Abstract
We have identified a previously unannotated catechol-O-methyltranferase (COMT), here designated COMT2, through positional cloning of a chemically induced mutation responsible for a neurobehavioral phenotype. Mice homozygous for a missense mutation in Comt2 show vestibular impairment, profound sensorineuronal deafness, and progressive degeneration of the organ of Corti. Consistent with this phenotype, COMT2 is highly expressed in sensory hair cells of the inner ear. COMT2 enzymatic activity is significantly reduced by the missense mutation, suggesting that a defect in catecholamine catabolism underlies the auditory and vestibular phenotypes. Based on the studies in mice, we have screened DNA from human families and identified a nonsense mutation in the human ortholog of the murine Comt2 gene that causes nonsyndromic deafness. Defects in catecholamine modification by COMT have been previously implicated in the development of schizophrenia. Our studies identify a previously undescribed COMT gene and indicate an unexpected role for catecholamines in the function of auditory and vestibular sense organs.
Keywords: deafness, ENU, genetics, hearing, positional cloning
Catecholamines such as epinephrine, norepinephrine, and dopamine have important functions as hormones and neuromodulators. Levels of catecholamines are tightly controlled through multiple pathways, including their enzymatic modification. A growing body of work suggests that a major function of catechol-O-methyltransferase (COMT) is to regulate epinephrine, norepinephrine, and dopamine levels in the brain, particularly in the prefrontal cortex (PFC) (1). In the dopamine catabolic pathway, COMT is the rate-limiting enzyme catalyzing the magnesium-dependent transfer of methyl groups from S-adenosyl methionine to a hydroxyl group on dopamine, converting it to 3-methoxytyramine. COMT also functions in the parallel monoamine oxidase-limited pathway to convert dopacetic acid (DOPAC) to homovanillic acid (HVA). A single gene encoding a COMT has been described on human chromosome 22. Hemizygous deletion of the COMT locus, observed in 22q11 microdeletion syndromes (velocardiofacial syndrome [VCFS] or DiGeorge syndrome [DGS]), is strongly associated with schizophrenia in humans (2, 3), as are specific COMT haplotypes (4, 5), though the pathogenic mechanisms have not been fully elucidated. Targeted deletion or chemical inhibition of COMT in rodents reveals relatively minor changes in locomotor behavior and dopamine levels in the brain under normal conditions and when the dopamine transporter (DAT) is inhibited (6–8). Furthermore, there is evidence to show that some COMT activity is retained in tissues of Comt-null mice (7), suggesting that additional COMT enzymes are encoded in the mammalian genome.
Pharmacological and immunohistochemical evidence suggests that dopamine may regulate the processing of auditory signals within the mammalian cochlea. The cochlea contains two types of sensory hair cells with different functions and innervation patterns. Outer hair cells (OHCs) are critical for the amplification of sound signals and are minimally innervated by afferent neurons. Inner hair cells (IHCs) transmit sound information to the central nervous system (CNS) and receive the preponderance of afferent innervation. The medial olivocochlear complex (MOC), which originates in the medial nucleus of the superior olivary complex, modulates OHC activity via numerous efferent fibers that directly synapse on OHCs. The lateral olivocochlear complex (LOC) sends efferents into the cochlea that synapse with the dendrites of afferent neurons that innervate IHCs (9, 10). Though the full complement of neurotransmitters expressed by efferent neurons projecting to OHCs and IHCs is not known, a small number of the LOC-derived efferent fibers have been shown to be dopaminergic (11). Dopamine can also modulate the activity of afferent neurons that synapse on IHCs (9, 10). However, a role for dopamine or other catecholamines in the control of OHC function has not been demonstrated. Likewise, there is little information on the function of catecholamines in vestibular hair cells and their innervating neurons during the detection of head movement.
