Abstract
Innate parental behaviors and adult social interactions are essential for survival of the individual along with the species as a whole. Because these behaviors require threat assessment of the environment, it is plausible that they are regulated by the amygdala-associated neural circuitry of fear. However, the amygdala is not a single anatomic and functional unit, and nuclei of the amygdala have multiple inter- and intra-connections. This poses a question as to the exact role of different amygdala nuclei in these behaviors and the mechanisms involved. The basolateral complex of the amygdala nuclei (BLA) is particularly interesting in this regard: although the BLA role in forming memories for learned fear is established, the BLA role in innate behaviors is not well understood. We recently demonstrated that mice without an inhibitor of microtubules, stathmin, a gene enriched in BLA-associated circuitry, have deficiency in innate and learned fear. Here we show that the deficiency in fear processing in stathmin−/− females leads to improper threat assessment, which in turn affects innate parental care and adult social interactions. Profound deficiency is observed in maternal behavior of stathmin−/− females: they lack motivation for retrieving pups and are unable to choose a safe location for nest-building. Remarkably, stathmin−/− females have an enhancement in social interactions. BLA lesions in WT mice produce similar effects in maternal and social behaviors, confirming vital BLA participation. The findings implicate stathmin as the critical molecular component linking the BLA-associated neural circuitry with innate parental behaviors and adult social interactions.
Keywords: amygdala-enriched genes, maternal behavior, fear, threat, pup retrieval
Among Niko Tinbergen's four “whys,” representing a framework for understanding animal behavior, the third why refers to the survival value of a behavior (1). As such, a proper threat assessment is crucial in guiding animal and human activities (2, 3). Danger detection is critically dependent on the amygdala (4) and its many nuclei have various neural connections throughout the brain (5–7). Recent work provides clear evidence that amygdala nuclei have different roles in fear-related and reward-related learned behaviors (8–12). Specifically, the basolateral complex of the amygdala nuclei (throughout the article we refer to the lateral, or LA, and basal, or BA, nuclei as the basolateral complex, or BLA) is thought to be the major area of the brain involved in learned fear. However, little is known about the role of the BLA in other behaviors where danger assessment is essential. Unraveling the molecular, cellular, and anatomic mechanisms underlying the relationship between the BLA-associated circuitry and behaviors involving threat assessment may help to better understand behavioral choices in animals and humans.
Here we examine the role of the BLA in two behaviors vital for individual safety and species survival: affiliative maternal care and adult social interactions. During maternal behavior, failure to keep progeny away from danger can be detrimental to their well being. Furthermore, threat assessment during social interactions is essential for the establishment of social hierarchy and for reproductive, immunological, and other functions in many species, including mice. The amygdala-mediated ability to detect danger is expected to have a strong influence on the ultimate success of these behaviors. To examine the impact of a BLA dysfunction, we used two approaches: WT mice with bilateral BLA lesions and mice deficient in the BLA-enriched gene stathmin. At the cellular level, stathmin−/− mice have increased microtubule stability in the amygdala. This leads to deficient amygdala long-term potentation (LTP), but does not affect basal synaptic transmission or dendritic morphology (13). Behavioral phenotype includes deficits in innate and learned fear, but normal pain sensitivity and hippocampus-dependent spatial memory in the water maze (13). Thus, changes in stathmin−/− mice appear to be specific to fear processing in the BLA and thus, stathmin−/− mice represent an attractive animal model to study the role of the BLA in behaviors dependent on threat assessment. In the work described here, we find that stathmin-enriched areas of the BLA-associated circuitry and the BLA itself are required for proper pup retrieval and adult social interactions. Interestingly, our results also show that the BLA role in strengthening maternal care is opposite to the previously established inhibitory role of the medial nucleus (MeA) of the amygdala (14–16). Thus, the BLA has a dual role in innate maternal care and adult social interactions.
Results
Stathmin−/− Females Are Deficient in Pup Retrieval.
