Abstract
Ribonucleotide reductase (RNR) catalyzes the conversion of nucleotides to deoxynucleotides and is essential in all organisms. Class I RNRs consist of two homodimeric subunits: α2 and β2. The α subunit contains the site of nucleotide reduction, and the β subunit contains the essential diferric-tyrosyl radical (Y•) cofactor. Escherichia coli contains genes encoding two class I RNRs (Ia and Ib) and a class III RNR, which is active only under anaerobic conditions. Its class Ia RNR, composed of NrdA (α) and NrdB (β), is expressed under normal aerobic growth conditions. The class Ib RNR, composed of NrdE (α) and NrdF (β), is expressed under oxidative stress and iron-limited growth conditions. Our laboratory is interested in pathways of cofactor biosynthesis and maintenance in class I RNRs and modulation of Y• levels as a means of regulating RNR activity. Our recent studies have implicated a [2Fe2S]-ferredoxin, YfaE, in the NrdB diferric-Y• maintenance pathway and possibly in the biosynthetic and regulatory pathways. Here, we report that NrdI is a flavodoxin counterpart to YfaE for the class Ib RNR. It possesses redox properties unprecedented for a flavodoxin (Eox/sq = −264 ± 17 mV and Esq/hq = −255 ± 17 mV) that allow it to mediate a two-electron reduction of the diferric cluster of NrdF via two successive one-electron transfers. Data presented support the presence of a distinct maintenance pathway for NrdEF, orthogonal to that for NrdAB involving YfaE.
Keywords: metallocofactor maintenance, NrdF, metallocofactor biosynthesis, iron homeostasis
Ribonucleotide reductases (RNRs) catalyze the conversion of nucleotides to deoxynucleotides in all organisms, providing the building blocks for DNA synthesis and repair and playing an essential role in controlling cellular deoxynucleotide pools (1). Class Ia and Ib RNRs in Escherichia coli consist of two homodimeric subunits: α2 and β2. The α subunit contains the site of nucleotide reduction and binding sites of the allosteric effectors that govern specificity and rate of substrate reduction. The β subunit contains a diferric-tyrosyl radical (Y•) cofactor. The essential Y• varies in stability from days in the purified β2 of E. coli to minutes in β2 of mouse. The concentration of the Y• is directly correlated with RNR activity in vitro and in vivo and hence Y• modulation (2, 3) potentially provides a method of RNR regulation. Our recent efforts have been focused on identifying factors involved in the assembly of the diferric-Y• cofactor in the class Ia β2 (biosynthetic pathway), factors that can reduce the Y• (regulatory pathway), and factors that can reactivate the Y•-reduced, diferric form of the protein (met-β2, maintenance pathway) (Fig. 1). Our studies have demonstrated the importance in E. coli of a [2Fe2S]-ferredoxin (Fd), YfaE, in the class Ia maintenance and possibly biosynthetic pathways (3, 4). We now report that NrdI is a flavodoxin (Fld), functioning as a two-electron reductant of NrdF (β2), and can play a role in the maintenance pathway of the E. coli class Ib RNR analogous to that of YfaE in the E. coli class Ia system.
Fig. 1.
Model for E. coli NrdB and NrdF biosynthesis, maintenance, and regulation. The model is based on recent studies of NrdB, the class Ia RNR (3, 4), and the present studies of NrdF, the class Ib RNR. Y122 (NrdB) and Y105 (NrdF) are the precursors to the Y•. The involvement of a metal (M) other than Fe in vivo has not been ruled out in the case of NrdF. M may be Fe (13), Mn (39), or Mn/Fe (40). O2 and an extra electron have been shown to be required for cluster assembly of NrdB. The substoichiometric assembly of cluster in NrdF suggests further investigation of cluster assembly is required. Fre may play an important role in the maintenance pathway and may be a reductase for YfaE (4, 41).
E. coli contains genes for three RNRs that have been classified based on their metallocofactors: two class I RNRs (Ia and Ib) and a class III RNR. The class Ia RNR is the workhorse enzyme and is expressed under normal aerobic, vegetative growth conditions, whereas the class Ib enzyme is expressed under oxidative stress and iron-limited growth conditions (5–8). The class III RNR is expressed only under anaerobic conditions and will not be discussed further. The E. coli class Ia RNR is composed of two gene products, NrdA (α) and NrdB (β), and the genes are found in an operon with a [2Fe2S]-Fd, YfaE. The E. coli class Ib RNR is composed of NrdE (α) and NrdF (β) and the genes are found in an operon (nrdHIEF) with two additional genes. NrdH is a thioredoxin-like protein that functions as the specific disulfide reductase for NrdE (9). NrdI has yet to be functionally characterized. However, NrdI is annotated in the genomic databases as a Fld and a recent structure of the Bacillus subtilis NrdI (Protein Data Bank ID code 1RLJ) shows a protein with a Fld fold and bound FMN, supporting this annotation. To understand the interplay of the class Ia and Ib RNRs and the mechanisms by which their metallo-Y• cofactors are biosynthesized and maintained, and by which the concentration of Y• is regulated, we have begun to examine the proteins found in the class Ib operon.
E. coli NrdE and NrdF have not been previously purified, although the equivalent proteins from Salmonella typhimurium, sharing 89 and 87% sequence identity, respectively, have been purified (10) and their structures have been determined (11, 12). Efforts to purify NrdI have been reported but were hampered by poor solubility. E. coli NrdI purified to 50% homogeneity was reported to stimulate by ≤2-fold the activity of S. typhimurium NrdEF (9). The basis for this observation has remained unexplored.
