Abstract
A major challenge for human allogeneic islet transplantation is the development of effective methods to induce donor-specific tolerance to obviate the need for life-long immunosuppression that is toxic to the insulin-producing β cells and detrimental to the host. We developed an efficient donor-specific tolerance therapy that utilizes infusions of ethylene carbodiimide (ECDI)-treated donor splenic antigen-presenting cells that results in indefinite survival of allogeneic islet grafts in the absence of immunosuppression. Furthermore, we show that induction of tolerance is critically dependent on synergistic effects between an intact programmed death 1 receptor–programmed death ligand 1 signaling pathway and CD4+CD25+Foxp3+ regulatory T cells. This highly efficient antigen-specific therapy with a complete avoidance of immunosuppression has significant therapeutic potential in human islet cell transplantation.
Keywords: anergy, programmed death-1, regulatory T cells, transplantation, islet transplantation
Allogeneic islet cell transplantation is a promising therapy for patients who have autoimmune diabetes (1, 2). However, as in solid-organ transplantation, robust donor-specific allogeneic responses (3–5) necessitate life-long immunosuppression that increases the risk for fatal opportunistic infections. In addition, the currently used immunosuppression regimen has intrinsic β-cell toxicity (6, 7). Therefore, a means of establishing donor-specific tolerance to obviate the need for immunosuppression is highly desirable for islet transplantation.
Strategies for inducing alloantigen-specific tolerance include mixed hematopoietic chimerism, costimulation blockade, peripheral T-cell depletion, and induction and expansion of regulatory T cells (Tregs) (8). Most regimens require myeloablation/cytoreduction and/or blocking antibodies targeting various T-cell signaling components, and these antibodies have significant toxicities. Using Tregs is less toxic, but the main challenge is to obtain sufficient numbers of alloantigen-specific Tregs (9, 10).
Previous studies have shown that i.v. injection of antigen-pulsed splenic antigen-presenting cells (APCs) chemically fixed with 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (ECDI) is a powerful and safe method to induce antigen-specific T-cell tolerance in vivo (11, 12). Specifically, myelin peptide–coupled, ECDI-fixed syngeneic APCs could effectively ablate induction and progression of experimental autoimmune encephalomyelitis (EAE), a murine Th1/17-mediated model of multiple sclerosis (13). Recent work using this tolerance method has defined the importance of cross-tolerance via host APCs and the role of specific Tregs (14–16). This protocol also is effective in preventing and treating autoimmune diabetes in nonobese diabetic (NOD) mice (ref. 17 and S.D.M., unpublished data).
We found that i.v. infusion of ECDI-treated donor splenocytes induced indefinite donor-specific tolerance in allogeneic islet cell transplantation. Here, the antigens of interest are mainly donor MHC class I and II molecules that are an integral surface component of donor lymphocytes, and ECDI treatment presumably interferes with costimulatory signals leading to tolerance induction to the membrane-bound allogeneic MHC antigens (18, 19). Two previous studies examined the efficacy of ECDI-treated donor dendritic cells or whole splenocytes in full MHC-mismatched heart and skin transplant models (20, 21). Transient graft protection was observed, but long-term donor specific tolerance was not achieved. Our protocol differs in the type of donor cells used and the number and timing of ECDI-fixed cell treatments and promotes indefinite acceptance of allogeneic islet grafts corresponding to markedly diminished donor-specific allo-responses. It induces a programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1)–dependent down-regulation of effector T-cell (Teff) activity and, independently, up-regulation of Tregs, which act synergistically to establish tolerance. Such differences may provide important clues for understanding mechanisms of tolerance by this protocol, thereby providing critical information for designing clinically relevant tolerance regimens for human applications.
Results
Repeated ECDI-Treated Donor Splenocyte Infusions Induce Indefinite Donor-Specific Tolerance in Allogeneic Islet Transplantation.
