Abstract
A key issue in understanding the pathogenic conditions associated with the aberrant aggregation of misfolded proteins is the identification and characterization of species formed during the aggregation process. Probing the nature of such species has, however, proved to be extremely challenging to conventional techniques because of their transient and heterogeneous character. We describe here the application of a two-color single-molecule fluorescence technique to examine the assembly of oligomeric species formed during the aggregation of the SH3 domain of PI3 kinase. The single-molecule experiments show that the species formed at the stage of the reaction where aggregates have previously been found to be maximally cytotoxic are a heterogeneous ensemble of oligomers with a median size of 38 ± 10 molecules. This number is remarkably similar to estimates from bulk measurements of the critical size of species observed to seed ordered fibril formation and of the most infective form of prion particles. Moreover, although the size distribution of the SH3 oligomers remains virtually constant as the time of aggregation increases, their stability increases substantially. These findings together provide direct evidence for a general mechanism of amyloid aggregation in which the stable cross-β structure emerges via internal reorganization of disordered oligomers formed during the lag phase of the self-assembly reaction.
Keywords: amyloid aggregation, amyloid oligomers, two-color coincidence spectroscopy, PI3-SH3 domain, neurodegenerative diseases
The tissue deposition of the β-sheet-rich, filamentous protein aggregates, amyloid fibrils, represents the common pathological hallmark of a range of degenerative disorders including Alzheimer's and Parkinson's diseases. However, the observation that many proteins unrelated to disease can also form amyloid fibrils suggests that the structural motif common to these aggregates is broadly accessible by polypeptide chains (1, 2). As such, developing an understanding of the mechanisms by which soluble protein molecules assemble into these fibrils is of fundamental and biomedical importance (3–7). Amyloid fibrils have been demonstrated to assemble typically via nucleation and growth kinetics, characterized by an initial lag phase before fibril elongation (8). A wealth of data indicates that species formed during this phase of the reaction are cytotoxic, and, furthermore, that soluble, oligomeric precursors to amyloid fibrils likely represent the critical pathological species in some amyloid disorders (7, 9–13). Probing the initial stages of the assembly process is, however, challenging because of the low populations of heterogeneous, unstable oligomeric species.
We have studied the early stages of amyloid fibril formation by the SH3 domain from bovine phosphatidylinositol-3′-kinase (PI3–SH3) using an approach involving both bulk and single-molecule techniques. PI3–SH3 has been shown to form highly ordered structures upon incubation at low pH and low ionic strength that have the key characteristics of amyloid fibrils associated with human disease (14, 15). The most well characterized example of the amyloid fibrils formed by PI3–SH3 comprises a double helix of protofilament pairs, in which the great majority of the 84 amino acid-residue protein is contained within the fibril structure, as shown from measurements of hydrogen/deuterium exchange kinetics and controlled proteolysis (16–18). Before fibril proliferation, however, PI3–SH3 forms smaller, granular aggregates that have been found to be as toxic as aggregates of the Aβ peptide associated with Alzheimer's disease (9, 19). These observations show that such toxicity is not simply restricted to those aggregates formed by disease-related peptides and proteins but, like the ability to form the fibrils themselves, could be a generic property of polypeptides (2, 9). Studies of PI3–SH3 can therefore provide valuable information not only on the nature of amyloid fibril formation, but also on the population of the cytotoxic oligomers that populate the assembly pathway.
Results and Discussion
Bulk Experiments.
