Abstract
One of the two principal hypotheses put forward to explain the primary magnetoreception event underlying the magnetic compass sense of migratory birds is based on a magnetically sensitive chemical reaction. It has been proposed that a spin-correlated radical pair is produced photochemically in a cryptochrome and that the rates and yields of the subsequent chemical reactions depend on the orientation of the protein in the Earth's magnetic field. The suitability of cryptochrome for this purpose has been argued, in part, by analogy with DNA photolyase, although no effects of applied magnetic fields have yet been reported for any member of the cryptochrome/photolyase family. Here, we demonstrate a magnetic-field effect on the photochemical yield of a flavin–tryptophan radical pair in Escherichia coli photolyase. This result provides a proof of principle that photolyases, and most likely by extension also cryptochromes, have the fundamental properties needed to form the basis of a magnetic compass.
Keywords: avian compass, cryptochrome, radical pair states, transient absorption spectroscopy, flavoprotein
Proteins in the photolyase/cryptochrome family share a high degree of sequence homology, a conserved three-dimensional fold, and the same redox-active cofactor, flavin adenine dinucleotide (FAD) (1, 2). Members of this family have been identified in organisms ranging from bacteria to plants and humans (3–5). The function of DNA photolyase is blue light-induced repair of UV-damaged DNA containing either cyclobutane pyrimidine dimers or (6-4) photoproducts (6). In contrast, cryptochromes are involved in entrainment of the circadian clock, control of photomorphogenesis in plants, and various other processes initiated by blue or UV (UV-A) light (3). Light-induced redox reactions appear to be key processes in the biochemical activity of both photolyases and cryptochromes (7–9). Recent studies on several mutant proteins suggest the existence of a triad of tryptophan residues that is highly conserved among cryptochromes and all structurally characterized photolyases (10–13). In Escherichia coli photolyase, intraprotein electron transfer via tryptophans W382, W359, and W306 (10) is believed to reduce the photoexcited FAD cofactor from the semiquinone (FADH•) or fully oxidized (FADox) state to the catalytically active FADH− state. In this light-induced reaction, an electron is transferred from W382 to the excited flavin, thus generating a tryptophanyl radical, which has a nanosecond lifetime (14), and a flavin species that is one-electron-reduced with respect to the initial redox state. The positive charge on W382 migrates via W359 to W306 where deprotonation to the neutral radical W306• takes place. Spin-correlated flavin-tryptophanyl radical pair states are generated by photoexcitation only if the flavin is initially in its FADox state (photoinduced electron transfer to the semireduced FADH• state generates a tryptophan radical and the diamagnetic, fully reduced flavin). The terminal radical pair comprising the flavin radical and W306• is stable for at least a few microseconds and decays by backward electron transfer to regenerate the ground state flavin (FADox) (15).
An intriguing function has recently been suggested for cryptochromes in the context of the ability of migratory birds to use the geomagnetic field for orientation (16, 17). Two competing hypothetical models for the primary detection mechanism of this magnetic compass have been proposed: one involving magnetic iron-containing particles (18–20), the other based on magnetic-field-sensitive radical pair chemistry (17, 21, 22). Cryptochrome, which has recently been discovered in the retina of migratory birds, is a candidate magnetoreceptor molecule, potentially able to form transient radical pairs on blue light photoexcitation (23). This hypothesis is supported by the observations that magnetic compass orientation of migratory birds can be disrupted by weak radiofrequency magnetic fields with frequencies in the 1- to 10-MHz range (21, 24) and that magnetoreception depends on the wavelength of the ambient light (25).
Spin-correlated radical pairs are typically the result of photo-induced electron transfer reactions starting from a molecular precursor in either an excited electronic singlet (S) or triplet (T) state. Under the influence of intramolecular electron nuclear hyperfine interactions or differences in the two electron Zeeman interactions of the two radicals, the radical pair undergoes coherent interconversion between its S and T states. Externally applied static and/or oscillating magnetic fields can affect both the extent and the frequency of S–T interconversion and hence alter the yields of the respective reaction products formed from S and T radical pairs (26–28). However, a significant effect is only expected if the lifetime of the radical pair is sufficiently long: assuming Earth-strength magnetic fields (≈50 μT), the radical pair lifetime would need to be of the order of a microsecond or longer (29).
