Abstract
In the model organism Caenorhabditis elegans, the dauer pheromone is the primary cue for entry into the developmentally arrested, dauer larval stage. The dauer is specialized for survival under harsh environmental conditions and is considered “nonaging” because larvae that exit dauer have a normal life span. C. elegans constitutively secretes the dauer pheromone into its environment, enabling it to sense its population density. Several components of the dauer pheromone have been identified as derivatives of the dideoxy sugar ascarylose, but additional unidentified components of the dauer pheromone contribute to its activity. Here, we show that an ascaroside with a 3-hydroxypropionate side chain is a highly potent component of the dauer pheromone that acts synergistically with previously identified components. Furthermore, we show that the active dauer pheromone components that are produced by C. elegans vary depending on cultivation conditions. Identifying the active components of the dauer pheromone, the conditions under which they are produced, and their mechanisms of action will greatly extend our understanding of how chemosensory cues from the environment can influence such fundamental processes as development, metabolism, and aging in nematodes and in higher organisms.
Keywords: ascaroside, natural product structure elucidation
The nematode Caenorhabditis elegans is well adapted to the “boom-and-bust” conditions that it likely encounters in nature. To exploit favorable growth conditions, C. elegans develops in 3 days through four larval stages (L1–L4) to the adult, which can then produce 300 progeny. However, if as an L1 or early L2, the nematode encounters a high population density, low food availability, or high temperature, it will instead develop into an alternative L3 larval stage, the dauer larva, which is specialized for survival under harsh conditions and for dispersal (1–3). During dauer development, C. elegans undergoes fundamental morphological and metabolic changes. In preparation for the dauer stage, the worm alters its energy metabolism and accumulates fat in its intestine and hypodermal cells. As a dauer, the worm does not feed. The dauer's pharynx is sealed from the environment and its cuticle is thicker, enabling it to survive desiccation and harsh chemical treatments. Whereas an adult-stage worm typically lives ≈2 weeks, a dauer can survive for several months, and then once it encounters better growth conditions, develop into a reproductive adult with a normal life span. In this way, the dauer is considered “nonaging” (4).
The primary trigger for dauer development is a set of small-molecule pheromones, known collectively as the dauer pheromone (2). Because the dauer pheromone is produced throughout the nematode life cycle and thus gradually accumulates, its concentration enables the nematode to sense its population density and modulate its development accordingly. More than 25 years ago, Golden and Riddle (2, 3, 5) first established the existence of a small-molecule dauer pheromone in C. elegans. They showed that culturing C. elegans for extended periods in liquid culture produced “conditioned” culture media that could be used to induce L1/L2 larvae to become dauers as well as to inhibit recovery of dauers to the L4 stage. By drying down the conditioned medium and extracting it with organic solvent, a crude dauer pheromone could be generated that replicated the effects of the conditioned media.
Using activity-guided fractionation of crude dauer pheromone and NMR-based structure elucidation, we have recently identified the chemical structures of several dauer pheromone components as structurally similar derivatives of the 3,6-dideoxysugar ascarylose (6). These components differ in the nature of the straight-chain substituent on ascarylose, and thus we refer to them based on the carbon length of these side chains as ascaroside C6 (1) and C9 (2) (Fig. 1A). These two ascarosides are approximately a hundredfold more active than a previously identified component of the conditioned media, ascaroside C7 (3), which has been interpreted to mean that ascaroside C7 is not a physiologically relevant component of the dauer pheromone (7). Ascaroside C7 may well have an unidentified role in C. elegans development or behavior.
Fig. 1.
Chemical structures of components of the dauer pheromone. (A) The chemical structures of ascarosides C6 (1), C9 (2), and C7 (3). (B) Key dqf-COSY and 1H-13C HMBC interactions for ascaroside C3 (4). (C) The chemical structure of ascaroside C3 (4). (D) The synthesis of ascaroside C3 (4) proceeded from dibenzoyl ascarylose (7). An excess of 3-buten-1-ol was first glycosylated with the dibenzoyl ascarylose in the presence of boron trifluoride diethyl etherate to afford the homoallyl ascaroside. The olefin was then cleaved with potassium permanganate to afford the carboxylic acid. Finally, ester hydrolysis with aqueous lithium hydroxide afforded the target compound (4).