Here, we report the cloning, overexpression, purification, and characterization of the E. coli class Ib RNR, NrdE, and NrdF and the Fld NrdI (and an N-terminally His6-tagged NrdI, HisNrdI). HisNrdI was overexpressed as inclusion bodies that were resolubilized and refolded in the presence of FMN, giving rise to large amounts of soluble NrdI containing noncovalently bound FMN. The three different redox states of FMN in HisNrdI were characterized by UV-visible (vis) absorption and EPR spectroscopies, and the reduction potentials governing their interconversions were determined. The unusual redox properties of HisNrdI allow it to function, anaerobically, as a two-electron reductant of the Y•-reduced diferric cluster in met-NrdF. Admission of O2 to diferrous NrdF results in rapid regeneration of the diferric-Y• cofactor. Our results support a role for NrdI in an in vivo maintenance pathway for NrdEF that is orthogonal to that for NrdAB involving YfaE (Fig. 1). They further point to the generality of maintenance pathways in RNRs to reactivate these essential enzymes when the Y• becomes reduced, whether by cellular regulatory mechanisms (e.g., cell cycle) or external environmental stresses (e.g., a host's immune response) (3, 4). Indeed, class Ib RNRs are present as the workhorse RNRs in many prokaryotes, including many eukaryotic pathogens, such as Bacillus anthracis and Staphylococcus aureus (http://rnrdb.molbio.su.se). We propose that the utility of the class Ib NrdHIEF system in E. coli and many bacteria under Fe-limiting and oxidative stress conditions is caused, at least in part, by the involvement of a Fld instead of a Fd in its cofactor maintenance pathway.
Results
Purification of HisNrdE and NrdF.
WT NrdF and HisNrdE were purified to 95% homogeneity by standard procedures [supporting information (SI) Text]. NrdF contained 0.33 Y• and 3.1 Fe/β2 when FeII and ascorbate were added to the crude extracts before purification, and 0.50 Y• and 3.6–3.8 Fe/β2 when β2 was purified in the apo form and reconstituted with FeII and O2. Its EPR spectrum (gav ≈2.0054; Fig. S1) was very similar to that previously reported for the S. typhimurium NrdF and other class Ib β2s (10, 13). The specific activities of E. coli HisNrdE and NrdF (0.33 Y•/β2), each determined with a 5-fold excess of the other subunit, using DTT as the reductant, were 110 and 195 nmol/min per mg, respectively. These numbers are similar to those reported for other class Ib subunits. In general, assembly of the diferric-Y• cofactor in class Ib β2s in vitro has resulted in 0.3–0.5 Y•/β2 (Table S1), distinct from the class Ia β2 where the levels are 1.2–1.3 Y•/β2. The inability to generate high levels of Y• and Fe/β2 emphasizes the importance of understanding cofactor generation in vivo.
Purification of HisNrdI.
Based on the presence of nrdI within the nrdHIEF operon and the induction of this operon under Fe-limited growth conditions and oxidative stress (5–7), we postulated that NrdI might replace the Fe-requiring, O2-labile, [2Fe2S]-Fd YfaE (4) in the maintenance pathway of the diferric-Y• cofactor for NrdF (Fig. 1). To test this hypothesis we cloned and expressed untagged NrdI (15.3 kDa) and HisNrdI (17.3 kDa). The untagged protein was poorly overexpressed, whereas HisNrdI was overexpressed to ≈30% of cellular protein but found predominantly in inclusion bodies. Small amounts of soluble untagged and His6-tagged NrdI were purified by conventional chromatographic methods. In each case, the vis spectrum exhibited features characteristic of a flavin. HPLC analysis of the small molecules isolated from the supernatant subsequent to protein denaturation revealed the bound cofactor to be FMN (Table S2). In neither case, however, was FMN incorporation stoichiometric.
Because of the high levels of expression of HisNrdI and previous reports that Flds can be refolded (14), the inclusion bodies became the focus of our attention. They were purified and solubilized in buffer containing 8 M urea and 10 mM DTT. The solubilized protein was then refolded by rapid dilution into buffer without urea in the presence of FMN. The protein was concentrated on an SP Sepharose column and eluted with 200 mM NaCl, yielding homogeneous HisNrdI as judged by SDS/PAGE. A typical yield of 50–60 mg HisNrdI per g of inclusion bodies was obtained.
Spectroscopic Characterization of HisNrdI.
The UV-vis absorption spectrum of oxidized (ox) HisNrdI is shown in Fig. 2 (solid line). At pH 7.0, it exhibits maxima at 275, 380, and 454 nm, with shoulders at ≈425 and ≈480 nm. The extinction coefficient of the oxidized, protein-bound FMN at 454 nm (ε454ox) was found to be 11.0 mM−1·cm−1 by the method of Mayhew and Massey (15) and was used to determine the concentration of HisNrdI in all experiments.
Fig. 2.
UV-vis spectra of HisNrdI in the ox (solid line), sq (dotted line), and hq (dashed line) forms, in units of mM−1·cm−1. The sq spectrum was estimated as described in Materials and Methods.
To determine the spectrum of the hydroquinone (hq) form of HisNrdI (Fig. 2, dashed line; ε454hq = 0.8 mM−1·cm−1), anaerobic titrations with sodium dithionite were carried out. Unusual stoichiometries caused us to examine HisNrdI by SDS/PAGE in the absence of β-mercaptoethanol, revealing a ≈35-kDa band in addition to the expected ≈17-kDa HisNrdI. The HisNrdI monomer thus appears to readily form an intermolecular disulfide. Upon preincubation with Tris(2-carboxyethyl)phosphine (5 mM for 5 min), the HisNrdI could be fully reduced by a stoichiometric amount of dithionite.
Typical Flds can stabilize near-stoichiometric amounts of the neutral semiquinone (sq) form of FMN, as the differences in reduction potential between the protein-bound ox/sq and sq/hq couples are on the order of 200–300 mV. To our surprise, however, reductive titration of NrdI revealed low amounts of neutral sq, detectable at 575 nm (Fig. S2). Attempts to obtain the sq spectrum and ε575sq from such a titration, either by a plot of A454 versus A575 (15) or spectral deconvolution using evolving factor analysis (16), failed because all three redox states coexisted in significant amounts throughout the titration. Ultimately, ε575sq was determined to be 3.4 mM−1·cm−1 by correlation of the vis and EPR spectra [acquired using an aqueous flat cell, with spin quantitation using NrdF Y• as a standard (17)] of HisNrdI, partially reduced with a defined amount of dithionite. Using ε575sq and titrations of HisNrdI with dithionite, the spectrum of the pure sq was extracted and is consistent with those reported for other Flds (Fig. 2). From these results, the maximum amount of sq stabilized by HisNrdI was calculated to be ≈28%, predicting that the reduction potential of the sq/hq couple (Esq/hq) is higher than that of the ox/sq couple (Eox/sq) by ≈14 mV at 25°C, using Eq. 1:
where K is the sq formation constant, defined as [sq]2/([hq][ox]).