Despite a preliminary characterization of cryptochrome from migratory birds (30), there have so far been no reports of in vitro studies of magnetic-field effects (MFEs) in cryptochromes. Here, because of its close similarity to cryptochrome and apparently greater stability in solution, we use catalytically competent DNA photolyase from E. coli as a paradigm system to test the hypothesis that an external magnetic field can alter the yields and kinetics of radicals formed in proteins of the photolyase/cryptochrome family. Optical transient absorption spectroscopy with submicrosecond time resolution is used to record light-induced decay signals with and without an applied magnetic field. Although a number of flash photolysis studies of E. coli DNA photolyase are reported in the literature (31–34), this article examines a photolyase in which the FAD cofactor is initially in the fully oxidized state.
Results
Transient Absorption Spectroscopy.
E. coli DNA photolyase contains two noncovalently bound cofactors, FAD and 5,10-methenyltetrahydrofolate (MTHF), which are involved in redox chemistry and light harvesting, respectively (2, 5). A non-MTHF-binding mutant of E. coli DNA photolyase (E109A) (EcPL) (35) was used in the present studies to avoid sample inhomogeneity arising from the low binding constant of MTHF to the wild-type enzyme, and to prevent MTHF photodecomposition as a result of prolonged blue light illumination (36). Recombinantly expressed, catalytically competent EcPL is typically isolated with its FAD cofactor in the blue-semiquinone radical state, FADH•. Here, we have oxidized the samples to the yellow FADox state before blue light illumination by using potassium ferricyanide as an external electron acceptor to generate the redox state of the FAD that is also found for cryptochromes in the dark state (37).
Blue light irradiation of EcPL converts FADox to FADH•; but in the presence of ferricyanide as electron acceptor, further photoreduction to the fully reduced, catalytically active form (FADH−) is inhibited. Acting as an electron donor, a tryptophan residue forms a transient, spin-correlated radical pair with the flavin. Two pathways for back-reaction are expected. First, the two radicals can recombine to regenerate the ground state of the protein, provided the pair is in its singlet state. Second, competing reactions with external electron donors/acceptors are feasible: the presence of ferricyanide ensures fast and complete reoxidation of the flavin (15) with concomitant reduction to ferrocyanide. However, the tryptophan radical can be reduced by an as yet unknown electron donor (most likely one of the buffer components, glycerol or Hepes) (see Fig. 2 and later for more details).
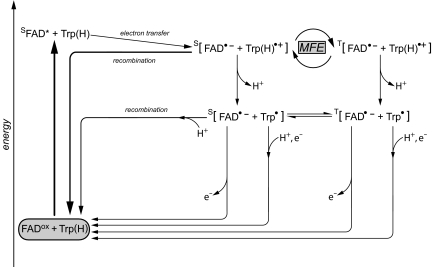
Fig. 2.
Formation of radical pairs in EcPL with the flavin initially in the fully oxidized redox state. FADox, chromophore in darkness (fully oxidized state); SFAD*, singlet excited state after absorption of blue light, FAD•−, flavosemiquinone anion radical; Trp(H)•+, tryptophanyl cation radical (W306); Trp•, neutral tryptophanyl radical (W306). Flavosemiquinone radicals and tryptophanyl radicals form spin-correlated radical pairs that exist either in the singlet (S) or in the triplet (T) state. Energies are not to scale. For further details, see Results.
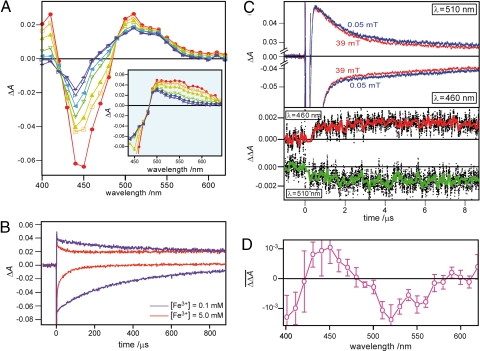
The recorded time-resolved (5–170 μs) transient absorption spectra (Fig. 1A) show a bleaching of the absorption bands of oxidized FAD in the 420- to 480-nm range and the formation of a broad absorption in the region above 500 nm. A second dataset obtained with an earlier detection time window (0.5–5 μs) is depicted in the Fig. 1A Inset. Their formation obscured by a strong fluorescence signal (500 ns), an absorption band at 400 nm and a broad and unresolved absorption ranging from 500 to 600 nm are observed concomitant with ground-state bleaching of FADox between 400 and 500 nm. This spectral pattern is in agreement with published spectra of the initially generated tryptophanyl radical cation and a flavin radical (33). No significant signals are observed from the excited triplet state of FAD, for which a broad absorption peaking ≈650 nm is typically expected (38). Within the first 3 μs (reciprocal decay rate constant, τ = 1.24 μs from biexponential fitting of the decay curves obtained without an applied magnetic field), this signal changes to a different spectral pattern with maxima at 500 and 540 nm. Changes within the next 150 μs include both the decay of the absorbance at 400 and 420 nm and the recovery of the ground state absorption. Two long-lived spectral components are observed, one with a maximum at 510 nm, the other with a minimum between 440 and 460 nm.