In an N-ethyl-N-nitrosourea (ENU) mutagenesis screen, we have now identified a mutation in a previously unannotated gene encoding a second COMT that we have named COMT2. In contrast to the previously described COMT gene that is widely expressed in many cell types and tissues, Comt2 is expressed in IHC and OHCs of the cochlea as well as in vestibular hair cells, without detectable expression elsewhere in the nervous system. Unexpectedly, mice with a point mutation in Comt2 show defects in cochlear and vestibular function. Based on the findings in mice, we have analyzed DNA from consanguineous families that suffer from autosomal recessive nonsyndromic deafness and identified homozygous mutations that segregate with the deafness phenotype. Our findings provide a direct link between COMT and the function of sensory hair cells, and indicate that defects in catecholamine signaling are not only linked to the development of schizophrenia but also to pathological changes that cause deafness and balance defects.
Results
Add, a neurobehavioral defect generated on a pure C57BL/6J background, was first observed in G3 mice descended from ENU-mutagenized G0 males and named in an allusion to its associated hyperkinesis. Add homozygotes exhibit circling, head tossing, and repetitive short-lasting arching of the neck (“stargazing”)—phenotypes that are observable as early as 3 weeks of age. Affected mice have normal fertility but are noticeably leaner than nonaffected littermates, probably as a result of excessive activity. They are also relatively aggressive, and when three or more homozygous males are confined in a single cage, they invariably attack one another, sustaining many wounds as a result. The phenotype is strictly recessive and fully penetrant on a mixed C3H/HeN × C57BL/6J background.
The add phenotype was mapped by backcross and intercross analysis with the C3H/HeN strain by scoring the behavioral phenotype of the mice (hyperkinesis, stargazing, circling). The mutation was initially assigned to distal chromosome 7 by low-resolution mapping (Fig. 1A). Suspecting a mutation in the Myo7a locus, mutated in shaker-1 mice (12) and in humans with Usher syndrome type Ib (13), we crossed homozygous Myo7ash1-11J mice to homozygous add mutants and observed full complementation. We also sequenced the Myo7a cDNA and found no mutation in add mice, which expressed the mRNA at normal levels (data not shown). We therefore mapped add to higher resolution, and on a total of 1,238 meioses, excluded Myo7a on genetic grounds, confining the mutation to a 1.1 Mb critical region ≈101 Mb from the centromere and bounded by two informative microsatellite markers, designated ADD_1_7 and ADD_1_15 (see Materials and Methods).
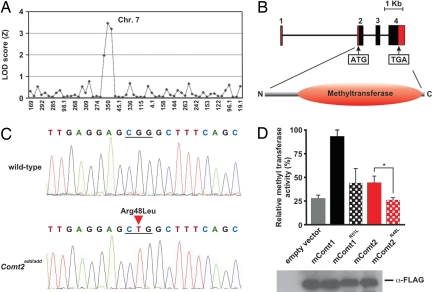
Fig. 1.
Add mice carry a mutation in COMT2 that affects catecholamine methyltransferase activity in vitro. (A) Coarse mapping of the add locus. The locus was mapped to distal chromosome 7 by 18 meioses with panels of 59 microsatellite markers. (B) The intron/exon structure of Comt2. The coding region is shown in black and the noncoding region in red. Sequence analysis using the SMART program (http://smart.embl-heidelberg.de/) identifies a methyltransferase domain between amino acids 55 and 247. (C) Identification of add mutation. A single nucleotide transversion (G→T) resulted in R48L of the polypeptide chain. (D) In vitro methyltransferase assay with cell lysates prepared from HEK 293 cells transfected with expression constructs for mouse COMT1, COMT2, COMT1R51L, and COMT2add (n = 4 separate transfections, mean ± SD). The methyltransferase activity of COMT2add is significantly diminished from that of COMT2 (P = 0.0346). At the bottom, a Western blot of COMT1, COMT2, COMT1R51L, and COMT2add expression in HEK 293 cells is shown.