Reduction Potential Determination.
The predicted similarity of Eox/sq and Esq/hq is unusual relative to generic Flds and essential to understanding NrdI's function. These potentials were thus measured spectrophotometrically by the xanthine oxidase (XO) method at 25°C, pH 7.0 (18) with data analysis using the Michaelis equation (Eq. 2 and Materials and Methods). This method uses catalytic, anaerobic oxidation of xanthine to urate by XO as a source of reducing equivalents, an indicator dye of known midpoint potential [phenosafranin (PS), Em = −252 mV at pH 7.0, 25°C (19)], and a low potential dye as a mediator [methyl viologen (MeV)] (Fig. S3). To enhance the sensitivity of the analysis because of the small amount of sq formed in the experiments, difference spectra were obtained by subtraction of the spectrum before XO addition from each subsequent spectrum (16). The difference spectra were then fit to dye (PSred − PSox) and protein (hq − sq, sq − ox) difference spectra by using multiple linear regression analysis.
Analysis of the datasets yielded an average redox potential (Em) of −260 ± 10 mV and a K value of 0.7 ± 0.2 (Fig. S4). Using Eq. 1, these values correspond to Eox/sq = −264 ± 17 mV and Esq/hq = −255 ± 17 mV. The large errors are primarily caused by the overlapping vis spectra of the oxidized PS and the sq, the substantial difference in their extinction coefficients, and the low amounts of sq formed during the experiment. However, Eox/sq and Esq/hq are consistent with the predicted difference in Eox/sq and Esq/hq based on the titrations with dithionite. These reduction potentials indicate that NrdI is able to transfer two electrons (one at a time in rapid succession), an unprecedented role for a Fld.
Titration of Met-NrdF with Reduced HisNrdI.
We hypothesized that NrdI plays a role in the NrdEF system analogous to YfaE's role in the NrdAB system (Fig. 1). To test this model, met-NrdF (≈0.002 Y•/β2) was prepared by reduction of the Y• of NrdF (0.33 Y•/β2) by hydroxyurea (HU). In the absence of O2, HisNrdI (≈100 μM, reduced to hq by titration with dithionite) was titrated into a cuvette containing met-NrdF (≈20 μM, 4.5 nmol), and spectra were recorded from 300–800 nm after each addition. The reduction of met-NrdF was monitored at 341 nm, the isobestic point of the hq and ox forms of HisNrdI. Representative spectra during the course of the titration are shown in Fig. 3. Upon reaching an endpoint, judged by the ability to attribute the full absorbance change at 341 nm to the amount of HisNrdI added, O2 was added to allow diferric-Y• cofactor assembly. The resulting difference spectrum (Fig. 3 Inset) demonstrates regeneration of Y•. The results of several titration experiments are summarized in Table 1. Stoichiometric reduction of met-NrdF was observed, with 1.9 Fe reduced/HisNrdI oxidized. The Y•/β2 recovered was similar to the Y• in the starting NrdF, even though ≈80% of the total met-NrdF-bound Fe was reduced. Similar titrations were carried out with NrdF (0.50 Y•/β2) reconstituted from apoprotein, with 1.8 Fe reduced/HisNrdI oxidized and 0.38 Y•/β2 recovered (Table 1). These results demonstrate that HisNrdI is chemically competent to carry out stoichiometric reduction of met-NrdF and suggest a role in NrdF maintenance.
Fig. 3.
Anaerobic titration of met-NrdF with reduced HisNrdI. Met-NrdF (4.4 nmol, 14.9 nmol Fe; thin line) was titrated with 2.5 and 5.0 nmol of reduced HisNrdI (dashed lines) and to an endpoint with 5.9 nmol of reduced HisNrdI (thick line). After addition of O2 to assemble diferric-Y• cofactor, a final spectrum was acquired (dotted line). (Inset) Difference spectrum between the endpoint of the titration with HisNrdI and addition of O2. The arrow indicates the sharp 408-nm feature associated with Y•. The feature at 450–500 nm is from oxidation of the small amount of excess HisNrdI added to verify that the endpoint had been reached.
Table 1.
Stoichiometry of Fe reduction in anaerobic titrations of met-NrdF with reduced HisNrdI
| Total Fe, nmol | Fe reduced, nmol | HisNrdI added, nmol | Fe reduced/HisNrdI added | Y•/β2 |
|---|---|---|---|---|
| 14.9* | 11.6 ± 0.4 | 6.2 ± 0.2 | 1.9 ± 0.1 | 0.32 ± 0.01 |
| 16.6† | 10.6 ± 0.4 | 5.8 ± 0.2 | 1.8 ± 0.1 | 0.38 ± 0.02 |
*Average (± SD) of four anaerobic titrations of met-NrdF (≈4.5 nmol), reconstituted in crude extracts, with reduced HisNrdI. O2 was added at the endpoint of each titration.
†Average of three anaerobic titrations of met-NrdF (≈4.4 nmol), reconstituted from apoprotein. Different batches of HisNrdI were used in titrations A and B.
Specificity of HisNrdI for Met-NrdF.
A number of control experiments were carried out to demonstrate the physiological importance of HisNrdI in met-NrdF reduction. First, met-NrdF was incubated anaerobically with stoichiometric or excess (relative to Fe) amounts of [2Fe2S]1+-YfaE or free FMNH2 (Table 2). At the end of the incubation, O2 was added and the samples were transferred to EPR tubes and frozen in liquid N2 for Y• quantitation. Analogous experiments with met-NrdB, using either reduced HisNrdI or FMNH2 as a reductant, were also performed. In all cases only 0.02–0.05 Y•/β2 were regenerated, which we attribute to dissociation of reduced FeII, followed by binding to apo-β2 and cluster assembly. The results argue that NrdI is involved in a specific reduction of NrdF, orthogonal to reduction of NrdB by YfaE (Fig. 1).