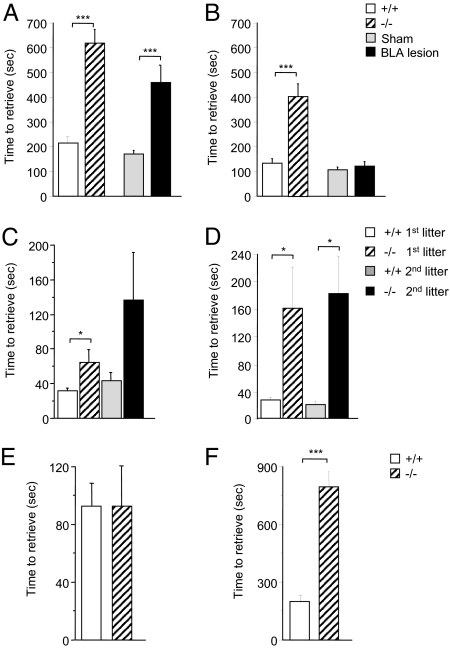
First we examined stathmin−/− mice in pup retrieval, which represents a motivational aspect of maternal care (17). Normally, virgin (nulliparous) laboratory mice inexperienced in pup retrieval immediately engage in this behavior when exposed to foster pups in their home cage (14). Pup retrieval was tested by placing three foster pups in three different corners of the home cage of a virgin female and monitoring the latency of retrieving the pups by the female to the nest. Stathmin−/− females showed a significant deficit in pup retrieval on the first (Fig. 1A) (F (3, 176) = 18.41, P < 0.001; Scheffe's F test P < 0.001) and second (Fig. 1B) (F (3, 176) = 14.67, P < 0.001; Scheffe's F test P < 0.001) days. The latency of pup approach and sniffing was normal (Fig. 2A) (P = 0.879), indicating that the females were aware of the presence of the pups. Other related functions, such as nest building (Fig. 2B) (P = 0.374), olfaction (Fig. 2C) (P = 0.776), and hoarding (Fig. 2D) (P = 0.345) were normal, suggesting the specificity of the maternal phenotype to the pup-retrieval function. Similarly, we have found deficits in pup retrieval in postpartum stathmin−/− females in both their first and second litters (Fig. 1 C and D). For the first litter, compared to the WT controls, stathmin−/− postpartum females had a deficit in pup retrieval on the first (F (3, 149) = 3.04, P < 0.03; Scheffe's F test P < 0.01) and second days (F (3, 149) = 5.43, P < 0.01; Scheffe's F test P < 0.02). On the second day, although the difference did not reach statistical significance (P > 0.1), the stathmin−/− females had longer duration of pup retrieval compared to the first day. For the second litter, stathmin−/− postpartum females had a deficit on the second day (see Fig. 1D), (F (3, 149) = 5.43, P < 0.01; Scheffe's F test P < 0.01). For the first day, although on average the time for the knockout females to retrieve pups was three times longer than for the WT controls, the numbers did not reach significant difference (see Fig. 1C, two right columns). The number of pups born and the pups' survival rate from day 1 to day 21 were decreased in the litters of stathmin−/− females [supporting information (SI) Fig. S1] (pups born, F (3, 58) = 5.83, P < 0.001; Scheffe's F test Ps < 0.05; pups' survival F (3, 58) = 10.58, P < 0.001; Scheffe's F test Ps < 0.05). Moreover, the weight of pups raised by the stathmin−/− females was decreased for the first 8 days, compared to pups raised by the WT females, irrespective of the progeny genotype (Figs. S2 and S3) (F (3, 45) = 13.19, P < 0.001; interaction days × genotype, F (21, 315) = 7.69, P < 0.001; Scheffe's F test Ps < 0.05). Thus, the deficiency in pups' birth and survival, observed in the litters of stathmin−/− mothers, is caused by the mother's genotype and not by the pups' genotype.
Fig. 1.
Analysis of pup retrieval in stathmin−/− females and in WT females with BLA lesions. (A and B) Pup retrieval of virgin stathmin−/− females and WT females with BLA lesions on day one (A) and day two (B). (C and D) Pup retrieval of the first and second litters in postpartum stathmin−/− females during the first (C) and second (D) days. (E and F) The pup retrieval deficit can be rescued by placing pups for 5 min in the nest of a virgin stathmin−/− female if the female is tested immediately (E), but not after a 1-h delay (F). Results are presented as mean ± SEM.
Fig. 2.
Analyses of pup approach, nest building, olfactory function, hoarding, and forced-swim test. (A) The latency of approach to sniff the pups is normal on both days in virgin stathmin−/− females and in virgin WT females with BLA lesions. (B) Nest building is normal in stathmin−/− females 90 min and 24 h after the introduction of the nest material. (C) The amount of time spent sniffing male urine and water is normal in stathmin−/− females. (D) Organizational skills as tested by hoarding behavior are normal in stathmin−/− females. (E) The general level of depression or motivation as analyzed in the Porsolt forced-swim test is normal in stathmin−/− females. Results are presented as mean ± SEM.