Genetic screens have identified Daf-c (dauer larva formation-constitutive) mutants that constitutively form dauers even under conditions of low pheromone and high food availability and Daf-d (dauer larva formation-defective) mutants that do not form dauers even under conditions of high pheromone and low food availability (8–13). Epistasis analysis has enabled these genes to be ordered into signaling pathways (8, 14–16). Currently, it is thought that the dauer pheromone is detected by exposed sensory neurons in the head of the worm (17–21) and signals through multiple signaling pathways, including the TGFβ pathway (20, 22–24) and the insulin/insulin-like growth factor-1 (IGF-1) pathway (25–29). These pathways converge on the nuclear hormone receptor DAF-12, which acts downstream of most Daf genes and which is expressed in many tissues that undergo remodeling during dauer formation (30). The TGFβ and insulin pathways are thought to control DAF-12 in part by regulating enzymes (31–34) involved in the biosynthesis of the DAF-12 steroid ligands (35, 36). In addition to controlling the developmental and metabolic changes important for dauer formation, the TGFβ and insulin pathways influence metabolism, stress resistance, and aging throughout the life cycle (27, 37–39). There is strong conservation of the TGFβ and insulin/IGF-1 pathways from C. elegans and to higher organisms, including humans, both in terms of the signaling proteins involved and the biological roles that they play (40–44). Dauer signaling provides insights into how environmental cues such as the dauer pheromone are sensed and how they control fundamental processes that are central to metabolism, development, and aging.
Here, we identify a component of the dauer pheromone that, under certain assay conditions, is more potent than any previously identified pheromone component. In addition, the activity of this component is synergistic with previously identified dauer pheromone components. Furthermore, we show that the conditions under which C. elegans is cultivated appear to influence the types and amounts of pheromone components that are produced. Previous work on pheromone-based communication in insects and fish has demonstrated that environmental conditions and the developmental status of the pheromone producer can influence the chemical identity and relative ratio of pheromone components (45, 46). In Drosophila, for example, the structures and relative ratios of the cuticular fatty acid-derived hydrocarbons that are used in mate attraction can vary depending on a number of nongenetic factors, including cultivation temperature, diet, developmental stage, and level of social interaction (45). The fact that the chemical components of the C. elegans dauer pheromone change depending on cultivation conditions suggests that the dauer pheromone may communicate more information about the environment than just population density.
Results and Discussion
At the start of this investigation, we set out to explore some of the differences between the methods that we used to identify the active pheromone components ascarosides C6 and C9 and the methods used by Jeong et al. (7) to identify the less potent ascaroside C7. One primary difference was that during activity-guided fractionation of the crude dauer pheromone, we used a dauer recovery assay to measure the activity of a fraction, whereas Jeong et al. used a dauer formation assay. In the dauer recovery assay, dauers are incubated in the presence of a fraction and a small amount of food (yeast extract), and the percentage of larvae that have exited dauer is measured after a 4-h period (2, 5). In the dauer formation assay, the fraction is incorporated into a minimal agar plate, onto which 50–100 eggs are laid and allowed to develop in the presence of a small amount of Escherichia coli food over the course of 3–4 d (6). Although the genetics of dauer formation and recovery are similar, some differences have been identified, such as the role of specific amphid chemosensory neurons (20) and certain G proteins (47) in the dauer formation and recovery processes. Thus, although it is likely that the small molecules that control dauer formation and recovery are similar, there may be differences between them. Additionally, the methods that we used differed from those used by Jeong et al. in that C. elegans was cultured under different culture conditions. We typically cultured C. elegans in 4-liter flasks at 22.5–25°C for 14–16 d, whereas Jeong et al. cultured C. elegans in a large-scale fermenter at 20°C for 20 d. Lastly, the purification methods that we used differed at several steps from those used by Jeong et al.