The add critical region contains 30 annotated genes (Ensembl release v41), all of which were fully sequenced at genomic and/or cDNA levels without finding a mutation. However, in earlier Ensembl releases, a total of 33 genes were listed, and one of these genes (denoted “similar to catecholamine O-methyltransferase” and hereafter called Comt2) was withdrawn from annotation for lack of evidence, although parts of the gene were represented in an EST clone derived from the inner ear (GenBank accession no. BY752782). The expression of the gene was verified by RT-PCR, and its full length was established by 3′ and 5′ RACE (GenBank accession no. DQ854743); no alternative splice forms were detected. The intron/exon structure and protein domains that were predicted by the SMART program, including the catalytic COMT domain, are shown in Fig. 1B. In add mice, the gene encoding COMT2 contains a single base pair transversion (G → T) in the second of four exons, which predicts substitution of an arginine for a leucine residue at position 48 of the 258 aa polypeptide chain (Fig. 1 B and C). The putative protein is conserved in all vertebrate species, and 92.64% sequence identity exists between human and mouse homologues (supporting information (SI) Fig. S1). In humans and in mice, classical COMT (hereafter called COMT1) and COMT2 share ≈35% sequence identity. Two isoforms of COMT1, a shorter soluble form (S-COMT) and a longer membrane-bound form (MB-COMT), are encoded by alternatively spliced transcripts (14). COMT2 is homologous to the longer COMT1 isoform that predominates in the brain. The residue corresponding to the add mutation is invariant among vertebrate COMT2 sequences collected to date, and is also conserved in COMT1 (Fig. S1).
Because of its similarity to COMT1, we generated expression constructs encoding Comt1 and both wild-type and mutant forms of the Comt2 cDNA, and compared their methyltransferase activity upon expression in vitro. We measured methyltransferase activity in cell lysates using ELISA to detect normetanephrine, the product of norepinephrine methylation. All constructs were expressed in HEK293 cells and produced soluble cytoplasmic products detectable by an N-terminal FLAG tag (Fig. 1D). COMT2-specific activity was ≈50% lower than that of COMT1 when data were normalized for the quantity of expressed protein with reference to the FLAG tag (Fig. 1D). Mutant COMT2 exhibited no methyltransferase activity toward norepinephrine (Fig. 1D). We also introduced the add mutation into the homologous site in the Comt1 cDNA and tested methyltransferase activity of the encoded protein. The add mutation substantially diminished COMT1 methyltransferase activity toward norepinephrine (Fig. 1D). These data show that COMT2 is a bona fide catecholamine methyltransferase.
Formal behavioral and electrophysiological tests of 8-week-old add mice revealed several abnormalities. A defective auditory startle response was noted (Fig. 2A), suggesting that the mice might be hearing impaired. We therefore established auditory thresholds by measuring the auditory brainstem response (ABR). Click stimuli were applied to mice starting with 90 db and then decreasing the intensity. ABR thresholds in wild-type mice were at about 40 dB and above 90 dB in the mutants, demonstrating that add mice are profoundly deaf (Fig. 2 B and C). We next measured the distortion product otoacoustic emission (DPOAE) at stimulus frequencies between 6 and 28 kHz and at an intensity range between 0 and 70 dB. Representative response spectra are shown in Fig. 2D for stimulus levels of 40 and 70 dB. Acoustic signals for the primary stimulus frequencies (f1, f2), but not the cubic distortion frequency (2f1-f2), could be recorded from the ear canal of mutant animals. The DPOAE levels of wild-type mice increased with the stimulus intensity at a given frequency, whereas DPOAE levels in add mutants were within noise level (shown for 16 kHz in Fig. 2E). Similar observations were made at all other frequencies analyzed (Fig. 2F). We therefore conclude that OHC function is drastically impaired in the mutant mice. Add homozygotes exhibited bidirectional lateralized circling as determined by quantitative analysis of the movement behavior in the open field test (Fig. 2G), and performed poorly in the horizontal beam test (Fig. 2H), indicative of vestibular defects.
Fig. 2.
Add mice are deaf and exhibit vestibular dysfunction. All experiments were carried out with mice ≈8 weeks of age. All values are given as mean ± SD. (A) Add mice demonstrated no acoustic startle response to a 120 dB sound burst (n = 10 mice for each genotype). (B) The ABR thresholds to a click stimulus were elevated in add mice (n = 4 for control mice; n = 20 for add). (C) Representative ABR recordings in response to click stimuli. ABR waves I–V are indicated for recordings obtained from a wild-type mouse. (D) Representative DPOAE response spectra for a wild-type and add mouse at 40 dB (top traces) and 70 dB (bottom traces). Note that the 2f1-f2 product (arrow) was absent in recordings from add mice. (E) DPOAE measurements were performed at 16 kHz using stimulus levels between 20 and 70 dB. Add mice were severely affected at all intensity levels (n = 4 wild-type mice; n = 6 add mice). (F) DPOAE thresholds in 8-week-old add mice were elevated at all frequencies analyzed (n = 4 wild-type mice; n = 6 add mice). (G) Add mice show increased small-diameter rotations (n = 14 wild-type mice; n = 5 add mice). (H) Horizontal beam test. None of the add mice tested were able to walk across the horizontal beam to reach the platform on one end (n = 6 mice for each genotype).
