Abstract
The ability to sense and respond to subtle variations in environmental temperature is critical for animal survival. Animals avoid temperatures that are too cold or too warm and seek out temperatures favorable for their survival. At the molecular level, members of the transient receptor potential (TRP) family of cation channels contribute to thermosensory behaviors in animals from flies to humans. In Drosophila melanogaster larvae, avoidance of excessively warm temperatures is known to require the TRP protein dTRPA1. Whether larval avoidance of excessively cool temperatures also requires TRP channel function, and whether warm and cool avoidance use the same or distinct TRP channels has been unknown. Here we identify two TRP channels required for cool avoidance, TRPL and TRP. Although TRPL and TRP have previously characterized roles in phototransduction, their function in cool avoidance appears to be distinct, as neither photoreceptor neurons nor the phototransduction regulators NORPA and INAF are required for cool avoidance. TRPL and TRP are required for cool avoidance; however they are dispensable for warm avoidance. Furthermore, cold-activated neurons in the larvae are required for cool but not warm avoidance. Conversely, dTRPA1 is essential for warm avoidance, but not cool avoidance. Taken together, these data demonstrate that warm and cool avoidance in the Drosophila larva involves distinct TRP channels and circuits.
Keywords: thermosensation, thermotaxis, TRPA1, TRPL, NORPA
Proper behavioral responses to temperature are critical for animal survival. At the molecular level, temperature perception in animals from flies to humans involves the action of transient receptor potential (TRP) family cation channels (1, 2). TRPs, a large family with 28 members in humans and 16 members in Drosophila, can be divided by sequence similarity into seven major subfamilies, including the TRPA, TRPC, TRPM, and TRPV subfamilies (3). Although TRPs participate in diverse sensory processes including the perception of odor, vibration, and chemicals, the activity of a subset of TRP channels (drawn from the TRPA, TRPM, and TRPV subfamilies) are highly sensitive to temperature and are dubbed “thermoTRPs” (2). ThermoTRPs are expressed in tissues known to be temperature responsive and are activated at characteristic temperatures (2). In mammals, for example, distinct thermoTRPs can be activated by painfully hot (noxious) temperatures (TRPV1 and TRPV2) (4, 5), by moderately warm (innocuous) temperatures (TRPV3, TRPV4) (6–10), by moderately cool temperatures (TRPM8) (11, 12), and possibly by painfully cold temperatures (TRPA1) (13–15). It has thus been proposed that a series of TRP channels mediate temperature perception in animals (1, 2).
Despite the identification of multiple thermal sensors, there remain important gaps in our understanding of thermosensation. In mice, for example, sensory neurons can respond to painfully high temperatures even without expressing TRPV1 or TRPV2 (16). In addition, how mammals perceive noxious cold, and whether TRPA1 is involved in cold sensation, remain controversial (17–20). Such findings indicate that additional regulators of thermosensation remain to be discovered.
Drosophila melanogaster larvae and adults exhibit robust behavioral responses to temperature, avoiding excessively warm or cool temperatures (21–23). To date, three putative Drosophila thermoTRPs, all of which are TRPA subfamily members, have been identified: namely, PYREXIA, PAINLESS, and dTRPA1. PYREXIA (PYX) is activated at temperatures greater than ≈35°C and has been proposed to protect flies from heat-induced paralysis (24). PAINLESS is required for larval and adult high-temperature nociception (25, 26) and is implicated in facilitating neuronal activation at temperatures greater than 42°C (25). Finally, dTRPA1 is activated by warming to more than ≈25°–29°C (27).
We have previously shown that RNA interference (RNAi)-mediated knockdown of dTRPA1 expression disrupts larval avoidance of moderately elevated temperatures (≈31–35°C) (22). Interestingly, the circuits for avoidance of excessively warm temperatures (herein referred to as warm avoidance) by larvae and high-temperature nociception appear distinct, as dTRPA1-expressing neurons in the central brain are required for warm avoidance but not high-temperature nociception, whereas multidendritic neurons within the PNS are required for nociception but not for warm avoidance (22). Larval sensation and avoidance of excessively cool temperatures requires yet another distinct group of neurons, which are located in the terminal organ at the larval anterior (23). However, the molecules that regulate such cold-dependent behavior have not been identified.
