Abstract
Cell-based therapies are attractive for revascularizing and regenerating tissues and organs, but clinical trials of endothelial progenitor cell transplantation have not resulted in consistent benefit. We propose a different approach in which a material delivery system is used to create a depot of vascular progenitor cells in vivo that exit over time to repopulate the damaged tissue and participate in regeneration of a vascular network. Microenvironmental conditions sufficient to maintain the viability and outward migration of outgrowth endothelial cells (OECs) have been delineated, and a material incorporating these signals improved engraftment of transplanted cells in ischemic murine hindlimb musculature, and increased blood vessel densities from 260 to 670 vessels per mm2, compared with direct cell injection. Further, material deployment dramatically improved the efficacy of these cells in salvaging ischemic murine limbs, whereas bolus OEC delivery was ineffective in preventing toe necrosis and foot loss. Finally, material deployment of a combination of OECs with another cell population commonly isolated from peripheral or cord blood, endothelial progenitor cells (EPCs) returned perfusion to normal levels in 40 days, and prevented toe and foot necrosis. Direct injection of an EPC/OEC combination was minimally effective in improving limb perfusion, and untreated limbs underwent autoamputation in 3 days. These results demonstrate that vascular progenitor cell utility is highly dependent on the mode of delivery, and suggest that one can create new vascular beds for a variety of applications with this material-controlled deployment of cells.
Keywords: biomaterial, cell therapy, ischemic diseases, neovascularization, regenerative medicine
Cell-based therapies are widespread in regenerative medicine (1–3), but clinical trials of stem cell transplantation have not resulted in consistent benefit (4–7). More specifically, the potential of progenitor cell populations for the treatment of ischemic diseases has been prominent recently, with >80 clinical trials during the past five years in which a patient's own cells have been isolated, often multiplied in vitro, and reinfused (e.g., into ischemic muscle) (8). These trials, while supporting the safety of these cells, indicate that simple infusions may have significant limitations (9). Work with many cell types indicates that the vast majority (typically >90%) of cells transplanted in this manner will rapidly die (10), and control over the fate of the cells is abandoned once they are placed in the body. One may bypass certain limitations of infusions by delivering the cells on sophisticated material carriers that promote tissue formation by the cells by using the material as a template (11–14). However, this approach does little to address vascularization in the host tissue, and integration of the new tissue mass and the host tissue is often problematic.
This study proposes a different approach in which a material system is used to create a depot of vascular progenitor cells in vivo that exit over time to repopulate the damaged tissue and participate in neovascularization. There is a compelling need for new strategies to revascularize ischemic tissues [e.g., in context of peripheral arterial disease (PAD)] (15), and a recent analysis of the tissue-engineering and regenerative medicine fields has suggested that understanding and controlling vascularization is the single most pressing issue in those broad fields (16). Alginate, a naturally occurring polysaccharide, which comprises α-l-guluronic and β-d-mannuronic acid sugar residues, was used to fabricate the scaffolds as it had been used extensively as a delivery vehicle for encapsulated cells in the past (17, 18). Peptides containing the arginine-glycine-aspartic acid (RGD) amino acid sequence, a ubiquitous cell-binding domain found in many extracellular matrix molecules, were covalently coupled to alginate as previously described (19–21). The RGD coupling confers a specific mechanism for integrin-mediated cell adhesion to the otherwise nonadhesive polymer, and the RGD-ligand density and distribution can be manipulated to provide control over cell adhesion, proliferation, and cell fate after transplantation (22–25). Vascular endothelial growth factor (VEGF) is a key regulator in new blood vessel formation, and was also investigated as a component of the material system because it regulates the survival, proliferation, and migration of endothelial cells (26). From mRNA alternative splicing of a single gene, several VEGF isoforms are generated, and VEGF121 and VEGF165 are the most commonly expressed (26, 27). VEGF 121 and VEGF 165 differ by the presence of a heparan sulfate binding site, with the result that VEGF 121 is a highly diffusible protein, in contrast to VEGF165, which bonds moderately to extracellular matrix (28). The utility of the two VEGF isoforms in the deployment of transplanted vascular progenitors was examined by VEGF immobilization in the alginate.