Bilateral BLA Lesions Inhibit Pup Retrieval.
We hypothesized that stathmin action in the BLA is required for normal pup retrieval. Using ibotenic acid, we made bilateral lesions in the BLA in virgin WT females (Fig. S4) and tested them for pup retrieval in the home cage. Pup retrieval was deficient in the lesioned animals on the first day (see Fig. 1A) (F (3, 176) = 18.41, P < 0.001; Scheffe's F test, P < 0.01), confirming the involvement of the BLA in this behavior. On the second day, the BLA-lesioned animals demonstrated full recovery in pup retrieval (see Fig. 1B). This suggests that the BLA is indeed critical for pup retrieval during an initial contact with the pups; however, on the second day other areas enriched with stathmin have an important role. It is also possible that the lesions did not target exactly the amygdala areas expressing stathmin.
Rescue of the Pup-Retrieval Deficit.
Remarkably, the pup-retrieval deficit can be completely rescued by a pre-exposure of stathmin−/− virgin females to foster pups for 5 min in the female's nest immediately before testing (Fig. 1E) (P = 0.996). A 1-h delay after the 5 min of the pre-exposure, on the other hand, does not rescue this deficit (Fig. 1F) (F (1, 20) = 20.31, P < 0.001). This short-term recovery suggests strongly that only the motivational aspect of maternal behavior is abnormal in virgin stathmin−/− mice, which is consistent with the notion that the BLA is involved in the motivational aspects of behavior (18–20). In the BLA-lesioned WT females, the pup-retrieval deficit was observed on the first but not on the second day of pup retrieval, confirming the motivational role of the BLA during maternal behavior (see Fig. 1 A and B). To determine whether the decrease in motivation in pup retrieval is specific to maternal care or reflects a general increase in helplessness or depression, we examined stathmin−/− females in the Porsolt forced-swim test (21). We found no differences between the mutants and WT controls (Fig. 2E) (P = 0.395), confirming that the decrease in motivation is specific to pup retrieval.
Stathmin Is Expressed in the BLA but not in the Main Anatomic Areas Associated with Maternal Behavior.
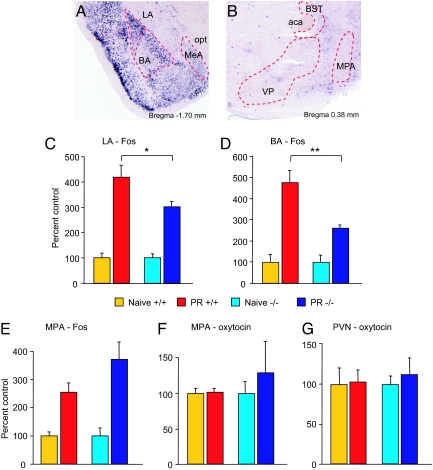
Using RNA in situ hybridization and immunohistochemistry, we have analyzed stathmin expression in the anatomic areas responsible for maternal care. Compared to the LA and BA, stathmin RNA and protein were negligible in the major areas involved in pup retrieval, including the medial preoptic area (MPA), bed nucleus of the stria terminalis (BST), MeA, and other related regions (Fig. 3 A and B; Fig. S5).
Fig. 3.
Expression of stathmin in the BLA and deficiency in Fos activity in the BLA during pup retrieval. (A and B) Stathmin RNA in situ hybridization in adult WT mice. (A) Stathmin is strongly expressed in the amygdala in the LA and BA but not in the MeA; opt, optical tract. (B) Stathmin is not expressed in other anatomic areas involved in pup retrieval. aca, anterior commissure; VP, ventral pallidum. (C and D) During pup retrieval, Fos is induced significantly less in the LA and BA in stathmin−/− females compared to WT mice as analyzed using immunohistochemical quantification. *, P < 0.05; **, P < 0.01. (E) During pup retrieval, Fos is induced normally in the MPA. (F and G) Oxytocin is induced normally in the MPA (F) and PVN (G). PR, pup retrieval; PVN, paraventricular hypothalamic nucleus. Results are presented as mean ± SEM.
Deficiency in Fos Induction Following Pup Retrieval in Stathmin−/− Females.