We next analyzed the expression of COMT2 by in situ hybridization on sagittal sections of animals at P4 using antisense and sense control probes. COMT2 was strongly and specifically expressed in OHCs and IHCs in the cochlea and vestibule (Fig. 3 A–E). No expression was observed in any other tissue, including the CNS (Fig. 3 F and G), although the transcript could be detected in the CNS by performing reverse transcription and PCR (RT-PCR) on RNA isolated from whole brain lysates (data not shown). Similar observations were made in adult mice (data not shown). Detailed histological analysis of the cerebrum in homozygous add mice disclosed no abnormalities at the light microscopic level (data not shown). Instead, in semithin sections, we observed progressive degeneration of the organ of Corti. Though inner ear morphology appeared normal in homozygous add mice at P5, severe degenerative changes, including the loss of OHCs and IHCs, were noted by 8 weeks of age (Fig. 4 A–D). Further analysis of hair cell morphology by scanning electron microscopy (SEM) revealed randomly oriented and disorganized stereociliary bundles by P4 (Fig. 4 E–J), indicating that hair cell defects were manifested before the degenerative changes in the organ of Corti. The density of spiral ganglion neurons was also reduced by 8 weeks of age, but not by P4 (data not shown). Based on the expression pattern of COMT2 and the histopathological findings, deafness and balance defects in add mice are therefore likely a consequence of degenerative changes in the inner ear.
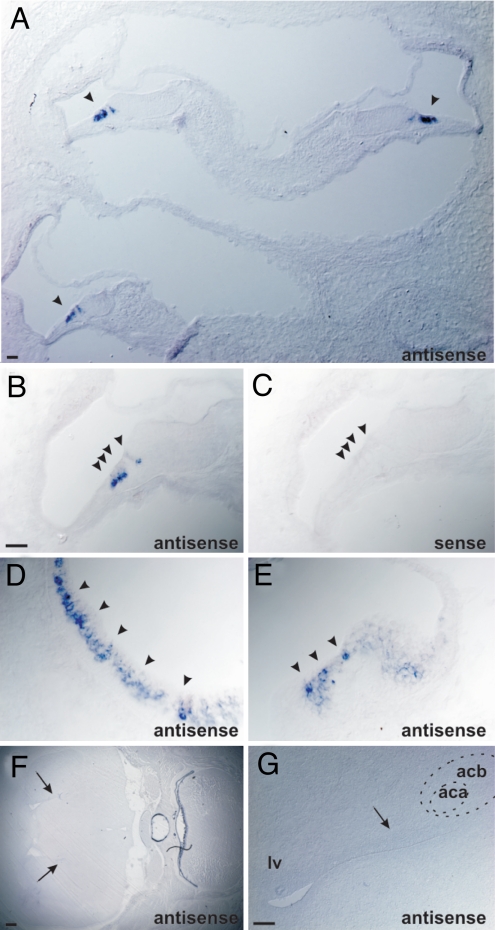
Fig. 3.
COMT2 is expressed in hair cells. Sagital section from P4 wild-type mice were analyzed for COMT2 expression by in situ hybridization. (A and B) COMT2 was expressed in the cochlea in IHCs and OHCs. (C) No signal was observed with the sense control probe. (D and E) COMT2 was also expressed in hair cells in the utricle (D) and cristae (E). (F and G) Coronal brain sections at P4. No expression was observed in the brain. Arrows point to ventricles. The anterior commissure (aca) and the accumbens nucleus (acb) are indicated. (Scale bars: A, 30 μm; B–E, 20 μm; F, 250 μm; G, 100 μm.)