Fig. 1.
Transient absorption spectroscopy of EcPL. (A) Light-induced electron transfer recorded on a microsecond time scale. Optical absorption spectra of the kinetic components in the wavelength region between 400 and 650 nm were recorded at the following times after pulsed-laser excitation: red, 5 μs; yellow, 10 μs; olive, 20 μs; green, 50 μs; light blue, 80 μs; blue, 130 μs and purple, 170 μs. (Inset) Optical absorption spectra of the kinetic components in the wavelength range 440–650 nm recorded at early times after the laser pulse (red, 0.5 μs; yellow, 0.7 μs; olive, 1 μs; green, 2 μs; blue, 3 μs and purple, 5 μs). (B) Absorbance decay curves at 460 nm (negative absorption) and 510 nm (positive absorption) recorded with samples containing 0.1 mM (purple) and 5.0 mM (red) potassium ferricyanide, respectively. (C) Absorption decay curves at 460 nm (negative absorption) and 510 nm (positive absorption) measured both with and without applied magnetic field (red curves, 39 mT; blue curves, Earth's magnetic field; ≈50 μT). Differences between red and blue curves are shown as red (460 nm) and green (510 nm) curves, respectively (dotted lines represent unsmoothed raw data). For calculation of the MFE, 25 transients (125 laser shots) at 510 nm and 36 transients (180 laser points) at 460 nm were averaged, respectively. The resulting MFEs (an average of 4,000 points between 1 and 9 μs) are −4.0% and +3.1% for 510 and 490 nm with calculated standard deviations of 1.8% and 1.7%, respectively. (D) MFE action spectrum observed in EcPL. The spectrum is calculated as the difference in the transient absorption signals, averaged over 0–60 μs after laser excitation, measured with (39 mT) and without (50 μT) an applied magnetic field. The error bars represent the standard deviations of the signals (0–60 μs) at each wavelength after smoothing with a 10-μs boxcar function.
Consideration of these absorbance changes in EcPL leads to an extended scheme of chemical reactions (Fig. 2) that is in general agreement with previous findings by Aubert et al. (33), although these authors have examined the photoreduction of the flavin starting out from the flavin in its radical form FADH•. A similar scheme is also used by Solov'yov et al. (22) for the discussion of calculated MFE in Arabidopsis thaliana.
FADox is excited by blue light to its excited state (FAD*). Subsequently, an electron is transferred to the flavin from a nearby amino acid thus forming a radical pair within a few picoseconds, which is far beyond the time resolution of our equipment. By individual replacement of each one of the 15 tryptophan residues in EcPL with phenylalanine, W306, which is situated ≈19 Å away from the flavin, was identified as the terminal electron donor (39). Two hypothetical pathways for the electron transfer between W306 and FADH• have been proposed: one is via the two other tryptophans of the triad, W382 and W359, which are located between W306 and FADH• (10, 14). The other putative pathway involves the α-helix (α-15) between residues D358 and the phenyl ring of F366 (34). Regardless of the pathway between the flavin chromophore and W306, we can conclude that the primary species detected here is a radical pair comprising a flavosemiquinone anion radical and the W306 cation radical: [FAD•− Trp(H)•+] based on the observation that two broad maxima located at ≈500 nm (40) (for a flavin anionic radical) and almost 600 nm (for a tryptophanyl cation radical) are expected for such a radical pair species (33). These spectral features are indeed observed at early times after the pulsed-laser excitation (see Fig. 1A Inset). It should be noted, however, that the electron spin configuration of this radical pair, singlet or triplet, cannot be assigned from the transient absorption measurements shown in Fig. 1A. The (nanosecond) time resolution of our spectrometer is much longer than the lifetime of the excited flavin singlet state, which is in the picosecond range for comparable flavoproteins (41). Whatever the spin state of the excited FAD, spin angular momentum is conserved during fast electron transfer, and the radical pair is formed with the same multiplicity as its precursor.