To resolve the role, if any, of these differences, we decided to generate two large-scale crude dauer pheromone preparations, one from worms cultured at 25°C and one from worms cultured at 20°C, and follow the activity during activity-guided fractionation using the dauer formation assay. In both cases, the conditioned media was separated from the worms by filtration and centrifugation and then freeze-dried, lyophilized, and extracted with ethanol to give the crude pheromone. The crude pheromone was then fractionated by reversed-phase (C18) chromatography and normal-phase silica gel chromatography. In the case of the crude dauer pheromone generated at 25°C, analysis of the active silica gel fractions by 1H-NMR and liquid chromatography-mass spectrometry indicated that the primary active components were ascarosides C6 and C9. A number of minor components that contributed to activity were also present, and preliminary characterization of these components suggested that they are all ascarosides (R.A.B. and J.C., unpublished results). Thus, the dauer formation assay appears to identify the same primary active components as the dauer recovery assay, namely ascarosides C6 and C9.
In the case of the crude dauer pheromone generated at 20°C, although there were two fractions with some activity that contained small amounts of ascarosides C6 and C9, the majority of the activity was associated with a much more polar fraction. Upon further purification, the primary active component in this fraction was identified as a novel ascaroside, ascaroside C3 (4) (see below). Interestingly, the crude dauer pheromone generated at 20°C contained significantly more ascaroside C7 than the crude dauer pheromone generated at 25°C, although these fractions were not active as judged by the dauer formation assay. This result is consistent with the fact that Jeong et al. (7) detected significantly more ascaroside C7 in the crude dauer pheromone generated at 20°C than we detected in the crude dauer pheromone generated at 22.5–25°C (6). All of these findings collectively indicate that the culture conditions used can have dramatic effects on the dauer pheromone components produced. Although temperature appears to be one factor determining what components are made and in what proportions, it may not be the only factor.
After two additional purifications of the more polar fraction by C18 high-pressure liquid chromatography (HPLC), the active component was isolated and analyzed by NMR, including dqfCOSY, gHMBC, gHMQC, and NOESY experiments, and by high-resolution mass spectrometry (HRMS) [Fig. 1B and supporting information (SI) Table S1 and Fig. S1 A–E). HMBC correlations established the connection between 3-hydroxypropionate and the ascarylose sugar, and coupling constants and NOESY correlations established the relative stereochemistry of the sugar. The active component was identified as ascaroside C3 (4) (Fig. 1C). Because it is possible that the isolated ascaroside C3 could contain contaminants that contributed to, or were even responsible for, the activity, ascaroside C3 was synthesized for comparison purposes (Fig. 1D and SI Text). The synthetically derived ascaroside C3 also provides further validation of the assigned structure as well as material for characterization of biological activity. The NMR spectra of the synthetic material matched the spectra of the natural material (see Table S1 and Fig. S2 A–D). In addition, the optical rotation of the synthetic material matched that of natural material, indicating that the absolute stereochemistry was correctly assigned (see Materials and Methods and SI Text). Furthermore, comparison of equivalent amounts of natural ascaroside C3 and synthetic ascaroside C3 in the dauer formation assay indicated that the two samples contained similar amounts of dauer-inducing activity (data not shown). The structure of ascaroside C3 differs from the previously reported dauer pheromone components in that its side chain has an ω alcohol instead of an ω-1 alcohol, and thus, its biosynthesis likely diverges from that of the remaining ascarosides.