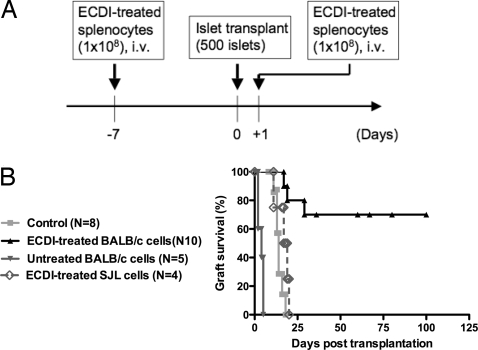
Streptozotocin-treated diabetic C57BL/6 recipients were injected i.v. with 108 ECDI-treated BALB/c splenocytes 7 days before and 1 day after grafting of BALB/c islets under the kidney capsule (Fig. 1A). Seventy percent of the recipients achieved indefinite (> 100 days) graft survival (Fig. 1B). To delineate the dose and timing requirement for effective tolerance induction, three parallel groups of mice received 108 ECDI-treated BALB/c splenocytes at (i) one dose on day −7; (ii) one dose on day +1; or (iii) two doses on days −8 and −7. None of the three treatment protocols led to significant prolongation of graft survival [supporting information (SI) Fig. S1], suggesting that repeated dosing and the interval between the doses are crucial for the efficacy of this regimen. Protection is donor specific and ECDI dependent, because injections of 108 ECDI-treated third-party strain SJL splenocytes on day −7 and day +1 did not protect transplanted BALB/c islets, and injection of untreated BALB/c splenocytes on day −7 and day +1 resulted in accelerated graft rejection (Fig. 1B).
Fig. 1.
Repeated infusions of ECDI-treated donor splenocytes induce significant graft protection in allogeneic islet cell transplantation. (A) Time line of the treatment protocols. (B) Graft survival. Day 0 indicates the day of islet transplantation. ECDI-treated BALB/c cells vs. control, P = 0.0036; ECDI-treated SJL cells vs. control, P = 0.2178; untreated BALB/c cells vs. control, P < 0.0001.
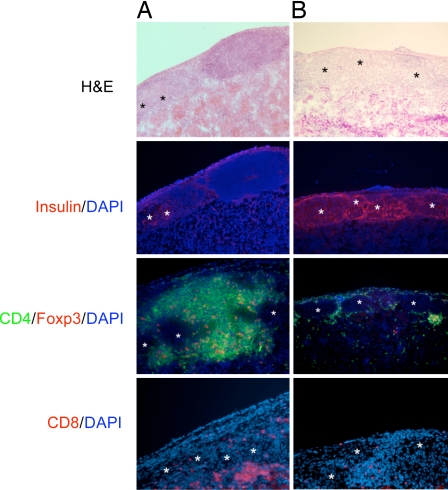
Acutely rejecting grafts showed dense lymphocytic infiltrates with destruction of islet structures and complete absence of insulin production by the β cells. Over time, islets were replaced progressively by fibrous tissue (data not shown). Protected grafts 14 days after transplantation (Fig. 2A) showed intense peri-islet lymphocytic infiltrates that did not enter the islets or disrupt the islet structure. Furthermore, insulin production by these islets was readily detectable. Similar peri-islet infiltration was observed in long-term protected grafts (70 days after transplantation) (Fig. 2B), but the number of infiltrating lymphocytes was considerably less than that observed in short-term protected grafts. Again, robust insulin secretion was maintained. Characterization of the infiltrating cells revealed the presence of CD4+, CD8+, and CD4+Foxp3+ T cells (Fig. 2 A and B).
Fig. 2.
Graft histology. Protected grafts at day 14 (A) and day 70 (B) after transplantation were stained with H&E, anti-insulin (red), CD4 (green), +Foxp3 (red), and CD8 (red). DAPI staining is shown in blue. Graphs are representatives of at least four sectioned and stained grafts of each group. Magnification × 100. Asterisks indicate intact islets.