The surface-exposed Trp(H)•+ in EcPL has been shown to release a proton (time constant, 0.3 μs) to generate the neutral Trp• radical (33). Whereas Trp(H)•+ has an absorption maximum at 570 nm, the neutral Trp• radical absorbs with a maximum at ≈500 nm. This shift is clearly observed in the spectra shown in Fig. 1A Inset. However, no (or only a minor amount of) proton transfer to FAD•− is detected on a microsecond time scale, a process that would yield the neutral flavin radical, FADH•, which typically shows a broad absorption above 550 nm (42). This result is unexpected because FADH• appears to be stabilized in E. coli photolyases (5). The secondary radical pair, [FAD•− Trp•], is stable for several hundred microseconds, and its decay to the ground state depends mainly on the presence of an external electron acceptor (the radical pair lifetime increases dramatically if this species is absent or present at a lower concentration; see Fig. 1B). Without an external electron donor, the stability of [FAD•− Trp•] depends mainly the recombination of the radical pair. Nevertheless, a minor fraction of the tryptophan radicals is believed to be reduced by transfer of an electron from an as yet unknown external electron donor, resulting in the ground-state of the tryptophan residue and FADH• (31).
In Fig. 1B, the two long-lived spectral components (the minimum at 460 nm and the maximum at 510 nm) are shown as transients on ≈100-μs time scale (red curves). After the initial decay of the signal components within 2 μs, signals that decay much more slowly are observed at both wavelengths. The decay constants are fairly similar (τ = 50 μs at 460 nm and τ = 40 μs at 510 nm) at a ferricyanide concentration of 5 mM. When the ferricyanide concentration was reduced 50-fold, the decay time constants increased to 330 μs and 340 μs, respectively (magenta curves in Fig. 1B), indicating that the slowly decaying components can be attributed to the reoxidation of the flavin anion radical by ferricyanide. A reoxidation lifetime of 17 ms was determined by Aubert et al. (33) in the absence of an external electron acceptor. Although that value was obtained starting from the neutral flavin radical FADH•, it supports our conclusion that the radical pair lifetime is determined mainly by the absence or presence of an external electron donor.
MFE and Potential Roles for Biological Magnetoreception.
Chemical magnetosensitivity via the radical pair mechanism requires in principle the following sequence of events: (i) generation of a radical pair species with correlated electron spins, either in an S or a T state; (ii) coherent evolution of the radical pair between the near-degenerate S and T spin states; and (iii) different reaction pathways of the S and T radical pairs. The frequency of S–T interconversion in the radical pair and hence the relative yields of reaction products and/or the lifetime of the radical pair depend on the strength of any applied magnetic field. MFEs in radical pairs are thus kinetic rather than thermodynamic in origin and may be detected for magnetic fields whose electron Zeeman energies are much smaller than the average thermal energy per molecule, kBT. The radical pair lifetime needs to be in the microsecond range (29) if a magnetic field of 50 μT is expected to have a significant effect.
To measure the influence of an external magnetic field on the absorption decay kinetics, transients at 460 and 510 nm were recorded with and without an applied 39-mT magnetic field. These time profiles are shown as red and blue curves in Fig. 1C Upper, respectively. Small but reproducible changes in the decay curves, which are clearly outside the experimental errors, are observed: the resulting MFEs (4,000 data points averaged over the interval 1–9 μs) are −4.0% and +3.1% for 510 and 460 nm, respectively. The decay time profiles observed at 510 nm were analyzed by biexponential curve fittings. The reciprocal decay rate of the fast component in a 50-μT field (1.20 μs) was indistinguishable from that in a 39-mT field (1.24 μs). The field-on/field-off difference signals at 460 and 510 nm are shown in Fig. 1C Lower: both deviate significantly from zero after the laser flash; it is noteworthy that the differences develop within the first 2 μs, whereas in the later part of the curve no additional changes are observed. Because the apparent time constant for the observed MFE and the time constant for the deprotonation of Trp(H)•+ are quite similar, we conclude that the primary radical pair [FAD•− Trp(H)•+] is mainly responsible for the MFE. Following this argument, the recombination rate of the secondary radical pair [FAD•− Trp•] should be much slower than its electron spin relaxation in order not to show a MFE. That the recombination of [FAD•− Trp•] is much slower than that of [FAD•− Trp(H)•+] is not unexpected because direct recombination is only possible for the primary species, whereas an additional proton transfer is required for the secondary species to return to the ground state.