Here we identify two TRP channels required for avoidance of excessively cool temperatures (herein referred to as cool avoidance), the Drosophila TRPC subfamily proteins Transient Receptor Potential-like (TRPL) and Transient Receptor Potential (TRP). We find that these two channels, previously shown to be critical for vision, are important for cool avoidance but are not necessary for warm avoidance. Interestingly, TRPL and TRP mediate cool responses independent of the phospholipase C NORPA, a molecule essential for vision. In contrast, we find that dTRPA1, previously implicated in warm avoidance, appears dispensable for cool avoidance. Thus, distinct TRP channels are required for warm and cool avoidance. At the circuit level, we also find distinctions between cool and warm avoidance, with the cold-activated neurons of the terminal organ specifically required for cool avoidance. These findings provide the first evidence for involvement of TRPC-family members in controlling temperature-dependent behaviors. They also provide initial insight into the molecules mediating cool avoidance in Drosophila and the functional relationship between warm and cool avoidance circuits.
Results
dTrpA1 Mutants Are Defective for Warm Avoidance Behavior.
We previously showed that RNA interference (RNAi)–mediated knockdown of dTRPA1 causes warm avoidance defects in Drosophila larvae (22). To further test the function of dTRPA1 in larval behavior, we examined animals containing loss-of-function mutations in the dtrpA1 gene. We recently described the creation of dtrpA1ins, a strong loss of function allele generated by homologous recombination-mediated ends-in gene targeting (28). Using dtpA1ins as a starting point, ICreI-based intragenic recombination was performed to create an additional allele, dtrpA1fs (29) (supporting information [SI] Fig. S1). dtrpA1fs is identical to the wild-type locus except for a 2–base pair insertion predicted to truncate the protein near its amino terminus, within the 3rd ankyrin repeat and before the transmembrane regions. In addition, the ICreI-based intragenic recombination also generated a genetically matched wild-type control strain (dtrpA1wtR) in which the wild-type dtrpA1 locus was regenerated from the dtrpA1ins starting chromosome (Fig. S1).
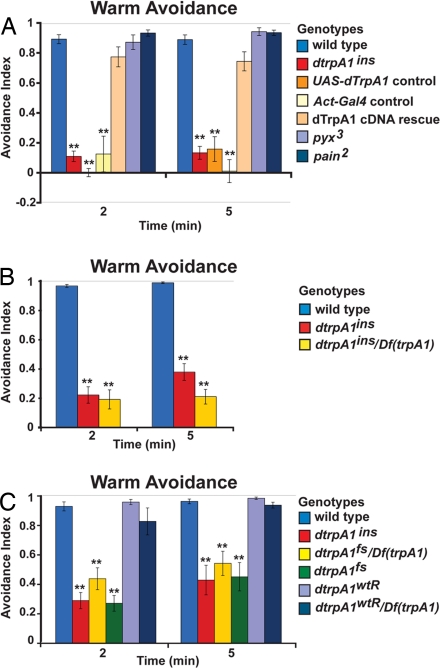
The warm avoidance behavior of dtrpA1 mutants was examined as previously described (22). Consistent with prior RNAi results (22), both dtrpA1ins and dtrpA1fs mutants exhibited severe warm avoidance defects (Figs. 1 A–C), whereas the dtrpA1wtR control strain exhibited wild-type avoidance (Fig. 1C). Consistent with dtrpA1ins and dtrpA1fs acting as strong loss-of-function alleles, similar phenotypes were observed when these alleles were either homozygous or in trans to a chromosomal deficiency lacking dtrpA1 (Fig. 1 B and C). The dtrpA1 mutant avoidance defect was largely rescued by expressing a dTrpA1 cDNA in the dtrpA1 mutant background (Fig. 1A). Together, these results confirm that dTRPA1 is required for larval warm avoidance behavior. In contrast, strong loss-of-function mutations in either pyrexia or painless, which encode the two other heat-activated Drosophila TRP channels, showed wild-type warm avoidance (Fig. 1A), indicating that dTRPA1 does not require these proteins to mediate the moderate warmth avoidance tested in this assay.
