Abstract
G protein-coupled receptors (GPCRs) are involved in various physiological processes, such as behavior changes, mood alteration, and regulation of immune-system activity. Thus, GPCRs are popular targets in drug screening, and a well-designed assay can speed up the discovery of novel drug candidates. The Promega cAMP-Glo Assay is a homogenous bioluminescent assay to monitor changes in intracellular cyclic adenosine monophosphate (cAMP) concentrations in response to the effect of an agonist, antagonist, or test compound on GPCRs. Together with the Labcyte Echo 555 acoustic liquid handler and the Deerac Fluidics Equator HTS reagent dispenser, this setup can screen compounds in 96-, 384-, and 1536-well formats for their effects on GPCRs. Here, we describe our optimization of the cAMP-Glo assay in 1536-well format, validate the pharmacology, and assess the assay robustness for HTS. We have successfully demonstrated the use of the assay in primary screening applications of known agonist and antagonist compounds, and confirmed the primary hits via secondary screening. Implementing a high-throughput miniaturized GPCR assay as demonstrated here allows effective screening for potential drug candidates.
Keywords: GPCR, HTS, bioluminescence, instrumentation
G protein-coupled receptors (GPCRs) are a large protein family of transmembrane receptors involved in regulation of various physiological processes, including behavior, mood, and immune-system activity. Ligands binding to their cognate receptors result in altered conformation of the receptors and the dissociation of the heterotrimeric G proteins, modulating activity of the effector molecules. This chain of molecular interactions culminates in changes in intracellular signal transduction pathways and cellular responses.
GPCRs represent one of the largest receptor families in the human genome and are, therefore, very popular targets in drug discovery. Nearly half of all prescription drugs are targeted toward GPCRs.1 GPCRs are currently under intense investigation in nearly all sectors of the scientific community, including pharma, biotech, and academic research institutions. Still, many more GPCRs are orphans and remain to be “deorphanized.”
Investigating GPCRs commonly occurs via assay miniaturization using high-throughput screening (HTS) platforms. HTS is a format that utilizes automated experimentation protocols in drug discovery and other fields of biology and chemistry research. HTS involves the use of robotic instrumentation capable of dispensing small volumes of compounds and buffers into microplates or other vessels, most commonly 384- and 1536-well plate formats. Reactions occur in individual wells, and subsequent detection and data analysis can be collected from thousands of samples at a time. This enables researchers to conduct thousands of biochemical or pharmacological tests rapidly in parallel.
HTS platforms enable researchers to quickly identify active compounds, antibodies, or genes that modulate a particular biomolecular pathway or cellular response. The results of these experiments provide starting points for drug design and for understanding the interaction or role of a particular biochemical process in biology.
GPCR-focused HTS assays are needed to identify novel ligand receptor modulators (agonist or antagonist compounds) both as potential drugs as well as pharmacological tools for understanding cellular physiology. Successful compound screening campaigns require precise liquid handling, robust assays, reliable plates, and sensitive detection methods. With the increasing number of options available to the screener, it is becoming more of a challenge to choose which components and screening system are best suited for a particular application.
Here, we present a unique miniaturized GPCR assay HTS application using an integrated system comprised of a bioluminescent assay, 1536-well microplates, two liquid-handling robotic platforms, and a detection instrument. By taking an integrated approach, it is possible to demonstrate the complementarity of diverse technologies for GPCR-focused HTS applications, and show that miniaturization does not compromise quality of assay performance.
INTEGRATED SYSTEM COMPONENTS
Bioluminescent Assay
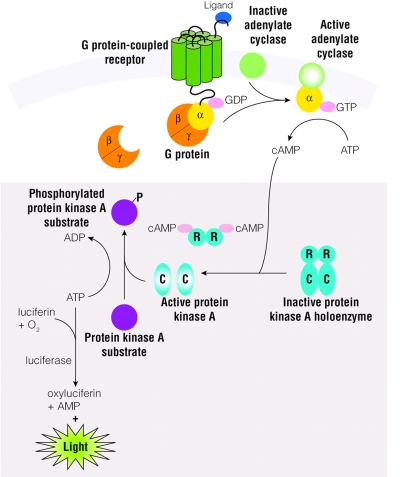
Assay principle (Figure 1A)
FIGURE 1.
