Abstract
Our sense of gravitation and linear acceleration is mediated by stimulation of vestibular hair cells through displacement of otoconia in the utricle and saccule (the gravity receptor organ). We recently showed that otoconin-90 (Oc90) deletion led to formation of giant otoconia. In the present study, we determined the extent to which the giant otoconia affected balance and gravity receptor sensory input and compared the findings with other otoconia mutants. We employed a wide spectrum of balance behavioral tests, including reaching and air-righting reflexes, gait, swimming, beam-crossing, rotorod latencies, and a direct measure of gravity receptor input, vestibular evoked potentials (VsEPs). All tests on homozygous adult mutants consistently ranked the order of imbalance as (from worst to best) Nox3het<otopetrin 1tlt<Oc90 null<Oc90 wild type and C57Bl/6 mice using systematic statistical comparisons of the frequency of occurrence or the severity of abnormal functions. This order coincides with the degree of otoconia deficiencies and is consistent with VsEP measures. Notably, all mice (except Nox3het) showed remarkable learned adaptation to peripheral vestibular deficits by staying on the rotating rod significantly longer in each successive trial, and the rate and extent of such learned improvements ranked the same order as their initial balance ability. Despite the vestibular morbidity, Oc90 null mice had normal hearing, as measured by auditory brainstem responses (ABRs) and distortion products of otoacoustic emissions (DPOAEs). The study demonstrates that the remnant otoconia mass in Oc90 nulls does stimulate the gravity receptor organs, which was likely responsible for the improved balance performance relative to strains with absent otoconia. Furthermore, the combination of direct electrophysiological measures and a series of behavioral tests can be used to interpret the imbalance severity arising from altered inputs from the gravity receptor end organ.
Keywords: otoconia, balance, vestibule, adaptation, compensation, hearing
The utricle and saccule of the vestibule specifically sense linear acceleration and gravity mediated by the inertial mass of otoconia displaced against the stereocilia of sensory hair cells under the stimuli of motion. The spatial arrangement and unique structures of the macula and otoconia are important to determine such specificity in motion detection. Indeed, electrophysiological and behavioral studies show that absent or drastically altered crystal mass invariably leads to balance deficits (Trune and Lim, 1983; Anniko et al., 1988; Ornitz et al., 1998; Kozel et al., 1998; Jones et al., 1999, 2004; Simmler et al., 2000; Sollner et al., 2003, 2004; Paffenholz et al., 2004). However, most of the mutants used in these reports may have potentially compromised hair cells, stereocilia or vestibular ganglia due to expression of the mutant genes in these structures. Studies using mutants that have deficits restricted to the otoconia would be useful to assess the contribution of the crystal mass alone on imbalance.
We have recently shown that deletion of otoconin-90 (Oc90), the predominant mammalian otoconial protein, leads to absent formation of the otoconial organic matrix and subsequent aggregation of the inorganic crystallites in mice (Zhao et al., 2007). The overall otoconia mass of these mutant mice is reduced by 50% on average, but the epithelial cells are morphologically and ultrastructurally normal. Oc90 is expressed in the squamous cells and transitional epithelium of the vestibule, but not in hair/supporting cells, stroma or ganglia (Zhao et al., 2007), therefore molecular or structural deficits of the sensory structures are not expected. We hypothesized that a partial loss of otoconia would not efficiently relay head motion to the sensory epithelium and balance deficits would result, but the balance deficits would be less severe than those reported previously for otoconia-deficient mutant mouse strains. Herein we assessed the extent of peripheral gravity receptor dysfunction and the severity of imbalance behaviors in Oc90 null mice and compared these findings with other mutants possessing severe otoconia deficiencies (NADPH oxidase 3 (Nox3)het and otopetrin 1 (Otop1)tlt). The results offer insights on the sensitivity of the gravity receptor organ to reduced otoconial mass and on the balance abilities of Oc90 mutants.
Both the Nox3het and Otop1tlt mice (previously known as the head-tilt or het mice, and the tilted or tlt mice, respectively) carry autosomal recessive, spontaneous mutations that lead to absent otoconia with no apparent abnormalities in other organs. The penetrance of both mutations is nearly 100%. The otoconia deficit results in head-tilting behavior and inability to swim. Both strains have absent vestibular evoked potentials (VsEPs) but normal thresholds for auditory brainstem responses (ABR) (Ornitz et al., 1998; Banfi et al., 2004; Jones et al., 1999, 2004). The Nox3 gene encodes NADPH oxidase 3 (Paffenholz et al., 2004), whose role in otoconia formation is still unclear. Deficits in signal transduction from hair cell to neuron cannot be ruled out as the gene is expressed in the vestibular sensory epithelium (both hair/supporting cells) and possibly ganglia (Banfi et al., 2004). However, Jones et al. (2007) demonstrated that the vestibular ganglia in Nox3het and Otop1tlt adult mice are spontaneously active with mature discharge rates and patterns, a phenomenon that presumably requires functional hair cell to afferent transmission. The Otop1 gene encodes a novel 10 transmembrane domain protein (Hurle et al., 2003) that influences Ca2+ fluxes (Hughes et al., 2007) and may be involved in protein secretion (Sollner et al., 2004). Otop1 is also expressed in the sensory epithelium of the inner ear, both hair and supporting cells (Hurle et al., 2003; Sollner et al., 2004). The mice occasionally have a few giant otoconia in the saccule (Ornitz et al., 1998). Other Otop1 mutants include the ethylnitrosourea-induced alleles Otop1mlh (Hurle et al., 2003) and Otop1ied (Besson et al., 2005).
The present study aims to compare the behavioral outcome of otoconia deficiencies in Oc90 null mice with the amount of sensory input relayed by the gravity receptor organ using VsEPs, a noninvasive method that directly measures the sensitivity of the gravity receptor organ (Jones and Jones, 2007; Jones, 2008).
Experimental Procedures
Mice
Oc90-targeted mutant mouse lines were recently generated (Zhao et al., 2007). The Otop1tlt and Nox3het mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and have been backcrossed to and maintained in the C57BL/6J (as C57) genetic background by the supplier. Unless otherwise indicated, the reported results were all obtained from homozygous mutants. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Boys Town National Research Hospital in accordance with federal and international guidelines on the ethical use of animals. Every effort was made to minimize the number of animals used and their suffering.
Mouse genotyping and histology
Mouse tail DNA was used for genotyping by multiplex PCR. The genotyping design for Oc90 knockout mice was previously described and validated by Southern blotting (Zhao et al., 2007). The other mutant mice were maintained by homozygous inbreeding as the heterozygous animals were initially assessed to be phenotypically normal. For histological examination, inner ears were dissected and fixed in paraformaldehyde (4%). Tissues were then embedded in paraffin for sectioning at 6 μm. Some inner ears were decalcified or partially decalcified in 0.1 M EDTA (pH7.4) for 2 h to overnight, depending on the age of the animals.
Phalloidin staining
Fixed and decalcified inner ears were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS), washed with PBS and stained with a 50 μg/ml FITC-conjugated phalloidin solution (Sigma, St. Louis, MO, USA) for 40 min at room temperature. Unbound phalloidin was washed with PBS and tissues were viewed using a Zeiss LSM 510 confocal microscope.
Scanning electron microscopy (SEM)
Otoconia morphology was visualized by SEM. Animals were anesthetized using a sub-lethal dose of ketamine (167 mg/kg) and xylazine (2 mg/kg), perfused through the left ventricle with 0.1 M sodium cacodylate (pH 7.2), followed by 20–30 ml 4% paraformaldehyde +2.5% glutaraldehyde+3 mM CaCl2 in 0.1 M sodium cacodylate (pH 7.2). Dissected utricles and saccules were immersion-fixed for 24 h in the same fixative, washed with 0.1 M sodium cacodylate (pH 7.2) and post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate (pH 7.2) for 1 h. Samples were then dehydrated, critical point dried in CO2, mounted, sputter coated with gold palladium and viewed on a JEOL JSM-848 scanning electron microscope at 8 kV.