Fig. 4.
Degeneration of the organ of Corti in add mice. (A–D) Semithin sections stained with toluidine blue. The organ of Corti appeared normal in add mice, and IHC and OHC could be detected at P5. By 8 weeks of age, the organ of Corti had degenerated. (E–J) SEM analyis revealed that stereociliar bundles of hair cells in the medial and basal part of the cochlea at P5 showed structural abnormalities (arrows). (Scale bars: A–D, 40 μm; E–J, 4 μm.)
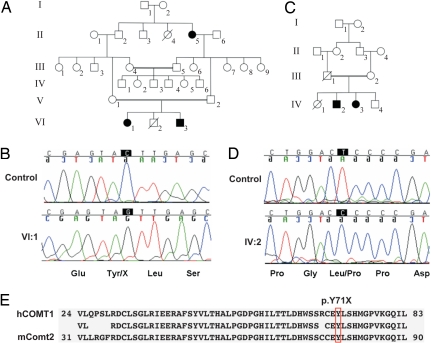
The human COMT2 gene is located at the DFNB63 locus on chromosome 11q13.4. To search for mutations in COMT2, we screened 192 unrelated congenitally deaf progeny of consanguineous Iranian parentage. Direct sequencing of the 5 exons of COMT2 identified a homozygous stop mutation, c.213C>G (p.Y71X), in exon 3 in family L-1013 (Fig. 5 A and B and Table S1) that is predicted to truncate the protein before the catalytic domain likely affecting methyltransferase activity. A homozygous missense mutation, c.47T>C (p.L16P), was identified in exon 2 in family L-714 (Fig. 5 C and D and Table S1), but the effect of the mutation on protein expression and methyltransferase activity is less clear. As indicated by the nomenclature DFNB assigned to the locus, these two human mutations are strictly recessive. Finally, two heterozygous nucleotide alterations (c.353G>A and c.503G>A) were identified in three other Iranian families (Table S1) that result in amino acid substitutions p.R118H and p.R168Q. None of these variants was identified in 192 (384 chromosomes) ethnically matched control individuals.
Fig. 5.
Mutations in the human COMT2 gene that cause deafness. (A and C) Pedigree of Iranian families L-1013 and L-714. (Open symbols, unaffected; filled black symbols, affected; double line, consanguineous event; diagonal line, deceased.) (B and D) Mutations in COMT2. (B) The c.213C>G (p.Y71X) stop mutation in homozygous state in affected individual VI:1 (family L-1013) compared with control. (D) The c.47T>C (p.L16P) missense mutation in affected individual IV:2 (family L-714) compared with control. (E) Alignment of amino acid sequences from human COMT1 and mouse COMT2. Identical residues are indicated between the two. Y71, mutated in family L-1013, is conserved in mouse and human COMT2.
Discussion
Previous studies have provided evidence that COMT1 regulates dopamine levels in the brain and that defects in COMT1 function lead to perturbations in neuronal circuits that predispose individuals to the development of schizophrenia (15). We have now identified a second gene encoding an enzyme with COMT activity that we have named COMT2. We demonstrate that COMT2 is essential for auditory and vestibular function in mice and humans. COMT2 is strongly expressed in sensory hair cells of the inner ear, suggesting that the deafness phenotype associated with mutations in COMT2 is a direct consequence of defects in the auditory sense organs and not of neuronal circuit dysfunction in the CNS. Consistent with this model, the organ of Corti degenerates in add mice, where degenerative changes in hair cells are observed before the degeneration of afferent neurons. COMT2 is also strongly expressed in vestibular hair cells, suggesting that the circling behavior in add mice is similarly caused by degeneration of the vestibular sensory epithelia. The molecular mechanism that causes the inner ear pathology in add mice still needs to be determined. Dopamine modulates the activity of OHC afferent neurons, and it has been proposed that this neuromodulatory role is necessary to protect the dendrites of IHC afferent neurons from degenerative changes triggered by overstimulation (9, 10). However, enhanced dopamine signaling in DAT-null mice (in which synaptic availability of dopamine is increased) does not cause deafness (16), and a function for catecholamines in regulating the function of OHCs or vestibular hair cells and their innervating neurons has not previously been demonstrated. The present data provide the strongest indication of such a function.