Using Fos as a marker of neuronal activity (14), we examined different nuclei of the amygdala and the anatomic areas responsible for maternal care (14) in stathmin−/− females following pup retrieval in the home cage. Fos induction was significantly reduced both in the LA and BA in stathmin−/− mice compared to control mice (for LA, Fig. 3C and Fig. S6 A and B) (F (1, 17) = 4.77, P < 0.05) (for BA, Fig. 3D and Fig. S6 A and B) (F (1, 17) = 5.01, P < 0.05; interaction genotype × group, F (1, 17) = 5.01, P < 0.05), confirming our hypothesis that the amygdala is involved, given its known role in motivation (8, 22) and processing of fearful events (10). Importantly, Fos expression was normal in the central nucleus (CeA) of the amygdala and in the MeA in stathmin−/− mice for both naïve animals and following pup retreival (Fig. S7 A and B), suggesting that the BLA is specifically impaired in stathmin−/− mice. Fos staining was also normal in the MPA in the mutant mice (Fig. 3E and Fig. S6 C and D) (P > 0.1), as expected, as stathmin is not normally expressed in this area (Fig. 3B). These experiments give additional support for the idea that the pup-retrieval deficit in stathmin−/− mice is a result of the BLA dysfunction.
No Differences Found in Oxytocin, Testosterone, or 17β-Estradiol in Stathmin−/− Females.
Because a number of studies have shown that oxytocin plays an important role in maternal behavior (14, 23), we also tested expression of this neuropeptide. No differences were found in oxytocin expression between the mutant and WT littermates in the MPA and paraventricular hypothalamic nucleus (PVN) (Fig. 3 F and G; Fig. S6 E–H) (Ps > 0.43) for both naïve condition and following pup retrieval. This suggests that stathmin modulates pup-retrieval behavior via mechanisms that do not involve oxytocin function. We have also tested whether behavioral deficits are accompanied by changes in the levels of testosterone and 17β-estradiol in the serum of stathmin−/− mice. We have found that these hormones were at normal levels in the knockout mice as compared to their WT counterparts (Table 1). We therefore conclude that changes in the testosterone and 17β-estradiol levels do not underlie the behavioral phenotype we have observed in the mutant mice.
Table 1.
Steroid hormone levels of WT and stathmin−/− females
| Steroid hormone level in blood | Stathmin+/+ | Stathmin−/− | P Value |
|---|---|---|---|
| Total testosterone, ng·ml−1 | <0.1 (n = 10) | <0.1 (n = 10) | |
| Free testosterone, pg·ml−1 | 0.73 ± 0.59 (n = 17) | 0.76 ± 0.59 (n = 15) | NS |
| 17β estradiol, pg·ml−1 | 30.21 ± 4.09 (n = 17) | 42.37 ± 7.84 (n = 14) | NS |
Values are mean ± SEM. NS = P > 0.1, Student's t test.
Stathmin−/− Females Are Deficient in Innate Fear.
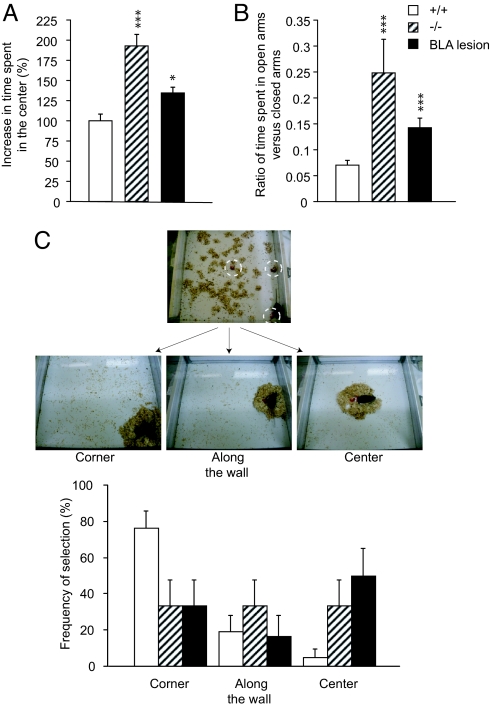
To examine the role of innate fear in maternal behavior, we first tested the basal anxiety levels in the stathmin−/− females in the open field and elevated plus maze. The mutant females showed less anxiety compared to the WT controls, as they spent significantly more time both in the center of the open field (Fig. 4A) (F (2, 39) = 23.49, P < 0.001; Scheffe's F test, P < 0.001) and in the open arms of the elevated plus maze (Fig. 4B) (F (2, 40) = 8.73, P < 0.001; Scheffe's F test, P < 0.001), confirming earlier results found in stathmin−/− males (13).