A variety of cell populations, including cardiac stem cells, natural killer cells, bone marrow cells, dendritic cells, and endothelial progenitor cells (EPCs) have been investigated in clinical revascularization trials (8, 29). Recent studies have revealed that EPCs can be isolated from umbilical human cord blood circulate in the peripheral blood in adults (30–33). EPCs were initially identified and isolated by their expression of CD34 and VEGFR-2, surface markers commonly found on hematopoietic cell populations, and likely contribute to adult neovascularization by supporting host cell angiogenesis (30, 33). Subsequent studies described another potentially therapeutic endothelial cell-like population, designated outgrowth endothelial cells (OECs), that could be isolated from mononuclear cells (31). These cells maintain a high proliferative potential and also present some endothelial cell markers, including CD31 and VEGFR-2 (31, 34). In this report, the ability of the material system to effectively deploy these two cell populations, examine their utility in combination, and reverse severe hindlimb ischemia was tested.
Results
Characterization of EPCs and OECs.
Both EPCs and OECs were isolated from human umbilical cord blood to analyze their potential utility in relieving ischemia and contributing to angiogenesis. In culture, the EPCs consisted of round cells forming colonies, and spindle-shaped cells at the periphery of the colonies forming cord-like structures (supporting information (SI) Fig. S1a), as noted in previous studies (30, 31). Distinctively, OECs exhibited a cobblestone-like morphology (Fig. S1a) similar to human microvascular endothelial cells (ECs), and multiple population doublings without senescence, again in agreement with past studies (31, 34, 35). OECs, in contrast to EPCs, also exhibited high telomerase activity (Fig. S2). Immunohistochemistry and FACS analysis confirmed that EPCs were monocyte/macrophage lineage cells and OECs were vascular endothelial lineage cells (Fig. S1b). Specifically, OECs expressed vascular endothelial cell surface antigens, including CD31, CD144, vWF, and VEGFR-2, but were negative for CD34 (Fig. S1b). EPCs also expressed CD31, CD144, VEGFR-2, and CD34, but revealed weak expression of vWF, because it was observed only in a small number of EPCs. CD14, the monocyte/macrophage cell surface antigen, was expressed only in EPCs. The role of EPCs and OECs in angiogenesis was investigated, via an in vitro cell sprouting assay (36). Human microvascular endothelial cells (ECs) exhibited significant sprouting from carrier beads, with formation of capillary-like extensions consisting of interconnected cells with central lumen (Fig. S1c). EPCs did not participate in sprout formation when cultured alone on beads, and the OECs alone revealed a highly migratory behavior. However, coculture of ECs with OECs on beads led to a significant increase in sprout formation, increased lumen formation in the sprouts, compared with ECs alone. Interestingly, coculture of EPCs, OECs, and ECs resulted in massive sprouting, compared with coculture of OECs and ECs, and contrasted with the absence of sprouts when EPCs were cultured alone. Interestingly, coculture of EPCs and OECs on top of the fibrin gel induced EC migration toward the EPCs and OECs. Further, the profile of protein secretion by these different endothelial cells populations was also investigated by using an angiogenesis antibody array (Fig. S1d). OECs and EPCs both secreted high levels of angiogenic factors FGF-α, IL-12, and IP-10. Leptin was exclusively secreted at high levels by EPCs, and only OECs expressed a high level of PlGF secretion.
Designing Scaffolds to Drive Outward Migration of Viable, Proliferative Cells.