Fig. 1.
dTRPA1 is required for warm avoidance. (A) Warm avoidance of wild-type (n = 10 assays), dtrpA1ins (n = 35), UAS-dTRPA1 control (UAS-dTRPA1/+; UAS-dTRPA1,dtrpA1ins/dtrpA1ins) (n = 7), Actin-Gal4 control (Actin-Gal4/+; UAS–mCD8-GFP,dtrpA1ins/dtrpA1ins) (n = 6), dTrpA1 cDNA rescue (Actin-Gal4/UAS-dTRPA1; UAS–mCD8-GFP,dtrpA1ins/UAS-dTRPA1, dtrpA1ins) (n = 9), pyx3 (n = 6) and pain2 (n = 6). (B) Warm avoidance of wild-type (n = 11), dtrpA1ins (n = 9) and dtrpA1ins/Df(trpA1) (n = 8). (C) Warm avoidance of wild-type (n = 6), dtrpA1ins (n = 7), dtrpA1fs/Df(trpA1) (n = 4), dtrpA1fs (n = 4), dtrpA1wtR (n = 4), and dtrpA1wtR/Df(trpA1) (n = 4). **, P < 0.01, significantly different from wild-type (Tukey-Kramer HSD).
dtrpA1 Is Specifically Required for Warm Avoidance.
Although dtrpA1 is required for warm avoidance, the molecular basis of cool avoidance has been unknown. To examine cool avoidance, we created an assay similar in design to that used to assess warm avoidance (see Materials and Methods) (Fig. 2A). When placed on a thermal gradient (within an ≈18–20°C release zone), wild-type 1st instar larvae exhibited robust cool avoidance, rapidly migrating away from the cooler half of the plate into the warmer zone of the thermal gradient. (Cool avoidance initiates in the low 20-degree range, with even a shallow 21.1° to 21.9°C release zone gradient promoting robust avoidance [AI ≥0.8, M.R., unpublished]). Although dtrpA1ins larvae showed slightly reduced cool avoidance at the 2-minute time point, the effect was not statistically significant and dtrpA1ins mutants went on to exhibit extremely robust cool avoidance as the assay progressed (Fig. 2B). The TRPAs painless and pyrexia were also not required for cool avoidance (Fig. 2B). Thus, dtrpA1 is essential for warm avoidance but dispensable for cool avoidance in our assay.
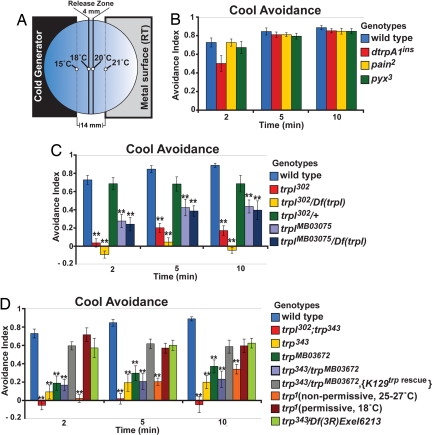
Fig. 2.
TRPL and TRP, but not dTRPA1, are required for cool avoidance. (A) Cool avoidance apparatus. (B) Cool avoidance of wild-type (n = 8), dtrpA1ins (n = 8), pain2 (n = 6) and pyx3 (n = 7). (C) Cool avoidance of wild-type (n = 8), trpl302 (n = 8), trpl302/Df(trpl) (n = 6), trpl302/+ (n = 4), trplMB03075 (n = 11) and trplMB03075/Df(trpl) (n = 7). (D) Cool avoidance of wild-type (n = 8), trpl302;trp343(n = 6), trp343 (n = 10), trpMB03672 (n = 11), trp343/trpMB03672 (n = 10), trp343/trpMB03672, [K129 trp rescue] (n = 8), trp1 at 25–27°C (n = 12), trp1 at 18°C (n = 7), and trp343/Df(3R)Exel6213 (n = 7). **, P < 0.01, significantly different from wild-type (Tukey-Kramer HSD).