Table 2.
Orthogonality of the NrdHIEF and NrdAB-YfaE systems
| Reductant | Oxidant | Fe, nmol | Reducing equiv, nmol | Y•/β2 |
|---|---|---|---|---|
| HisNrdI | met-NrdF | 16.3 | 18.6 | 0.29 |
| HisNrdI | met-NrdB | 16.3 | 18.6 | 0.02 ± 0.01 |
| 16.3 | 55.6 | 0.02 ± 0.01 | ||
| YfaE | met-NrdF | 16.3 | 16.3 | 0.05 ± 0.01 |
| 16.3 | 48.9 | 0.04 ± 0.01 | ||
| FMNH2 | met-NrdF | 24.5 | 24.4 | 0.03 ± 0.01 |
| 24.5 | 122 | 0.04 ± 0.01 | ||
| FMNH2 | met-NrdB | 24.5 | 24.4 | 0.03 ± 0.01 |
| 24.5 | 122 | 0.04 ± 0.01 |
A control titration of met-NrdB with YfaE (not shown) gave results as described (4).
Discussion
Initially as a method to judge the success of the refolding of HisNrdI, we focused on characterizing the redox properties of the bound FMN. Typically, the protein environment of Flds stabilizes near-stoichiometric amounts of neutral FMN sq by shifting Esq/hq from −172 mV for free FMN (20) to between −370 and −450 mV for bound FMN (21) and Eox/sq from −238 mV to between −50 and −220 mV for free and bound FMN, respectively. Thus the physiological role of typical Flds is as a one-electron reductant. Our studies indicate that HisNrdI's Eox/sq and Esq/hq values are roughly equivalent: −264 and −255 mV, respectively. This behavior is, to our knowledge, unprecedented for a Fld.
Two arguments suggest that the unusual reduction potentials of NrdI are physiologically interesting. The first is based on an examination of sequence alignments and structures of WT and mutant Flds in comparison with sequence alignments of NrdIs (Fig. S5) and a structure of B. subtilis NrdI. The second is the ability of reduced NrdI to specifically reduce met-NrdF.
Flds have been categorized into two classes, short chain and long chain, which differ by an insertion of ≈20 residues interrupting the final β-strand (22). Structures of both classes of Flds in the three different oxidation states have been determined. Additional structures in which residues suggested to be involved in redox perturbation have been mutated (21, 23), combined with reduction potential measurements of these mutants (23, 24), have given us a framework to think about the unusual properties of NrdI.
The basis for the large perturbation of the sq/hq equilibrium in Flds relative to free FMN is proposed to be largely electrostatic. The reduced FMN is bound in the anionic form with N(1) deprotonated, there is often a D within ≈6 Å of N(1), and there are additional, uncompensated negatively charged residues within the vicinity of the flavin. The negative electrostatic environment around the FMN is proposed to hinder reduction of the sq to the hq, thus lowering Esq/hq (24). In Desulfovibrio vulgaris Fld, for example, seven acidic residues, without compensating positively charged residues, are within 13 Å of the FMN N(1). A homology model (25, 26) of E. coli NrdI based on the structure of B. subtilis NrdI suggests a more neutral or positively charged environment in B. subtilis and E. coli NrdIs, respectively.
In oxidized D. vulgaris Fld, D95, 6.3 Å from the flavin N(1), is particularly interesting. Mutation of this residue to N increased Esq/hq by 46 mV (24). This D95 is conserved in many, although not all, Flds, but the corresponding residue is an N in all NrdIs (Fig. S5). We propose that the D → N substitution in NrdIs and the general, neutral electrostatic environment around the FMN (presuming the hq is anionic in NrdIs as well) may play important roles in the destabilization of the sq form observed in E. coli NrdI relative to other Flds.
The perturbation of the ox/sq equilibrium in generic Flds is proposed to be associated with conformational changes of a flexible loop near the N(5) of FMN (the 50s loop, E. coli NrdI G50GGG53; see Fig. S5) (21, 22, 27). Both long- and short-chain Flds can stabilize the sq and hq forms by protein backbone O-HN(5) interactions. The ability also to form a hydrogen bond in the ox form between N(5) and a protein backbone amide, absent in short-chain Flds, is thought to contribute to the lower Eox/sq values of long-chain Flds (21, 27). Therefore, Eox/sq for NrdI, low for a short-chain Fld, may be explained by the presence of a flexible, G-rich loop in the vicinity of N(5) in most NrdIs. This loop could be responsible for hydrogen-bonding with the N(5) position in all three FMN oxidation states, either as hydrogen bond donor (ox) or acceptor (sq and hq). Thus, although studies need to be carried out to examine the hypotheses raised above, our knowledge of Flds in general allows us to rationalize the differences observed for NrdI and suggest that the unusual reduction potentials of its FMN are physiologically interesting.
The most compelling support that the reduction potentials observed are not an artifact of refolding or the His6 tag is the demonstration that NrdI can reduce NrdF. Anaerobic titration of met-NrdF with reduced HisNrdI resulted in reduction of ≈80% of its Fe and upon admission of O2, diferric-Y• cofactor was generated to the same level as the starting NrdF (Table 1). Our inability to generate higher ratios of Y•/β2, as we observed with the class Ia system (4), suggests that despite its reduction, much of the Fe is not chemically competent in cofactor assembly. Control experiments with FMNH2 yielded ≈10% the levels of Y• observed with reduced NrdI, similar to the results with met-NrdB and FMNH2. These results demonstrate that FMNH2 can only inefficiently reduce met-NrdF (and met-NrdB) to form cofactor and that dissociation of FMNH2 from reduced NrdI does not account for Y• formation. Thus, NrdI can specifically reduce NrdF and is likely involved in diferric-Y• maintenance of the class Ib RNR, analogous to the role proposed for YfaE in the class Ia RNR (Fig. 1). The presence of NrdI and NrdF within the same operon and the fact that Flds in a number of systems have been observed to substitute for Fds in vitro and in vivo under Fe-limited growth conditions (28–30) provide additional support for this proposal.