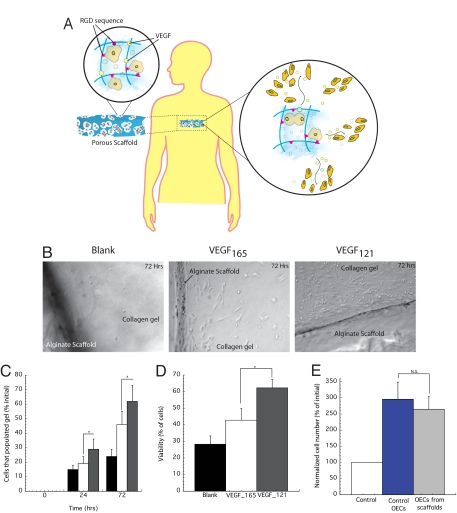
The ability of scaffolds fabricated with RGD adhesive ligands and VEGF (Fig. 1a) to maintain cell viability, proliferation, and outward migration from the scaffolds was first examined in vitro by using OECs. Scaffolds were partially embedded in collagen gels (Fig. S3) to mimic placement in tissue, and outward cell migration quantified. Three days after cell seeding, very few OECs (<0.5%) migrated out of alginate scaffolds not containing coupled RGD peptides, and this condition was discontinued from all further studies. Scaffolds with RGD but not containing VEGF resulted in an order of magnitude increase in cells migrating out of the scaffolds (Fig. 1 B and C). Inclusion of VEGF165 further doubled the number of cells that migrated outward from the RGD-coupled scaffolds, and inclusion of VEGF121 led to even higher cell migration out of scaffold. ≈60% of the cells that migrated out from scaffolds presenting VEGF121 were viable, whereas only 28% of cells migrating from blank scaffolds remained viable (Fig. 1D). Subsequently, the proliferation capacities of the cells that migrated out of VEGF121 scaffolds was tested, and compared with cells that never were placed in contact with scaffolds. Both OECs populations displayed the same rate of proliferation when stimulated with VEGF165 (Fig. 1E).
Fig. 1.
Proposed cell delivery approach, characterization of cell migration from macroporous alginate scaffolds. (A) Diagram of approach to present cell adhesion ligands (RGD-containing peptides) and local morphogens (VEGF) in the material to maintain cell viability, and to activate and induce cell migration out of scaffold. (B) Phase-contrast micrographs of OECs that have migrated out from scaffolds that contain no VEGF (blank), VEGF121, or VEGF165 and populated the surrounding tissue mimic (collagen gel) after 72 h. (C) Quantification of OECs populating the collagen matrix when VEGF121 was incorporated into scaffolds (gray filled bar), compared with the presentation of VEGF165 (open bar), or no VEGF (black filled bar). Values were normalized to the initial cell number placed in scaffolds. (D) Viability of the cells that migrated out from scaffolds with no VEGF (blank), VEGF121 or VEGF165 in the scaffolds. (E) Proliferation of OECs that had migrated out of scaffolds and were subsequently recovered and placed in culture on tissue culture dishes in the presence of VEGF in the medium. Control OECs that had never been placed in scaffolds were cultured in parallel, maintained in culture in the absence of VEGF stimulation (control) for comparison. Values in C–E represent mean and standard deviations (n = 6). Magnification 200× for all photomicrographs.
Enhancing Angiogenesis in Ischemic Hindlimbs with OECs.
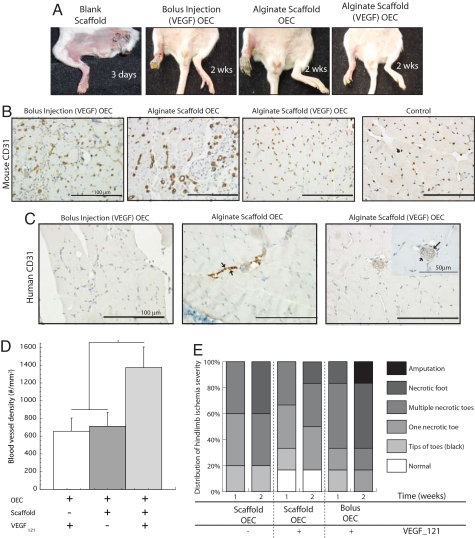