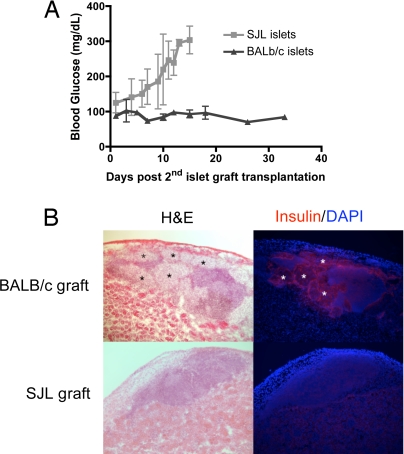
Graft nephrectomy was performed to establish that long-term normoglycemia was caused by functioning grafts rather than incomplete streptozotocin. Within 48 hours after graft removal, the mice became hyperglycemic (data not shown). Long-term tolerized recipients (60–90 days after initial transplantation) also were transplanted with a second same-donor (BALB/c) graft or with a third-party (SJL) graft without further treatment. As shown in Fig. 3A, these recipients accepted the BALB/c grafts without further manipulation but rejected the SJL grafts within the expected time frame. Histology of the second BALB/c graft showed similar peri-islet infiltrates but intact islet architecture and robust insulin production (Fig. 3B). Collectively, infusion with ECDI-treated donor cells resulted in durable donor-specific unresponsiveness.
Fig. 3.
Long-term tolerized recipients show spontaneous acceptance of a second same-donor islet graft without further intervention. (A) Long-term tolerized B6 recipients (60–90 days after the first transplantation) were nephrectomized to remove the first graft and were transplanted with a second same-donor (BALB/c, n = 3) or third-party (SJL, n = 3) graft. Day 0 indicates the day of the second islet graft transplantation. (B) Graft histology of the second islet graft. Upper panels: a protected same-donor BALB/c graft; Lower panels: a rejected third-party SJL graft. Insulin is shown in red; DAPI is shown in blue. Magnification × 100.
Allogeneic Graft Protection Is Associated with Absence of Anti-Donor Responses.
Delayed type-hypersensitivity.
We examined delayed-type hypersensitivity (DTH) responses around the time of expected acute allograft rejection (15 days after transplantation). Rejecting hosts showed robust DTH responses upon ear challenge with donor BALB/c cells (Fig. 4A), but tolerized recipients showed a complete absence of DTH.
Fig. 4.
Diminished alloantigen-specific T-cell and antibody responses are associated with protection of allogeneic islet grafts. (A) DTH responses. P = 0.0018, rejecting vs. tolerized recipients. (B) Specific anti-donor antibodies were measured for IgG1, IgG2a, IgG2b, and IgG3. The top two rows of histograms are results from two control (rejected) recipients. The bottom two rows of histograms are results from two long-term tolerized recipients. Data are representative of two individual experiments. Shaded histogram indicates syngeneic cells. (C) Mixed lymphocyte reaction and IFN-γ production. Thy1.2+ T cells from the spleens and peripheral lymph nodes from control and tolerized recipients were used. P values are indicated in the graphs. Data are representative of three separate experiments.
Donor-specific antibodies.
We next examined the effect of tolerance on allo-specific β-cell activity by measuring anti-donor antibodies of the IgG1, IgG2a, IgG2b, and IgG3 subclasses. Long-term (90 days after transplantation) tolerized recipients showed complete absence, whereas control rejected recipients showed robust antibody productions of all IgG subclasses (Fig. 4B).
Mixed lymphocyte reactions and cytokine production.
Compared with control recipients, T cells from the spleen or peripheral lymph nodes of tolerized recipients showed significantly diminished mixed lymphocyte reactions at the time of expected graft rejection (15–20 days after transplantation) as measured by 3H-thymidine uptake (Fig. 4C). Furthermore, IFN-γ production was significantly inhibited (Fig. 4C). No differences were observed in the production of IL-17, IL-10, or IL-4 (data not shown).
Collectively, these data suggest that multiple effector functions of alloantigen-specific T and β cells are suppressed by this tolerance protocol.
Tolerance Induction, but Not Maintenance, Is Dependent on CD4+CD25+ Regulatory T Cells.