Tests for imbalance behaviors
The following complementary tests are commonly used to detect balance deficits manifested in postural control, spatial orientation and sensorimotor functions and coordination (Trune and Lim, 1983; Sondag et al., 1995, 1996, 1998, 1999; Ying et al., 1999; Rampello and Drago, 1999; Hamm, 2001; de Caprona et al., 2004; Jones et al., 2004; Besson et al., 2005; Walton et al., 2005; Sajdel-Sulkowska et al., 2005). These tests can involve multiple systems (i.e. peripheral inputs and central integration), but can also specifically reveal the behavioral outcome of a particular lesion when the lesion is known to be localized to a single organ or system. Severe abnormalities in the gravity receptor organ often lead to abnormal air-righting reflexes, irregular and wider gaits, compromised ability to swim or shortened stay on the rotorod (Trune and Lim, 1983; Ying et al., 1999; Rampello and Drago, 1999; Hamm, 2001; Jones et al., 2004; Besson et al., 2005). We assessed the grip-strengths of the animals to rule out neuromuscular weakness as a possible secondary outcome of the vestibular sensory deficits. Further, we directly measured the input by the peripheral gravity receptor organ using VsEP recordings. The age, genotype and number of mice tested are summarized in the corresponding tables presented in the Results section.
Air-righting reflex
The test was performed on P9–P11 pups, a time window that is ideal for observing abnormal righting responses because vestibular behavioral adaptation is minimal. The pups were suspended by their tails several inches above a foam cushion and released in such a way that they would make a backward somersault and land on the foam. The position with which the pups landed and the time they took to right their body positions were recorded. Each pup was tested three times in a row on each day from P9–P11.
Reaching response
When suspended by the tails, animals that extend their forelimbs and try to reach a surface are normal whereas animals with vestibular deficits often clasp their forelimbs or have head-bobbing behavior. The initial reaction of the animal at suspension was recorded. Both adults (2–8 months old) and pups (2–3 weeks old) were tested.
Gait
Animals' gaits were recorded on a white paper after applying Indian ink to the paws. Regularity of the gait was observed and measurements of the stride length and width were compared between mutants and wild type (wt), heterozygous or C57 age-matched control mice. The measurements were obtained according to published methods (Klapdor et al., 1997; Clarke and Still, 1999) and were adapted from a standard protocol used by the Charles River Laboratories (Wilmington, MA, USA). Findings with P16 (Oc90 null and wt) and P20 pups (C57, Otop1tlt and Nox3het) are reported in this paper.
Swimming test
Adult mice (2–6 months old) were placed in a sterilized container filled with lukewarm water and swimming behavior was observed for 15–30 s. The test was repeated three times on different days and the following scoring system was used: 0, the animal was totally submerged, could not maintain orientation at the surface of the water and required immediate rescue; 1, the animal could swim to the surface if submerged, but did not maintain a horizontal body line at the surface and may re-submerge briefly; 2, the animal stayed at the surface but hesitated for a few seconds before swimming almost normally; 3, the animal swam at the surface immediately and maintained horizontal body position at the surface. Due to some ambiguity of scores 2 and 3, we ultimately categorized the mice as poor (score 0), intermediate (score 1) or good swimmers (scores 2 and 3).
Rotorod test
Adult animals (7 to 10 mice at 2–6 months old for each genotype) were placed on a 2 cm-diameter stationary and then rotating rod (Rotamex-5, Columbus Instruments, Columbus, OH, USA). The rotation was initially set at a constant speed of 5 r.p.m./min as a test run. Once each mouse was able to maintain balance for 1 min in each trial, it was then tested on the accelerating rod (5–20 r.p.m. over 3 min) for three consecutive trials per day for five consecutive days. At the end of each trial, animals that stayed on the rod were left on until they fell or until 10 min had passed. A laser sensor detected if the animal fell from the rod and the time and speed of rotation at falling were recorded. The average time the mice remained on the rotorod (mean latency) was calculated from 7 to 10 animals and plotted for each trial, each genotype and each age group. The mean values of all mice in one genotype were compared with those of Oc90 wt or C57 controls in the same trial using Student's t-test assuming similar variances. All tests were carried out within the same time window of the day (between 2 and 4 pm) to control for possible variations introduced by circadian rhythms.
Beam-crossing
A round wood bar of 2.5 cm in diameter was attached to two Styrofoam platforms at the ends and the length of the bar to be crossed was adjustable at the ends. Before the test, adult mice (2–6 months old) were fasted for a maximum of 4 h (water was provided ad libitum) in order to use food (rodent chow) as an effective incentive for the animals to cross the beam. Pre-training of mice on the bar at 5 cm long was performed first, followed by three consecutive trials of crossing the bar at 30 cm long. The test (including pre-training) was repeated on three consecutive days. Time spent in crossing the bar at 30 cm was recorded; however, it was not used for quantitative purposes because wt mice often paused on the beam to explore at some point. Rather, the posture (i.e. lowered body position) and behavior (i.e. reluctance, nervousness) on the beam were observed.
Grip-strength test
Mutant and control mice were made to hang in a supine position on an inverted metal meshwork, and the length of time the animal remained on the meshwork was recorded.
Data analysis
Data are presented as mean±standard deviation (S.D.) where applicable. Balance behaviors (except rotorod tests) of mutant strains were compared with those of Oc90 wt or C57 controls using chi-square analysis. Latencies before falling off the rotorod were compared as described above. P values of less than 0.05 were considered statistically significant.
Electrophysiological measures VsEPs, ABRs and distortion product otoacoustic emissions (DPOAEs)
Animal preparation
The use of animals for VsEPs and hearing tests was also approved at East Carolina University and the University of California at Davis. Mice were anesthetized with a ketamine (120 mg/kg) and xylazine (13 mg/kg) solution and core body temperature was maintained at 37.0±0.1 °C using a homeothermic heating blanket system (FHC, Inc., Bowdoin, ME, USA). VsEPs were measured from two age groups (4 and 10 months old) and hearing tests were performed on young mice (2–4 months old) because the parental strains hosting the genetic mutations, C57BL/6J and 129Ola/Hsd, display early onset age-related hearing loss.
Vestibular stimulus coupling
VsEP recording procedures followed methods previously published (Jones et al., 1999, 2002, 2004; Jones and Jones, 1999). Herein, we used a noninvasive method (a spring clip) to couple the head to the voltage-controlled mechanical shaker that delivered the motion stimuli. Linear acceleration pulses (17 pulses/s, 2 ms duration) were presented to the cranium in the naso-occipital axis using two stimulus polarities, normal and inverted. Stimulus amplitude ranged from +6 dB to −18 dB re: 1.0 g/ms (where 1 g=9.8 m/s2) adjusted in 3 dB steps.
ABR stimulus coupling
Tone burst stimuli (17 stimuli/s) were generated and controlled using custom software and Tucker Davis Technologies (TDT, Gainesville, FL, USA) modules (TG6, DA3-2, PA4), and were calibrated using a Bruel & Kjaar ¼″ microphone and Nexus amplifier. Stimuli were calibrated in decibel peak equivalent sound pressure level (dB peSPL) and were presented via high frequency transducers (TDT ED1 driver, EC1 speakers) coupled at the left ear via PE tubing. Tone bursts at 8, 16, 32 and 41 kHz had 1.0 ms rise-fall times with 1.0 ms plateau (3 ms total duration).
VsEP and ABR recording parameters
Ongoing electroencephalographic activity was amplified (200,000×), filtered (300–3000 Hz, −6 dB amplitude points) and digitized (1024 points, 10 μs/pt). Two hundred fifty-six primary responses were averaged and replicated for each VsEP or ABR waveform. VsEP recordings began at the maximum stimulus intensity (i.e. +6 dB re: 1.0 g/ms) with and without acoustic masking, then intensity was dropped to −18 dB and raised in 3 dB steps to complete an intensity profile. A broad-band forward masker (50–50,000 Hz, 97 dB SPL) was presented during VsEP measurements to verify absence of cochlear responses (Jones and Jones, 1999). ABR intensity series were collected with a descending series of stimulus intensities (12 dB steps) beginning at approximately 100 dB peSPL.
DPOAE recording protocol
Methods for recording DPOAEs were similar to Jimenez et al. (1999) and Guimaraes et al. (2004). Stimuli for DPOAEs were generated and controlled with modules from TDT (TG6, PA4) and calibrated in a 0.1 ml coupler, which simulates the mouse ear canal volume. Pure tone frequencies (f1, f2, f2/f1 ratio=1.25), 150 ms duration at equal levels (L1=L2=60 dBSPL), were generated with independent sources (two HP Agilent signal generators) and routed through separate drivers to mix acoustically in the ear canal. Stimulus frequencies for the primaries were such that geometric mean (GM=(f1×f2)0.5) frequencies ranged from 6.0–48.5 kHz (at least eight frequencies per octave). Ear canal sound pressure levels were recorded with a low noise probe microphone (Etymotic ER 10B+), whose output was amplified and input to a dynamic signal analyzer (Stanford Research Systems SR785, Stanford Research Systems Inc., Sunnyvale, CA, USA) for sampling (at 200 kHz) and fast Fourier transform (FFT). The amplitude of f1, f2, and the 2f1–f2 distortion product were measured from the FFT waveform. The noise floor was measured from the amplitudes in the five frequency bins above and below the 2f1–f2 component. The recording system was also tested periodically in the 0.1 ml coupler to rule out the presence of artifact distortion products.