The fact that 192 of 210 bacterial genomes containing annotated nrdEF genes also contain a nrdI (http://rnrdb.molbio.su.se) strongly suggests that the maintenance function of NrdI will be conserved among organisms encoding a class Ib RNR. Indeed, while our manuscript was in preparation, Sjöberg and coworkers (31) published the results of a study in which the nrdI gene from Streptococcus pyogenes was shown to be essential for activity of the NrdEF system in a heterologous complementation assay in E. coli. This in vivo requirement for NrdI suggests that the specific role we have identified in vitro is applicable in vivo.
Our experiments also suggest explanations for the expression of NrdEF under oxidative stress and Fe-limited growth conditions. Reactive oxygen species are known to degrade Fe-S clusters, and YfaE is, in fact, very O2-sensitive. Thus YfaE may be unable to fulfill its role in NrdB maintenance under these conditions, even if NrdAB is still expressed. Response to Fe limitation would involve decreasing synthesis of nonessential Fe-requiring proteins (32), and thus an RNR using a Fld (NrdI) rather than a [2Fe2S]-Fd would also be more favorable. Furthermore, this switch provides a rationalization for the regulation of nrdHIEF by Fur (8).
Rapid reduction of diferric, met-NrdF to the diferrous state to initiate its reactivation requires two electrons, one at a time. A number of strategies are possible to achieve this goal. The reduction potentials of E. coli HisNrdI appear to have been tuned to ensure rapid transfer of the second electron to mixed-valent (FeIIFeIII) NrdF, assuming that this state is more susceptible to reduction than met-NrdF, as is the case in the E. coli class Ia NrdB (33). An alternative strategy has evolved to reduce met-NrdB. The [2Fe2S]-Fd YfaE is a one-electron reductant. Thus, for YfaE to function efficiently in the delivery of the second electron, the catalytic involvement of a Fd reductase (Fre) (Fig. 1) is likely required, or two YfaEs must be able to bind to a single β. Whether NrdI is present in stoichiometric amounts relative to NrdF, or a Fld reductase such as Fre allows NrdI to function catalytically, remains to be established. Understanding the interactions of NrdI and NrdF and modulation of Y• and thus RNR activity in vivo through model studies in E. coli will hopefully shed light on how the NrdEF system is involved in permitting survival of E. coli and other prokaryotes, including many human pathogens, under oxidative stress and Fe-limited conditions.
Materials and Methods
Materials and General Methods.
See SI Text for details.
Cloning, Expression, and Purification of N-Terminally His-Tagged NrdE and NrdF.
Details are provided in SI Text. nrdE and nrdF were obtained by PCR from WT E. coli K-12 (Yale E. coli Genetic Stock Center) and were cloned into pET-28a (HisNrdE) and pET-24a (NrdF) vectors (Novagen). HisNrdE was purified by Ni-NTA (Qiagen) and Q Sepharose chromatography. NrdF was purified according to previously published procedures for NrdB (SI Text). The concentration of HisNrdE was determined by using ε280 = 177 mM−1·cm−1, estimated by ExPaSy (34). Its purification resulted in ≈1 mg/liter culture (≈95% pure by SDS/PAGE). The concentration of NrdF was determined by using ε280 = 132 mM−1·cm−1 (34). Its purification resulted in ≈120 mg/liter culture (≈95% pure by SDS/PAGE), with 3.1 Fe/β2 and 0.33 Y•/β2.
In Vitro Reconstitution of NrdF.
The diferric-Y• cofactor was also assembled from apo-NrdF as described (SI Text for details). The purification procedure for apo-NrdF resulted in ≈25 mg/liter culture (≈95% pure by SDS/PAGE), and upon reconstitution, 3.6–3.8 Fe/β2 and 0.50 Y•/β2.
EPR Spin Quantitation of Y•.
EPR spectra were recorded at 77 K on a Brüker ESP-300 X-band spectrometer (9.3 GHz, 50-μW power, 2.52 × 103 gain, 1.5-G modulation amplitude). A CuSO4 standard solution was used for spin quantitation (35), with analysis performed by using Win-EPR software (Brüker). The ε408 of Y• (3.1 mM−1·cm−1) was determined by using the dropline method as described (13).
Activity Assays.
The reaction mixture contained in a final volume of 170 μl: 0.2 μM NrdF (or HisNrdE) and 1.0 μM HisNrdE (or NrdF), 0.3 mM dATP, 20 mM DTT, and 0.5 mM [3H]CDP (3,857 cpm/nmol), 50 mM Hepes, 15 mM MgSO4, pH 7.6, at 37°C (13). Analysis was carried out by the method of Steeper and Steuart (36).
Cloning, Expression, and Purification of HisNrdI.
Cloning and expression.
Cloning and expression of E. coli nrdI was carried out as described for nrdE (SI Text), using the primers 5′-GCGGCCAGCATATGAGCCAGCTCGTCTACTTCTC-3′ and 5′-CGTTTGGATCCTCAGGCATTCTGCGGTTGTC-3′, and Taq polymerase (Promega). nrdI was cloned into pET-28a. HisNrdI was overexpressed by induction with isopropyl β-D-thiogalactoside for 4 h at 30°C to isolate soluble protein or at 37°C to isolate inclusion bodies. In both cases, typical yields of cells were ≈2.8–2.9 g of cell paste per liter culture.
Purification of soluble HisNrdI.
Purification was effected by using Ni-NTA affinity and Q Sepharose anion exchange columns as described in SI Text. The yield was ≈30 μg/liter culture (>95% purity), based on 18.5 mM−1·cm−1 (34).
Purification of HisNrdI from inclusion bodies.