To examine the potential role of CD4+CD25+ Tregs in the induction and maintenance of tolerance using ECDI-treated donor-cell infusions, transplant recipients were treated with anti-CD25 mAb (PC61) to deplete/inactivate Tregs either before or after establishment of tolerance. Impairment of Tregs (by treatment with PC61 on days −9 and −7) around the time of initial tolerance induction (day −7) completely blocked tolerance induction, resulting in rejection of islet allografts in all six recipients, whereas the three Ig-treated tolerized control mice maintained glucose homeostasis for > 60 days (Fig. 5A). Thus, functional CD25+ Tregs seem to be essential for tolerance initiation. This probability is supported further by data showing a greater number of CD4+CD25+Foxp3+ Tregs in the spleen of tolerized mice as than in controls (Fig. 5B). Correspondingly, PC61 treatment resulted in significant depletion in the number of CD4+CD25+Foxp3+ cells in the spleen, peripheral lymph nodes, and lymph nodes draining the transplanted kidney (Fig. 5B), concomitant with its ability to inhibit tolerance induction. Immunohistochemical analysis also showed the presence of significant numbers of CD4+Foxp3+ T cells in the peri-islet infiltrates in tolerized hosts, indicating that Tregs in the graft site may mediate local regulation of alloantigen-specific Teffs (Fig. 2A). Interestingly, donor antigen-specific IFN-γ response still was suppressed in PC61-treated mice, although these animals rejected the grafts (Fig. 5C). This finding is consistent with our previous observations that tolerance induced with peptide-coupled syngeneic APCs for treatment of EAE induces both direct anergy in autoreactive CD4 T cells and activation of Tregs (16).
Fig. 5.
CD4+CD25+ Tregs are required for tolerance induction by i.v. treatment with ECDI-treated donor splenocytes but not for tolerance maintenance. (A) PC61 treatment at the time of tolerance induction abrogated graft protection in recipients receiving ECDI-treated donor cell infusions. Day 0 indicates the day of islet transplantation. The dotted line indicates the blood glucose level of 250 mg/dl, which was used to diagnose graft rejection. (B) Quantification of the CD4+CD25+Foxp3+ T-cell population in peripheral lymphoid organs from rejecting, tolerized, or PC61-treated recipients on day 15 after transplantation. Data were expressed as the percentage of total CD4+ T cells that were Foxp3+ cells. dLNs = draining lymph nodes; pLNs = peripheral lymph nodes. *Rejecting vs. tolerized, P = 0.0076; **, PC61-treated vs. tolerized, P = 0.0013; #PC61-treated vs. tolerized, P = 0.0086; &PC61-treated vs. tolerized, P = 0.026. (C) Anti-donor IFN-γ production by rejecting, tolerized, or PC61-treated recipients. *Rejecting vs. tolerized, P = 0.0009; **rejecting vs. PC61-treated, P = 0.0012. (D) PC61 treatment in long-term tolerized recipients (n = 3). Treatment was given on day 118 and day 120 after islet transplantation as indicated by the arrows. Blood glucose levels were followed for an additional 50 days after PC61 treatment. (E) Anti-TGF-β treatment at the time of tolerance induction abrogated graft protection in recipients receiving infusions of ECDI-treated donor cell.
In contrast, PC61 treatment in long-term tolerized recipients (120 days after transplantation) did not break the established tolerance, because all treated mice remained normoglycemic during the ensuing observation period (Fig. 5D) despite evidence showing an absence of CD25+ cells in peri-islet infiltrates after PC61 treatment (Fig. S2). These results indicate that CD4+CD25+ Tregs are critical for the initial establishment of the donor-specific tolerance, but active regulation is less important once tolerance has been established. Thus, other mechanisms, such as anergy, may be required for long-term maintenance of donor-specific unresponsiveness.
A recent study (22) of CD3-specific antibody-induced immune tolerance revealed an important role of TGF-β in the in vivo induction of CD4+Foxp3+ regulatory T cells. We next examined whether TGF-β plays a role in tolerance by our protocol. As shown in Fig. 5E, treatment with anti-TGF-β completely abolished tolerance induction, resulting in three of three grafts being rejected between posttransplantation day 6 and day 14. Therefore, TGF-β plays an obligatory role in the tolerance induced by ECDI-treated donor cells.