Initially, we performed bulk kinetic studies of the formation of amyloid fibrils by wild-type PI3–SH3 at room temperature and pH 2.0 by monitoring the fluorescence of the amyloidophilic dye, thioflavin T (ThT). These data yield a sigmoidal kinetic profile that could be well fitted by using a simple logistic model, where the overall aggregation rate decreases as soluble precursors are consumed and that has been used previously to describe PI3–SH3 aggregation kinetics (see Fig. 2A) (20). Based on this fit, the initial lag phase for fibril proliferation (assigned here as the time until the ThT fluorescence has increased by 5% of its maximal increase) of 18.4 ± 2.3 h is followed by an exponential fibril growth phase with a rate constant of 0.16 ± 0.02 h−1. The presence of a lag phase is typical of the conversion of soluble proteins into amyloid fibrils and is generally considered to represent the nucleation phase for ordered aggregation (8). Characteristically, this early stage of fibril formation also corresponds to the highest population of toxic prefibrillar aggregates (9–13). Indeed, aggregates were shown to form during this stage of the reaction by determination of the fraction of PI3–SH3 that remained soluble subsequent to ultracentrifugation of the solution at various time points (see Fig. 2A).
Fig. 2.
Determination of aggregation kinetics. (A) Bulk aggregation kinetics of PI3–SH3 were measured by the increase in the fluorescence of Thioflavin T (squares) and the increase in the fraction of protein sedimentable by ultracentrifugation (circles). The ThT trace showed a lag phase of 18.4 ± 2.3 h, in contrast to the absence of an observable lag phase in the sedimentation data, indicating the formation of aggregated prefibrillar species in the early stages of the reaction that do not bind ThT. (B) Single-molecule TCCD measurement of blue (squares) and red (circles) monomer event rates (corrected for variations in dilution), proportional to monomer concentration, at different incubation times. The traces were globally fitted to a single exponential function, giving a decay time of 3.1 ± 0.4 h. (C) Association quotient variation with incubation time. The traces were fitted to a sum of exponential functions, showing a fast rise-time (1.9 ± 1.0 h) and a longer decay time (16.3 ± 1.3 h) related to the initial accumulation and subsequent growth of the oligomers.
In contrast to the ThT data, the fraction of soluble protein decreased without a lag phase and with a single exponential rate constant of 0.033 ± 0.006 h−1 (see Fig. 2A); ≈40% of the protein became sedimentable during the ThT-determined lag phase for fibril proliferation. From the centrifugation parameters used in this experiment, particles with a sedimentation coefficient of >38 S are expected to have been cleared from these solutions. Although the sedimentation coefficient of an oligomeric assembly is highly dependent on particle shape and its degree of hydration, by simple comparison, 40 S particles correspond approximately to biomolecular complexes with diameters in the range of 10–20 nm (21). This value falls within the broad range of aggregate sizes observed by TEM in the early stages of the reaction [see supporting information (SI) Text and Figs. S1–S4] for this and other systems (9, 22–25). Taken together, the marked differences in the kinetic profiles of the aggregation reaction acquired from the ThT and sedimentation data strongly suggest the existence of multiple processes on the reaction pathway. To resolve the individual species present during the reaction and, hence, to probe the nature of these processes, we have applied a single-molecule fluorescence technique that employs simultaneous two-color detection (26).
Single-Molecule Experiments.
To enable these single-molecule experiments, which were otherwise performed under identical conditions to the bulk studies, an N-terminal cysteine mutation (M1C) was introduced into the polypeptide sequence of PI3–SH3 to allow site-specific labeling. Batches of protein were labeled separately with either Alexa Fluor 488 or Alexa Fluor 647 fluorophores. Fibrils produced from a mixture of these labeled proteins were found by TEM to be morphologically similar to those formed by unmodified PI3–SH3 under the same conditions (see SI Text and Fig. S4). For the single-molecule experiments, equimolar mixtures of Alexa Fluor 488- and Alexa Fluor 647-labeled PI3–SH3 were incubated at room temperature for 4 d, corresponding to the lag and growth phases of aggregation detected by ThT fluorescence, during which time aliquots were taken at different time points for analysis. The aliquots were then rapidly diluted by a factor of (1–2) × 105 to enable the detection of single-molecule fluorescence bursts and were subsequently subjected to single-molecule analysis using the two-color coincidence detection (TCCD) technique.