Cell paste (≈12 g) was suspended in 60 ml of 50 mM sodium phosphate (pH 7.6), 10% glycerol, and 1 mM PMSF, and passed through a French pressure cell once at 14,000 psi. The lysate was centrifuged at 30,000 × g for 20 min. The pellet was resuspended in 60 ml of 100 mM Tris·HCl, 4% (vol/vol) Triton X-100, 2 M urea, pH 8.0 (4), by vortexing and sonication on ice (7 W for 4 × 1-min increments, with 1 min rest in between), and the suspension was centrifuged at 17,000 × g for 20 min. The resuspension and centrifugation were repeated once. The pellet was washed twice with 60 ml of water, resuspended, and centrifuged at 17,000 × g for 20 min. The procedure yielded ≈0.2 g inclusion bodies/g of cell paste, which were stored at −20°C.
Solubilization, refolding, and purification.
HisNrdI inclusion bodies (200 mg) were solubilized in 80 ml of 50 mM sodium phosphate, 8 M urea (pH 7.0), and 10 mM DTT for 5 h at 25°C. All subsequent operations were performed at 4°C. The solution was added dropwise to a stirring solution of 560 ml of 50 mM sodium phosphate, 20% glycerol, pH 7.0 (buffer A), containing 200 μM FMN and 1 mM EDTA. After being stirred for 4 h in the dark, 6 ml of SP Sepharose Fast Flow resin (preequilibrated with buffer A) was incubated with the refolding solution with stirring for 1 h. The column (1.5 × 4 cm) was packed and washed with 20 column volumes of buffer A. HisNrdI was eluted with 50 mM sodium phosphate, 20% glycerol, 200 mM NaCl, pH 7.0 (buffer B). HisNrdI-containing fractions were identified by their yellow color and the Bradford reagent, pooled, and concentrated with a Millipore Amicon Ultra-15 5K MWCO centrifugal filter. SDS/PAGE (17%) established that the protein was purified to homogeneity. Typical yields were ≈11 mg/g cell paste.
Characterization of HisNrdI.
The identity of the flavin cofactor was determined by the method of Birch et al. (37) as described in SI Text. The ε454ox for HisNrdI was determined by the method of Mayhew and Massey (15), and the hq spectrum and ε454hq were determined by anaerobic titration with a solution of sodium dithionite in buffer B, standardized using a solution of potassium ferricyanide. The ε575sq was determined (SI Text) from correlation of the vis spectrum of a sample of HisNrdI partially reduced with dithionite and the X-band EPR spectrum acquired anaerobically at 20°C in an aqueous flat cell (Wilmad). From the amount of dithionite added and the concentration of sq (using ε575sq), the concentrations of ox and hq HisNrdI were determined. The spectral contributions of the ox and hq forms, scaled by concentration, were subtracted from the total spectrum to give the spectrum of the pure sq.
Determination of Eox/sq and Esq/hq of HisNrdI (18).
HisNrdI was made anaerobic on a Schlenk line, and the other reagents were brought into a glovebox at 4°C in solid form. HisNrdI (≈30 μM), ≈25 μM PS (λmax = 524 nm, ε524 = 34.6 mM−1·cm−1 for oxidized PS), 2 μM MeV, 250 μM xanthine in buffer B, pH 7.0, in a final volume of 400 μl, were placed in an anaerobic cuvette (SI Text). XO (from buttermilk, 0.6 units/mg protein, 1 unit = 1 μmol xanthine oxidized per min at pH 7.5, 25°C; Sigma Aldrich) was also added to the cuvette (at 150 nM), but not in contact with the other reagents. A vis spectrum was acquired from 360–800 nm at 25°C. The concentrations of HisNrdI and PS were determined by fitting this spectrum as a linear combination of the spectra of oxidized HisNrdI and oxidized PS in Matlab (MathWorks). The cuvette was then inverted to add the XO and initiate the reaction and spectra were acquired every 2–4 min until A454 was ≈10% of its initial value. The spectra (≈80–100) were collected for analysis.
Difference spectra for each redox couple (PSox/red, FMNox/sq, and FMNsq/hq) were calculated by subtracting εs of the oxidized from the reduced forms at each wavelength (380 nm ≤ λ ≤ 800 nm). The spectra and εs of the oxidized and reduced PS were determined by titration with a standardized solution of sodium dithionite in buffer B, pH 7.0, at 25°C.
Each dataset was analyzed by subtraction of the average absorbance between 750 and 800 nm, followed by subtraction of the initial oxidized spectrum from each subsequent spectrum (16) between 380 nm ≤ λ ≤ 800 nm. Each difference spectrum was fit as a linear combination of component difference spectra by using multiple linear regression analysis in Matlab. The concentrations of the ox, sq, and hq forms of protein and ox and red forms of PS were calculated from the outputs of the fits. The solution potential (Eh) at each point in the titration was determined from the concentrations of ox and red PS by the Nernst equation, using Em = −252 mV for PS (19). The number of oxidizing equivalents (ξ) present at a given point in the titration was calculated from the concentrations of ox, sq, and hq HisNrdI at that point. Eh was plotted against ξ, and the data points for which Eh was within 30 mV of −252 mV were fit to Eq. 2 (38):
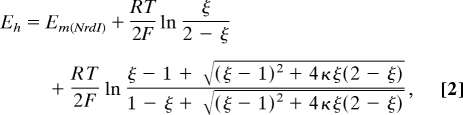 |
where Em(NrdI) = Eh at ξ = 1 and κ = 1/K, where K is the sq formation constant, from which Eox/sq and Esq/hq can be determined by using Eq. 1.
Titration of Met-NrdF with Reduced HisNrdI.
Titration experiments at 25°C were modeled after those described for met-NrdB and YfaE (4). NrdF was reduced with HU to produce met-NrdF, and HisNrdI was reduced with sodium dithionite by standard procedures (SI Text). There was <5% excess dithionite present after reductive titration of HisNrdI, as judged from the 312-nm region (λmax of dithionite) of the UV-vis spectrum of the HisNrdI hq. Protein was degassed on a Schlenk line (evacuation followed by five to six cycles of filling with Ar for 3–5 min) and brought into a 4°C glovebox immediately before use.