We examined whether dtrpA1 is required for other sensory behaviors such as chemotaxis and phototaxis. dtrpA1ins mutant larvae avoided the repellent odor n-octyl acetate at least as well as wild-type (Fig. S2A) and showed no significant defects in responses to the attractant odor propionic acid (Fig. S2B). Furthermore, phototaxis of late 1st and early 2nd dtrpA1ins instar larvae was indistinguishable from the wild-type (Fig. S2C). These data support a selective requirement for dtrpA1 in warm avoidance.
TRPL and TRP Are Involved in Cool Avoidance.
As dTRPA1 function was largely dispensable for cool avoidance, we examined the involvement of other TRP family proteins. We found that larvae mutant for two TRP channels with partially redundant functions in phototransduction, TRPL and TRP, were severely defective for cool avoidance (Fig. 2 C and D). Unlike wild-type larvae, which robustly avoided the cooler regions, trpl302;trp343 double mutant larvae migrated into both cooler and warmer regions (Fig. 2 C and D).
Both trpl and trp contributed to cool avoidance, as single mutations in either gene disrupted cool avoidance behavior. Multiple trpl allelic combinations were used to test the requirement for trpl in cool avoidance. trpl302 homozygotes and trpl302/Df(trpl) larvae showed cool avoidance defects similar in severity to trpl;trp double mutants (Fig. 2 C and D). Furthermore, trplMB03075 homozygotes, which contain a Minos transposon insertion in the 1st intron of trpl, and trplMB03075/Df(trpl) animals were also significantly defective in cool avoidance (Fig. 2C). These data indicate a significant and nonredundant requirement for trpl in cool avoidance.
Animals mutant for trp also exhibited significant cool avoidance defects, although the defects were less severe than in trpl single mutants or trpl;trp double mutants (Fig. 2D). Animals homozygous for either trp343 or trpMB03672 (which contains a Minos insertion within the 4th exon of trp) were defective in cool avoidance (Fig. 2D); trp343/trpMB03672 animals also exhibited significant defects, which could be rescued using a trp minigene ([K129 trp rescue]) (30) (Fig. 2D). In addition, the temperature-sensitive trp allele trp1 showed significant cool avoidance defect when grown at the restrictive temperature (25–27°C) but not when grown at the permissive temperature (18°C) (Fig. 2D). Together these data support a role for trp in cool avoidance. Surprisingly, trp343/Df(3R)Exel6213 animals did show significant cold avoidance, even though Df(3R)Exel6213 lacks the trp gene and failed to complement the visual phototransduction phenotype of trp343 (K.J.K. and P.A.G. unpublished work). However, as Df(3R)Exel6213 deletes 24 or more genes in addition to trp, these additional genetic alterations may ameliorate the trp mutant phenotype. Nonetheless these data indicate that, at least in some genetic backgrounds, trpl can support significant cool avoidance without assistance from trp. Taken together, our data indicate that trpl is essential for cool avoidance, whereas trp also exerts a significant effect.
Cool Avoidance and Phototaxis Are Mechanistically Distinct Behaviors.