The principles of oligomer detection by TCCD are shown in Fig. 1. TCCD is capable of detecting oligomeric complexes even in the presence of monomers, based on a molecule-by-molecule analysis of the species as they diffuse through a confocal volume excited by overlapped red and blue lasers (26, 27). The converged lasers allow for the independent detection of Alexa Fluor 488- or Alexa Fluor 647-labeled monomers as they pass through the confocal volume, as well as the detection of coincident bursts of fluorescence from mixed-label complexes. Therefore, oligomeric species formed by equimolar mixtures of Alexa Fluor 488- and Alexa Fluor 647-labeled PI3–SH3 can be readily distinguished from monomeric protein molecules containing a single fluorophore. In the analysis of these data, we have applied methods developed to account for the chance coincidental background events that occur when two noninteracting PI3–SH3 molecules with different fluorophores pass through the confocal volume simultaneously; through this approach we can determine the fraction of oligomers in solution with greater accuracy (27).
Fig. 1.
Principle of the TCCD method to detect oligomeric aggregates. (A) Detection of oligomer events in PI3–SH3 aggregation. The coincident fluorescent bursts on both channels show the presence of oligomers (marked as asterisks). (B) Expansion of fluorescence bursts in A. Comparison of the intensity of bursts from monomers and oligomers: The monomer events are not coincident and are much less intense than those due to oligomers.
Representative results from the single-molecule (TCCD) measurements are shown alongside data from the bulk kinetic measurements in Fig. 2. The TCCD data can be analyzed to define either the changes in the fluorescent burst rate arising from monomeric molecules as aggregation proceeds (low intensity, noncoincident fluorescence bursts), or the changes in rate of coincident fluorescence bursts arising from oligomeric species. We determined first the changes in monomer burst rate, a parameter that is directly correlated with the concentration of monomeric protein molecules in solution. We observe that the concentration of monomers decreases rapidly by ≈90% in the initial stages of the reaction, as they coalesce into aggregates (Fig. 2B). The measured decay time of 3.1 ± 0.4 h falls well within the lag time for fibril growth (18.4 ± 2.3 h), as determined by ThT measurements (Fig. 2A), and indicates that these aggregated species are rapidly formed before the proliferation of fibrillar species. The inability of aggregates formed at this stage of the reaction to bind ThT strongly suggests that they do not possess a well defined cross-β structural motif common to mature amyloid fibrils but, instead, possess a less regular structure.
Next, we determined the changes in the population of oligomeric species during the aggregation reaction. For each time point, the association quotient—a value describing the fraction of all bursts that are coincident, arising from associated oligomers (27)—was determined (Fig. 2C). This analysis reveals a rapid increase in the fraction of oligomeric species in the early stages of PI3–SH3 aggregation, with an average rate constant of the same order of magnitude as that of the decay in monomer concentration (0.53 ± 0.20 h−1 and 0.32 ± 0.04 h−1, respectively). The quantitative agreement between these rate constants is close enough to identify the two observations as the same physical process—the aggregation of monomers into oligomers. Subsequently, the association quotient decreases slowly with a rate constant of 0.061 ± 0.005 h−1. This value is intermediate to the aggregation rates determined from the sedimentation and ThT measurements (0.033 ± 0.006 h−1 and 0.16 ± 0.02 h−1, respectively). Taken together, these data suggest that PI3–SH3 rapidly forms oligomers at low pH and low ionic strength, which are then subsequently and more slowly consumed as the fibrils proliferate.
The apparent disappearance of these species is likely to result from the decreasing efficiency of their detection by TCCD as they grow into larger aggregates, because larger aggregates diffuse more slowly and encounter the confocal volume less frequently than smaller species. This notion is supported by our determination of the TCCD detection efficiency for simulated oligomer populations with a Gaussian distribution of sizes whose average molecular weight incrementally increases as the reaction time progresses (see SI Text and Figs. S1 and S2). When these oligomer size distributions are corrected by the detection efficiency, a maximum in the association quotient is predicted for early reaction times as the monomers aggregate, followed by a decrease in this quantity as oligomers grow increasingly large at later reaction times. The form of the observed TCCD data (Fig. 2C) is in excellent agreement with that predicted from simulation (see Fig. S1F).