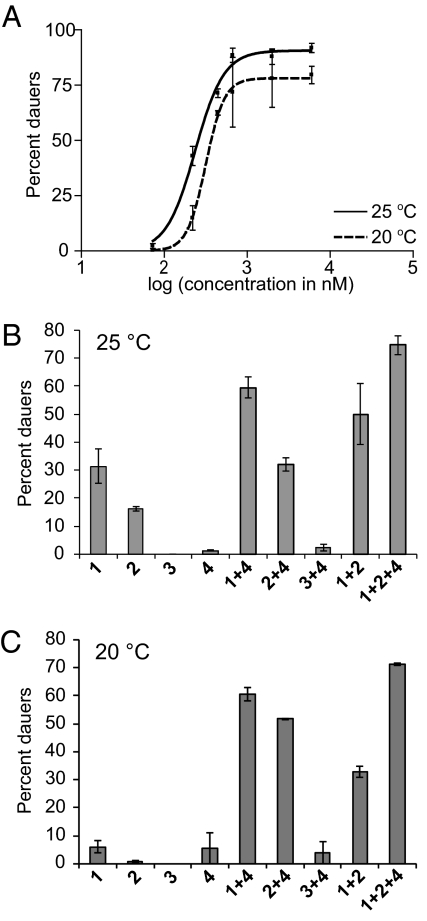
To compare the biological activity of ascaroside C3 (4) with that of the other ascarosides, synthetic ascaroside C3, and the other ascarosides were titrated in the dauer formation assay. Because temperature appears to be at least one factor that modulates the production of ascaroside C3, we tested the activity of ascaroside C3 at both 20°C and 25°C (Fig. 2A). High temperature is thought to favor dauer formation, independent of pheromone. Importantly, Daf-c null mutations cause temperature-sensitive arrest at the dauer stage, even in the absence of pheromone, and this temperature-dependence requires a functional AIY interneuron that is important for the C. elegans thermotactic response pathway (3, 9, 48). In addition, mutant (daf-22) worms that do not produce dauer pheromone are as likely as wild-type worms to enter dauer at higher temperature (11, 49). Thus, as one might expect, all of the ascarosides (C3, C6, C9 and C7) are more effective at 25°C than at 20°C. However, C3 is almost as effective at 20°C (EC50 = 320 nM) as it is at 25°C (EC50 = 240 nM). C6, C9, and C7, on the other hand, are much less effective at 20°C (C6 EC50 = 510 nM, C9 EC50 = 3,900 nM, C7 EC50 = 74,000 nM) than at 25°C (C6 EC50 = 110 nM, C9 EC50 = 700 nM, C7 EC50 = 33,000 nM). In addition to being relatively more potent at lower temperature, ascaroside C3 demonstrates a much sharper titration curve than any of the other ascarosides.
Fig. 2.
Biological characterization of synthetic ascaroside C3 (4). (A) Titration of ascaroside C3 (4) in the dauer formation assay at 20°C and 25°C. The data represent the average of two experiments (± 1 SD). (B) Effect of ascarosides C6 (1), C9 (2), C7 (3), and C3 (4) (each at 73 nM), alone or in combination, in the dauer formation assay in wild-type worms at 25°C. The data represent the average of two experiments (± 1 SD). (C) Effect of ascarosides C6 (1), C9 (2), C7 (3), and C3 (4) (each at 220 nM), alone or in combination, in the dauer formation assay in wild-type worms at 20°C. The data represent the average of two experiments (± 1 SD).
To test whether ascaroside C3 works additively or synergistically with the dauer pheromone components, we tested its activity in the dauer formation assay alone or in combination with the other ascarosides. The dauer pheromone components have been reported to work additively (5). We have previously shown that the activities of ascaroside C6 and C9 are additive at 25°C in wild-type worms but are mildly synergistic at 25°C in a mutant strain that does not produce dauer pheromone (daf-22) (6, 49). Interestingly, ascaroside C3 synergistically induces dauer formation with ascarosides C6 and C9 at 25°C, even in wild-type worms (Fig. 2B). Ascaroside C3 also synergistically induces dauer formation with ascarosides C6 and C9 at 20°C in wild-type worms (Fig. 2C). Additionally, at this lower temperature, there is some synergism between ascarosides C6 and C9 (see Fig. 2C). This synergism between C6 and C9 is possibly apparent at 20°C (Fig. 2C), but not at 25°C (Fig. 2B), because of differential natural ascaroside production (by the worms participating in the assay) at the two temperatures. Indeed, this result is consistent with our previous result that C6 and C9 did not show synergism in wild-type worms, but did show mild synergism in the daf-22 mutant strain (6, 49). In addition to structural differences between the ascarosides, which make ascaroside C3 much more polar than the other ascarosides, and differences in temperature dependence between the ascarosides, ascaroside C3's synergistic activity provides evidence that ascaroside C3 may target a different target receptor than either ascaroside C6 or C9. To test whether ascarosides behave differently in different dauer mutant backgrounds, we tested the activities of ascarosides C3, C6, and C9 in a number of Daf-c and Daf-d mutants (Table S2). In the dauer formation assay, the ascarosides behave similarly in the different mutant backgrounds, suggesting that they target similar dauer signaling pathways or that the mutations tested occur too far downstream in the signaling pathways to distinguish differences in the pheromone components.