Data analysis
The first three positive and negative response peaks were scored for VsEPs. Response peak latencies (measured in milliseconds), peak-to-peak amplitudes (measured in microvolts) and thresholds (measured in dB re: 1.0 g/ms) were quantified. Descriptive statistics were generated for each genotype. Multivariate analysis of variance (MANOVA) was used to compare response peak latencies and amplitudes between genotypes and independent samples t-test was used to compare VsEP thresholds. ABR thresholds and DPOAE amplitudes were compared between wt (or C57) controls and mutant genotypes.
Results
Otoconia deficiencies correlate with imbalance behaviors
In all experiments, Oc90 null mice were compared with Oc90 wt mice, and Nox3het and Otop1tlt mice with C57 mice, to obtain P values. Heterozygous and wt Oc90 mice had normal otoconia and were phenotypically similar to C57 mice.
Oc90 null pups showed delayed development of the air-righting reflex (Fig. 1A and Table 1). Between P9 and P11, Oc90 wt and heterozygous pups developed a normal air-righting reflex: they could instantly right their body positions (<0.5 s at P9) and land in an upright position when held by their tails and suspended to make a backward somersault. During the same time window, however, 60% of Oc90 null littermate pups took longer (average 1.8 s, occasionally >10 s at P9) to right their body positions and often landed in a supine position rather than the normal prone position. Such deficits in the righting reflex were more severe in homozygous Nox3het and Otop1tlt mice, 91–100% of which were abnormal and required an average of 2 s for Nox3het and 3 s for Otop1tlt pups to right their body positions at P9. By P13-14, the Oc90 null pups showed righting responses similar to Oc90 wt mice, suggesting compensation from the maturing CNS, visual systems and proprioceptors. This also suggests little or no weakness in the muscular system. It took Otop1tlt and Nox3het mice at least 1 day longer to show normal righting reflexes. Although Oc90 null pups occasionally had abnormal reaching responses (one of six), all the adult null mice were normal (Table 1). When suspended by the tails, the mice extended the forelimbs like C57 and Oc90 wt mice and did not bob their heads when suspended. In contrast, 70% of Otop1tlt and Nox3het mice had abnormal reaching responses; the mice bobbed their heads and/or clasped their limbs.
Fig. 1.
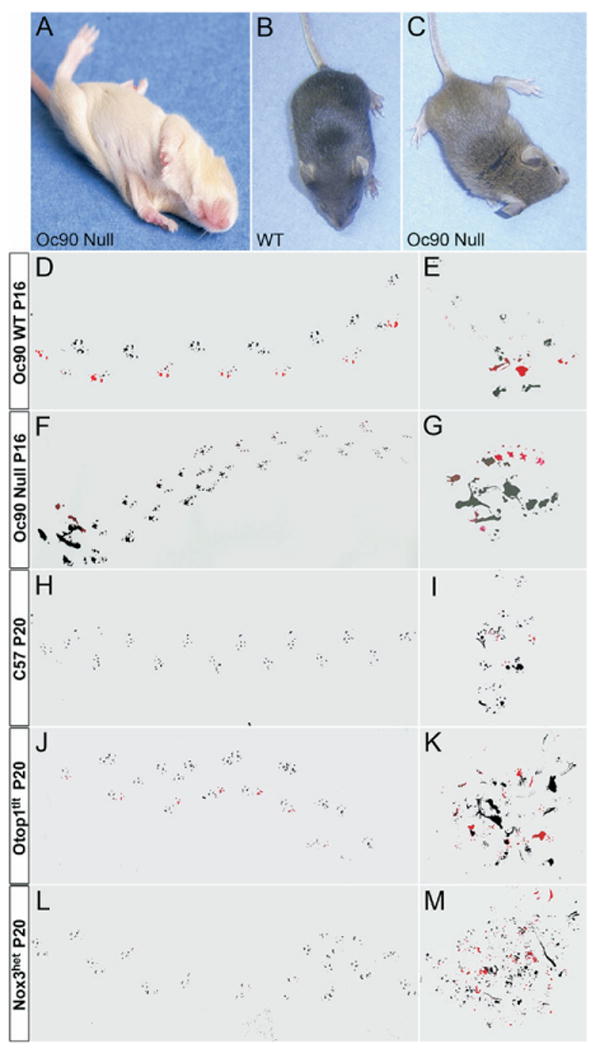
Imbalance behaviors of Oc90 null mice as compared with other otoconia mutants. Statistical comparisons are presented in Table 1 and Fig. 2. (A) Delayed development of the air-righting response of Oc90 null mice (P11 shown). At this age, wt pups right their body positions instantly, and it is not possible to photograph the landing process. (C) Oc90 null pups (P16) hold their heads and bodies in a tilted position. Compared with wt mice (D), Oc90 null mice (F) walk with ataxic gaits that have irregular shorter strides, wider stances and a non-linear curved pattern compared with wt littermate controls. Homozygous Otop1tlt (J) and Nox3het (L) mice have worse ataxic gaits than Oc90 null mice. (G) Oc90 null pups sometimes walk in a circular pattern, i.e. when they are put in a new environment and become disoriented. Otop1tlt (K) and Nox3het (M) mice show worse circular patterns under the same conditions.
Table 1.
Imbalance behaviors of Oc90 null mice in comparison with some otoconia mutants
| Abnormal/total | WT | Oc90 Null | Otop1tlt | Nox3het |
|---|---|---|---|---|
| Head-tilting (2–3 wk) | 0/64 | 37/161*** | 10/12*** | 5/8*** |
| Head-tilting (adult) | 0/12 | 1/15 | 7/10*** | 7/9*** |
| Air-righting (P9–10) | 0/10 | 10/17** | 9/9*** | 10/11*** |
| Reaching (2–3 wk) | 0/10 | 1/6 | 12/12*** | 8/8*** |
| Reaching (adult) | 0/12 | 0/15 | 7/10*** | 6/9** |
The number of mice showing imbalance behaviors is shown as the numerator and the total number of mice tested as the denominator. P values were obtained using chi-square analysis. Adult ages tested were 2–8 month homozygous mutants or wt. Age-matched C57 mice were all normal and are not listed in the table.
P<0.01.
P<0.001.
From P16, 23% of Oc90 null animals walked with ataxic gaits that had wider stances of the hind limbs and irregular (or shuffling) shorter strides compared with wt controls (Figs. 1D, F and 2B). The null mice also had a smaller left-right alternation (LRA) and a larger overlap (OL). The most severe gait patterns had even shorter strides than those shown. Starting from 3 months of age, one of six Oc90 null mice walked with a lowered body posture, but assessment of grip-strengths showed normal muscle tone, suggesting that the lowered posture and other observed balance deficits was not due to muscular weakness. The lowered body posture is probably the consequence of a wider gait. Nox3het and Otop1tlt mice had wider and worse shuffling strides than Oc90 null mice (Figs. 1H, J, L and 2C). Compared with age-matched C57 mice (n=18, P20), the gaits of Otop1tlt mice (n=6, P20) were significantly different in all measurements including wider stances and shorter strides of all limbs, a smaller LRA and a larger OL. The gaits of Nox3het mice (n=6, P20) had similar strides and LRA compared with those of C57 mice, but showed wider stances of all limbs and a larger OL.
Fig. 2.