A: Schematic diagram of cAMP production in cells and the cAMP-Glo Assay (shaded box). B: Schematic diagram showing the cAMP-Glo Assay protocol.
The cAMP-Glo Assay (Promega Corporation, Madison, WI) is a bioluminescent assay for monitoring changes in intracellular cyclic adenosine monophosphate (cAMP) concentrations. As the concentration of cellular cAMP increases, cAMP binds to the exogenously added cAMP-dependent protein kinase or protein kinase A (PKA). Since PKA is composed of two catalytic subunits and two regulatory subunits in its inactive heterotetrameric form, cAMP binds to the regulatory subunits and dissociates the holoenzyme, releasing the free and active catalytic subunits. The free catalytic subunits then catalyze the transfer of the terminal phosphate of ATP to a PKA substrate, and thus decrease ATP concentration in the process. The level of remaining ATP after the kinase reaction is determined using the luciferase-based Kinase-Glo Reagent (Promega Corporation). Relative luminescence output is inversely proportional to cAMP levels. Because modulation of certain GPCRs leads to alteration in intracellular cAMP concentration, the cAMP-Glo assay can be used to measure the level of intracellular cAMP and to assess the functional activity of GPCRs that are coupled to G proteins that activate adenylate cyclase (Gs) or inhibitors (Gi).
Assay protocol (Figure 1B)
The cAMP-Glo Assay protocol is quick and easy to perform due to its homogeneous nature and simple procedure. First, cells are induced with a test compound for an appropriate period of time to modulate cellular cAMP levels. After induction, cells are lysed to release cAMP, and then the cAMP Detection Solution, which contains protein kinase A (PKA) and kinase substrate (Leu-Arg-Arg-Ala-Ser-Leu-Gly), is added. The Kinase-Glo Reagent is next added to terminate the PKA reaction and detect the remaining ATP via a luciferase reaction. Plates are read using a microplate-reading luminometer. Luminescence can be correlated to the cAMP concentrations by using a cAMP standard curve. The assay is very sensitive (30 fmol ± 5 SEM cAMP/well) and reproducible (Z′ values > 0.8). Also, the assay can be used with adherent, suspension, or frozen cells to measure cAMP concentration in a single tube, multiwell plates, or in other HTS formats, and is less prone to compound interference than fluorescence-based assays. The half-life for the luminescent signal is >4 h, thus eliminating the need for luminometers with reagent injectors, and allows batch-mode processing of multiple plates.
Liquid-Handling Instrumentation
Reagent dispenser (Figure 2)
FIGURE 2.
A: Deerac Fluidics Equator HTS reagent dispenser. B: Schematic diagram of the dispensing tip function. c:Active Wash Station.
The Equator HTS (Deerac Fluidics, Ireland) is used for low- to mid-volume reagent dispensings. The instrument has an eight-tip pipetting system capable of handling volumes ranging from 50 nL to 50 μL. Each pipetting tip acts as a fast-actuating valve that is connected to a pressure or vacuum source to allow air and the sample liquid through the tip. The tip consists of a PEEK body in which is contained a magnetic boss. The boss when resting against the capillary creates a seal and can be used as a valve. This valve opens by raising the boss, which creates an opening to the capillary for the fluid to flow. The boss movement is controlled by the passage of current through an electronic coil surrounding the lower section of the tip (Figure 2B). Precise control of the dispense volume is further enabled by sensing the level of magnet actuation via a coil and real-time feedback. Reagents can be quickly and thoroughly washed from the system between dispensings via the Active Wash Station (Figure 2C). Active washing pumps clean wash solution to the individual tips in the wash station. The tips may also be washed between dispensings, if desired, using a Fast Wash feature, which sends a purge of wash through the tubing and tips to clean them from the inside.
Ultra-low-volume compound dispenser (Figure 3)
FIGURE 3.
A: Labcyte Echo 555 acoustic liquid handler. B: Schematic diagram of the acoustic drop ejection process. c: Time lapsed photographs of the 2.5-nL droplet ejection.