Fig. 4.
Deficiency in innate fear leads to deficits in risk assessment during pup retrieval in stathmin−/− females. (A and B) Innate fear is deficient in stathmin−/− females and in BLA-lesioned WT females. In the open field (A), the mutants and lesioned animals spent more time in the center of the arena; in the elevated plus maze (B), the mutants and lesioned animals spent more time in the open arms compared to the control mice. *, P < 0.05; ***, P < 0.001. (C) Pup retrieval in the open field. Stathmin−/− females and BLA-lesioned WT females choose in random fashion the place to rebuild the nest and retrieve the pups, in contrast to control animals, who prefer corners of the open field. Upper photograph shows the view of the open field with the pieces of the nest broken apart, three scattered pups (dashed circles), and the female at the beginning of the experiment. Three photographs in the middle show the final stage of the experiment with three possible choices that the females make for the locations of the nest and the pups. Results are presented as mean ± SEM.
We have also tested BLA-lesioned WT females in the open field and elevated plus maze. The lesions caused a decrease in anxiety in the open field (see Fig. 4A) (F (2, 39) = 23.49, P < 0.001; Scheffe's F test, P < 0.05) and in the elevated plus maze (see Fig. 4B) (F (2, 40) = 8.73, P < 0.001; Scheffe's F test, P < 0.001), confirming previous studies (24).
Deficiency in Threat Assessment During Pup Retrieval in Stathmin−/− Females and in WT Females with BLA Lesions.
Next, we investigated maternal care in the presence of a potential threat. We developed a paradigm to test pup retrieval in the open field: a virgin female was placed in the open field with foster pups dispersed (one in the middle of the wall, one in the center of the field, and one in the corner of the field) and the nest broken apart and its pieces scattered across the field (Fig. 4C). Then, the female was videotaped while she was rebuilding the nest and retrieving the pups to the nest. In a striking difference to the WT mice, stathmin−/− mice selected at random either the corner, the middle of the wall, or the center of the arena (see Fig. 4C and Movie S1 Video file) (P > 0.166). However, as expected, the WT mice preferred safe locations for the nest: the majority selected a corner (76.2%) and the rest chose the middle of the wall (19%) or the center of the arena (4.8%), which is the most exposed and dangerous place for pups in the open field (see Fig. 4C and Movie S2) (P < 0.01).
To confirm that the maternal care deficits in stathmin−/− mice are caused by the dysfunction of the BLA, we examined WT females with ibotenic acid-induced bilateral lesions to the BLA. Pup retrieval in the open field by the BLA-lesioned WT mice showed the same random pattern as observed with stathmin−/− mice (see Fig. 4C) (P > 0.166).
Social Behavior Is Enhanced in Stathmin−/− Females and in WT Females with BLA Lesions.
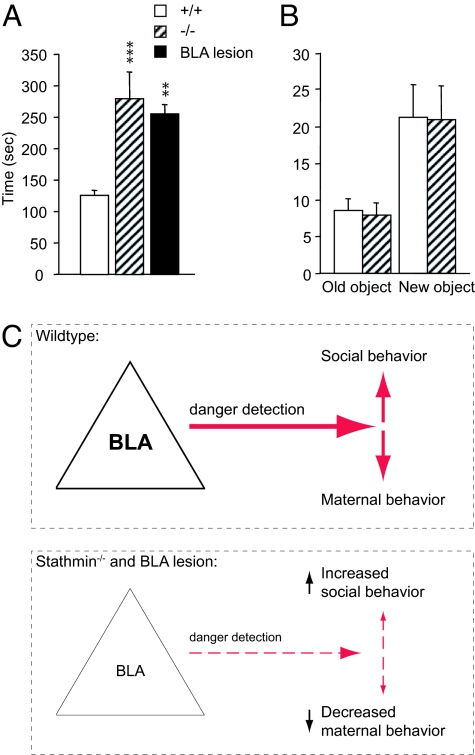
To test another behavior critical for individual safety and well being, we turned to social interactions and examined adult stathmin−/− females as hosts in the host-intruder paradigm. In contrast to the deficit in maternal behavior, host stathmin−/− mice demonstrated an increase in female-female social interactions (Fig. 5A) (F (2, 37) = 11.67, P < 0.001; Scheffe's F test, P < 0.001). Compared to the WT controls, they spent significantly more time following a WT intruder female around the cage; no aggressive behaviors were observed. Interestingly, object recognition was normal in stathmin−/− mice (Fig. 5B) (P = 0.290), suggesting a specificity of the phenotype to social interactions. To examine the contribution of the BLA to the social abnormality found in stathmin−/− females, the BLA-lesioned WT females were also subjected to the host-intruder paradigm. Similar to the stathmin−/− females, the lesioned animals showed an increase in social interactions with WT intruders when used as hosts, compared to the control animals (Fig. 5A) (F (2, 37) = 11.67, P < 0.001; Scheffe's F test, P < 0.001).