Oligomer Size Distribution.
Our next objective was to characterize the nature of the soluble oligomeric species observed during the aggregation reaction. Oligomer size information was extracted from the relative intensities of the blue and red fluorescence bursts arising from individual oligomers compared with the average intensity value of a monomer. This approach is justified because the fluorescence lifetimes of fluorescently labeled monomers, oligomers, and fibrils showed no significant difference (see SI Text), indicating the absence of quenching or environmental effects on the dyes upon aggregation. The fluorescence intensities of the oligomers were also corrected for their slower diffusion relative to monomers, because these latter species spend more time in the excitation volume and thus emit more photons per fluorescent burst event (see SI Text). By using this method of analysis, 2D surface histograms of the number of blue- and red-labeled monomers per oligomer were calculated for each time point sampled, as illustrated in Fig. 3A for a sample taken after 8.9 h of incubation.
Fig. 3.
Distribution of oligomer sizes. (A) A 2D contour plot of the number of red- and blue-labeled PI3–SH3 monomers per oligomer, from an aliquot taken after 8.9 h of incubation. (B) Histogram of the total number of monomers per oligomer, corrected for the different oligomer detection efficiency, from an aliquot taken after 8.9 h of incubation. (C) Median of the total number of monomers per oligomer as a function of incubation time.
At this time point, the majority of the observed oligomers were calculated to contain between 5 and 20 red- and blue-labeled molecules, which strongly indicates that the aggregation process generates species containing both types of dye and does not segregate into oligomers containing only red or only blue dye molecules. These data also demonstrate the highly heterogeneous nature of the oligomer size distribution, a feature that can more readily be quantified by conversion of these data into a simple histogram of the total number of molecules per oligomer, as shown in Fig. 3B. The distribution of sizes for detected oligomers in solution at this time point is revealed to be very broad and to follow a log-normal function, peaking at an average size of 30 monomers with a median of 38 ± 10 monomers (SD). This represents the oligomers detectable by TCCD, which are those that remain in solution. As discussed in SI Text, there is a decrease in detection efficiency with increasing oligomer size, due to their slower diffusion. The experimental distributions were corrected for this effect so the observation of a reduced number of larger oligomers in the TCCD experiment is due to their reduced concentration in solution. Remarkably, independent studies using very different measurement techniques have shown that oligomers with average sizes similar to the value observed here are crucial for fibril formation by the yeast prion, Sup35, and are considered to represent the most infective particles formed by the human prion protein (28).
The shape of the PI3–SH3 oligomer distribution, as well as the median oligomer size described above, remained relatively constant at all time points throughout the aggregation reaction (Fig. 3C). Because smaller oligomers comprising 10–40 monomers are observed to be more numerous than larger species, we infer that oligomeric species above a critical size of ≈40 monomers are likely to be consumed more readily than smaller species during the aggregation reaction, growing into larger, undetectable species. This implies that larger oligomers may play a more critical role in fibril proliferation than do smaller ones. This notion is supported by a previous study in which only amorphous aggregates comprising >20 molecules of a tandem repeat construct of PI3–SH3 (equal to >40 individual domains), were found to be efficient in promoting ordered fibril formation (20). Furthermore, a recent molecular simulation study has shown that for a small fragment from the β-amyloid peptide (Aβ16–22), the barrier for the conformational conversion of disordered oligomers into β-sheet-rich structures is lower for larger species (29, 30). Taken together, these findings indicate that although a broad ensemble of oligomer sizes may populate the pathway for amyloid fibril formation, oligomers within a specific range of sizes are required for efficient conversion into more ordered species.