The Echo 555 (Labcyte, Sunnyvale, CA) is used for ultra-low-volume compound dispensing for primary screening applications, hit confirmation, and dose-response curve preparation. The instrument performs direct plate-to-plate transfers of aqueous fluids and DMSO via acoustic droplet ejection technology, a touchless transfer technology based on focused acoustic energy. The instrument monitors acoustic energy reflected from fluids inside each plate well to determine DMSO hydration and well fluid depth. The measured amplitude of the echo returned by the interface between the plastic of the well and the fluid it contains determines the level of DMSO hydration. Fluid depth is assessed by measuring the time of flight of the echo from the meniscus of the liquid. The system transfers compound droplets from one multiwell source plate in 2.5-nL increments directly into multiwell destination assay plates (Figures 3B, 3C). Thus, intermediate dilutions of active compounds are not needed.
RESULTS
Adaptation for HTS Format
Assay optimization
Initial optimization experiments were performed with the cAMP-Glo Assay to determine the optimal cell density and induction time to modulate cellular cAMP levels. To use the assay with Gαs-protein-coupled receptors, D293 cells expressing a dopamine (D1) receptor were created. In Costar #3937 1536-well plates (Corning, Inc., Corning, NY), a dose-dependent time-course study was performed in which D1 D293 cell number was varied (1000, 2000, or 3000 cells per well) against a titration of a known D1 agonist SKF38393, with induction time ranging from 10 to 30 min. IBMX was added to the induction media to minimize breakdown of cAMP by inhibiting endogenous cAMP phosphodiesterases. Luminescence was measured on a PHERAstar plate reader (BMG Labtech, Durham, NC). One-thousand D1 D293 cells per reaction and 30 min for induction were found to be the optimal parameters for the system based on EC50 values (data not shown), matching reported literature values at these conditions.2
Validation of pharmacology
The assay was further validated by confirming selectivity and potency of known agonist and antagonist compounds that modulate GPCRs, which alter adenylate cyclase activity (Gαs and Gαi). Different concentrations of agonists and antagonists of the D1 receptor were tested using the cAMP-Glo Assay to generate dose-response curves and subsequent EC50 and IC50 values for each compound. Compounds tested included known D1 agonists (dopamine, apomorphine, and SKF38393), activators (forskolin), and antagonists (SCH23390). All assays were performed in Costar #3937 1536-well plates using the optimal assay conditions described above. Luminescence was measured on a PHERAstar plate reader. EC50 and IC50 values compared favorably with published values for agonists and antagonists using competitive radiolabel binding assays3,4 (data not shown).
Robustness in HTS format (Figure 4)
FIGURE 4.
A: z’ factor for cAMP-Glo Assay in 1536-well format determined using 1000 D1 D293 cells per well, with half the cells treated with medium only, and the other half with 300 nM SKF38393 agonist. B: z’ factor for cAMP-Glo Assay in 1536-well format determined using 1000 D1 D293 cells per well, with half the cells treated with 300 nM SKF38393 agonist only, and the other half treated with 300 nM SKF38393 agonist and 10 μM SCH23390 antagonist. Both assays show z’ values >0.5.
Z′-factor analyses, statistical measures of reproducibility and robustness for both optimized agonist and antagonist assays, were performed in Costar #3937 1536-well plates. Based on the SKF38393 titrations results, an approximate EC80 of 300 nM SKF38393 was used for induction in the agonist analysis. One thousand D1 D293 cells per well were treated with 300 nM SKF or with induction buffer alone. For the antagonist analysis, 300 nM of the agonist SKF was used for induction, then cells were treated with 10 μM SCH23390 antagonist or induction buffer for control. Luminescence was measured on a PHERAstar plate reader. Z′-factor values for both assays were >0.5, indicating excellent assay quality.5 Additionally, signal-to-background (S/B) ratios for both assays were found to be higher than 10, indicating a respectable dynamic range between sample populations.
Screening for Agonists
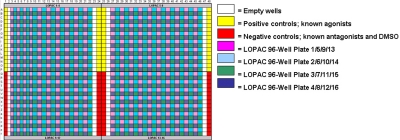
The LOPAC1280 library of pharmacologically active compounds (Sigma-Aldrich, St. Louis, MO) was used for agonist screening applications. Compound plates were stored at −20ºC until use, upon which they were thawed for 1 h before exposure to cells. Compounds were transferred using the Echo 555 acoustic dispenser into quadrants of Costar #3937 1536-well plates as shown in Figure 5. The library was diluted 10-fold in DMSO to achieve a 10 μM final concentration with 10 nL compound per well. Additional controls including known agonists, antagonists, and DMSO were added along the sides of the 1536-well plate for additional reference.