To characterize the MFE more fully, transients were measured across the wavelength range 400–650 nm, and the field-on/field-off difference signals were calculated. The result is depicted in Fig. 1D. This curve deviates significantly from zero and shows a characteristic peak pattern with negative values at 400, 520, and 550 nm. A positive MFE is observed at 450 nm. The wavelength dependence of the MFE resembles the transient absorption spectra shown in Fig. 1A (e.g., green and yellow spectra) except for a sign inversion. The MFE observed here can be explained by the “hyperfine” radical pair mechanism. The presence of a magnetic field hinders the S–T mixing process because the Zeeman interaction isolates the T+1 and T−1 spin states from the S and T0 states of the radical pair. Charge recombination to the ground-state reactants is allowed for the S state of the radical pair, but not for its T state. Because the recombination pathway of the primary radical pair [FAD•− Trp(H)•+] is in direct competition with the spin-independent deprotonation step leading to the secondary radical pair [FAD•− Trp•], the reduction in the efficiency of S–T interconversion caused by applying a magnetic field leads to more efficient recombination and a concomitant reduction in the radical concentration. This effect can indeed be observed in Fig. 1D: the applied magnetic field reduces the transient concentration of radical pairs. Hence, negative and positive MFEs on both the radical pair and the ground-state bleaching are only consistent for the case of a singlet-born radical pair.
Discussion
Time-resolved electron paramagnetic resonance spectroscopy (tr-EPR) has shown that radical pairs can be created photochemically in photolyases with the electron spin correlation that is a necessary, but not sufficient, condition for the yields and rates of the subsequent reactions to be sensitive to applied magnetic fields (15, 43). The experiments reported here demonstrate that, in contrast to published data (15), radical pairs are formed in EcPL from a singlet-state precursor. Additionally, EcPL is shown here to have the magnetic and kinetic properties that are essential for magnetosensitivity. Specifically, it has been demonstrated that competition between spin-dependent recombination and spin-independent deprotonation of the primary radical pair, together with magnetic field-dependent S–T interconversion of the primary radical pair, cause the yield of the long-lived secondary radical pair to depend on the presence or absence of a weak Zeeman interaction that is orders of magnitude smaller than kBT. Even though the detected change in radical yield is small and is observed for an applied magnetic field much stronger than that of the Earth, these findings constitute a proof of principle that photoinduced electron transfer reactions in photolyase and most probably by extension the closely related cryptochromes, respond to magnetic interactions in a manner that could form the basis of a biological magnetic compass.
As noted above, the reaction scheme in Fig. 2 bears considerable similarities to that discussed by Solov'yov et al. (22). It differs in the following respects: (i) we could not detect the participation of amino acid residues other than the tryptophan we presume to be W306, most likely because of the limited time resolution; (ii) proton transfer to the flavin radical was not observed; (iii) we would not expect the radical pair precursors of [FAD•− Trp(H)•+] in the Trp-triad chain to experience significant S–T interconversion because of their large anticipated exchange and dipolar interactions (44).