Programmed Death-1/Programed Death Ligand-1 Signaling Is Crucial for Donor-Specific Tolerance Induced by Ethylene Carbodiimide-Treated Donor Splenocytes.
We next examined whether cell-surface inhibitory molecules play a role in tolerance. The PD-1 pathway is up-regulated upon T-cell activation and has been implicated in controlling intrinsic T-cell function under tolerogenic conditions (17). We thus tested the efficacy of infusing ECDI-treated donor splenocytes in diabetic PD-L1–deficient mice receiving BALB/c islet grafts. Untreated diabetic PD-L1−/− mice rejected BALB/c islet grafts with kinetics (Fig. 6A) similar to those in untreated wild-type B6 recipients (Fig. 1B). Interestingly, long-term donor-specific tolerance was not induced in diabetic PD-L1−/− mice tolerized with ECDI-treated donor BALB/c splenocytes, because the majority of recipients rejected islet allografts within 10 to 20 days. These data suggest that the PD-1/PD-L1 signaling plays an important role in this tolerance regimen.
Fig. 6.
Absence of PD-L1 signaling impairs tolerance induction mediated by the infusions of ECDI-treated donor cells. (A) Islet graft survival in PD-L1−/− recipients with or without ECDI treatment. Day 0 indicates the day of islet transplantation. (B) Anti-donor IFN-γ production by PD-L1−/− vs. wild-type recipients receiving ECDI treatment. *, PD-L1−/− ECDI-treated vs. wild-type ECDI-treated; P = 0.0005. (C) Quantification of the CD4+CD25+Foxp3+ T-cell population in peripheral lymphoid organs from PD-L1−/− vs. wild-type recipients receiving ECDI-treated cells.
Interestingly, production of IFN-γ by splenic T cells from ECDI-treated PD-L1−/− recipients was enhanced significantly in comparison with T cells from wild-type tolerized recipients and even in comparison with T cells from wild-type untreated mice (Fig. 6B). However, compared with untreated rejecting recipients (Fig. 5B), the increase in the number of CD4+CD25+Foxp3+ Tregs in the peripheral lymphoid organs of PD-L1−/− recipients of ECDI-treated cells and in wild-type tolerized hosts is similar (Fig. 6C). Collectively, these data suggest that, whereas PD-1/PD-L1–negative signaling plays a crucial role in the initial induction of tolerance by down-regulating donor-specific IFN-γ responses, the expansion and/or induction of the Treg population by ECDI treatment is independent of the PD-1/PD-L1 pathway.
Discussion
Here, we show that two infusions of ECDI-treated donor splenocytes lead to permanent acceptance of allogeneic islet grafts in fully immunocompetent recipients without the need for immunosuppression. The tolerance state induced by the ECDI-treated donor cells is donor-specific and is associated with markedly diminished donor-specific allo-responses. Tolerance by this protocol is mediated through a PD-1/PD-L1–dependent down-regulation of Teff activity and an independent up-regulation of Tregs. However, once tolerance is established, Treg impairment does not break the tolerant state, and the hosts accept a second same-donor graft indefinitely without further intervention.
The critical difference between this tolerance protocol and others using infusions of donor cells (e.g., donor-specific transfusion) is that efficient induction of tolerance is achieved in the complete absence of immunosuppression, including transient cell depletion, antibody-mediated blockade of costimulatory signals, or peri-transplantation application of immunosuppressive drugs. Cell ablation around the time of transplantation is thought to debulk alloreactive T cells, which are present at high frequencies (23, 24), inducing a favorable ratio of regulatory cells to effector cells. Increasing understanding of Treg biology has resulted in cell therapy using allogeneic transplantation of ex vivo expanded CD4+CD25+ Tregs in animal models, and this strategy now is being tested clinically (25). However, several rounds of ex vivo stimulation are required to obtain sufficient numbers of Tregs for in vivo suppression (26), and initial depletion of recipient T cells still is required for its success. Several other approaches are now in human trials in solid-organ transplantation, including infusion of donor bone marrow stem cells with or without induction of mixed chimerism (27, 28). Similarly, these approaches also require initial myeloablation, which is associated with significant comorbidities. The fact that infusion of ECDI-treated donor cells induces durable tolerance in the absence of any immunosuppression makes this potential therapy highly desirable.