COMT1 inhibition or targeted deletion has surprisingly mild effects on extracellular and tissue dopamine levels. The mild phenotype of Comt1−/− mice has been variously attributed to compensatory changes in dopamine metabolism, neurotransmission, or signal transduction, or to changes in morphology or density of dopamine-producing or -responsive neurons that may be induced by the chronic absence of COMT during development (7, 8), although none of these possibilities have been substantiated. Though we have observed COMT2 expression only in sensory hair cells of the inner ear by in situ hybridization, we note that it is possible to recover the COMT2 mRNA from whole brain lysates, indicating that the gene is expressed in the CNS, perhaps in small groups of cells or at very low levels. Add mice appear to be more aggressive than their wild-type littermates, indicating potential defects outside the inner ear. It will be important to determine the precise function of COMT2 in the CNS, and equally important to address whether COMT1 and COMT2 may have redundant functions in the degradation of dopamine, and that one COMT gene may be ectopically upregulated and compensate when the other is lacking. DAT-deficient mice display increased persistent levels of synaptic dopamine and increased spontaneous locomotor activity compared with wild-type mice (16). It is possible that the lack of increased locomotor activity in COMT1-null compared with wild-type mice may be explained by the presence of a redundant COMT enzyme. In future studies it will be essential to examine the phenotypes of double-mutant COMT1- and COMT2-deficient mice. Finally, our data raise the possibility that the mixed findings either implicating or excluding various COMT1 SNPs as causal for schizophrenia may be related to the genotype of the COMT2 locus, such that a particular COMT1 genotype may be linked with disease only when associated with specific COMT2 haplotypes.
Materials and Methods
Mice.
C57BL/6J mice were used for N-ethyl-N-nitrosourea (ENU) mutagenesis to generate the add strain, as described (17). All studies were conducted in conformity with ILAR regulations. Euthanasia, when performed, was by CO2 narcosis. The add strain is described at http://mutagenetix.scripps.edu.
Mapping and DNA Sequencing.
Initial confinement of the mutation was made by outcrossing add homozygotes to C3H/HeN mice, and intercrossing the F1 hybrids or backcrossing them to the mutant stock. Linkage was measured with respect to a panel of 59 markers evenly distributed across the genome. In fine mapping studies, the essential microsatellites that defined the critical region were amplified using the primer pairs:
ADD_1_15: 5′-ttggacagtaagttctcctctgctc-3′; 5′-ctgggttcaaatccaaacaatacc-3′
ADD_1_7: 5′-agggaaagtgagcgagcataccttaatgg-3′; 5′-tttaaacggaggcggtaagatactggg-3′.
Coverage of candidate genes and the region subtended by the BAC clone used for phenotypic correction was accomplished using an ABI 3730 XL sequencer. Alignments were made using the programs Phred and Phrap. All target regions were covered to a Phred score >30, and all sequences were examined using the program Consed.
RT-PCR.
Tissue RNA was extracted using TRIzol Reagent (Invitrogen) and reverse transcribed by RETROscript™ First Strand Synthesis Kit (Ambion). The primers used to amplify Comt2 cDNA are 5′-tgctgagaggatttcgagactgcctgtc-3′ and 5′-tcctttaggtagggagcgtctggcagtg-3′.
Comt1 and Comt2 Constructs.
The mouse Comt1 and Comt2 cDNAs and the mutant Comt2add version of the sequence were cloned into the vector p3xFLAG-CMV-7.1 (HindIII to EcoRI). The expression construct for COMT1R51L was generated with the Phusion™ Site-Directed Mutagenesis Kit (New England Biolabs) using the wild-type Comt1 expression construct as a template.
In Vitro Catechol O-methyltransferase Activity Assay.