A radical pair reaction must satisfy a number of quite stringent conditions if it is to show a strong response to an external magnetic field. Three such prerequisites in particular may be mentioned. (i) The spin–spin coupling of the two electrons must be weak enough to allow hyperfine and Zeeman interactions to drive S–T interconversion. (ii) Electron spin relaxation must be slow compared with the radical pair reaction rates. (iii) The spin-dependent and spin-independent reactions must have comparable rates to give rise to the kinetic competition essential for a MFE. Had any one of these conditions not been approximately satisfied, the MFE in EcPL would almost certainly have been too small to be detected with the techniques described here. The degree to which the three conditions are met could be very different in vivo. (i) The FAD and W306 radicals in EcPL are ≈1.9 nm apart. Calculations suggest exchange and dipolar interactions of the order of 1 mT (44), which are far from negligible and likely to interfere significantly with S–T interconversion. However, if the protein were immobilized, because it would have to be if it were to act as a directional sensor, the dipolar coupling would not be averaged by molecular tumbling, and the two interactions, being of similar size, would have more-or-less equal and opposite effects. Estimates suggest that this cancellation, which allows some S–T interconversion to proceed unimpeded, should occur for radical–radical separations (2.0 ± 0.2 nm) that are close to that of the FAD–W306 radical pair (44). (ii) The relaxation rates of the electron spins are determined in part by molecular motions. It is entirely possible therefore that relaxation is slower in vivo if the internal motions and the overall rotational motion of the protein molecule are slower than in vitro. (iii) Both the back electron transfer rate of the primary radical pair and the deprotonation of the tryptophan cation radical are likely to be sensitive to the environment of the protein and binding to signaling partners and so could be quite different in vivo. Because no structural information for any avian cryptochrome is available, the amino acid residue that could form the spin-correlated radical pair with the FAD is as yet unknown. Although the chain of tryptophan residues is conserved in avian cryptochromes, there is some evidence from experiments with other photolyases (43, 45) that a tyrosine residue can also act as the electron-donating amino acid. Information on the distance and orientation of this amino acid with respect to the flavin chromophore is lacking but is crucial because subtle differences in these properties can strongly influence the magnetic interaction parameters and therefore alter the extent of the MFE.
In short, there are several possible reasons why the MFE observed here for EcPL is small and only detectable at magnetic fields much stronger than the geomagnetic field and why such effects could be much larger in a magnetic-field-sensing system exposed to much weaker magnetic fields. It is relevant to note that careful optimization of experimental conditions has very recently allowed an intramolecular photoinduced electron transfer reaction, chosen as a model chemical compass, to show a detectable response in a magnetic field of ≈50 μT (46).
Methods
Protein Preparation.
The expression and purification of EcPL are described elsewhere (35). Enzyme activity was monitored following the procedure developed by Jorns et al. (47). To oxidize the protein, potassium ferricyanide was added to a final concentration of 10 mM, and the mixture was incubated at 278 K for 3 days. The progress of the oxidation reaction was monitored by optical spectroscopy. Excess ferricyanide was removed by three consecutive ultrafiltation steps using 30-kDa membranes (Amicon Ultra; Millipore).
Transient Absorption Spectroscopy.
Samples containing 50 mM Hepes (pH 7.0), 100 mM KCl, 20% glycerol (vol/vol), 5 mM potassium ferricyanide, and 0.2 mM EcPL were excited with a dye laser (Sirah Cobra) pumped by a Nd:YAG laser (Continuum Surelite-1). The wavelength and the pulse width were 450 nm and 5 ns, respectively. The pulse energy was 5 mJ. Absorbance changes were recorded with a time resolution of 50 ns or 10 μs as described elsewhere in more detail (48). At each wavelength, transient signals were recorded at a repetition rate of 0.03 Hz alternately with (39 mT) and without (Earth's magnetic field: ≈50 μT) an applied magnetic field. At each wavelength selected by a monochrometer (Oriel 77250), transient signals were recorded by a digital oscilloscope (Iwatsu–LeCroy LT342) connected to a photomultiplier tube (Hamamatsu R928) at a repetition rate of 0.03 Hz alternately with (39 mT) and without (Earth's magnetic field: ≈50 μT) an applied magnetic field.
Acknowledgments.