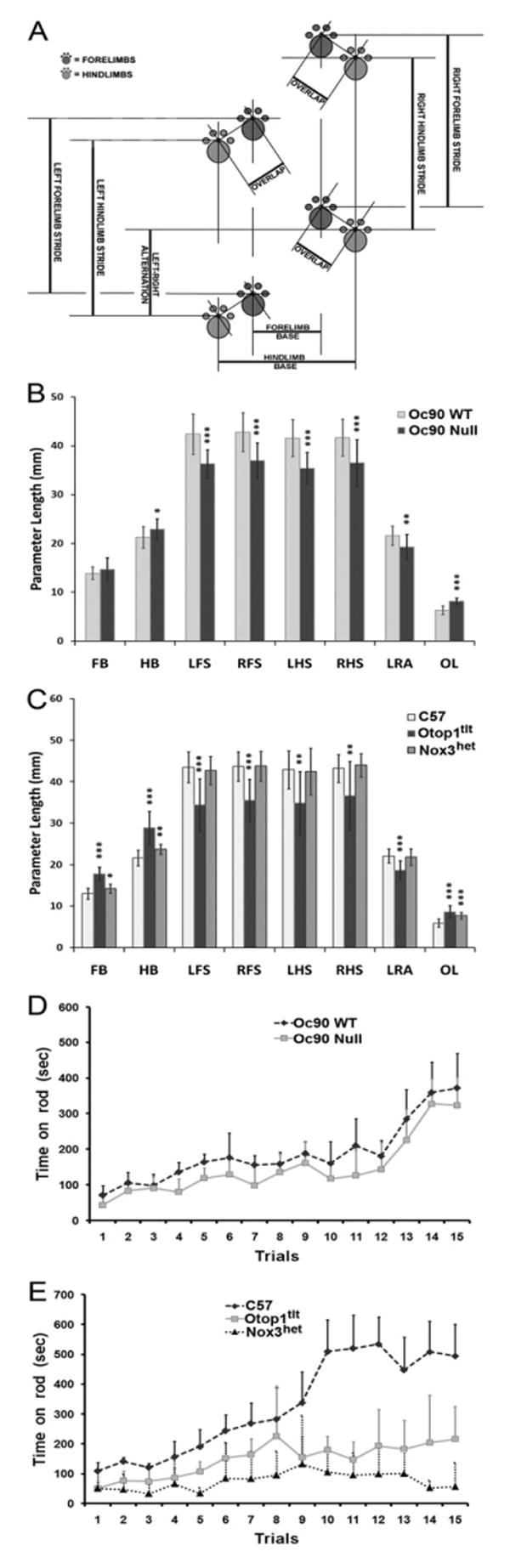
(A–C) Gait analysis of otoconia mutant mice. Compared with wt mice (n=18, P16), Oc90 null mice (n=16, P16) have wider stances of the hind limbs (hind limb base or HB), shorter strides of all limbs, a smaller LRA and a larger OL. Compared with age-matched C57 mice (n=18, P20), the gaits of homozygous Otop1tlt mice (n=6, P20) show significant differences in all measurements including wider stances and shorter strides of all limbs, a smaller LRA and a larger OL. In comparison, the gaits of homozygous Nox3het mice (n=6, P20) have similar strides and LRA compared with those of C57 mice, but show wider stances of all limbs and a larger OL. FB and HB, forelimb and hind limb base. LFS and RFS, left and right forelimb strides. LHS and RHS, left and right hind limb strides. * P<0.05; ** P<0.01; *** P<0.001. (D, E) Poor balance and coordination of Oc90 null mice on the accelerating rod as compared with other otoconia mutants (2–6 months old). The latencies on the rotorod are compared in Table 3. (D) Oc90 wt (n=7) and null (n=9) mice. (E) C57 (n=7), homozygous Otop1tlt (n=8) and Nox3het (n=8) mice. Oc90 null mice stayed on for a significantly shorter time than wt mice, Otop1tlt mice even shorter and Nox3het the shortest. All mice stayed longer with the progressing of each trial, with Oc90 wt and C57 improved the most (P<0.001). The rate and extent of learned adaptation for each strain of mice ranked the same order as their initial balance ability, with Nox3het mice showing only limited improvement (E).
When put in a new environment, some Oc90 null and most of Otop1tlt and Nox3het mice initially became disoriented, moved around slowly and walked around in a circular pattern (Fig. 1G). The circular pattern was worse (Fig. 1 K, M) and more frequently seen with Otop1tlt and Nox3het mice. Although control mice hesitated under the same situation, they did not walk around in circles (Fig. 1E, I). Such circular pattern displayed by the Oc90 nulls is different than the hyperactive, incessant circling behavior observed in mice with labyrinthine dysfunction affecting the semicircular canals (Wenngren and Anniko, 1989; Holme et al., 2002; Kiernan et al., 2002; Cryns et al., 2004) and/or central deficits (Shima and Hassler, 1982). In fact, the homozygous mutants in our study were usually less active. None of the otoconia mutants circled in their home cage. After the initial disorientation, the mutants started to walk forward but with a non-linear curved pattern (Fig. 1F, J, L), whereas controls walked forward in an almost linear pattern (Fig. 1D, H). Oc90 null mice crossed the balance beam on the abdomen and were clearly nervous about being on the beam, Otop1tlt mice sometimes fell off the beam and Nox3het frequently did so.
Twenty-three percent of Oc90 null pups held their heads in a tilted position (Fig. 1B, C) and 10% of the mice tilted their heads prominently. The head-tilting behavior coincided with the reduction of otoconia; the severely tilted mice all had complete absence of otoconia in the utricle in both ears. Only one saccule had absent otoconia out of nearly 100 examined. The less severe head-tilting features of Oc90 null mice were usually self-corrected by P21. This timeline coincides with the development of central compensation. The more severe head-tilting behavior in Oc90 nulls only improved slightly, resembling the Otop1tlt phenotype. Otop1tlt mice tilted their heads the most severely and frequently, with a frequency of 83% among 2–3 week-old pups and 70% among adults. Nox3het mice ranked next, with a frequency of ∼62% among pups and interestingly, 78% among adults. The small number of animals tested may have contributed to the higher percentage among adults artificially suggesting a more frequent occurrence of head tilting among adults. Alternatively, one might speculate that some neuronal degeneration occurred due to a loss of functional Nox3het protein in the vestibular ganglia. However, preliminary ultrastructural examination showed no reduction in neuronal numbers (Hoffman et al., 2006; Jones et al., 2007). With Otop1tlt and Nox3het mice, the head-tilting behavior always coincided with the individual's abnormal reaching response. As these mice have bilateral absence of otoconia, such head-tilting behavior probably does not arise from asymmetric sensory reduction. There may, however, be asymmetric sensory reduction in Oc90 nulls as the otoconial loss can vary between ears.
Most of Oc90 null mice (85%) swam normally (score 3) or almost normally (score 2), 5% completely failed the swimming test and required immediate rescue and 10% performed very poorly (Table 2). Occasionally the poor swimmers improved with time; however, the Oc90 nulls with severely tilted heads (which had absent otoconia) always failed and never improved. The normal or near normal swimmers had remnant otoconia present in the utricle and/or saccule. Nox3het mice showed the worst swimming ability (Table 2), nearly 90% of which required immediate rescue. Otop1tlt mice performed slightly better, with only 50% requiring immediate rescue, 25% performed poorly and another 25% swam almost normally. In the absence of neuromuscular abnormalities, inability to swim is usually a good indicator for severe deficits in the gravity receptor organ. However, normal swimming behavior does not necessarily indicate normal gravity receptor function due to the ability of an individual to adapt to peripheral deficits. A previous study showed that as long as otoconia-deficient mutant mice had some crystals, they could swim nearly normally (Jones et al., 2004). While the poor swimmers always had absent VsEPs, occasionally a few mice with absent VsEPs could swim almost normally.
Table 2.
Swimming behaviors of Oc90 null mice in comparison with some otoconia mutants (2–6 month-old homozygous mice)
| Strain | Poor | Intermediate | Normal | P< |
|---|---|---|---|---|
| Oc90 WT | 0/14 | 0/14 | 14/14 | |
| Oc90 Null | 3/52 | 5/52 | 44/52 | |
| Otop1tlt* | 4/8 | 2/8 | 2/8 | 0.001 |
| Nox3het* | 7/8 | 0/8 | 1/8 | 0.001 |
The number of mice showing abnormal swimming behaviors is shown as the numerator and the total number of mice tested as the denominator. Age-matched C57 mice all showed normal swimming behavior and are not listed.
Strain showed significant difference compared with controls using chi-square analysis (poor and intermediate swimmers were pooled as the abnormal group to obtain P values).