Fig. 5.
Social interaction and object recognition in stathmin−/− females and in BLA-lesioned WT females. (A) When used as hosts, stathmin−/− mice and WT females with BLA lesions show enhancement in social interaction in the host-intruder test. **, P < 0.01; ***, represents P < 0.001. (B) Object recognition is normal in stathmin−/− mice. Results are presented as mean ± SEM. (C) A model of how the elimination of stathmin and the BLA lesions negatively regulate affiliative maternal behavior and enhance adult social interactions.
Discussion
The present study shows that the BLA-associated neural circuitry of fear enriched in stathmin is able to control, via threat assessment, affiliative maternal care and adult social behavior. Studying maternal behavior, we found that virgin stathmin−/− females have strong deficits in pup retrieval. Our next series of experiments has suggested that stathmin expression in the BLA is responsible for the observed phenotype: stathmin is enriched in the BLA, but is excluded from the brain areas thought to be associated with maternal care. Consistent with this expression pattern, the immediate-early gene c-Fos is strongly induced in the BLA in WT mice, but not in the stathmin mutants following pup retrieval. Moreover, the stathmin−/− females show less innate fear in the open field and elevated plus maze. As a result of this deficiency in threat assessment, they are unable to make proper safety choices during pup retrieval in the open field, which is a more threatening environment than the home cage. During pup retrieval in the open field, the stathmin−/− females do not perceive the center of the arena as dangerous for nest selection, which is opposite to their WT counterparts, preferring consistently the corner to build the nest and retrieve the pups. Normal performance in food hoarding and in the forced-swim test indicates that the observed deficits are specific to maternal care.
Based on these results, we hypothesize that the lack in threat assessment leads to deficiency in motivation to protect pups from danger and in turn causes inefficient and slow retrieval. A lack in sensory perception is unlikely to cause the deficit because stathmin−/− females are normal in recognizing pups during the first encounter and normal in olfactory function (Fig. 2 A and C). Unexpectedly, we found that the pup-retrieval deficit can be rescued by placing pups in the nest of an inexperienced female for 5 min and then immediately testing the female for pup retrieval, which suggested further the motivational cause. If the idea that the lack of threat assessment leads to a deficiency in motivation for “child care” is true, this scenario should work well only when danger to the parents is less than to their young. With more danger perceived, the parents would stop caring for their young and flee, saving their own lives so they can have another chance to reproduce in the future.
Consistent with the BLA role in danger detection, stathmin−/− mice demonstrate enhancement in female-female adult social interactions. However, their behavior in the object recognition task is normal, suggesting that the deficiency observed is specific to innate affiliative and adult social interactions. Given our current results, it would be of interest to test stathmin−/− mice and mice with BLA lesions in other reproductive and social behaviors, such as male-female and male-male interactions.
Importantly, the bilateral BLA injections in WT mice replicated results obtained with stathmin−/− females both in maternal and social behaviors, confirming involvement of the BLA-associated neural circuitry. However, the possibility exists that other stathmin-positive areas of the BLA-associated circuitry may contribute to the phenotype.
These findings reveal several major points. First, they expose new dissociable roles of the BLA-associated neural circuitry in two behaviors essential for survival: maternal care and social interactions (Fig. 5C). Second, this work provides genetic evidence that stathmin is required for this function. Third, the neural circuitry expressing stathmin controls these behaviors via threat assessment. The fact that the maternal behavior is deficient but the social interactions are enhanced suggests that both stathmin and the BLA modulate these behaviors with high specificity. This is consistent with the previous work that has shown that stathmin−/− mice are deficient in amygdala-dependent innate and learned fear, but are normal in hippocampus-dependent spatial memory (13). At the cellular level, stathmin is a negative regulator of microtubule formation (25) and in the amygdala of stathmin−/− mice a decrease in microtubule dynamics leads to deficits in synaptic plasticity (13), suggesting that the deficiency at the BLA synapses may be responsible for effects described here.