Like most other pheromones, the C. elegans dauer pheromone is a mixture. We have identified a component of the dauer pheromone, ascaroside C3, that at lower temperatures is the most potent component of the dauer pheromone yet identified. Intriguingly, the types of dauer pheromone components that are produced by C. elegans appear to vary depending on the culture conditions that are used. It will be interesting to define the factors that determine what types of components are produced, and this problem is one that we are actively pursuing. Although we have identified at least one factor that influences what dauer pheromone components are made (temperature), this factor could well interact with other environmental factors to modulate pheromone production. The variable composition of the dauer pheromone indicates that the dauer pheromone conveys not only information on population density but also additional information about the environment of the pheromone producers. In addition, we have shown that ascaroside C3 acts synergistically with previously identified dauer pheromone components, suggesting that it has a different mechanism of action. To clarify the mechanistic differences between the ascarosides, their corresponding receptor(s) must be identified. The dauer pheromone is thought to target a G protein-coupled receptor(s) in the amphid neurons, and multiple G proteins and cGMP-gated channels have been implicated in the dauer formation process (47, 50–52). Unlike the mammalian odor receptor system in which each neuron contains a single type of GPCR, in the C. elegans chemosensory system, multiple types of receptors are located in an individual neuron (53). Thus, the different ascarosides may target multiple receptors and their associated G proteins in a given neuron, and these multiple inputs may be necessary for activation of the neuron and signaling to downstream pathways. An understanding of the dauer pheromone will require not only a cataloging of all of the dauer pheromone components produced and the conditions that induce their production, but also identification of their target receptors and downstream effects.
Materials and Methods
General Procedures.
NMR spectra were recorded on a Varian VNMRS 600 NMR (600 MHz for 1H, 151 MHz for 13C) for natural and synthetic ascaroside C3 (4). NMR spectra were recorded on a Bruker Avance 300 NMR (300 MHz for 1H, 75 MHz for 13C) for intermediates 5 and 6. Optical rotations were measured on an Autopol IV Automatic Polarimeter (Rudolph Research Analytical). HRMS was performed at the University of Illinois at Urbana–Champaign Mass Spectrometry Facility.
Strains and Culture Conditions.
C. elegans variety Bristol, strain N2 (wild type), and mutant worms were grown at room temperature or 16°C on NGM agar plates that were made with granulated agar (BD Biosciences) and seeded with OP50 bacteria.
Generation of Crude Dauer Pheromone.
Crude dauer pheromone was prepared essentially according to the method of Golden and Riddle (2). N2 worms were cultured in 16 liters of S medium for 16–18 d at 20°C or 25°C on a rotary shaker. Eight liters of OP50 was resuspended in a small volume of S medium and added as a food source at day 1 and then again at day 6. Conditioned media was centrifuged, filtered, and freeze-dried. The solids were extracted with 95% aqueous ethanol (1.6 liters, three times) to afford crude dauer pheromone.
Dauer Formation Assay.
The dauer formation assays were performed as described (6) on NGM-agar plates made with Noble agar (BD Biosciences).
Purification of Ascaroside C3 (4).