In islet cell transplantation, another concern is recurrent autoimmunity toward the transplanted β cells. Similar to our published work in EAE (18), our preliminary data indicate that tolerance induced with ECDI-fixed syngeneic APCs coupled with either the immunodominant insulin peptide InsB9–23 or intact insulin prevents onset of diabetes or induces remission in new-onset disease, respectively, in NOD mice (S.D.M., unpublished data). This finding confirms earlier data showing that InsB9–23 probably is the initiating diabetogenic epitope in NOD (29). Therefore, ECDI-treated cells potentially can induce tolerance in both alloantigens and the insulin autoantigen, thereby preventing rejection of the allogeneic islet graft and recurrence of autoimmunity in patients who have type 1 diabetes.
The exact mechanism with which ECDI-treated cells induce donor-specific tolerance is not completely understood. Recent studies indicate that ECDI treatment induces the cells to undergo rapid apoptosis and that tolerance is induced by both direct and indirect antigen presentation (18). Cell tracking indicates that ECDI-treated cells distribute widely, but intact cells disappear within 48 hours (S.D.M., unpublished observations). Therefore, although direct presentation may play a role, this mechanism probably is transient. In contrast, indirect presentation of alloantigens by host regulatory APCs probably is the predominant tolerance mechanism. Other models of allogeneic transplantation also indicate that the indirect pathway plays a critical role in donor-specific tolerance (30, 31). Recent data in the EAE model suggest that host plasmacytoid dendritic cells are crucial in this tolerogenic cross-presentation and that the tolerogenic interactions probably occur in the spleen (S.D.M., unpublished observations).
The depletion of Tregs around the time the first injection of the ECDI-treated donor cells abolished tolerance induction suggests that initial presence of Tregs is crucial for conferring the tolerant state. However, as seen in the control animals, once the process of rejection begins, the number of Tregs observed both in the peripheral lymphoid organs and in the rejecting grafts increases as compared with naïve hosts. This phenomenon has been observed previously in other models of graft rejection (32, 33) and may represent an intrinsic attempt by the host to control inflammation that ultimately fails, probably because of an unfavorable Treg:Teff balance. Infusion of ECDI-treated donor cells probably promotes early establishment of a favorable Treg:Teff ratio and possibly enhances Treg function. It therefore is intriguing that, once tolerance is established in our model, depletion of Tregs does not ameliorate the tolerant state. It is possible that donor-specific Teff cells are kept effectively in a state of anergy by continuous interaction with the tolerated graft. The anergy hypothesis is supported by persistently depressed proliferation and IFN-γ production by T cells in mixed lymphocyte reaction cultures (data not shown) and by the significantly fewer numbers of Foxp3+ T cells in the graft bed observed in the long-term tolerized hosts (compare Fig. 2 A and B) and by similarly depressed T-cell responses in hosts tolerized after Treg depletion (Fig. 5C). Therefore, anergy may be the major mechanism for tolerance maintenance, as suggested in other models of tolerance (34).
The importance of PD-1/PD-L1–negative signaling unresponsiveness induced in ECDI-treated cells was substantiated further by the observation that tolerance induction was disrupted significantly in PD-L1−/− recipients. PD-L1 is widely expressed on leukocytes and in nonlymphoid tissues including the pancreatic islets (35), although PD-1/PD-L1 signaling in the graft site is clearly not sufficient for induction of tolerance because PD-L1–sufficient mice were used as islet donors. It is interesting that ECDI-treated donor cell infusions induced a similar increase in Tregs in both wild-type and PD-L1−/− recipients, suggesting that the induction and/or expansion of Tregs by this protocol is independent of PD-L1 signaling. In addition, suppression of IFN-γ production was abolished in PD-L1−/− recipients, suggesting a direct effect of PD-1 signaling on Teff cells. Because ECDI-fixed cell tolerance also was prevented by depletion of Tregs, our data collectively suggest that both T-cell anergy and T-cell regulation are involved independently in the process of tolerance induction. During the initial phase of tolerance, it is critical to establish a favorable Treg:Teff cell balance, both in numbers and in function. Consequently, impairment of such a balance, either by impairing Tregs with anti-CD25 antibody or by blocking Teff cell anergy via the PD-1/PD-L1 pathway, results in failure of tolerance induction.