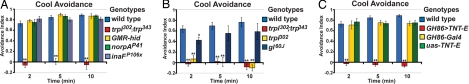
The cells and the molecular pathways through which TRPL and TRP contribute to phototransduction have been extensively studied. To test whether the same cells mediate phototransduction and cool avoidance, the effects of eliminating the larval photoreceptor neurons of the Bolwig Organ were examined. Larvae that express the pro-apoptotic protein Hid in their photoreceptors (GMR-Hid animals) or are mutant for the transcription factor GLASS (e.g., gl60J mutants) lack larval photoreceptors and are defective for phototaxis (31–36). However, GMR-Hid larvae exhibited wild-type cool avoidance (Fig. 3A). The gl60J larvae were also largely normal for cool avoidance (Fig. 3B), although gl60J mutants showed a modest deficit at the two minute time point (P = 0.024). The small, early thermotaxis defect in gl60J mutants may reflect the effects of GLASS expression in cells outside the visual system (33). These data suggest that larval light sensing organ is not required for temperature perception.
Fig. 3.
GH86-Gal4-expressing neurons contribute to cool avoidance. (A) Cool avoidance of wild-type (n = 8), trpl302;trp343 (n = 8), GMR-Hid (n = 7), norpAP41 (n = 7), and inaFP106X (n = 8). (B) Cool avoidance of wild-type (n = 8), trpl302;trp343(n = 6), trpl302 (n = 8), and gl60J (n = 8). (C) Cool avoidance of wild-type (n = 8), GH86-Gal4/Y or +;UAS-TNTE/+ (n = 7), GH86-Gal4 (n = 8), and UAS-TNTE (n = 8). *, P < 0.05, **, P < 0.01, significantly different from wild-type (Tukey-Kramer HSD).
At the molecular level, activation of TRPL and TRP during phototransduction depends on NORPA, a phospholipase C which acts downstream of fly opsins (37, 38). However, animals homozygous for the norpA null allele norpAP41 exhibited no deficit in cool avoidance (Fig. 3A). In addition, the transmembrane protein INAF is important for normal TRP expression and phototransduction in the adult photoreceptors (39, 40). However, animals homozygous for the inaF null allele inaF P106x (Fig. 3A) also failed to show cool avoidance defects. These data indicate that TRPL and TRP mediate cool avoidance by functioning outside the larval photoreceptor neurons and without relying on these critical elements of the phototransduction cascade.
TRP and TPRL Are Not Required for Warm Avoidance.
To further examine the molecular relationship between cool avoidance and warm avoidance, we tested whether trpl and trp were required for warm avoidance. In contrast to their robust defect in cool avoidance, trpl302;trp343 double mutant animals were normal for warm avoidance (Fig. 4A). Thus, TRPL and TRP are required for cool avoidance, but not warm avoidance.
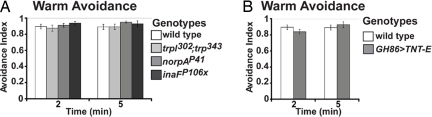
Fig. 4.
TRPL, TRP, and GH86-Gal4-expressing neurons are not required for warm avoidance. (A) Warm avoidance of wild-type (n = 9), trpl302;trp343 (n = 6), norpAP41 (n = 8), and inaFP106X (n = 6). (B) Warm avoidance of wild-type (n = 10), GH86-Gal4/Y or +;UAS-TNTE/+ (n = 9).
We also noticed that warmth avoidance of norpAP41 and inaFP106x mutant larvae was normal (Fig. 4A), suggesting that visual system signal transduction is dispensable for thermotaxis at elevated temperatures. The lack of requirement of NORPA for warmth avoidance is particularly intriguing as NORPA is important for the synchronization of the Drosophila circadian clock by elevated temperature (41).
Although we found no requirement for larval photoreceptor neurons in cool avoidance, prior work has identified a set of cold-activated thermosensors located in the larval terminal organ (23). Consistent with a role for these terminal organ cold sensors in cool avoidance behavior, expression of tetanus toxin light chain (TNT-E), which blocks synaptic vesicle release (42) in the terminal organ neurons under the control of GH86-Gal4 (23, 43), caused a robust cool avoidance defect in our assay (Fig. 3C). However, the function of these neurons appears specific for cool avoidance, as GH86-Gal4>UAS-TNTE animals showed normal warm avoidance (Fig. 4B). Thus, distinct circuits are involved in warm and cool avoidance.