Reduction of met-NrdF to diferrous NrdF.
Anaerobic reduced HisNrdI (≈100 μM in buffer B) was loaded into a 100-μl gas-tight syringe, and the needle was inserted through the septum in a 0.5-ml cuvette that contained 240 μl of ≈20 μM met-NrdF in 50 mM Hepes, 5% glycerol, pH 7.6. Reduced HisNrdI was added in 2- or 4-μl aliquots, and a spectrum was recorded after each addition. Titrations were monitored at 341 nm, the isosbestic point of the ox and hq forms of HisNrdI. The amount of Fe(III) in nmol present at a given point in the titration, Nx, was calculated after each addition of reduced HisNrdI as described in SI Text, and the endpoint was judged to have been reached when there was no significant change in Nx upon a further addition of 2 μl of HisNrdI.
Reassembly of diferric-Y• NrdF.
At the endpoint of the titration, O2 was blown over the solution for 5 s, the sample was mixed, and a spectrum was recorded. Repetition of the procedure resulted in no further Y• formation. The sample was transferred to an EPR tube and frozen in liquid N2. The quantity of Y• regenerated was determined by EPR spectroscopy.
Reduction of Met-NrdF and Met-NrdB by NrdI, FMNH2, and [2Fe-2S]1+-YfaE.
HisNrdI (≈100 μM) and FMN (≈450 μM) were prereduced anaerobically with sodium dithionite as described above. [2Fe-2S]1+-YfaE and met-NrdB were prepared as described (4), and met-NrdF was prepared as above. In a glovebox at 4°C in reaction volumes of 240 μl, met-NrdF (20 μM) was mixed with [2Fe-2S]1+-YfaE in the ratios of one or three [2Fe-2S]1+-YfaE per Fe in met-NrdF; met-NrdF (30 μM) with one or five FMNH2 per Fe; met-NrdB (20 μM) with one or three HisNrdI per Fe; and met-NrdB (30 μM) with one or five FMNH2 per Fe. After anaerobic incubation for 5 min at 25°C, oxidation to generate cluster was carried out as above. The solutions were transferred to EPR tubes and frozen in liquid N2 for Y• quantitation.
Supplementary Material
Acknowledgments.
We thank Chia-Hung Wu for cloning nrdI, purifying YfaE, and general assistance and Prof. Catherine Drennan for helpful discussions regarding structural features of Flds. This work was supported by National Institutes of Health Grant GM29595 (to J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807348105/DCSupplemental.
References
- 1.Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 2.Barlow T, Eliasson R, Platz A, Reichard P, Sjöberg BM. Enzymic modification of a tyrosine residue to a stable free radical in ribonucleotide reductase. Proc Natl Acad Sci USA. 1983;80:1492–1495. doi: 10.1073/pnas.80.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hristova D, Wu C-H, Stubbe J. Importance of the maintenance pathway in the regulation of the activity of Escherichia coli ribonucleotide reductase. Biochemistry. 2008;47:3989–3999. doi: 10.1021/bi702408k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CH, Jiang W, Krebs C, Stubbe J. YfaE, a ferredoxin involved in diferric-tyrosyl radical maintenance in Escherichia coli ribonucleotide reductase. Biochemistry. 2007;46:11577–11588. doi: 10.1021/bi7012454. [DOI] [PubMed] [Google Scholar]
- 5.McHugh JP, et al. Global iron-dependent gene regulation in Escherichia coli: A new mechanism for iron homeostasis. J Biol Chem. 2003;278:29478–29486. doi: 10.1074/jbc.M303381200. [DOI] [PubMed] [Google Scholar]
- 6.Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C. Expression analysis of the nrdHIEF operon from Escherichia coli: Conditions that trigger the transcript level in vivo. J Biol Chem. 2001;276:18031–18037. doi: 10.1074/jbc.M011728200. [DOI] [PubMed] [Google Scholar]
- 7.Gon S, Beckwith J. Ribonucleotide reductases: Influence of environment on synthesis and activity. Antioxid Redox Signal. 2006;8:773–780. doi: 10.1089/ars.2006.8.773. [DOI] [PubMed] [Google Scholar]
- 8.Vassinova N, Kozyrev D. A method for direct cloning of Fur-regulated genes: Identification of seven new Fur-regulated loci in Escherichia coli. Microbiology. 2000;146:3171–3182. doi: 10.1099/00221287-146-12-3171. [DOI] [PubMed] [Google Scholar]
- 9.Jordan A, Aslund F, Pontis E, Reichard P, Holmgren A. Characterization of Escherichia coli NrdH: A glutaredoxin-like protein with a thioredoxin-like activity profile. J Biol Chem. 1997;272:18044–18050. doi: 10.1074/jbc.272.29.18044. [DOI] [PubMed] [Google Scholar]
- 10.Jordan A, et al. A second class I ribonucleotide reductase in Enterobacteriaceae: Characterization of the Salmonella typhimurium enzyme. Proc Natl Acad Sci USA. 1994;91:12892–12896. doi: 10.1073/pnas.91.26.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson M, Jordan A, Eklund H. Structure of Salmonella typhimurium nrdF ribonucleotide reductase in its oxidized and reduced forms. Biochemistry. 1998;37:13359–13369. doi: 10.1021/bi981380s. [DOI] [PubMed] [Google Scholar]
- 12.Uppsten M, et al. Structure of the large subunit of class Ib ribonucleotide reductase from Salmonella typhimurium and its complexes with allosteric effectors. J Mol Biol. 2003;330:87–97. doi: 10.1016/s0022-2836(03)00538-2. [DOI] [PubMed] [Google Scholar]
- 13.Huque Y, et al. The active form of the R2F protein of class Ib ribonucleotide reductase from Corynebacterium ammoniagenes is a diferric protein. J Biol Chem. 2000;275:25365–25371. doi: 10.1074/jbc.M002751200. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Recio J, Genzor CG, Sancho J. Apoflavodoxin folding mechanism: An α/β protein with an essentially off-pathway intermediate. Biochemistry. 2001;40:15234–15245. doi: 10.1021/bi010216t. [DOI] [PubMed] [Google Scholar]