In summary, we report a simple but highly effective donor-specific tolerance protocol for promoting successful allogeneic islet cell transplantation. The lack of a need for any immunosuppression and the avoidance of associated toxicities make this protocol a highly attractive potential therapy for human islet cell transplantation.
Materials and Methods
Mice.
Eight- to 20-week-old male BALB/c (H2d) and C57BL/6 (H2b) mice were purchased from the Jackson Laboratory. Male PD-L1−/− mice on C57BL/6 background were obtained from Lieping Chen, Johns Hopkins University. All mice were housed under specific pathogen-free conditions at Northwestern University (NU). Protocols were approved by the NU Institutional Animal Care and Use Committee.
Antibodies.
APC-conjugated anti-CD25 (PC61) and FITC-conjugated anti-CD25 (7D4), anti-CD4 (GK1.5), and PE-conjugated anti-CD4 (L3T4; GK1.5) were from BD Biosciences. PE-conjugated anti-mouse Foxp3 (FJK-16a) was from eBiosciences. PC61 antibody (rIgG1, given at 0.5 mg per mouse every other day for two doses) was from Bio Express. Anti-TGF-β antibody (rIgG1, given at 0.3 mg per mouse every other day for six doses) was from R&D.
Coupled Cell Tolerance.
Tolerance was induced by i.v. injection of ECDI-treated splenocytes as described in ref. 36. Briefly, spleens were processed into single-cell suspensions. Red blood cells were lysed, and splenocytes were incubated with ECDI (Calbiochem, 150 mg/ml per 3.2 × 108 cells) on ice for 1 hour with agitation followed by washing; 108 ECDI-treated splenocytes in 200 ml of PBS were used for each injection.
Diabetes Experiments.
Mice were treated with streptozotocin (Sigma Aldrich) at 170 mg/kg. Two consecutive glucose readings (1 day apart) >250 mg/dl were used to diagnose diabetes. The protocol for islet isolation and transplantation has been described previously in ref. 10. Approximately 500 islets were implanted under the kidney capsule of recipient mice. Graft rejection was determined by two consecutive blood glucose readings > 250 mg/dl.
Delayed-Type Sensitivity, Mixed Lymphocytic Reactions, Cytokine, and Anti-Donor Antibody Measurement.
Ear thickness was measured at baseline with a Mitutoyo engineer's micrometer (Schlesinger's Tool). A total of 107 irradiated BALB/c and control B6 splenocytes in 10 μl PBS were injected into either ear, and swelling was determined 24 hours later. Results were reported as the difference in mean swelling between the donor- and recipient-challenged ears in units of 10−4 inches. For mixed lymphocyte reactions, a total of 105 cells per well of Thy1.2+ T cells from B6 islet recipients were cultured in a 96-well plate with irradiated splenocytes from donor BALB/c mice at a 5:1 APC/T-cell ratio. Cultures were pulsed with 1 μCi per well of [3H]thymidine (PerkinElmer) during the last 18 hours of a 5-day culture. Culture supernatants were analyzed with the Liquichip Mouse 10-cytokine assay kit (Qiagen) and confirmed with ELISA for IFN-γ (BD Biosciences). For donor-specific antibody measurement, blood was collected with heparin, lysed, and incubated with splenocytes from BALB/c mice for 1 hour. Cells then were washed and stained with PE-conjugated anti-B220 mAb and with FITC-conjugated anti-IgM, anti-IgG1, anti-IgG2a, anti-IgG2b, and anti-IgG3 mAbs (BD PharMingen). Syngeneic cells were used as negative control.
Immunohistochemistry and Immunofluorescence.