The utility of this approach in enhancing the efficacy of transplanted OECs was next assessed in SCID mice that were subjected to femoral artery and vein ligation. VEGF121-presenting scaffolds loaded with OECs, blank scaffolds (neither cells nor VEGF), infusion of a solution of OECs and VEGF121 (same quantities as placed in scaffolds), and scaffolds with no VEGF but loaded with OECs were all tested. Placement of scaffolds without cells had little benefit, because autoamputation of the ischemic limbs was noted in three days (Fig. 2A), and this control condition was not used in any further analysis. The infusion of OECs and VEGF121 resulted in relatively high levels of limb necrosis, and transplanting OECs on scaffolds without VEGF revealed some degree of limb necrosis. In contrast, transplantation of OECs on scaffolds presenting VEGF121 prevented autoamputation and the levels of necrosis were reduced (Fig. 2A). Examination of tissue sections suggested that bolus injection of cells plus VEGF led to a modest density of host cell-derived capillaries in the muscle. Cell delivery with a scaffold system lacking VEGF led to a low and comparable density of capillaries as found with bolus injection, and both control conditions resulted in the formation of large disorganized capillaries with an erratic distribution, compared with healthy limbs (Fig. 2B). In contrast, delivery of cells in the scaffold system (containing VEGF) led to a significant increase in the muscle capillary densities, and capillaries exhibited a normal size and spatial distribution (Fig. 2B). Quantification confirmed that the capillary density increased more than twofold when cells were delivered from the scaffolds instead of bolus injection (Fig. 2D). To investigate the engraftment of OECs, sections were also immunostained with human-specific antibodies. Bolus injection showed little engraftment, and OECs transplanted in scaffolds without VEGF revealed limited engraftment and only contributed to the formation of small vessels (Fig. 2C). In contrast, OECs transplanted on VEGF-containing scaffolds displayed significant engraftment, resulting in the formation of functional human-murine chimeric blood vessels (Fig. 2C). Finally, OEC delivery on VEGF-containing scaffolds led to a significant decrease in tissue necrosis, whereas bolus OEC delivery was ineffective in preventing toe necrosis and foot loss (Fig. 2E).
Fig. 2.
Analysis of angiogenesis in ischemic hindlimbs after OEC transplantation. (A) Implantation of blank scaffolds, bolus injection of OECs and VEGF (same quantities as placed in scaffolds), transplantation of OECs on scaffolds lacking VEGF (alginate scaffold OEC), and transplantation of OECs on scaffolds presenting VEGF121 [alginate scaffold (VEGF) OEC]. Photomicrographs of tissue sections from ischemic hindlimbs of SCID mice at postoperative day 15, immunostained for the mouse endothelial cell marker CD-31 (B), and human CD-31 (C). (D) Quantification of the total blood vessel densities in hindlimb muscle tissue after 2 weeks with bolus injection of VEGF121 and OECs (+ − +), scaffold delivery (no VEGF121) of OECs (+ + −), or scaffold delivering OECs with VEGF121 (+ + +) in SCID mice. (E) Hindlimbs subjected to surgery were also visually examined, and grouped as normal (displaying no discrepancy in color or limb integrity from nonischemic hindlimbs of the same animal), or presenting one necrotic toe, multiple necrotic toes, or a complete necrotic foot. Mean values are presented with standard deviations (n = 6) in both graphs. *, P < 0.05 between conditions.
Cotransplantation of EPC and OEC Enhances Neovascularization in Vivo.
