbons M. Structured clinical interview for DSM-IV -Patient version. NY State Psychiatric Institute; New York: 1995. [Google Scholar]
- Friedmann PD, Herman DS, Freedman S, Lemon SC, Ramsey S, Stein MD. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. J Addict Dis. 2003;22:91–103. doi: 10.1300/J069v22n02_08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeringer KE, Raymon L, Logan BK. Postmortem forensic toxicology of trazodone. J Forensic Sci. 2000;45:850–856. [PubMed] [Google Scholar]
- Haffmans PM, Vos MS. The effects of trazodone on sleep disturbances induced by brofaromine. Eur Psychiatry. 1999;14:167–171. doi: 10.1016/s0924-9338(99)80736-6. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Hansten ML, Downey L, Rosengren DB, Donovan DM. Relationship between follow-up rates and treatment outcomes in substance abuse research: more is better but when is “enough” enough? Addiction. 2000;95:1403–1416. doi: 10.1046/j.1360-0443.2000.959140310.x. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Barlas S. RMASS2: Repeated Measures with Attrition: Sample Sizes for 2 Groups [Computer software] University of Illinois at Chicago, Division of Epidemiology & Biostatistics; Chicago, IL: 1999. [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- Le Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, Dupont P, Lion K, Pelc I, Verbank P. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol. 2003;23:377–383. doi: 10.1097/01.jcp.0000085411.08426.d3. [DOI] [PubMed] [Google Scholar]
- Liebowitz NR, El-Mallakh RS. Trazodone for the treatment of anxiety symptoms in substance abusers. J Clin Psychopharmacol. 1989;9:449–451. [PubMed] [Google Scholar]
- Longo LP, Johnson B. Treatment of insomnia in substance abusing patients. Psychiatric Annals. 2000;28:154–159. [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O'Brien CP. A new measure of substance abuse treatment. Initial studies of the Treatment Services Review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66:469–476. doi: 10.4088/jcp.v66n0409. [DOI] [PubMed] [Google Scholar]
- Miller I, Bishop S, Norman W, Dow M. The modified scale for suicidal ideation: reliability and validity. J Consult Clin Psychol. 1986;54:724–725. doi: 10.1037//0022-006x.54.5.724. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, MacHen MA, Perie SR, Ritenour AM. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- O'Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med. 1998;338:592–602. doi: 10.1056/NEJM199802263380907. [DOI] [PubMed] [Google Scholar]
- Parrino L, Spaggiari MC, Boselli M, Di Giovanni G, Terzano MG. Clinical and polysomnographic effects of trazodone CR in chronic insomnia associated with dysthymia. Psychopharmacology (Berl) 1994;116:389–395. doi: 10.1007/BF02247467. [DOI] [PubMed] [Google Scholar]
- Roccatagliata G, Albano C, Maffini M, Farelli S. Alcohol withdrawal syndrome: treatment with trazodone. Int Pharmacopsychiatry. 1980;15:105–110. doi: 10.1159/000468420. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology (Berl) 1999;144:220–233. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. Chapman & Hall; Boca Raton, FL: 1997. [Google Scholar]
- Sobell LC, Sobell MB. Self-report issues in alcohol abuse: state of the art and future directions. Behav Assess. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: psychological and biological methods. Humana Press; New Jersey: 1992. pp. 41–72. [Google Scholar]
- Stein MD, Friedmann PD. Disturbed sleep and its relationship to alcohol use. Subst Abus. 2006;26:1–13. doi: 10.1300/j465v26n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Swift RM. Medications and alcohol craving. Alcohol Res Health. 1999;23:207–213. [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV. Sleep, alcohol and alcohol abuse. Addiction Biology. 1997;2:151–158. doi: 10.1080/13556219772697. [DOI] [PubMed] [Google Scholar]
- Wagman A, Allen RP. Effects of alcohol ingestion and abstinence on slow wave sleep of alcoholics. Adv Exp Med Biol. 1975;59:453–466. doi: 10.1007/978-1-4757-0632-1_32. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Schweitzer PK. Ten-year trends in the pharmacological treatment of insomnia. Sleep. 1999;22:371–375. [PubMed] [Google Scholar]
- Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry. 1990;51(Suppl):18–22. [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. How to Score the SF-12 Physical & Mental Health Summary Scales. Second edition ed. The Health Institute, New England Medical Center; Boston, MA: 1995. [Google Scholar]
- Yamadera H, Suzuki H, Nakamura S, Endo S. Effects of trazodone on polysomnography, blood concentration and core body temperature in healthy volunteers. Psychiatry Clin Neurosciences. 1999;53:189–191. doi: 10.1046/j.1440-1819.1999.00531.x. [DOI] [PubMed] [Google Scholar]