Oligomer Stability.
To examine whether the PI3–SH3 oligomers are capable of undergoing such a conformational conversion, we examined the changes in oligomer stability over the course of the aggregation reaction. These measurements were based on our observation that a marked increase in the frequency of noncoincident blue and red bursts occurs during the course of a single TCCD measurement, a result that can be attributed to the dissociation of oligomers at the low protein concentrations required for single-molecule analysis. Fig. 4A shows these data for a sample that had been incubated under aggregation conditions for 2 h and subsequently diluted. Fitting the increases in burst rate to single exponential functions for each sampled time point yielded rise times between (0.1–2.5) × 103 s. Despite the range of rise times observed, these values are significantly lower than the dissociation time constant determined for the liberation of PI3–SH3 molecules from mature fibrils (7.2 × 103 s) (18), indicating that the oligomers formed initially are substantially less stable than mature fibrils.
Fig. 4.
Blue- and red-labeled monomer burst rates during TCCD measurements. (A) Aliquot taken after 2 h of incubation of PI3–SH3, showing an increase in the monomer burst rate due to oligomer dissociation. The traces were fitted to exponential functions with the same time constant, giving a rise-time value of 1,000 ± 56 s. (B) An aliquot taken after 72 h of incubation showing the constant rates of monomer events.
We do observe, however, a shift toward slower dissociation of the oligomeric species as the aggregation reaction progresses (see SI Text and Fig. S3), until, after 48 h, dissociation of the oligomers becomes too slow to be detectable by TCCD. At incubation times longer than 48 h, the monomer concentration remains constant during the TCCD measurement period, as shown in Fig. 4B for a sample incubated under aggregation conditions for 72 h. A plausible cause of this enhancement in stability is an internal conformational reorganization of the oligomeric species populated during the reaction that leads to fibril formation. Interestingly, such a process has been predicted in computer simulations of the early stages of amyloid formation and attributed to the nucleation of the cross-β structure within species whose coalescence is initially driven by the rapid formation of less stable hydrophobic interactions (29).
Conclusions
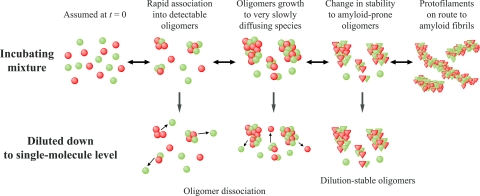
The use of the TCCD single-molecule strategy described in this article has provided unique insights into the biologically important early stages of aggregation by a protein that forms very well ordered amyloid fibrils under the conditions used in this study. The data suggest that fibril formation is preceded by the accumulation of oligomeric species whose stability increases with time before their consumption by the fibril proliferation reaction, as depicted in Fig. 5. Our detection of a transient population of oligomeric species early in the fibril formation reaction is consistent with the emerging view that the origin of cytotoxicity in disorders such as Alzheimer's and Parkinson's diseases could result from the existence of small aggregates formed before the appearance of mature amyloid fibrils (2). The successful detection of single particles in this study has revealed that, although the median size of these oligomers in the case of PI3–SH3 is ≈40 molecules, they constitute a highly heterogeneous ensemble of species, indicative of a stochastic polymer-like assembly process. The approach described here should be widely applicable to comparative studies of other peptide and protein systems; extension of its scope should permit the investigation not only of the size and stability of oligomeric species but also their interactions with other molecular species of biological or pharmaceutical importance, ranging from molecular chaperones and antibodies to small-molecule inhibitors of fibril formation.
Fig. 5.
PI3–SH3 aggregation process as detected by TCCD. Single-molecule fluorescence two-color coincidence detection (TCCD) provides information concerning PI3–SH3 oligomerization prior to amyloid fibril formation and about the size of the oligomers, their growth, stability, and molecular rearrangement.
Materials and Methods
Protein Expression and Purification.