FIGURE 5.
LOPAC1280 library compound dispensing layout in a 1536-well assay plate. Compounds were dispensed using the Echo 555.
One thousand D1 D293 suspension cells (990 nL) per well were dispensed into the prepared 1536-well assay plates containing compounds using the Equator HTS. After the cell addition, plates were allowed to incubate for 30 min at room temperature. One microliter per well of cAMP-Glo Lysis Buffer was added to the treated cells and allowed to incubate for 15 min. After lysis, 2 μL per well of cAMP-Glo Detection Solution was added to the lysed cells and allowed to incubate for 20 min. Next, 4 μL per well of Kinase-Glo Reagent was added to the mixture and allowed to incubate for 10 min. The 1536-well assay plates were then read on a PHERAstar luminometer using a 0.25-sec integration time per well. All incubations and reagent additions were carried out at room temperature.
Agonist-screening results using the cAMP-Glo Assay with D1 D293 cells and LOPAC1280 library in 1536-well plates are shown in Figure 6. Compounds determined to be hits in the agonist screen were identified as those compounds whose relative light units (RLU) fell below 3 SD of the DMSO control mean (Figure 6, purple line, 11,699 RLU). Approximately 166 compounds fell below this threshold. SKF38393 and apomorphine (Figure 6, circled in orange), two known dopamine receptor agonists in the LOPAC1280 library, were fittingly found as hits in this assay, as well as the supplied known agonist controls (Figure 6, circled in black). The large majority of remaining hits were characterized in the dopamine compound class (77%), with others from classes including adrenoreceptor (12%), serotonin (3%), cyclic nucleotides (2%), and adenosine (1%).6 Seven of the 166 hits, including forskolin (Figure 6, light blue data points), were possible false positives (i.e., endogenously activating compounds) as determined by comparison to the same screening experiment repeated with D293 cells lacking the D1 receptor. The remaining two agonist-like hits (Figure 6, pink data points) may have been caused by toxicity, as assessed by performing a cell viability experiment using the CellTiter-Glo Assay (Promega Corporation) on D1 D293 cells after a 30-min treatment with the LOPAC1280 library compounds.
FIGURE 6.
cAMP-Glo Assay agonist screening results with 1000 D1 D293 cells per well and 10 μM LOPAC1280 library compounds in a 1536-well plate. Hits are identified as those compounds whose relative light units fall below 3 SD of the DMSO control (purple line). SKF38393 and apomorphine are known dopamine receptor agonists.
Screening for Antagonists
The LOPAC1280 library was also used for antagonist screening applications. Compounds were transferred and stored using the same methodology as described above for the agonist screening. One thousand D1 D293 suspension cells (500 nL) per well plus 300 nM SKF38393 agonist (490 nL) were dispensed into the prepared 1536-well assay plates containing compounds using the Equator HTS. After the cell addition, the plates were allowed to incubate for 30 min at room temperature. One microliter per well of cAMP-Glo Lysis Buffer was added to the treated cells and allowed to incubate for 15 min. After lysis, 2 μL per well of cAMP-Glo Detection Solution was added to the lysed cells and allowed to incubate for 20 min. Next, 4 μL per well of Kinase-Glo Reagent was added to the mixture and allowed to incubate for 10 min. Lastly, the 1536-well assay plates were read on a PHERAstar luminometer using a 0.25-sec integration time per well. All incubations and reagent additions occurred at room temperature.
Antagonist screening results using the cAMP-Glo Assay with D1 D293 cells and LOPAC1280 library in 1536-well plates are shown in Figure 7. Compounds determined to be hits in the antagonist screen were identified as those compounds whose RLUs fell above 3 SD of the agonist control mean (Figure 7, pink line, 4219RLU). Approximately 112 compounds fell above this threshold. As anticipated, SCH23390, butaclamol, and chlorpromazine (Figure 7, circled in green), three known dopamine receptor antagonists in the LOPAC1280 library, were found in this hit pool along with the supplied known antagonist controls (Figure 7, yellow data points). The overwhelming majority of the remaining hits were found to be characterized in the dopamine compound class (98%).6
FIGURE 7.
cAMP-Glo Assay antagonist screening results with 1000 D1 D293 cells per well and 10 μM LOPAC1280 library compounds in a 1536-well plate. Hits are identified as those compounds whose relative light units fall above 3 SD of the agonist control (pink line). SCH23390, butaclamol, and chlorpromazine are known dopamine receptor antagonists.