Crude dauer pheromone from conditioned media generated at 20°C was fractionated by C18 column chromatography with stepwise gradient of aqueous methanol (0–100%). The active fractions were followed with the dauer formation assay. The most active fractions, which eluted with 10–70% methanol, were combined and further fractionated on a silica gel column with chloroform and methanol solvent mixtures (10:1 to 1:5), followed by a chloroform, methanol, and water solvent mixture (3:7:0.5). This polar wash fraction contained the majority of activity and was further purified by reversed-phase HPLC on a C18 column (Supelco) using an aqueous acetonitrile gradient (0–30%), followed by reversed-phase HPLC on a C18 column (Supelco) using an aqueous acetonitrile gradient (5–40%) with 0.1% trifluoroacetic acid. Approximately 0.36 mg of pure ascaroside C3 (4), isolated as a colorless oil, was obtained from every 10 liters of conditioned media. [α]D20 = −60.7, c 0.03 (methanol); HR-ESIMS (m/z): [M+Na]+ calculated for C9H16O6Na 243.0845, found 243.0835; for 1H and 13C NMR data, see Table S1 and Fig. S1.
Synthesis of Ascaroside C3 (4).
Experimental details on the synthesis of ascaroside C3 (4) can be found in SI Text.
Data Analysis.
EC50 values were determined by using Prism software. Each titration curve was fit with a sigmoidal curve with variable slope, in which the lower limit was set at 0, and the upper limit was not defined. The EC50 was defined as the concentration at which each ascaroside reached half its maximal activity (as calculated by Prism).
Supplementary Material
Acknowledgments.
We thank Cori Bargmann (Rockefeller University) for worm strains and advice, Gary Ruvkun (Massachusetts General Hospital) for worm strains and use of a microscope, and Piali Sengupta (Brandeis University) for advice. Some worm strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. This work was supported by NIH Grant CA24487 (to J.C.). R.A.B. was the recipient of a Kirschstein National Service Research Award from the NIH. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806676105/DCSupplemental.
References
- 1.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 2.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 3.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 4.Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 5.Golden JW, Riddle DL. A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol. 1984;10:1265–1280. doi: 10.1007/BF00988553. [DOI] [PubMed] [Google Scholar]
- 6.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 7.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 8.Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 9.Golden JW, Riddle DL. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci USA. 1984;81:819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malone EA, Thomas JH. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics. 1994;136:879–886. doi: 10.1093/genetics/136.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue T, Thomas JH. Suppressors of transforming growth factor-β pathway mutants in the Caenorhabditis elegans dauer formation pathway. Genetics. 2000;156:1035–1046. doi: 10.1093/genetics/156.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ailion M, Thomas JH. Isolation and characterization of high-temperature-induced Dauer formation mutants in Caenorhabditis elegans. Genetics. 2003;165:127–144. doi: 10.1093/genetics/165.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: Genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- 18.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 19.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 20.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 21.Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics. 1994;138:303–316. doi: 10.1093/genetics/138.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- 23.Estevez M, et al. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- 24.Ren P, et al. Control of C. elegans larval development by neuronal expression of a TGFβ homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce SB, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 28.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 29.Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 30.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 31.Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- 33.Mak HY, Ruvkun G. Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development. 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 34.Rottiers V, et al. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 38.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGFβ Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190:191–202. doi: 10.1677/joe.1.06856. [DOI] [PubMed] [Google Scholar]
- 40.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 41.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 42.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 43.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 44.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 45.Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in. Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen PW, Christensen TA, Stacey NE. Discrimination of pheromonal cues in fish: Emerging parallels with insects. Curr Opin Neurobiol. 1998;8:458–467. doi: 10.1016/s0959-4388(98)80032-9. [DOI] [PubMed] [Google Scholar]
- 47.Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hobert O, et al. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 49.Golden JW, Riddle DL. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol Gen Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 50.Birnby DA, et al. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lans H, Jansen G. Multiple sensory G proteins in the olfactory, gustatory and nociceptive neurons modulate longevity in Caenorhabditis elegans. Dev Biol. 2007;303:474–482. doi: 10.1016/j.ydbio.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 52.Coburn CM, Mori I, Ohshima Y, Bargmann CI. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: A distinct pathway for maintenance of sensory axon structure. Development. 1998;125:249–258. doi: 10.1242/dev.125.2.249. [DOI] [PubMed] [Google Scholar]
- 53.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. www.wormbook.org. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.