One microgram of COMT1, COMT1R51L, COMT2, or COMT2add expression construct was transfected into 293 cells. Cells were lysed 40 h later. Forty microliters of cell lysate was added to 160 μl of sample buffer (50 mM sodium phosphate buffer [pH 7.8], 2 mM MgCl2, 200 μM S-adenosyl-L-methionine and 1.5 mM norepinephrine) and incubated at 37°C for 2 h. Four hundred millimolars perchloric acid was added to the mixture to stop the reaction, which was kept on ice for 10 min before centrifugation and collection of the supernatant. Normetanephrine (NMN) in the supernatant measured by ELISA using the Normetanephrine Plasma EIA Kit (Immunobiological Laboratories, Inc.). The quantity of NMN produced was normalized to the protein expression level (determined by quantitative Western blot) and used to calculate catechol O-methyltransferase activity for each sample.
Analysis of Auditory and Vestibular Function.
The measurement of the auditory startle response, ABR, DPOAE, and circling behavior were carried out as described previously (18). For the horizontal beam test, mice were placed on the midpoint of a stationary 64 cm-long horizontal wooden beam wrapped in aluminum foil (2.5 cm width × 4 cm height) with platforms 26 cm in diameter at both ends (54 cm apart). The beam was positioned 47 cm above the floor so the total height of the beam was 51 cm (47 + 4 cm). Measures taken were the number of mice successfully walking the beam and stepping off onto either platform, the amount of time spent on the beam, and the number of mice falling off the beam. Each mouse was tested three times.
Histology and in Situ Hybridization.
The inner ear was embedded into soft plastic, and 5–10 μm sections were made. The sections were stained with toluidine blue.
SEM and in situ hybridization was carried out as described (18). As an in situ probe, a DNA fragment ranging from nucleotide 37 to 774, counting from the ATG start codon, was cloned into pBluescript (Stratagene) and in vitro transcribed as a sense control and antisense probe.
Family Report.
The screening cohort comprised 192 probands with autosomal recessive nonsyndromic hearing loss who were progeny of consanguineous parentage. In families L-1013 and L-714, all hearing-impaired persons had prelingual severe-to-profound hearing loss. Physical examination by an otolaryngologist and clinical geneticist excluded syndromic hearing loss. All participants in this study donated 10 ml of whole blood, which was used as a DNA source. Human Research Institutional Review Boards at the Welfare Science and Rehabilitation University and the Iran University of Medical Sciences (Tehran, Iran) and the University of Iowa (Iowa City, IA) approved all procedures.
Sequencing of the Human COMT2 Gene.
COMT2 gene (UCSC Genome Browser accession code uc001ors.1) was amplified using gene-specific primers (Table S2). Amplification reactions were cycled using a standard protocol on a GeneMate Genius thermocycler (ISC BioExpress). Sequencing was completed with BigDye™ v3.1 Terminator Cycle Sequencing Kit (Applied Biosystems), according to the manufacturer's instructions. Sequencing products were read using an ABI 3730s Sequencer (Perkin-Elmer). All sequencing chromatograms were compared with published cDNA sequence; nucleotide changes were detected using Sequencher v4.5 (Gene Code Corp.).
Supplementary Material
Acknowledgments.
Our sincere thanks to the families who participated in this study. This research was funded by the National Institute of Deafness and Other Communicative Disorders Grants R01-DC02843 (to R.J.H.S.), R01-DC007704, and R01-DC005965 (to U.M.), the Skaggs Institute for Chemical Biology (U.M.), the National Institutes of Health Grants AI070167 and GM067756, and BAA Contract (HHSN272200700038C) (to B.B.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. BY752782 and DQ854743) and the University of California, Santa Cruz Genome Browser, http://genome.ucsc.edu (accession code uc001ors.1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807219105/DCSupplemental.
References
- 1.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Karayiorgou M, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 4.Shifman S, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SG, et al. Association of Ala72Ser polymorphism with COMT enzyme activity and the risk of schizophrenia in Koreans. Hum Genet. 2005;116:319–328. doi: 10.1007/s00439-004-1239-y. [DOI] [PubMed] [Google Scholar]
- 6.Huotari M, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 7.Gogos JA, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huotari M, et al. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- 9.Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995;47:449–476. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 11.Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: Evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson F, et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 13.Weil D, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 14.Tenhunen J, et al. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 15.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 17.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 18.Schwander M, et al. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci. 2007;27:2163–2175. doi: 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.