- 15.Mayhew SG, Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969;244:794–802. [PubMed] [Google Scholar]
- 16.Blazyk JL, Lippard SJ. Expression and characterization of ferredoxin and flavin adenine dinucleotide binding domains of the reductase component of soluble methane monooxygenase from Methylococcus capsulatus (Bath) Biochemistry. 2002;41:15780–15794. doi: 10.1021/bi026757f. [DOI] [PubMed] [Google Scholar]
- 17.Liu A, et al. The tyrosyl free radical of recombinant ribonucleotide reductase from Mycobacterium tuberculosis is located in a rigid hydrophobic pocket. Biochemistry. 1998;37:16369–16377. doi: 10.1021/bi981471p. [DOI] [PubMed] [Google Scholar]
- 18.Massey V. In: Flavins and Flavoproteins. Curtis B, Roncho S, Zanetti G, editors. Berlin: de Gruyter; 1991. pp. 59–66. [Google Scholar]
- 19.Clark WM. Oxidation-Reduction Potentials of Organic Systems. Baltimore: Williams and Wilkins; 1960. [Google Scholar]
- 20.Draper RD, Ingraham LL. A potentiometric study of the flavin semiquinone equilibrium. Arch Biochem Biophys. 1968;125:802–808. doi: 10.1016/0003-9861(68)90517-1. [DOI] [PubMed] [Google Scholar]
- 21.Hoover DM, et al. Comparisons of wild-type and mutant flavodoxins from Anacystis nidulans: Structural determinants of the redox potentials. J Mol Biol. 1999;294:725–743. doi: 10.1006/jmbi.1999.3152. [DOI] [PubMed] [Google Scholar]
- 22.Drennan CL, et al. Refined structures of oxidized flavodoxin from Anacystis nidulans. J Mol Biol. 1999;294:711–724. doi: 10.1006/jmbi.1999.3151. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy AA, et al. Crystallographic investigation of the role of aspartate 95 in the modulation of the redox potentials of Desulfovibrio vulgaris flavodoxin. Biochemistry. 2002;41:10950–10962. doi: 10.1021/bi020225h. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Swenson RP. Electrostatic effects of surface acidic amino acid residues on the oxidation-reduction potentials of the flavodoxin from Desulfovibrio vulgaris (Hildenborough) Biochemistry. 1995;34:3183–3192. doi: 10.1021/bi00010a007. [DOI] [PubMed] [Google Scholar]
- 25.Bennett-Lovsey RM, Herbert AD, Sternberg MJE, Kelley LA. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins. 2008;70:611–625. doi: 10.1002/prot.21688. [DOI] [PubMed] [Google Scholar]
- 26.Kelley LA, MacCallum RM, Sternberg MJ. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J Mol Biol. 2000;299:499–520. doi: 10.1006/jmbi.2000.3741. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig ML, et al. Control of oxidation-reduction potentials in flavodoxin from Clostridium beijerinckii: The role of conformation changes. Biochemistry. 1997;36:1259–1280. doi: 10.1021/bi962180o. [DOI] [PubMed] [Google Scholar]
- 28.Laudenbach DE, Reith ME, Straus NA. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988;170:258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razquin P, Schmitz S, Fillat MF, Peleato ML, Böhme H. Transcriptional and translational analysis of ferredoxin and flavodoxin under iron and nitrogen stress in Anabaena sp. strain PCC 7120. J Bacteriol. 1994;176:7409–7411. doi: 10.1128/jb.176.23.7409-7411.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancho J. Flavodoxins: Sequence folding, binding, function, and beyond. Cell Mol Life Sci. 2006;63:855–864. doi: 10.1007/s00018-005-5514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roca I, Torrents E, Sahlin M, Gibert I, Sjöberg BM. NrdI essentiality for class Ib ribonucleotide reduction in Streptococcus pyogenes. J Bacteriol. 2008;190:4849–4858. doi: 10.1128/JB.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews SC, Robinson AK, Rodriguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 33.Atta M, Andersson KK, Ingemarson R, Thelander L, Gräslund A. EPR studies of mixed-valent [FeIIFeIII] clusters formed in the R2 subunit of ribonucleotide reductase from mouse or herpes simplex virus: Mild chemical reduction of the diferric centers. J Am Chem Soc. 1994;116:6429–30. [Google Scholar]
- 34.Gasteiger E, et al. In: The Proteomics Protocols Handbook. Walker JM, editor. Totowa, NJ: Humana; 2005. pp. 571–607. [Google Scholar]
- 35.Malmström BG, Reinhammar B, Vanngard T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970;205:48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- 36.Steeper JR, Steuart CD. A rapid assay for CDP reductase activity in mammalian cell extracts. Anal Biochem. 1970;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- 37.Birch OM, et al. MioC is an FMN-binding protein that is essential for Escherichia coli biotin synthase activity in vitro. J Biol Chem. 2000;275:32277–32280. doi: 10.1074/jbc.M004497200. [DOI] [PubMed] [Google Scholar]
- 38.Michaelis L. Semiquinones, the intermediate steps of reversible organic oxidation-reduction. Chem Rev. 1935;16:243–286. [Google Scholar]
- 39.Willing A, Follmann H, Auling G. Ribonucleotide reductase of Brevibacterium ammoniagenes is a manganese enzyme. Eur J Biochem. 1988;170:603–611. doi: 10.1111/j.1432-1033.1988.tb13740.x. [DOI] [PubMed] [Google Scholar]
- 40.Jiang W, et al. A manganese(IV)/iron(III) cofactor in Chlamydia trachomatis ribonucleotide reductase. Science. 2007;316:1188–1191. doi: 10.1126/science.1141179. [DOI] [PubMed] [Google Scholar]
- 41.Covès J, Nivière V, Eschenbrenner M, Fontecave M. NADPH-sulfite reductase from Escherichia coli: A flavin reductase participating in the generation of the free-radical of ribonucleotide reductase. J Biol Chem. 1993;268:18604–18609. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