Discussion
Drosophila melanogaster exhibit strong temperature preferences and robustly avoid thermal environments that are too warm or too cool (21–23, 44). Here we demonstrate that larvae avoid environments that are too warm or too cool using distinct molecules and circuits. As we previously reported using RNAi (22), we confirm here using classical genetic mutations that dTRPA1 is essential for larval warm avoidance. We extend these previous findings to show that cool avoidance does not require dTRPA1. Rather, we discover that cool avoidance depends on the TRPC family members TRPL and TRP. Although these two TRP channels also have critical functions in phototransduction, larval cool avoidance and phototransduction are distinct, as neither larval photoreceptors nor the phototransduction molecules NORPA and INAF are required for cool avoidance.
TRPL and TRP belong to the TRPC family of TRP channels. The TRPC family is evolutionarily conserved, with seven members in mammals (45, 46). Although individual members of the TRPV, TRPM, and TRPA families are known to be temperature-activated ion channels, an involvement for TRPC proteins in mediating temperature perception has not been previously demonstrated, and it will be interesting to learn whether mammalian TRPCs also contribute to thermosensation. Here we demonstrate a clear requirement for the Drosophila TRPCs TRPL and, to a lesser extent, TRP in cool avoidance. However, in contrast to classic thermoTRPs such as dTRPA1, which exhibits strong warmth activation when ectopically expressed in oocytes (27), neither TRPL nor TRP showed detectable cool activation in oocytes in our hands (K.J.K. and P.A.G., unpublished). In addition, whereas expression of TRPL and TRP was readily detected in the larval photoreceptors using RNA in situ hybridization, expression could not be detected in the putative cold receptor neurons of the terminal organ (M.R., unpublished). Thus, whether these TRPC proteins participate directly in thermotransduction or affect thermosensory behavior by acting at a downstream step remains to be determined. Although the mechanism by which TRPL and TRP mediate cool avoidance is not clear, it appears distinct from the mechanisms by which TRPL and TRP channels mediate visual system signal transduction, as the latter rely on NORPA (37, 38) and INAF (39, 40), which are dispensable for cool avoidance (Fig. 3C).
We have also found that that the neural pathways for cool and warm avoidance are distinct. Whereas the larval cold sensors, located in the terminal organ, are essential for larval cool avoidance, they are not necessary for warm avoidance. As for the larval warm sensors, we previously implicated a set of dTRPA1-expressing neurons in the brain in warm avoidance in third instar larvae (22). Unfortunately, it is not yet technically possible to assess the function of these cells in cool avoidance, as available promoters for manipulating these dTRPA1-expressing neurons are expressed too late to effectively manipulate neuronal function in first and second instar larvae, the stages at which cold avoidance is most robust (M.R. and P.A.G., unpublished).
Together our data indicate that Drosophila use distinct TRP channels and neurons to respond to different, discrete ranges of temperature. The channels TRPL and TRP and the neurons of the terminal organ are specifically involved in the avoidance of cool temperatures, whereas dTRPA1- and dTRPA1-expressing neurons are required for the avoidance of moderately warm temperatures. At even higher temperatures, PAINLESS mediates avoidance by acting in multiple-dendritic neurons, whereas PYREXIA has a potentially general neuroprotective effect possibly reflecting its broad neuronal expression (24–26). Thus, Drosophila melanogaster possesses a suite of thermosensory detection pathways, each of which responds at specific temperatures and promotes a characteristic set of behavioral responses, ranging from gradual migration away from moderately warm or cool temperatures to immediate withdrawal from extreme temperatures that cause rapid tissue damage. As mammals also use distinct sensors for detecting different portions of the thermal spectrum (1, 2), these studies support a fundamental similarity in the logic of thermosensation in both mammals and insects, with both types of animals sensing the range of temperatures they encounter using a series of TRPs and thermosensory cells, with different sensors tuned to different portions of the temperature spectrum.