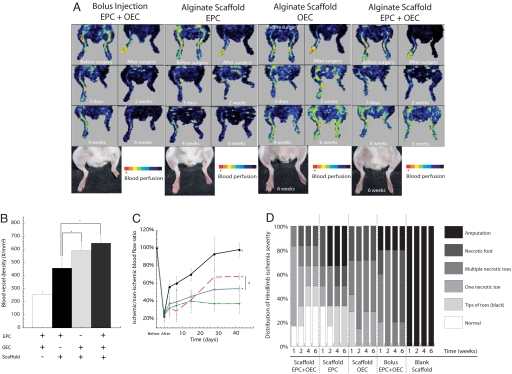
Cotransplantation of EPCs with OECs was next examined to investigate the potential of these two cell populations together to orchestrate in vivo neovascularization. A bolus infusion of EPCs and OECs was used as a control condition for these studies, and this treatment salvaged the limbs, as did sustained scaffold delivery of each cell population alone and in combination (Fig. 3A). The transplantation of EPCs and OECs alone on scaffolds (containing VEGF121) induced an ≈2-fold increase in vessel density, compared with bolus delivery of both cell populations, and the density was 2.5-fold higher when both cell populations were transplanted on scaffolds (Fig. 3B). Femoral artery and vein ligation led to a rapid loss in perfusion to the ligated limbs (Fig. 3 A and C), and animals treated with blank scaffolds (VEGF121 loaded but no cells) again rapidly suffered from extreme necrosis and loss of the ischemic hindlimbs and were not further analyzed. Animals treated with a bolus injection of both cell types demonstrated a marginal recovery of regional blood flow (Fig. 3 A and C). In contrast, animals treated with scaffolds delivering cells and VEGF121 showed a marked increase in blood flow over time (Fig. 3 A and C). Transplanting a combination of EPCs and OECs on the scaffolds led to a markedly superior perfusion recovery, resulting in normal perfusion levels by 4 weeks. Necrosis of toes or foot loss was also decreased by EPC and OEC codelivery from scaffolds, as 30% of mice in this group displayed normal limbs 6 weeks after surgery. In contrast, bolus EPC and OEC delivery was ineffective in preventing or reversing necrosis (Fig. 3D and Fig. S3). Perhaps most notably, cell deployment by using this material system led to significant recovery of limb locomotion (Fig. S4, Movie S1). Finally, histologic analysis indicated that animals transplanted with scaffolds delivering EPCs alone displayed significant levels of adipose tissue in hindlimbs, whereas animals treated with codelivery of EPCs and OECs by using implantable scaffolds revealed normal tissue organization (Fig. S5).
Fig. 3.
Gross photographs and perfusion images of ischemic hindlimbs as a function of time postsurgery. (A) Limbs with no treatment (blank scaffold), demonstrated precocious and rapid limb necrosis (<3 days) (left-most column), and no perfusion images were obtained. For other conditions, hindlimbs were maintained over time, and perfusion images could be obtained. In all of these conditions, scaffolds presenting RGD ligands and VEGF121 were used. The normal baseline (before) perfusion was immediately reduced after unilateral femoral artery ligation (after), and subsequent recovery was tracked as a function of time postsurgery. (B) Total blood vessel densities in hindlimb muscle tissue at six weeks postligation for the various experimental groups. (C) Quantification of hindlimb perfusion for the conditions, including bolus injection of EPC and OEC (inverted filled triangle), EPC transplantation with scaffolds (open square), OEC transplantation with scaffolds (open triangle), and EPC and OEC combined transplantation on scaffolds (filled circle) in SCID mice. (D) Quantification and distribution of hindlimb ischemia severity observed in different experimental groups over time. Mean values are presented with standard deviations (n = 6). *, statistically significant difference (P < 0.05), as compared with control (EPC and OEC bolus injection); #, statistically significant difference (P < 0.05) between conditions.
Discussion
The results of this study suggest a radically different approach for endothelial progenitor cell-based therapies that may be applicable in the treatment of ischemic diseases and more broadly in regenerative medicine, in which vascular progenitor cell populations are delivered on a bioactive material that provides a microenvironment enhancing cell survival, and the sustained release and repopulation of the surrounding tissue by outwardly migrating cells. The feasibility of this approach was examined by using endothelial progenitors isolated from human cord blood to treat ischemic muscle tissue. This system was demonstrated to dramatically improve vascularization and perfusion of ischemic murine hindlimb musculature, and prevented toe and foot necrosis. These results suggest that endothelial progenitor cell utility highly depends on the mode of delivery and control over cell fate after transplantation.
A macroporous polymer scaffold (37) provided the delivery vehicle, and the inclusion of cell adhesion anchors and morphogens was explored to create a microenvironment to maintain the viability of resident cells and increase their outward migration. The polysaccharide used to form the scaffolds, alginate, does not mediate cell adhesion itself, and in accordance with previous reports of the importance of cell adhesion in migration (38), very few OECs migrated out of devices formed from the native polymer in vitro. Several recent studies have evaluated the utility of various materials for the delivery of progenitors for endothelial cells (39–41); however, all of these former approaches had as a goal the creation of new vascular beds within the material, not the mobilization of the cells to repopulate and revascularize the host tissue. The results of this study demonstrate that coupling of an appropriate density of adhesion ligands to the polymer chains dramatically increased OEC outward migration, and VEGF inclusion further improved outward cell migration. Endothelial cells are known to be both activated by VEGF165 and to migrate up gradients of this factor established as a result of its ECM binding (26), and the VEGF165 in the scaffold in this system likely traps a high percentage of the activated cells. VEGF121 appears to be more useful to activate and drive cells out of a material, likely because of its lack of ECM binding (42) and resultant more even spatial distribution. Altogether, these results indicate that one can create a 3D material niche for vascular progenitor cell populations that directs their outward migration over time, and the cell adhesion ligand RGD and morphogen VEGF121 is particularly useful for OECs.