A single-cysteine mutant of the SH3 domain from the α-subunit of bovine phosphatidylinositol-3′-kinase with the N-terminal methionine residue substituted for a cysteine (termed M1C PI3–SH3) was expressed in Escherichia coli [strain BL21(DE3)] as a fusion protein with a 6× Histidine tag and purified via nickel-affinity chromatography, followed by gel filtration (26/60 Superdex 75 column; GE Healthcare) in a similar manner to that described previously (15, 31). Preparations of M1C PI3–SH3 were labeled with either maleimide-modified Alexa Fluor 647 or Alexa Fluor 488 fluorescent dye (Invitrogen), via the cysteine thiol moiety, purified by gel filtration on a 16/60 Superdex 75 column (GE Healthcare), lyophilized to dryness and stored at −20°C. The degree of labeling was determined by both UV-Vis spectroscopy and mass spectrometry measurements to be ≈90%.
Sample Preparation.
For the aggregation reactions, 0.6 mg each of the Alexa-647- and Alexa-488-labeled M1C PI3–SH3 were dissolved in 120 μl of 0.01 M HCl (pH 2.0), giving a final protein concentration of 1 mM. The pH of the solution was adjusted to 2.00 by the addition of 1.0 M HCl. The solution was then centrifuged (15 min, 16,000 × g) to remove any preexisting fibrillar species. This supernatant was subsequently incubated in the dark at room temperature, during which time, aliquots were taken for TCCD analysis. Each time point was diluted a total of 105-fold by serial dilution with pure deionized water (MilliQ), with the final dilution being made on the well of a chambered cover glass to a final volume of 1.0 ml.
The incubations were repeated three times, and consistent results were found. Nevertheless, the preliminary centrifugation step makes it impossible to control the final concentration of peptide exactly. Therefore, because the aggregation behavior is concentration dependent, direct comparison of the quantitative results between experiments is not feasible. We found the main features of the curves to be reproducible, but there were appreciable differences in the numerical values between experiments because of differences in the initial (postcentrifugation) concentration.
Bulk Kinetics Measurements.
Incubations for bulk measurements were prepared exactly as for the single-molecule measurements by using 1 mM wild-type PI3–SH3. Aliquots were taken for analysis by thioflavin T fluorescence using a Cary Eclipse fluorescence spectrophotometer (Varian) or by UV-vis spectroscopy using a Cary 400 UV-vis spectrophotometer (Varian) after ultracentrifugation (avg. 290,000 × g, 45 min, 4°C; Beckman Coulter Optima TLX).
TCCD Instrument and Data Analysis.
The instrumentation for TCCD measurement has been reported in detail (27). Two overlapped Gaussian laser beams, at 488 nm, argon ion (35LAP321–230; Melles Griot), and 633 nm, He:Ne laser (25LHP151; Melles Griot), were directed to the back port of an inverted microscope (Nikon Eclipse TE2000-U). The beams were focused 6 μm into the solutions in a Lab-TeK chambered cover glass (Scientific Laboratory Suppliers Ltd.) through a high numerical aperture oil-immersion objective (Apochromat 60X, NA 1.40; Nikon). Fluorescence was collected by the same objective and imaged onto a 50-μm pinhole (Melles Griot) and then separated in two different channels by using a dichroic mirror (585DRLP; Omega Filters) and sent to two avalanche photodiodes (APD) (SPCM AQR-14; Perkin–Elmer Optoelectronics). The cross-talk (detection of one fluorophore emission in the other channel) was calculated to be 1% from the blue channel into red channel, whereas it was negligible from the red into blue channel. The laser powers were 300 and 80 μW for the blue and red excitation, respectively. For all of the single molecule experiments, data were collected at 20°C with a 1-ms bin time over 8,000 channels on both MCS cards. Typically, 400 frames of 8000 ms were collected for a total measurement time of 1 h per aliquot.