Snap-frozen sections of islet grafts were blocked with peroxidase (Dako). Anti-insulin rabbit IgG antibody (Dako) was used to detect islets. For detection of CD4+Foxp3+ T cells, sections were blocked with anti-CD16/32 and incubated with anti-Foxp3 mAb (rIgG2a clone FJK-16; eBiosciences). CD4 and CD8 staining were accomplished by anti-CD4-FITC mAb (rIgG2a clone RM4–5; eBiosciences) and biotinylated anti-mouse CD8a; BD PharMingen). Antibody binding was visualized using a secondary antibody or streptavidin conjugated to HRP.
Statistical Analysis.
Statistical analysis was performed using Student's unpaired t test for DTH, mixed lymphocyte reactions, and cytokine assays. ANOVA was used to analyze allogeneic islet graft survival. P values of < 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Joseph Gugenheim, Mr. and Mrs. V. Robert Rottering, and Susan Mardell for support of our research through private gifts. This work was supported in part by the Juvenile Diabetes Research Foundation (1–2007-1055), the National Institutes of Health (NS-026543, K08 DK-070029), the National Multiple Sclerosis Society (RG 3965-A-8), and the Myelin Repair Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805204105/DCSupplemental.
References
- 1.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Mohanakumar T, et al. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006;82:180–187. doi: 10.1097/01.tp.0000226161.82581.b2. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PM, et al. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7:2311–2317. doi: 10.1111/j.1600-6143.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 5.Lobo PI, et al. Development of anti-human leukocyte antigen class 1 antibodies following allogeneic islet cell transplantation. Transplant Proc. 2005;37:3438–3440. doi: 10.1016/j.transproceed.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyder A, Laue C, Schrezenmeir J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005;19:541–546. doi: 10.1016/j.tiv.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation—how much of the promise has been realized? Nat Med. 2005;11:605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 9.Long ET, Wood KJ. Regulatory T cells—a journey from rodents to the clinic. Front Biosci. 2007;12:4042–4049. doi: 10.2741/2370. [DOI] [PubMed] [Google Scholar]
- 10.Luo X, et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25− T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy MK, et al. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. J Immunol. 1990;144:909–915. [PubMed] [Google Scholar]
- 12.Tan LJ, Kennedy MK, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. II. Fine specificity of effector T cell inhibition. J Immunol. 1992;148:2748–2755. [PubMed] [Google Scholar]
- 13.Vanderlugt CL, et al. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 14.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 15.Kohm AP, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 16.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 17.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 20.Elliott C, Wang K, Miller S, Melvold R. Ethylcarbodiimide as an agent for induction of specific transplant tolerance. Transplantation. 1994;58:966–968. doi: 10.1097/00007890-199410270-00023. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko K, Morelli AE, Wang Z, Thomson AW. Alloantigen presentation by ethylcarbodiimide-treated dendritic cells induces T cell hyporesponsiveness, and prolongs organ graft survival. Clin Immunol. 2003;108:190–198. doi: 10.1016/s1521-6616(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 22.Perruche S, et al. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 23.Bushell A, Morris PJ, Wood KJ. Transplantation tolerance induced by antigen pretreatment and depleting anti-CD4 antibody depends on CD4+ T cell regulation during the induction phase of the response. Eur J Immunol. 1995;25:2643–2649. doi: 10.1002/eji.1830250936. [DOI] [PubMed] [Google Scholar]
- 24.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008;8:298–306. doi: 10.1111/j.1600-6143.2007.02088.x. [DOI] [PubMed] [Google Scholar]
- 25.Bluestone JA. Regulatory T-cell therapy: Is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 26.Koenen HJ, Joosten I. Antigen-specific regulatory T-cell subsets in transplantation tolerance regulatory T-cell subset quality reduces the need for quantity. Hum Immunol. 2006;67:665–675. doi: 10.1016/j.humimm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scandling JD, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 31.Joffre O, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthukumar T, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 33.Schneider TM, et al. Development of suppressor lymphocytes during acute rejection of rat cardiac allografts and preservation of suppression by anti-IL-2-receptor monoclonal antibody. Transplantation. 1986;42:191–196. doi: 10.1097/00007890-198608000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Cook CH, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180:3103–3112. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 36.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.