We find that transplantation of EPCs and OECs increases neovascularization of ischemic muscle tissue, consistent with previous reports (32–34, 43), but also find a critical role for sustained delivery of appropriately activated progenitor cells, in place of bolus injections, and a significant benefit of transplanting these two cell populations together. The material system used to deliver vascular progenitors made possible therapeutic angiogenesis, reversal of ischemia, and prevention of necrosis and autoamputation. These results indicate that the clinical utility of these cell populations can likely be dramatically improved by delivering the cells in a sustained and viable fashion over time from a material system, in a manner that guides the function of the exogenous cells and their integration with native cells to together orchestrate tissue regeneration. EPCs and OECs each provided benefit when delivered individually, but together they provided a greater benefit in this particular model of PAD. These results suggest this approach will be useful to treat cardiac infarction (44), and other situations in which vascularization is lacking (e.g., wound healing) (45). More broadly, this may provide a core technology for the entire field of regenerative medicine, because of the need to create new vascular beds in most situations of regeneration and tissue engineering (16). The observed benefit in vivo of cotransplanting EPCs and OECs also suggests that providing together cell populations with complementary functions may have broad benefit in vascularization strategies. The results of the in vitro sprouting assay supported a synergistic effect of the two cell populations, likely because of the distinct participation of these two cell populations in the angiogenic process. EPCs appear to mainly contribute to cytokine production, whereas OECs also directly interact with native EC, supporting new blood vessel formation, as suggested by recent work in this field (34, 46, 47). OECs and EPCs both secrete a variety of angiogenic factors, and this likely causes the massive increase in EC migration when OECs and EPCs were cultured on top of gels containing ECs. This finding, together with the finding that transplantation of OECs alone increased the density of mouse vessels in ischemic tissue, suggests that, although OECs can contribute directly to vascular formation, their role in promoting host angiogenesis is also significant. EPCs alone exhibited high levels of leptin secretion. Leptin coordinates the levels of fat tissue (48, 49) and stimulates angiogenic activity (49, 50), and the finding that limbs treated with scaffold delivery of EPCs exhibited significant adipose tissue may relate to the leptin secretion by these cells. OECs were also noted to express PlGF (Fig. S1d), which is a potent angiogenic factor (51), and may partially underlie the ability of OECs to mobilize a host cell angiogenic response. It was notable that the density of blood vessels in limbs treated with OEC delivered from scaffolds actually decreased from 2 to 6 weeks. The increase in perfusion over this same time frame suggests that the decrease in vessel density is related to remodeling processes that lead to a more functional vascular network, and the factors secreted by OECs, specifically PlGF, may contribute to the vascular maturation process.
The cell delivery approach described in this report may be broadly useful to solve some of the fundamental problems associated with current vascular cell-based therapies—the rapid loss of cell viability, low engraftment efficiency, and absence of control over cell fate after introduction into the body. Some of the failures experienced in clinical cell transplantation (4, 5, 29) may arise directly from the manner of administration of the cells, rather than a lack of intrinsic bioactivity of the cells. Our findings clearly support the potential of progenitor cells, and stem and differentiated cell populations, if their delivery and in vivo fate is appropriately regulated. Whereas the specific cues will likely be distinct for different cell populations, the importance of locally regulating cell activation, migration, and tissue engraftment is anticipated to remain constant by using material systems.
Materials and Methods
Macroporous Alginate Scaffolds.