The photon time traces were analyzed as follows. We first set an optimized threshold value for each channel, donor and acceptor channels, as described (32) using a model sample, a dual-labeled 40-bp double-stranded DNA. Once the thresholds are applied to the SH3 dilutions, the coincidence events are counted and analyzed (after correction for coincident events due purely to chance) following the methodology previously reported (27). The association quotient shown in Fig. 2C, a measurement of the fraction of dual-labeled molecules, is obtained from:
where rB and rR are the burst rates in the blue and red channel respectively. The significant coincident event rate, rS, is obtained by subtracting the expected rate of coincident events due to chance (rE) from the total coincident events rate (rC).
The frames of fluorescence bursts were filtered to count events due only to monomers (Fig. 2B) by only analyzing frames with a Q value <0.01. This ensures the absence of coincidence events, and thus no oligomers were present. In this way, the monomer concentration during the TCCD measurement could be monitored for each aliquot analyzed (Fig. 4), and thus information on the stability of oligomers could be obtained.
The third step was the analysis of oligomer frequency rate and size. Because the oligomers diffuse very slowly due to their size, the photon traces were rebinned using a bin time that allows the detection of oligomer events into single bins. By analyzing oligomeric burst duration we found the optimum bin size to be 20 ms. Then, TCCD analysis was performed again, by using a large threshold value of 300 photons per bin, 20 times higher than the threshold used for the single-molecule analysis with 1 ms binned data. This method allows us to only count events due to oligomers, which have a high fluorescence intensity. Based on the average intensity from a monomer (blue- or red-labeled), the approximate number of monomers per oligomer event can be extracted. The number of monomers per oligomer was corrected for the fact that the oligomer diffuses much more slowly, spending more time in the excitation volume, and thus each monomer fluorophore emits more photons per event and has a higher average brightness. This was an iterative process as follows: The number of monomers per oligomer for each oligomeric event was first estimated by using the average brightness of the free monomers, and then the average brightness values of the monomers were corrected for the slower diffusion (considering the diffusion coefficient proportional to the cube root of the molecular weight); with the new monomeric brightness values, the number of monomers per oligomer was calculated again. This was repeated until a convergent solution was reached. The overall histogram shape and median oligomer size for each aliquot (Fig. 3) did not significantly depend on the threshold value used.
Supplementary Material
Acknowledgments.
We thank Prof. Alvarez-Pez for providing access to the time-resolved fluorescence instrument and acknowledge the contribution of Drs. Cait MacPhee and Haitao Li to the initial experiments that led to this work. We also thank Dr. Giorgio Favrin, Sarah Shammas, and Albert Chiou for insightful discussions. A.O was funded by a Marie Curie Intra-European (6th Framework) Fellowship, G.L.D. is a C. J. Martin Fellow of the National Health and Medical Research Council, Australia, and D.K. gratefully acknowledges the Biotechnology and Biological Sciences Research Council for a research development Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803086105/DCSupplemental.