Rotorod tests showed the same outcome as the above observations. Oc90 null mice (n=9) stayed on the accelerating rod for a significantly shorter length of time than age-matched wt mice in all trials (Fig. 2D and Table 3) from days 1–4 (P<0.05). On day 5 the difference between null and wt mice did not reach the significant level. Otop1tlt mice (n=8) stayed on the rod for an even shorter time (Fig. 2E), with a P<0.001 when compared with age-matched C57 controls (n=7) on all days. Again, Nox3het mice (n=8) performed the worst (Fig. 2E) (P<0.001 vs. C57 on all days), with latencies significantly shorter than those for Otop1tlt mice as well (P<0.05–0.001 on all days). Nox3het mice frequently fell even on the stationary rod. The poor performers could not balance their body positions on the rod, which apparently caused them to fall. Some mice walked backwards on the rod. Notably, all the mice, except Nox3het, showed remarkable change with experience by staying on longer with each subsequent trial. Interestingly, the rate and extent of improvement for each strain ranked the same order as their initial balance functions, with C57 and Oc90 wt improving the most, Oc90 nulls next, followed by Otop1tlt mice and ending with Nox3het showing little improvement across trials. The nearly fourfold increase in latencies on the rotorod on day 5 as compared with day 1 (P<0.001 except for Nox3 that had a P=0.07) demonstrates a great degree of learned adaptation.
Table 3.
Rotorod tests of homozygous otoconia mutant mice (2–6 months old)
| Oc90 WT | Oc90 Null | C57 | Otop1tlt | Nox3het | |
|---|---|---|---|---|---|
| Day 1 | 91.7±31.6 | 72.5±35.0* | 124.3±22.6 | 67.7±36.4*** | 43.2±51.0*** |
| Day 5 | 338.6±187.2 | 292.1±164.3 | 496.9±157.1 | 234.1±157.1*** | 70.2±70.9*** |
| N | 7 | 9 | 7 | 8 | 8 |
Oc90 nulls were compared with Oc90 wt and Otop1tlt and Nox3het were compared with C57. All P values were generated using Student's t-test assuming equal variances. Detailed mean latencies for each trial are plotted in Fig. 2D and 2E.
P<0.05.
P<0.001.
To conclude, the Oc90 null mice have significant deficits in air-righting reflexes, head-tilting behavior and rotorod performances, with the former two behaviors manifesting mainly during the postnatal developmental stage and the latter behavior improving over repeated trials. The Oc90 behaviors contrast with the Otop1tlt and Nox3het mice that have severe imbalance in all categories examined and less improvement over repeated trials, demonstrating a concurrence of otoconia deficiencies and imbalance behaviors. VsEPs (below) confirm that gravity receptors in Oc90 nulls are functional but with reduced sensitivity due to the abnormal otoconia.
The mutant mice have deficits in afferent neuronal relay that originate from and correlate with otoconia deficiencies
VsEPs are compound action potentials generated by the vestibular nerve and its central relays in response to linear acceleration pulses applied to the cranium. They are critically dependent upon the utricle and saccule and provide a direct measure of their functional status (Jones and Jones, 2007; Jones, 2008). Representative VsEP waveforms for Oc90 null and wt genotypes are shown in Fig. 3. There were no differences in response parameters across the two ages tested (4 null and 3 wt mice at 4 months old, and 9 null and 8 wt mice at 10 months old) within each genotype; therefore all animals were pooled into their respective genotypes. Descriptive statistics for Oc90 VsEP response parameters are listed in Table 4. Evident from the figure and the table is that VsEP thresholds for Oc90 null mice were significantly higher than those for the age-matched wt (t= −2.836, df=1, 22, P=0.01). On average, peak-to-peak amplitudes for P1-N1 and P2-N2 were significantly smaller for the null mice (MANOVA, P=0.007 and P=0.014 respectively, Table 4). There were no significant differences between the null and wt groups for peak latencies, suggesting that synaptic timing and neural conduction times are similar for the two genotypes.
Fig. 3.
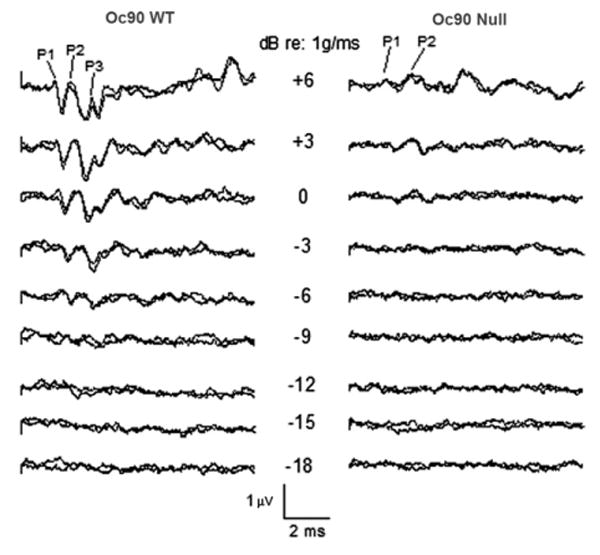
Representative VsEP intensity series for Oc90 wt (left) and null (right) mice (4 months old). Response peaks P1, P2 and P3 are labeled where present. Stimulus intensity is described in dB re: 1.0 g/ms. As stimulus amplitude decreases, response peak latencies increase and peak amplitudes decrease until no response is seen at levels below threshold. Clearly evident here are the reduced response peak amplitudes (P1, P2 and P3) for the null mouse and elevated threshold. Thresholds for these animals were scored at −12.5 dB for the wt and at −1.5 dB for the KO. Total time represented for each waveform is 10 ms.
Table 4.
VsEP thresholds, peak latencies and peak-to-peak amplitudes at +6 dB
| Group | n | Threshold (dB re: 1 g/ms) | P1 (ms) | P2 (ms) | P1/N1
(μV) |
P2/N2
(μV) |
|---|---|---|---|---|---|---|
| Oc90 Null | 13 | −5.65±2.88 | 1.33±0.10 | 2.18±0.19 | 0.53±0.23 | 0.70±0.21 |
| Oc90 WT | 11 | −8.60±2.00 | 1.35±0.07 | 2.13±0.13 | 0.82±0.24 | 0.95±0.26 |
Values are means±S.D. Two age groups (4 and 10 months old) showed no significant difference in all parameters and were thus pooled. The threshold was significantly increased and amplitudes for both P1-N1 and P2-N2 were significantly smaller for the Oc90 null mice.
The presence of VsEP responses suggests the presence of some otoconia in Oc90 null mice, which is consistent with the histological observation. The elevated thresholds and reduced amplitudes, however, indicate that the remnant otoconia are not sufficiently transferring the mechanical force to the entire sensory epithelium. Indeed, examination of functionally measured temporal bones confirmed that only remnants of otoconia were present in Oc90 null mice and those with the greatest otoconial loss demonstrated the smallest VsEP peak amplitudes and most elevated thresholds.
While we cannot rule out subtle hair cell, stereocilia or neural deficits, significant abnormalities in these structures would likely have led to prolonged latencies in conjunction with elevated VsEPs thresholds as was seen in ATP2b2dfw mice (S. M. Jones, unpublished observations) and demyelinating mutants (Jones et al., 2004, 2005). On average, no significant prolonged latencies were seen in Oc90 null mice (Table 4) although three animals showed somewhat prolonged latencies. In contrast, Nox3het and Otop1tlt homozygotes (n=3) had absent VsEPs, which confirms an earlier report (Jones et al., 2004).
To conclude, the elevated VsEP thresholds and reduced amplitude with normal latencies are consistent with a primary dysfunction in otoconia. The VsEP amplitude coincides with the otoconia deficiency (i.e. smaller amplitudes for more severe otoconial loss) and is absent when otoconia are absent.
Histological and ultrastructural examinations confirm that the imbalance behaviors arise from and correlate with otoconia deficiencies
SEM confirmed that Otop1tlt and Nox3het homozygotes lacked otoconia in the utricle and saccule (Fig. 4C, D), accounting for the absent VsEPs. These mice had normal vestibular cellular morphology and ultrastructure (Ornitz et al., 1998; Hoffman et al., 2006), including the epithelia and ganglia, as is true with Oc90 null mice which had giant otoconia (Fig. 4B, also Zhao et al., 2007). Phalloidin staining of whole-mounted vestibules showed the appearance of normal density and organization of hair cells and stereocilia in all mice (Fig. 5, 3 months old) despite the severe imbalance in some strains. SEM revealed normal bundle size and organization (insets in Fig. 5) in all mice tested.
Fig. 4.