Alginate molecules rich in guluronic acid blocks (LF 20/40, FMC Biopolymer) were first oxidized by using sodium periodate (NaIO4) (54) to generate hydrolytically labile polymers. Oxidized alginates were coupled with oligopeptides containing the Arg-Gly-Asp cell adhesion sequence (Commonwealth Biotech) following aqueous carbodiimide chemistry (38). Hydrogels were prepared by mixing the alginate solution with a calcium sulfate slurry and the mixture was injected between glass plates with a spacer of 1 mm. After curing for 20 min, gel disks with diameter of 10 mm were punched out. These gel disks were frozen and stored at −20°C, and after 24 h, gel disks were lyophilized to yield macroporous materials (55) (for more detail, see SI Text).
In Vitro Cell Assays.
The ability of cells to migrate outward from macroporous alginate scaffolds with no VEGF, or from scaffolds containing VEGF121 or VEGF165 (1 μg of total incorporated per scaffold, respectively), was analyzed by seeding 5 × 105 of OECs (passage 3) into the scaffolds, and then placing the scaffolds in contact with a collagen gel (3.0 mg/ml) (PureCol) (Fig. S3). At different experimental time points, the scaffold was removed and the cells that had populated the collagen gel were obtained by washing the collagen gels, and dissolving the gels, and counting cells in a Coulter Counter (Beckman). The viability of the cells populating the collagen gel was quantified by trypan blue exclusion with a Viacell Counter (Beckman).
The ability of the EPCs and OECs to modulate angiogenesis was analyzed in vitro by using an endothelial cell sprouting assay (36) (for more detail, see SI Text).
Ischemic Hindlimb Model in SCID Mouse.
All procedures were approved by the Experimental Animal Committee of Harvard University. For evaluation of in vivo angiogenesis, surgery to induce hindlimb ischemia was performed and the cell-loaded alginate scaffolds (5 × 106 cells per scaffold) were implanted on the medial side of thigh muscle or 5 × 106 cells in 50 μl of serum-free EBM medium were injected into the hindlimb intramuscularly. The groups (n = 6 per condition) were as follows: (i) blank scaffold, (ii) bolus (containing 3 μg of VEGF121) intramuscular injection of OEC (5 × 106 cells), (iii) OEC-loaded scaffolds (without VEGF121) (5 × 106 cells), and (iv) OEC-loaded scaffolds (with VEGF121, 3 μg per scaffold) (5 × 106 cells). These animals were humanely euthanized two weeks after surgery. A different group of animals was subjected to hindlimb ischemia surgery and euthanized six weeks postoperatively. After the vessel ligation, mice were injected intramuscularly with a total volume of 50 μl of a solution (containing 3 μg of VEGF121) of EPCs and OECs (5 × 106 cells total in a 1:1 ratio), EPC-loaded scaffolds (5 × 106 cells), OEC-loaded scaffolds (5 × 106 cells), and EPC- and OEC-loaded scaffolds (5 × 106 cells total per scaffold in a 1:1 ratio). All scaffolds also contained 3 μg of total VEGF121 in this experiment. Before surgery, and 0, 1, 3, and 7 days, and 2, 4, and 6 weeks postsurgery, measurements of the ischemic/normal limb blood flow ratio were performed on anesthetized animals (n = 6/time point/experimental condition) by using a Periscan system blood perfusion monitor laser Doppler equipment (Perimed).
After euthanization, hindlimb muscle tissues (n = 6/time point/experimental condition) were immunostained for mouse CD31 (BD Biosciences PharMingen), or human CD31 (Dako). Sections from each sample were visualized at 200× and 400× with an Olympus-IX81 light microscope connected to an Olympus DP70 digital image capture system and analyzed by using IPLab 3.7 software (Scanalytics). Vessel quantification was determined by using ImageJ (National Institutes of Health) software (for more detail, see SI Text).
Statistical Analysis.
All statistical analysis was performed by using Student's t test (two-tail comparisons), and analyzed by using InStat 2.01 (Graphpad) software. Differences between conditions were considered significant if P < 0.05.
Supplementary Material
Acknowledgments.