References
- 1.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Gertz H-J, Siegers A, Kuchinke J. Stability of cell size and nucleolar size in Lewy body containing neurons of substantia nigra in Parkinson's disease. Brain Res. 1994;637:339–341. doi: 10.1016/0006-8993(94)91257-2. [DOI] [PubMed] [Google Scholar]
- 4.Lue L-F, et al. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean CA, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Serio TR, et al. Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science. 2000;289:1317–1321. doi: 10.1126/science.289.5483.1317. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DM, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 8.Harper JD, Lansbury PT. Models of amyloid seeding in Alzheimer's disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 9.Bucciantini M, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 10.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 11.Bucciantini M, et al. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J Biol Chem. 2004;279:31374–31382. doi: 10.1074/jbc.M400348200. [DOI] [PubMed] [Google Scholar]
- 12.Demuro A, et al. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 13.Zamotin V, et al. Cytotoxicity of albebetin oligomers depends on cross-β-sheet formation. FEBS Lett. 2006;580:2451–2457. doi: 10.1016/j.febslet.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 14.Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. Amyloid fibril formation by an SH3 domain. Proc Natl Acad Sci USA. 1998;95:4224–4228. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zurdo J, Guijarro JI, Jimenez JL, Saibil HR, Dobson CM. Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J Mol Biol. 2001;311:325–340. doi: 10.1006/jmbi.2001.4858. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez JL, et al. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999;18:815–821. doi: 10.1093/emboj/18.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polverino de Laureto P, et al. Protein aggregation and amyloid fibril formation by an SH3 domain probed by limited proteolysis. J Mol Biol. 2003;334:129–141. doi: 10.1016/j.jmb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Carulla N, et al. Molecular recycling within amyloid fibrils. Nature. 2005;436:554–558. doi: 10.1038/nature03986. [DOI] [PubMed] [Google Scholar]
- 19.Baglioni S, et al. Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci. 2006;26:8160–8167. doi: 10.1523/JNEUROSCI.4809-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bader R, Bamford R, Zurdo J, Luisi BF, Dobson CM. Probing the mechanism of amyloidogenesis through a tandem repeat of the PI3–SH3 domain suggests a generic model for protein aggregation and fibril formation. J Mol Biol. 2006;356:189–208. doi: 10.1016/j.jmb.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S, Verschoor A, Radermacher M, Grassucci R, Frank J. Three-dimensional reconstruction of mammalian 40 S ribosomal subunit embedded in ice. J Mol Biol. 1995;245:461–466. doi: 10.1006/jmbi.1994.0037. [DOI] [PubMed] [Google Scholar]
- 22.Bitan G, et al. A molecular switch in amyloid assembly: Met35 and amyloid β-protein oligomerization. J Am Chem Soc. 2003;125:15359–15365. doi: 10.1021/ja0349296. [DOI] [PubMed] [Google Scholar]
- 23.Ellisdon AM, Thomas B, Bottomley SP. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J Biol Chem. 2006;281:16888–16896. doi: 10.1074/jbc.M601470200. [DOI] [PubMed] [Google Scholar]
- 24.Pieri L, et al. The yeast prion Ure2p native-like assemblies are toxic to mammalian cells regardless of their aggregation state. J Biol Chem. 2006;281:15337–15344. doi: 10.1074/jbc.M511647200. [DOI] [PubMed] [Google Scholar]
- 25.Habicht G, et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc Natl Acad Sci USA. 2007;104:19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li HT, Ying LM, Green JJ, Balasubramanian S, Klenerman D. Ultrasensitive coincidence fluorescence detection of single DNA molecules. Anal Chem. 2003;75:1664–1670. doi: 10.1021/ac026367z. [DOI] [PubMed] [Google Scholar]
- 27.Orte A, Clarke R, Balasubramanian S, Klenerman D. Determination of the fraction and stoichiometry of femtomolar levels of biomolecular complexes in an excess of monomer using single-molecule, two-color coincidence detection. Anal Chem. 2006;78:7707–7715. doi: 10.1021/ac061122y. [DOI] [PubMed] [Google Scholar]
- 28.Silveira JR, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheon M, et al. Structural reorganisation and potential toxicity of oligomeric species formed during the assembly of amyloid fibrils. PLoS Comput Biol. 2007;3:1727–1738. doi: 10.1371/journal.pcbi.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheon M, Favrin G, Chang I, Dobson CM, Vendruscolo M. Calculation of the free energy barriers in the oligomerisation of Abeta peptide fragments. Front Biosci. 2008;13:5614–5622. doi: 10.2741/3104. [DOI] [PubMed] [Google Scholar]
- 31.Booker GW, et al. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell. 1993;73:813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- 32.Clarke RW, Orte A, Klenerman D. Optimized threshold selection for single-molecule two-color fluorescence coincidence spectroscopy. Anal Chem. 2007;79:2771–2777. doi: 10.1021/ac062188w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.