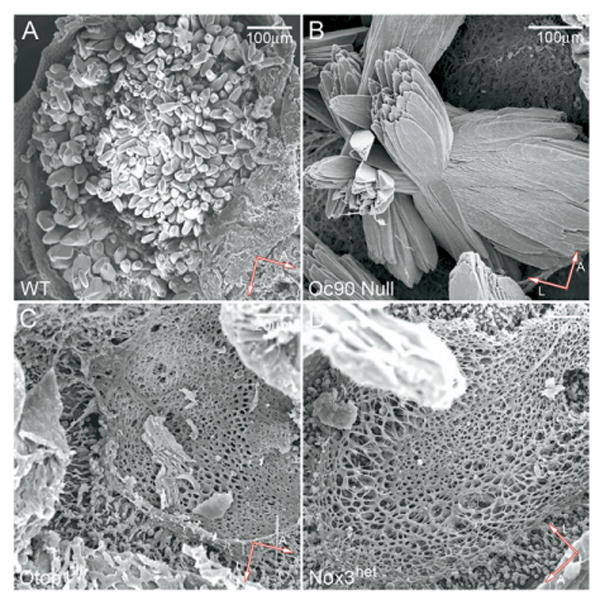
SEM confirmation of giant otoconia in Oc90 null mice (P20) (B) or absent otoconia in homozygous Otop1tlt (C) and Nox3het mice (D) (P20) used in behavioral and VsEPs tests.
Fig. 5.
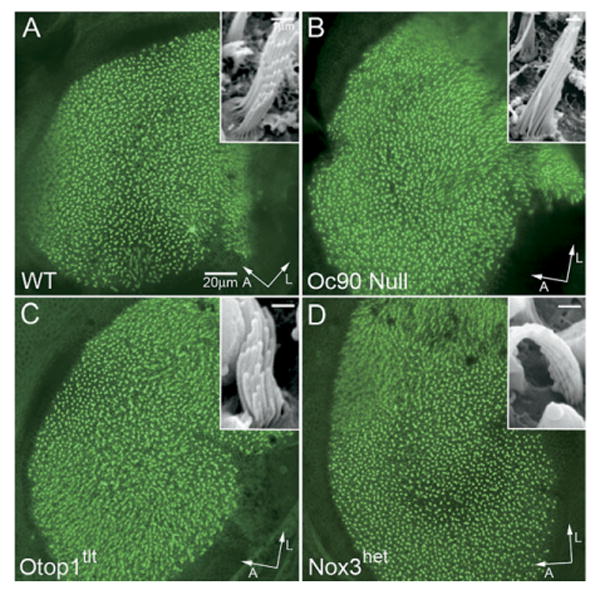
Normal density, organization and appearance of stereocilia in the adult vestibule (the utricle is shown) of homozygous otoconia mutants as demonstrated by phalloidin-stained whole-mount vestibules (3 months old). The Oc90 null vestibule has been previously shown (Zhao et al., 2007). Insets show normal hair bundle size and organization in the homozygous mutant mice as demonstrated by SEM. Scale bars= 20 μm (A—D); 1 μm (insets). A, anterior; L, lateral.
Similar to other otoconia mutant mice, Oc90 null mice have unaffected hearing
The thinner tectorial membrane in Oc90 null mice (Zhao et al., 2007) may lead to sub-optimal mechanotransduction of hair cells. A severe reduction in the tectorial membrane in α-tectorin null mice led to undetectable DPOAEs (Legan et al., 2000). We therefore measured both ABRs and DPOAEs in the Oc90 nulls. We also tested the Otop1tlt mice, whose DPOAEs were not previously measured. Otop1 may affect mechanotransduction as a regulator of Ca2+ fluxes (Hughes et al., 2007), events that are known to affect the function of the cochlear amplifier (Bobbin, 2002). ABRs assess the peripheral auditory sensitivity and DPOAEs assess the outer hair cell integrity. DPOAEs are mechanical distortions created in the inner ear when two primary tones (f1 and f2) are presented.
Despite the high level of Oc90 expression in the stria vascularis and Reissner's membrane of the developing and postnatal wt cochlea (Wang et al., 1998; Verpy et al., 1999), Oc90 null mice had normal hearing (Fig. 6A, B; 4 months old). As previously presented (Zhao et al., 2007), the mutant cochlea has normal cellular morphology including normal hair/supporting cells. Apparently, the reduction in the tectorial membrane does not alter hearing significantly at this age in mice. When compared with other findings (Legan et al., 2000; Russell et al., 2007), the data suggest that only severe abnormalities in the tectorial membrane result in detectable changes in otoacoustic emissions. ABR thresholds and DPOAE amplitudes were also normal for the Otop1tlt genotypes (2–3 months old) at all frequencies tested (data not shown) despite expression of the gene in the cochlea (Hurle et al., 2003). Our ABR data confirm what has been reported previously (Ornitz et al., 1998). Similarly, the Nox3het mice reportedly also have normal hearing (Paffenholz et al., 2004). The data suggest that, despite their Ca2+-binding capacities (Pote and Ross, 1991; Hughes et al., 2007), Oc90 and Otop1 do not play a significant role in maintaining endolymph Ca2+ or in influencing auditory mechanotransduction; or if they do, the function can be compensated by another unknown protein in the cochlea.
Fig. 6.
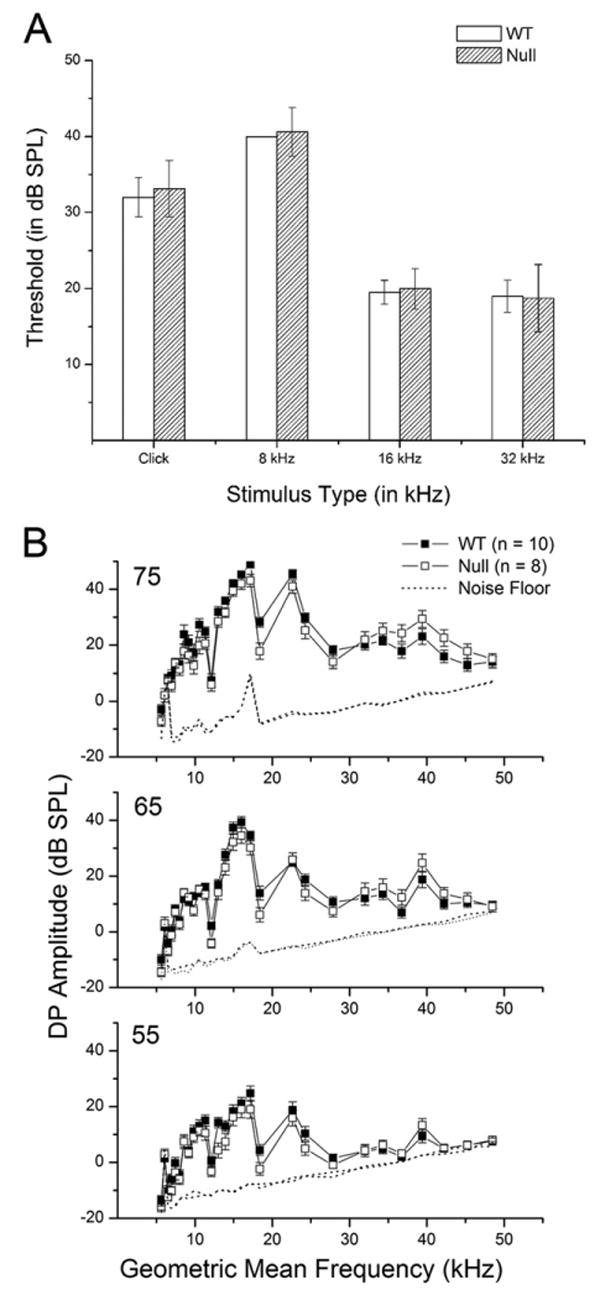
Normal hearing of Oc90 null mice (n=10 for wt, 8 for null, 4 months old). (A) Normal ABR thresholds of Oc90 null mice (only a selected set of frequencies is presented). (B) Normal DPOAE amplitudes of Oc90 null mice.
Discussion
This is the first study to utilize a wide spectrum of balance tests along with the direct measure of gravity receptor input in order to analyze imbalance that occurs as a consequence of variable changes in otoconia. We reveal that the otoconia mass has a direct impact on the amount of stimulus transduced and relayed to the ganglia, and that the presence of some otoconia, whether giant or normal in size, can relay some mechanical force albeit with reduced efficiency as determined by VsEP measurements. Notably, the residual sensory input in Oc90 nulls can lead to greater compensation, as demonstrated by the mild and less frequent occurrence of imbalance behaviors and greater improvement of performance over trials. This demonstrates that, despite the limitations of each behavioral test, they can jointly provide information on balance functions and can be useful to evaluate imbalance severity by examining the prevalence of the abnormal occurrence and improvement in performance over time. The present study provides such quantitative behavioral data for Nox3het and Oc90 mice and expands the behavioral dataset previously available for Otop1tlt mice.