We thank the Biological Resources Branch of National Cancer Institute for generously providing VEGF165 used in our studies. This work was supported by National Institutes of Health Grant R01 HL069957. E.A.S. is a student of the Gulbenkian PhD Program in Biomedicine, Portugal, and was supported by Fundacao para Ciencia e Tecnologia Predoctoral Fellowship SFRH/BD/9613/2002.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803873105/DCSupplemental.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Couzin J, Vogel G. Cell therapy. Renovating the heart. Science. 2004;304:192–194. doi: 10.1126/science.304.5668.192. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 4.Assmus B, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 5.Lunde K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 6.Hou D, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112:I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 7.March KL, Johnstone BH. Cellular approaches to tissue repair in cardiovascular disease: The more we know, the more there is to learn. Am J Physiol. 2004;287:H458–H463. doi: 10.1152/ajpheart.00343.2004. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Trials. [Accessed August 18, 2008]; Available at http://clinicaltrials.gov/
- 9.Rosenzweig A. Cardiac cell therapy–mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann M, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 11.Akar AR, Durdu S, Corapcioglu T, Ozyurda U. Regenerative medicine for cardiovascular disorders-new milestones: Adult stem cells. Artif Organs. 2006;30:213–232. doi: 10.1111/j.1525-1594.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 13.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 14.Sales VL, et al. Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation. 2007;116:I55–I63. doi: 10.1161/CIRCULATIONAHA.106.6806637. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PC, Mikos AG, Fisher JP, Jansen JA. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827–2837. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs JR, Hannouche D, Terada S, Vacanti JP, Fauza DO. Fetal tracheal augmentation with cartilage engineered from bone marrow-derived mesenchymal progenitor cells. J Pediatr Surg. 2003;38:984–987. doi: 10.1016/s0022-3468(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 18.Koch RJ, Gorti GK. Tissue engineering with chondrocytes. Facial Plast Surg. 2002;18:59–68. doi: 10.1055/s-2002-19828. [DOI] [PubMed] [Google Scholar]
- 19.Hubbell JA. Biomaterials in tissue engineering. Biotechnology (N Y) 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 20.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 21.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 22.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci USA. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong HJ, Boontheekul T, Mooney DJ. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci USA. 2006;103:18534–18539. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrie TA, Capadona JR, Reyes CD, Garcia AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 29.Welt FG, Losordo DW. Cell therapy for acute myocardial infarction: Curb your enthusiasm? Circulation. 2006;113:1272–1274. doi: 10.1161/CIRCULATIONAHA.105.613034. [DOI] [PubMed] [Google Scholar]
- 30.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 31.Ingram DA, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 32.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 33.Shi Q, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 34.Yoder MC, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 36.Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 37.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 39.Kanczler JM, et al. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29:1892–1900. doi: 10.1016/j.biomaterials.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Krenning G, Dankers PY, Jovanovic D, van Luyn MJ, Harmsen MC. Efficient differentiation of CD14+ monocytic cells into endothelial cells on degradable biomaterials. Biomaterials. 2007;28:1470–1479. doi: 10.1016/j.biomaterials.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Thebaud NB, et al. Human endothelial progenitor cell attachment to polysaccharide-based hydrogels: A pre-requisite for vascular tissue engineering. J Mater Sci Mater Med. 2007;18:339–345. doi: 10.1007/s10856-006-0698-1. [DOI] [PubMed] [Google Scholar]
- 42.Simons M. Angiogenesis: Where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 43.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 44.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 45.Suh W, et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 46.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 47.Woywodt A, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: Proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–677. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 48.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sierra-Honigmann MR, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 50.Wolk R, Deb A, Caplice NM, Somers VK. Leptin receptor and functional effects of leptin in human endothelial progenitor cells. Atherosclerosis. 2005;183:131–139. doi: 10.1016/j.atherosclerosis.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 51.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 52.Murohara T, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 54.Bouhadir KH, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 55.Thornton AJ, Alsberg E, Albertelli M, Mooney DJ. Shape-defining scaffolds for minimally invasive tissue engineering. Transplantation. 2004;77:1798–1803. doi: 10.1097/01.tp.0000131152.71117.0e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.