Our findings suggest a clear distinction between the roles of the different nuclei of the amygdala in maternal behavior. In contrast to the inhibitory function of the MeA (14–16), our work illustrates the positive involvement of the BLA. Therefore, the maternal deficit in the stathmin−/− mice may be different from that in other mutant mice described so far (14, 16, 26). Also, to our knowledge, the rescue experiment using pup pre-exposure has not been described before. Therefore, it would be interesting to observe if other mutant mouse lines deficient in pup retrieval can improve their performance, and that their deficit can be transiently rescued by a brief pre-exposure to pups, similar to the stathmin−/− virgin females.
Our earlier work has shown a decrease in long-term memory in fear conditioning in the stathmin−/− mice (13); it is possible that this deficiency can underlie the persistence of the pup retrieval deficit on the second day. However, innate responses must underlie the deficits on the first day, because virgin females have no prior experience in pup retrieval. The pups in our experiments are weaned from their mother before the next litter is born and thus they do not see their mother retrieving pups from another litter. The notion that the lack of motivation is part of the pup-retrieval deficit in stathmin−/− mice is in agreement with the hypothesis that the BLA is involved in the motivational aspects of maternal care (17). The lack of motivation may also explain the deficits in the decreased weight of pups and the amount of pups survived. The weight of pups raised by the stathmin−/− postpartum females was lower only during the first 8 days, when the pups are particularly dependent on their mother for all aspects of maternal care. The deficiency in the weight of cross-fostered WT pups raised by the stathmin−/− females was similar to that of the pups born from and raised by the stathmin−/− females. Although, we cannot exclude other possibilities, these data suggest that maternal care is responsible for the effect. It is unclear what mechanism is responsible for the decrease in pups born and survived in the litters of the stathmin−/− mice.
These findings have interesting parallels with recent work in humans and non-human primates, which suggests that the amygdala functions as a danger detector and, through the control of fear, modulates behaviors where an assessment of a potential threat is important (27–29). Similarly, the stathmin−/− females and the mice with the BLA lesions show an increase in social interactions, confirming the notion that the amygdala regulates social behavior via threat assessment.
Because the role of the BLA is essential to behaviors related to safety, the identification and characterization of amygdala-enriched genes, as well as the molecular signaling cascades these genes are involved in, can shed light on the mechanisms specifically devoted to the role of the BLA in health and disease. Our earlier and present work on amygdala-enriched genes, including gastrin-releasing peptide (GRP), zinc transporter 3 (ZnT3), and stathmin, has shown that these genes, although originally isolated from the same cDNA library derived from an individual amygdala principal neuron (30), have distinct functions in fear processing. GRP and its receptor GRPR serve as a inhibitory constraint on amygdala LTP and learned fear, but not innate fear; ZnT3 is thought to be involved in the cortical but not auditory sensory inputs to the LA; finally, stathmin, in addition to the behaviors described here, controls amygdala LTP as well as innate and learned fear (13, 30, 31).
Our results demonstrate a spectrum of dissociable roles that the basolateral nucleus of the amygdala plays in affiliative and social interactions, and pinpoints stathmin as a potential molecular target for designing pharmacological interventions to treat mental disabilities where threat assessment and social behavior are affected, including anxiety disorders and autism.
Materials and Methods
In Situ Hybridization.
RNA in situ hybridization was performed as described (32) (see SI Text).
Behavioral Testing.
Behavioral experiments were performed between 0900 and 1800 h.
Pup retrieval in the home cage.
Females were housed individually 1 week before the experiment with the nest material placed in the cage. Each behavior was videotaped with minimal disturbance and later analyzed offline by an observer blind to the genotype.
On the day of the experiment, three pups (foster pups for virgin females and the female's own pups for postpartum females) were placed in three corners away from the nest in the home cage. Each mouse was tested for 20 min. The latencies to sniff and retrieve each pup were recorded. Retrieval was defined as a female picking up a pup in her mouth and transporting it to the nest. The mice used included 22 WT mice, 21 stathmin−/− mice, 8 sham- and 12 amygdala-lesioned mice. Two sham-lesioned mice were excluded because of infanticide.
Rescue of the pup-retrieval deficit.