Our finding on the effect of the otoconia mass on balance is not surprising. Absent or grossly abnormal otoconia/otolith has been known to cause imbalance behaviors in both mice and zebrafish (see introduction for references). However, either the severity of such behaviors has not been quantified or other types of deficits (i.e. deficits of sensory hair cells or ganglia) have not been ruled out. Our data show that the gravity receptor is quite sensitive to changes in otoconia, even though the CNS and peripheral nervous system (PNS) can compensate such changes to a certain degree. Thus, significantly altered otoconia under the conditions of diseases, ototoxic drugs and aging will affect balance, and therapeutic intervention may be necessary and helpful. Moreover, the finding that residual sensory input due to remnant otoconia can lead to better improvement in performance over time supports early therapeutic intervention in related balance disorders.
Behavioral tests assess functions of the PNS, the CNS and the neuromuscular system. While the mutant genes studied herein are inner ear-specific (Wang et al., 1998; Verpy et al., 1999; Hurle et al., 2003; Banfi et al., 2004), alterations in the CNS due to chronic loss of peripheral sensory input (i.e. sensory deprivation) are likely (Wiesel and Hubel, 1963; Anniko, 1990; Woolsey, 1990; Walton, 1998). Several factors may account for the consistent ranking of imbalance behaviors of various otoconia mutant strains by different methods. (1) The balance-related behaviors reflect the combined input from the utricle, saccule and the semi-circular canals. (2) The anatomic organization of the vestibular organs is such that the utricular macula and the lateral canal are not strictly in the horizontal plane, and the saccular macula and the superior and posterior canals are not strictly 90° perpendicular to the lateral canal, etc., therefore the accelerating force administered by each test may stimulate multiple end organs simultaneously. (3) The mechanics in these balance-behavioral experiments included both linear and angular forces. Neuronal recordings such as VsEPs are advantageous in this regard as one can selectively measure the gravity receptor organ by administering linear acceleration, in addition to the ability to control and alter the amount of stimulus. Moreover, the P1 response peak of VsEPs, which is generated by the peripheral afferent nerve, presumably would not be affected by the CNS and thus account for the smaller phenotypic variations compared with behavioral tests. P2 and P3 response peaks are generated by central relays and may be influenced by higher centers.
Evident from the data are that behavioral tests do not detect peripheral vestibular deficits in the same capacity and indeed, may not detect mild peripheral deficits at all. Behavioral tests, however, are useful to provide initial evidence of imbalance for further investigation of animal models and are important prognosis indicators for clinical vestibular rehabilitation. Our study shows that some tests may be more useful than others. For example, the swimming and rotorod tests produced the most consistent result from an animal across trials whereas the reaching reflex did the least. The swimming test may give the best indication of possible severe abnormalities in the gravity receptor organ since the task substantially reduces, if not eliminates, the animal's reliance on somatosensory and visual inputs and requires gravity perception to discern which way is up. Advantages of the rotorod test include the ability to quantify the balance function and to ascertain improvement in performance over time.
Behavioral (de Caprona et al., 2004; Besson et al., 2005) and optokinetic compensation (Andreescu et al., 2005) has been noted with the Otop1tlt and Otop1ied mutants. Several factors may have caused the Otop1tlt mice to perform better than the Nox3het mice in most of the tests. (1) The Otop1tlt mice occasionally have a few giant otoconia in the saccule (Ornitz et al., 1998). (2) Otop1 is primarily expressed at embryonic and postnatal stages in mice (Kim et al., 2008), but Nox3 is also expressed in the sensory epithelium at adult stages (Banfi et al., 2004). Therefore, Otop1 may be primarily involved in otoconia formation whose growth stops postnatally, whereas Nox3 may also carry out critical cellular functions similar to its other family members. Subsequently, the lack of Nox3 may have resulted in more extensive peripheral deficits that were difficult to overcome despite the fact that otoconia deficiencies were affected to the same degree. (3) Nox3 mice have reduced capacity in the CNS to utilize other sensory inputs (visual, somatosensory and proprioceptive) to improve balance performance over repeated trials. The current lack of detailed information on the two proteins in inner ear functions and the sequelae of the sensory deprivation in each strain make it difficult to fully appreciate the underlying cause for the difference in Nox3 and Otop1 behavioral performances.
Intriguingly, a number of vestibular mutant mice have normal hearing even when the mutant genes are expressed in hair cells (Otop1tlt and Nox3het) and ganglia (Nox3het) of the auditory system. This suggests substantial differences in the molecular machineries that participate directly or indirectly in the mechanosensory processes of the two systems. Although the possibility has not been ruled out that redundant proteins may have compensated for their functions in the cochlea but not in the vestibule, it is unlikely that this is the case for all these unrelated proteins. Additional evidence for possible differences between the cochlea and the vestibule comes from a recent study in which the otoferlin gene is deleted (Roux et al., 2006). The null mice are profoundly deaf but show normal balance behaviors despite the fact that the gene is expressed in both cochlear and vestibular hair cells.
Conclusion
In summary, this study describes the structure and function of the inner ear for mice lacking the Oc90 protein and elucidates the contribution of the otoconia mass to the amount of stimulus transduced to the sensory epithelium and ultimately relayed to the CNS. Additional insights regarding the use of behavioral tests in evaluating imbalance severity have also been provided. The study confirms and extends the functional and structural analyses of otoconia mutants, and demonstrates that animals can quickly adapt to peripheral deficits in a task-specific way and the rate and extent of such adaptation are influenced by the degree of the peripheral deficit as well as the balance function of the animal.
Acknowledgments
We thank B. Mock for assistance with data collection and Drs. Richard Hallworth and Heather Jensen-Smith from the Nebraska Center for Cell Biology at Creighton University for help with the confocal microscope. Part of the work was supported by grants from the National Institute on Deafness and Other Communication Disorders (DC008603 to Y.W.L., DC006443 to S.M.J. and DC007592 and DC003826 to E.N.Y.) and the National Center for Research Resources (1P20RR018788 to Y.W.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Abbreviations
- ABRs
auditory brainstem responses
- dB peSPL
decibel peak equivalent sound pressure level
- DPOAEs
distortion product otoacoustic emissions
- EDTA
ethylene diamine tetra acetate
- FFT
fast Fourier transform
- LFA
left-right alternation
- MANOVA
multivariate analysis of variance
- Nox3
NADPH oxidase 3
- Oc90
otoconin-90
- OL
overlap
- Otop1
otopetrin 1
- PBS
phosphate-buffered saline
- PNS
peripheral nervous system
- SEM
scanning electron microscopy
- VsEPs
vestibular evoked potentials
- wt
wild type
References
- Andreescu CE, De Ruiter MM, De Zeeuw CI, De Jeu MT. Otolith deprivation induces optokinetic compensation. J Neurophysiol. 2005;94:3487–3496. doi: 10.1152/jn.00147.2005. [DOI] [PubMed] [Google Scholar]
- Anniko M. Development of the vestibular system. In: Coleman JR, editor. Development of sensory systems in mammals. New York: John Wiley & Sons; 1990. pp. 341–400. [Google Scholar]
- Anniko M, Wenngren BI, Wroblewski R. Aberrant elemental composition of otoconia in the dancer mouse mutant with a semidominant gene causing a morphogenetic type of inner ear defect. Acta Otolaryngol. 1988;106:208–212. doi: 10.3109/00016488809106427. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Besson V, Nalesso V, Herpin A, Bizot JC, Messaddeq N, Romand R, Puech A, Blanquet V, Herault Y. Training and aging modulate the loss-of-balance phenotype observed in a new ENU-induced allele of Otopetrin1. Biol Cell. 2005;97:787–798. doi: 10.1042/BC20040525. [DOI] [PubMed] [Google Scholar]