The Oxford group was supported by the Electromagnetic Fields Biological Research Trust, the Engineering and Physical Sciences Research Council, the Royal Society, the Human Frontier Science Program, and International Association for the promotion of cooperation with scientists from the New Independent States of the former Soviet Union. R.B, S.W., and E.S. were supported by Deutsche Forschungsgemeinschaft Grants SfB-498/A2 and B7. A.B. was supported by Deutsche Forschungsgemeinschaft Grant SfB-533/A5, the Hans-Fischer Gesellschaft, and the Fonds der Chemischen Industrie.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Todo T. Functional diversity of the DNA photolyase/blue light receptor family. Mutat Res. 1999;434:89–97. doi: 10.1016/s0921-8777(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 2.Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 3.Lin C, Shalitin D. Cryptochrome structure and signal transduction. Annu Rev Plant Biol. 2003;54:469–496. doi: 10.1146/annurev.arplant.54.110901.160901. [DOI] [PubMed] [Google Scholar]
- 4.Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6:220.1–220.9. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber S. Light-driven enzymatic catalysis of DNA repair: A review of recent biophysical studies on photolyase. Biochim Biophys Acta. 2005;1707:1–23. doi: 10.1016/j.bbabio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Sancar A. No “end of history” for photolyases. Science. 1996;272:48–49. doi: 10.1126/science.272.5258.48. [DOI] [PubMed] [Google Scholar]
- 7.Bouly JP, et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- 8.Merrow M, Roenneberg T. Circadian clocks: Running on redox. Cell. 2001;106:141–143. doi: 10.1016/s0092-8674(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 9.Sancar A. Structure and function of DNA photolyase. Biochemistry. 1994;33:2–9. doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- 10.Park HW, Kim ST, Sancar A, Deisenhofer J. Crystal structure of DNA photolyase from Escherichia coli. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- 11.Froy O, Chang DC, Reppert SM. Redox potential: Differential roles in dCRY and mCRY1 functions. Curr Biol. 2002;12:147–152. doi: 10.1016/s0960-9822(01)00656-x. [DOI] [PubMed] [Google Scholar]
- 12.Zeugner A, et al. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J Biol Chem. 2005;280:19437–19440. doi: 10.1074/jbc.C500077200. [DOI] [PubMed] [Google Scholar]
- 13.Essen LO. Photolyases and cryptochromes: Common mechanisms of DNA repair and light-driven signaling? Curr Opin Struct Biol. 2006;16:51–59. doi: 10.1016/j.sbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Byrdin M, Eker APM, Vos MH, Brettel K. Dissection of the triple tryptophan electron transfer chain in Escherichia coli DNA photolyase: Trp382 is the primary donor in photoactivation. Proc Natl Acad Sci USA. 2003;100:8676–8681. doi: 10.1073/pnas.1531645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gindt YM, et al. Origin of the transient electron paramagnetic resonance signals in DNA photolyase. Biochemistry. 1999;38:3857–3866. doi: 10.1021/bi981191+. [DOI] [PubMed] [Google Scholar]
- 16.Wiltschko W, Wiltschko R. Magnetic compass of European robins. Science. 1972;176:62–64. doi: 10.1126/science.176.4030.62. [DOI] [PubMed] [Google Scholar]
- 17.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschvink JL, Gould JL. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems. 1981;13:181–201. doi: 10.1016/0303-2647(81)90060-5. [DOI] [PubMed] [Google Scholar]
- 19.Shcherbakov VP, Winklhofer M. The osmotic magnetometer: A new model for magnetite-based magnetoreceptors in animals. Eur Biophys J. 1999;28:380–392. [Google Scholar]
- 20.Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 21.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 22.Solov'yov IA, Chandler DE, Schulten K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys J. 2007;92:2711–2726. doi: 10.1529/biophysj.106.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouritsen H, et al. Cryptochromes and neuronal activity-markers colocalize in the retina of migratory birds during magnetic orientation. Proc Natl Acad Sci USA. 2004;101:14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thalau P, Ritz T, Burda H, Wegner RE, Wiltschko R. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J R Soc Interface. 2006;3:583–587. doi: 10.1098/rsif.2006.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiltschko W, Möller A, Gesson M, Noll C, Wiltschko R. Light-dependent magnetoreception in birds: Analysis of the behaviour under red light after pre-exposure to red light. J Exp Biol. 2004;207:1193–1202. doi: 10.1242/jeb.00873. [DOI] [PubMed] [Google Scholar]