The procedure was similar to that described above for pup retrieval in the home cage. However, before the experiment, pups were placed for 5 min in the nest of a virgin female to be tested (with the female present). Immediately or 1 h after the 5-min pup exposure, the three pups were removed from the nest and placed in three corners of the same cage, and pup retrieval was recorded as described for the procedure of pup retrieval in the home cage. Seven WT females and 10 stathmin−/− mice were used to test the rescue of pup retrieval deficit immediately after the pup exposure. For the rescue of the pup retrieval deficit 1 h after the pup exposure, 11 WT and 11 stathmin−/− mice were used.
Pup retrieval in the open field.
Females were already pre-exposed to the open field to reduce their exploratory behavior and were experienced in pup retrieval so that the initial deficit in pup retrieval in the knockout mice would not affect their strategy. The nest, previously made by the female, was broken apart and spread evenly throughout the open field. Three pups were placed in each of the three positions: in the corner, in the middle of the wall, and in the center of the arena. Then, the female was introduced and observed for place preference in nest building and pup retrieval. All mice that were used rebuilt the nest and retrieved the pups in the nest at the end of the experiment. For this experiment, 14 WT females, 12 stathmin−/− mice, 7 sham- and 12 amygdala-lesioned mice were used. Three sham-lesioned females were excluded because of infanticide.
Statistical Analysis.
Statistical analyses were run by way of SAS (SAS Institute) and Systat 9 software. Statistical analyses of immunohistochemical data and behavioral experiments were performed using ANOVAs and subsequent post hoc tests (Scheffe F test). For the assessment of the nest location during pup retrieval in the open field, statistical analyses were run by way of Systat 9 software. χ2 (χ2) analyses with Yates corrections (a conservative adjustment allowing comparisons of cells with frequencies of less than five) were conducted to determine if any genotype exhibited a significant tendency to build the nest in a particular location. Within-group comparison between the percentages of each location used paired t tests to determine which location type was preferred.
Supplementary Material
Acknowledgments.
We thank V.Y. Bolshakov, K. Herrup, E.R. Kandel, L.D. Matzel, M. Mayford, M. Numan, and M.R. Plummer for comments on the manuscript. We thank Jamie Joseph for help with genotyping and behavioral experiments; Andrew Hoffman for help with genotyping; Alisa Chen, Youngjin Jung, and Trevor Baybutt for help with oxytocin, Fos, and stathmin immunohistochemistry; and Guohui Pan for help with hoarding behavior and radioimmunoassays. This work was supported by the Charles and Johanna Busch Memorial Fund (to G.P.S.), the New Jersey Governor's Council on Autism (to G.P.S.); the National Alliance for Research on Schizophrenia and Depression (to G.P.S.); and the Japan Society for the Promotion of Science (to A.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807507105/DCSupplemental.
References
- 1.Tinbergen N. On aims and methods of ethology. Z f Tierpsychol. 1963;20:410–433. [Google Scholar]
- 2.Tinbergen N. The Study of Instinct. London: Oxford Univ Press; 1951. [Google Scholar]
- 3.Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–131. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- 4.Fanselow MS, Lester LS, Helmstetter FJ. Changes in feeding and foraging patterns as an antipredator defensive strategy: a laboratory simulation using aversive stimulation in a closed economy. J Exp Anal Behav. 1988;50:361–374. doi: 10.1901/jeab.1988.50-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitkanen A. In: The Amygdala: A Functional Analysis. Aggleton JP, editor. New York: Oxford Univ Press; 2000. pp. 31–116. [Google Scholar]
- 6.Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 7.McDonald JA. In: The Amygdala: Neurological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- 8.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 10.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 11.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 12.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 13.Shumyatsky GP, et al. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer; 2003. [Google Scholar]
- 15.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 16.Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- 17.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 18.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 19.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 23.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Sargolini F, Roullet P, Oliverio A, Mele A. Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience. 1999;93:855–867. doi: 10.1016/s0306-4522(99)00259-6. [DOI] [PubMed] [Google Scholar]
- 25.Curmi PA, et al. Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999;24:345–357. doi: 10.1247/csf.24.345. [DOI] [PubMed] [Google Scholar]
- 26.Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biol Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- 27.Mobbs D, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 29.Amaral DG, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 30.Shumyatsky GP, et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 31.Kodirov SA, et al. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc Natl Acad Sci USA. 2006;103:15218–15223. doi: 10.1073/pnas.0607131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.