- Bobbin RP. Caffeine and ryanodine demonstrate a role for the ryanodine receptor in the organ of Corti. Hear Res. 2002;174:172–182. doi: 10.1016/s0378-5955(02)00654-8. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Cryns K, van Alphen AM, van Spaendonck MP, Van de Heyning PH, Timmermans JP, De Zeeuw CI, Van CG. Circling behavior in the Ecl mouse is caused by lateral semicircular canal defects. J Comp Neurol. 2004;468:587–595. doi: 10.1002/cne.10975. [DOI] [PubMed] [Google Scholar]
- de Caprona, Beisel KW, Nichols DH, Fritzsch B. Partial behavioral compensation is revealed in balance tasked mutant mice lacking otoconia. Brain Res Bull. 2004;64:289–301. doi: 10.1016/j.brainresbull.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hoffman LF, Ross MD, Varelas J, Jones SM, Jones TA. Afferent synapses are present in utricular hair cells from otoconia-deficient mice. Hear Res. 2006;222:35–42. doi: 10.1016/j.heares.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Holme RH, Kiernan BW, Brown SD, Steel KP. Elongation of hair cell stereocilia is defective in the mouse mutant whirler. J Comp Neurol. 2002;450:94–102. doi: 10.1002/cne.10301. [DOI] [PubMed] [Google Scholar]
- Hughes I, Saito M, Schlesinger PH, Ornitz DM. Otopetrin 1 activation by purinergic nucleotides regulates intracellular calcium. Proc Natl Acad Sci U S A. 2007;104:12023–12028. doi: 10.1073/pnas.0705182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle B, Ignatova E, Massironi SM, Mashimo T, Rios X, Thalmann I, Thalmann R, Ornitz DM. Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in otopetrin 1. Hum Mol Genet. 2003;12:777–789. doi: 10.1093/hmg/ddg087. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear Res. 1999;138:91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM. Vestibular evoked potentials. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory evoked potentials: basic principles and clinical applications. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 622–650. [Google Scholar]
- Jones TA, Jones SM, Hoffman LF. Spontaneous discharge patterns of vestibular neurons in the otoconia-deficient mouse. Assoc Res Otolaryngol Abst. 2007;328 [Google Scholar]
- Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res. 1999;135:56–60. doi: 10.1016/s0378-5955(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Jones SM, Johnson KR, Yu H, Erway LC, Alagramam KN, Pollak N, Jones TA. A quantitative survey of gravity receptor function in mutant mouse strains. J Assoc Res Otolaryngol. 2005;6:297–310. doi: 10.1007/s10162-005-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Subramanian G, Avniel W, Guo Y, Burkard RF, Jones TA. Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J Neurosci Methods. 2002;118:23–31. doi: 10.1016/s0165-0270(02)00125-5. [DOI] [PubMed] [Google Scholar]
- Jones SM. Vestibular sensory evoked potentials. In: Jacobson G, Gans R, Shepard N, editors. Balance function assessment and management. San Diego, CA: Plural Publishing; 2008. pp. 379–404. [Google Scholar]
- Jones TA, Jones SM. Short latency compound action potentials from mammalian gravity receptor organs. Hear Res. 1999;136:75–85. doi: 10.1016/s0378-5955(99)00110-0. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Erven A, Voegeling S, Peters J, Nolan P, Hunter J, Bacon Y, Steel KP, Brown SD, Guenet JL. ENU mutagenesis reveals a highly mutable locus on mouse chromosome 4 that affects ear morphogenesis. Mamm Genome. 2002;13:142–148. doi: 10.1007/BF02684018. [DOI] [PubMed] [Google Scholar]
- Kim E, Lundberg YW, Salles F, Kachar B, Warchol ME, Ornitz DM. Generation and characterization of Otopetrin 1 knockout mice. Assoc Res Otolaryngol Abst. 2008;576 [Google Scholar]
- Klapdor K, Dulfer BG, Hammann A, Van der Staay FJ. A low-cost method to analyse footprint patterns. J Neurosci Methods. 1997;75:49–54. doi: 10.1016/s0165-0270(97)00042-3. [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Kossi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Bohne BA, Thalmann I, Harding GW, Thalmann R. Otoconial agenesis in tilted mutant mice. Hear Res. 1998;122:60–70. doi: 10.1016/s0378-5955(98)00080-x. [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pote KG, Ross MD. Each otoconia polymorph has a protein unique to that polymorph. Comp Biochem Physiol B. 1991;98:287–295. doi: 10.1016/0305-0491(91)90181-c. [DOI] [PubMed] [Google Scholar]
- Rampello L, Drago F. Nicergoline facilitates vestibular compensation in aged male rats with unilateral labyrinthectomy. Neurosci Lett. 1999;267:93–96. doi: 10.1016/s0304-3940(99)00328-6. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Legan PK, Lukashkina VA, Lukashkin AN, Goodyear RJ, Richardson GP. Sharpened cochlear tuning in a mouse with a genetically modified tectorial membrane. Nat Neurosci. 2007;10:215–223. doi: 10.1038/nn1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Nguon K, Sulkowski ZL, Rosen GD, Baxter MG. Purkinje cell loss accompanies motor impairment in rats developing at altered gravity. Neuroreport. 2005;16:2037–2040. doi: 10.1097/00001756-200512190-00014. [DOI] [PubMed] [Google Scholar]
- Shima F, Hassler R. Circling behavior produced by unilateral lesions of the central vestibular system. Appl Neurophysiol. 1982;45:255–260. doi: 10.1159/000101609. [DOI] [PubMed] [Google Scholar]
- Simmler MC, Cohen-Salmon M, El-Amraoui A, Guillaud L, Benichou JC, Petit C, Panthier JJ. Targeted disruption of otog results in deafness and severe imbalance. Nat Genet. 2000;24:139–143. doi: 10.1038/72793. [DOI] [PubMed] [Google Scholar]
- Sollner C, Burghammer M, Busch-Nentwich E, Berger J, Schwarz H, Riekel C, Nicolson T. Control of crystal size and lattice formation by starmaker in otolith biomineralization. Science. 2003;302:282–286. doi: 10.1126/science.1088443. [DOI] [PubMed] [Google Scholar]
- Sollner C, Schwarz H, Geisler R, Nicolson T. Mutated otopetrin 1 affects the genesis of otoliths and the localization of Starmaker in zebrafish. Dev Genes Evol. 2004;214:582–590. doi: 10.1007/s00427-004-0440-2. [DOI] [PubMed] [Google Scholar]
- Sondag HN, de Jong HA, Oosterveld WJ. Behaviour of adult hamsters subjected to hypergravity. J Vestib Res. 1999;9:13–18. [PubMed] [Google Scholar]
- Sondag HN, de Jong HA, van Marle J, Oosterveld WJ. Effects of sustained acceleration on the morphological properties of otoconia in hamsters. Acta Otolaryngol. 1995;115:227–230. doi: 10.3109/00016489509139297. [DOI] [PubMed] [Google Scholar]
- Sondag HN, de Jong HA, van Marle J, Oosterveld WJ. Behavioural changes in hamsters with otoconial malformations. Acta Otolaryngol. 1998;118:86–89. doi: 10.1080/00016489850155189. [DOI] [PubMed] [Google Scholar]
- Sondag HN, de Jong HA, van Marle J, Willekens B, Oosterveld WJ. Otoconial alterations after embryonic development in hypergravity. Brain Res Bull. 1996;40:353–356. doi: 10.1016/0361-9230(96)00127-x. [DOI] [PubMed] [Google Scholar]
- Trune DR, Lim DJ. The behavior and vestibular nuclear morphology of otoconia-deficient pallid mutant mice. J Neurogenet. 1983;1:53–69. doi: 10.3109/01677068309107072. [DOI] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Petit C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc Natl Acad Sci U S A. 1999;96:529–534. doi: 10.1073/pnas.96.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. Postnatal development under conditions of simulated weightlessness and space flight. Brain Res Brain Res Rev. 1998;28:25–34. doi: 10.1016/s0165-0173(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Walton KD, Benavides L, Singh N, Hatoum N. Long-term effects of microgravity on the swimming behaviour of young rats. J Physiol. 2005;565:609–626. doi: 10.1113/jphysiol.2004.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kowalski PE, Thalmann I, Ornitz DM, Mager DL, Thalmann R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc Natl Acad Sci U S A. 1998;95:15345–15350. doi: 10.1073/pnas.95.26.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenngren BI, Anniko M. Vestibular hair cell pathology in the dancer mouse mutant. Acta Otolaryngol. 1989;107:182–190. doi: 10.3109/00016488909127497. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman JR, editor. Development of sensory systems in mammals. New York: John Wiley & Sons; 1990. pp. 461–516. [Google Scholar]
- Ying HC, Hurle B, Wang Y, Bohne BA, Wuerffel MK, Ornitz DM. High-resolution mapping of tlt, a mouse mutant lacking otoconia. Mamm Genome. 1999;10:544–548. doi: 10.1007/s003359901041. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang H, Yamoah EN, Lundberg YW. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev Biol. 2007;304:508–524. doi: 10.1016/j.ydbio.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