Confirmation with Secondary Screening
In addition to using the cAMP-Glo Assay for primary screening with the LOPAC1280 library, the assay was also used for follow-up secondary testing to evaluate the potency of each hit compound. Secondary screening was performed on hit compounds found from the agonist and antagonist primary screening results. Compounds selected include known D1 receptor agonists (dopamine, apomorphine, and SKF38393), and known D1 receptor antagonists (SCH23390, trifluoperazine, haloperidol, butaclamol, and chlorpromazine).
The Echo 555 cherry-picked and dispensed the select hit compounds into rows of Costar #3937 1536-well plates. Compounds were arrayed in replicates of four in each assay plate (Figure 8; each color represents a different compound). A 16-point dose range was tested, starting at 10 μM, titrating 1:2 with a no-compound control. D1 D293 cells, SKF38393 agonist (antagonist screen only), and cAMP-Glo reagents were added sequentially using the same methodology as described above for the primary screening assays.
FIGURE 8.
Secondary screening in a 1536-well assay plate layout for 12 hit compounds. Compounds were titrated using the Echo 555.
Secondary hit titration results using the cAMP-Glo Assay with D1 D293 cells and select hit compounds in 1536-well plates are shown for agonist and antagonist hits in Figure 9. All compounds tested were known to be agonists or antagonists for the D1 receptor. EC50 and IC50 values obtained were agreeable with reported literature values.4
FIGURE 9.
Secondary screening titration results with D1 D293 cells and hit compounds from the primary screens. a: Agonist dose-response curves. B: Antagonist dose-response curves. EC50 and IC50 values obtained are agreeable with reported literature values.
CONCLUSIONS
In this article, we have shown that bioluminescent GPCR-assay screenings can be performed on a single 1536-well plate, with only one endpoint readout needed to capture data. This HTS methodology enables researchers to study thousands and even millions of test compounds simultaneously, simplifying data analysis and sample tracking.
The homogeneosity and simplicity of the cAMP-Glo Assay protocol made it well-suited for use on automated platforms, particularly in 1536-well HTS format. Excellent Z′-factor results and appropriate AC50 values highlighted the assay performance in primary and secondary screening applications.
The two liquid-handling instruments used in this application, the Equator HTS and Echo 555, were particularly well suited due to their speed (Equator HTS: <1 min per 1536-well plate; Echo 555: <90 sec per 1536-well plate), precision, minimal compound sample required (Echo 555: 10 nL per assay well), and overall ease of use.
The combination of liquid-handling instrumentation and a robust cAMP-Glo Assay makes rapid primary and secondary screening in HTS formats a reality. Implementing this high-throughput miniaturized GPCR assay using the tools demonstrated in this application allows effective screening to identify lead modulators of GPCR signaling.
REFERENCES
- 1.Ellis C. The state of GPCR research in 2004. Nat Rev Drug Discovery. 2004;3:577–626. doi: 10.1038/nrd1458. [DOI] [PubMed] [Google Scholar]
- 2.Kumar M, Hsiao K, Vidugiriene, Goueli SA. A Bioluminescent-Based, HTS-Compatible Assay to Monitor G-Protein-Coupled Receptor Modulation of Cellular Cyclic AMP. Assay Drug Dev Technol. 2007;5(2):237–245. doi: 10.1089/adt.2006.055. [DOI] [PubMed] [Google Scholar]
- 3.Ryman-Rasmussen JP, Nichols DE, Mailman RB. Differential Activation of Adenylate Cyclase and Receptor Internalization by Novel Dopamine D1 Receptor Agonists. Mol Pharmacol. 2005;68:1039–1048. doi: 10.1124/mol.105.012153. [DOI] [PubMed] [Google Scholar]
- 4.Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- 5.Zhang JH, Chung TDY, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomolecular Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 6.LOPAC1280™. Compound List; Sigma-Aldrich, USA: [Google Scholar]