Materials and Methods
Fly Strains and Molecular Genetics.
dtrpA1ins was recently described (28) and contains two mutant copies of dtrpA1 flanking targeting vector sequences (Fig. S1): one copy is deleted for the ion pore, 6th transmembrane domain and C terminus, whereas the other copy lacks the putative promoter, exon 1 (including predicted translation start site), 1st intron, part of exon 2 and contains a 2–base pair insertion creating an early translational frame-shift (predicted D183->A, 27aa, STOP). Both dtrpA1fs and dtrpA1wtR were generated from dtrpA1ins as previously described (29). The original dtrpA1ins strain was backcrossed to Canton S control for five generations before the cool avoidance assays. Warm avoidance of original and backcrossed dtrpA1ins strains were examined and were indistinguishable (Fig. S2D). Fig. 1 experiments used original dtrpA1ins strain. UAS-dTRPA1 (22) used for rescue contains dTRPA1H408R cDNA (28). [K129 trp rescue] (gift of W. Pak) contains a trp cDNA flanked by trp genomic sequences, corresponding to P[trp(124)] in (30). Targeting strains, norpAP41 (phenotype confirmed by electroretinogram), trplMB03075, Df(3R)Exel6213, trpMB03672, trp1, Df(trpl)(w1118;Df(2R)BSC132,P+Pbac[XP5.WH5]BSC132), GMR-hid, and Actin-Gal4 were obtained from Bloomington Stock Center. Deficiencies uncovering dtrpA1 (Df(dtrpA1): Df(3L)ED4415 and Df(3L)ED4416) were obtained from Szeged Stock Center.
Behavioral Assays.
white;Canton S was control. Larvae for behavioral assays were raised on molasses plates. Warm avoidance examined as described (22), except with a release zone of ≈33.5° to 30.2°C rather than ≈35° to ≈31°C (≈140 late 1st/early 2nd instar larvae used per assay). Cool avoidance assays were similar to (23) but with different temperatures and 1st instar larvae (23). Cool avoidance was assayed on 2% agarose (25 ml) in lids from 150 × 15–mm Petri plates (VWR 25384–139) covered with a glass slab. The release zone was positioned midway between a room temperature metal slide warmer and a cold plate (set for 10°C; AHP-301CP, TECA), which were ≈14 mm apart. The cold plate surface was covered with black plastic to ease scoring (34.3-μm-thick RNW 4050 bags [Office Depot]). Agarose surface was equilibrated >60 min before assay (covered by a glass slab). As described (22), at each time point, larvae migrating out of the release zone were counted to calculate an avoidance index (AI) = (number of larvae on RT side − number of larvae on cooled side)/(number of larvae on the RT side + number of larvae on cooled side). Surface temperatures were monitored with Fluke 52II thermometer with dual K-type flat probes (VWR). Each cool assay used ≈100 to 150 1st larvae, and assays were performed in the dark. Further details are available upon request. Larvae distributed evenly in the absence of a temperature gradient.
Chemotaxis assays were as described (22, 43). Phototaxis was assayed on 90-mm plastic Petri dish with four light/dark quadrants as described (47), except for using clear 2% agarose with dark quadrants generated using black electrical tape on dish bottom. Light was applied from underneath with TW-26 transilluminator (VWR), and plate was raised ≈12.5 cm above the light to minimize heating. Late 1st/early 2nd instar larvae were assayed. The number of larvae migrating out of the release zone to stimulus zone (odor or light) and no stimulus zone (no odor or dark) were counted to calculate AI.
Data are mean ± SEM and were analyzed with one-way analysis of variance (ANOVA) followed by Tukey-Kramer HSD tests using JMP 5.0 software (SAS), with P ≤ 0.05 considered significant. Where differences between genotypes are noted, one-way ANOVA probability >F was <0.0001.
Supplementary Material
Acknowledgments.