- 26.Brocklehurst B. Magnetic fields and radical reactions: Recent developments and their role in nature. Chem Soc Rev. 2002;31:301–311. doi: 10.1039/b107250c. [DOI] [PubMed] [Google Scholar]
- 27.Steiner UE, Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem Rev. 1989;89:51–147. [Google Scholar]
- 28.Schulten K. In: Festkörperprobleme. Treusch J, editor. Braunschweig: Vieweg; 1982. pp. 61–83. [Google Scholar]
- 29.Cintolesi F, Ritz T, Kay CWM, Timmel CR, Hore PJ. Anisotropic recombination of an immobilized photoinduced radical pair in a 50-μT magnetic field: A model avian photomagnetoreceptor. Chem Phys. 2003;294:385–399. [Google Scholar]
- 30.Liedvogel M, et al. Chemical magnetoreception: Bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE. 2007;2:e1106. doi: 10.1371/journal.pone.0001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heelis PF, Sancar A. Photochemical properties of Escherichia coli DNA photolyase: A flash photolysis study. Biochemistry. 1986;25:8163–8166. doi: 10.1021/bi00373a006. [DOI] [PubMed] [Google Scholar]
- 32.Heelis PF, Okamura T, Sancar A. Excited-state properties of Escherichia coli DNA photolyase in the picosecond to millisecond time scale. Biochemistry. 1990;29:5694–5698. doi: 10.1021/bi00476a008. [DOI] [PubMed] [Google Scholar]
- 33.Aubert C, Vos MH, Mathis P, Eker APM, Brettel K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature. 2000;405:586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 34.Saxena C, Sancar A, Zhong D. Femtosecond dynamics of DNA photolyase: Energy transfer of antenna initiation and electron transfer of cofactor reduction. J Phys Chem B. 2004;108:18026–18033. [Google Scholar]
- 35.Schleicher E, et al. Light-induced reactions of Escherichia coli DNA photolyase monitored by Fourier transform infrared spectroscopy. FEBS J. 2005;272:1855–1866. doi: 10.1111/j.1742-4658.2005.04617.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamm-Alvarez S, Sancar A, Rajagopalan KV. Role of enzyme-bound 5,10-methenyltetrahydropteroylpolyglutamate in catalysis by Escherichia coli DNA photolyase. J Biol Chem. 1989;264:9649–9656. [PubMed] [Google Scholar]
- 37.Lin C, et al. Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science. 1995;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 38.Swartz TE, et al. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem. 2001;276:36493–36500. doi: 10.1074/jbc.M103114200. [DOI] [PubMed] [Google Scholar]
- 39.Li YF, Heelis PF, Sancar A. Active site of DNA photolyase tryptophan-306 is the intrinsic hydrogen-atom donor essential for flavin radical photoreduction and DNA repair in vitro. Biochemistry. 1991;30:6322–6329. doi: 10.1021/bi00239a034. [DOI] [PubMed] [Google Scholar]
- 40.Ehrenberg A, Müller F, Hemmerich P. Basicity, visible spectra, and electron spin resonance of flavosemiquinone anions. Eur J Biochem. 1967;2:286–293. doi: 10.1111/j.1432-1033.1967.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 41.Gauden M, et al. Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor. Proc Natl Acad Sci USA. 2006;103:10895–10900. doi: 10.1073/pnas.0600720103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller F, Brustlein M, Walker WH, Massey V, Hemmerich P. Light-absorption studies on neutral flavin radicals. Eur J Biochem. 1972;25:573–580. doi: 10.1111/j.1432-1033.1972.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 43.Weber S, et al. Photoactivation of the flavin cofactor in Xenopus laevis (6-4) photolyase: Observation of a transient tyrosyl radical by time-resolved electron paramagnetic resonance. Proc Natl Acad Sci USA. 2002;99:1319–1322. doi: 10.1073/pnas.032469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efimova O, Hore PJ. Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys J. 2008;94:1565–1574. doi: 10.1529/biophysj.107.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubert C, Mathis P, Eker APM, Brettel K. Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. Proc Natl Acad Sci USA. 1999;96:5423–5427. doi: 10.1073/pnas.96.10.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda K, et al. Chemical compass model of avian magnetoreception. Nature. 2008;453:387–390. doi: 10.1038/nature06834. [DOI] [PubMed] [Google Scholar]
- 47.Jorns MS, Sancar GB, Sancar A. Identification of oligothymidylates as new simple substrates for Escherichia coli DNA photolyase and their use in a rapid spectrophotometric enzyme assay. Biochemistry. 1985;24:1856–1861. doi: 10.1021/bi00329a008. [DOI] [PubMed] [Google Scholar]
- 48.Henbest KB, et al. Photoionization of TMPD in DMSO solution: Mechanism and magnetic field effects. Mol Phys. 2006;104:1789–1794. [Google Scholar]