We thank the following individuals for their contribution to this work: J. D. Kaplan and C. Rex for technical assistance; H. Nash, C. Montell, M. Rosbash, D. Tracey, S. Tsunoda, W. Pak and R. Stocker for fly strains; R. Teodoro and T. Schwarz for assistance with electroretinograms; and J. Agosto, R. Bohm, J. Hall, T. Orr-Weaver, C. Quinn, J. Whited, and Garrity laboratory for discussions. Supported by National Institute for Neurological Disorders and Stroke (NINDS) (PO1 NS044232, P30 NS045713S10 and RR16780) and National Eye Institute (NEI) (RO1 EY13874, P.A.G.). M.R. also supported by Centocor fellowship, National Science Foundation predoctoral grant and National Institutes of Health predoctoral training grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805041105/DCSupplemental.
References
- 1.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 2.Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 3.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 6.Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 8.Smith GD, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 9.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel. TRPV4 J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talavera K. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 11.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 12.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 14.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 15.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Woodbury CJ, et al. Nociceptors lacking TRPV1 and0 TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid G. ThermoTRP channels and cold sensing: What are they really up to? Pflugers Arch. 2005;451:250–263. doi: 10.1007/s00424-005-1437-z. [DOI] [PubMed] [Google Scholar]
- 19.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci USA. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci. 2003;6:267–273. doi: 10.1038/nn1009. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 25.Tracey WD, Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 26.Xu SY, et al. Thermal nociception in adult Drosophila: Behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 27.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 28.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong YS, et al. Targeted mutagenesis by homologous recombination in (D) melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong YS, et al. Single amino acid change in the fifth transmembrane segment of the TRP Ca2+ channel causes massive degeneration of photoreceptors. J Biol Chem. 2002;277:33884–33889. doi: 10.1074/jbc.M204075200. [DOI] [PubMed] [Google Scholar]
- 31.Moses K, Ellis MC, Rubin GM. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989;340:531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- 32.Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443–1453. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- 33.Hassan J, Busto M, Iyengar B, Campos AR. Behavioral characterization and genetic analysis of the Drosophila melanogaster larval response to light as revealed by a novel individual assay. Behav Genet. 2000;30:59–69. doi: 10.1023/a:1002090627601. [DOI] [PubMed] [Google Scholar]
- 34.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Sawin-McCormack EP, Sokolowski MB, Campos AR. Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J Neurogenet. 1995;10:119–135. doi: 10.3109/01677069509083459. [DOI] [PubMed] [Google Scholar]
- 37.Bloomquist BT, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 38.McKay RR, Chen DM, Miller K, Kim S, Stark WS, Shortridge RD. Phospholipase C rescues visual defect in norpA mutant of Drosophila melanogaster. J Biol Chem. 1995;270:13271–13276. doi: 10.1074/jbc.270.22.13271. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Nash HA. Drosophila TRP channels require a protein with a distinctive motif encoded by the inaF locus. Proc Natl Acad Sci USA. 2007;104:17730–17734. doi: 10.1073/pnas.0708368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Geng C, Leung HT, Hong YS, Strong LL, Schneuwly S, Pak WL. INAF, a protein required for transient receptor potential Ca(2+) channel function. Proc Natl Acad Sci USA. 1999;96:13474–13479. doi: 10.1073/pnas.96.23.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glaser FT, Stanewsky R. Temperature synchronization of the Drosophila circadian clock. Curr Biol. 2005;15:1352–1363. doi: 10.1016/j.cub.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 43.Heimbeck G, Bugnon V, Gendre N, Haberlin C, Stocker RF. Smell and taste perception in Drosophila melanogaster larva: Toxin expression studies in chemosensory neurons. J Neurosci. 1999;19:6599–6609. doi: 10.1523/JNEUROSCI.19-15-06599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zars T. Two thermosensors in Drosophila have different behavioral functions. J Comp Physiol [A] 2001;187:235–242. doi: 10.1007/s003590100194. [DOI] [PubMed] [Google Scholar]
- 45.Hardie RC. TRP channels and lipids: From Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 47.Lilly M, Carlson J. smellblind: A gene required for Drosophila olfaction. Genetics. 1990;124:293–302. doi: 10.1093/genetics/124.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.