Abstract
Mutations in PTEN-induced kinase 1 (pink1) or parkin cause autosomal-recessive and some sporadic forms of Parkinson's disease. pink1 acts upstream of parkin in a common genetic pathway to regulate mitochondrial integrity in Drosophila. Mitochondrial morphology is maintained by a dynamic balance between the opposing actions of mitochondrial fusion, controlled by Mitofusin (mfn) and Optic atrophy 1 (opa1), and mitochondrial fission, controlled by drp1. Here, we explore interactions between pink1/parkin and the mitochondrial fusion/fission machinery. Muscle-specific knockdown of the fly homologue of Mfn (Marf) or opa1, or overexpression of drp1, results in significant mitochondrial fragmentation. Mfn-knockdown flies also display altered cristae morphology. Interestingly, knockdown of Mfn or opa1 or overexpression of drp1, rescues the phenotypes of muscle degeneration, cell death, and mitochondrial abnormalities in pink1 or parkin mutants. In the male germline, we also observe genetic interactions between pink1 and the testes-specific mfn homologue fuzzy onion, and between pink1 and drp1. Our data suggest that the pink1/parkin pathway promotes mitochondrial fission and/or inhibits fusion by negatively regulating mfn and opa1 function, and/or positively regulating drp1. However, pink1 and parkin mutant flies show distinct mitochondrial phenotypes from drp1 mutant flies, and flies carrying a heterozygous mutation in drp1 enhance the pink1-null phenotype, resulting in lethality. These results suggest that pink1 and parkin are likely not core components of the drp1-mediated mitochondrial fission machinery. Modification of fusion and fission may represent a novel therapeutic strategy for Parkinson's disease.
Keywords: mitofusin, drp1, opa1, parkin-pink1
Parkinson's disease (PD), the second most common neurodegenerative disorder, is characterized by degeneration of dopaminergic neurons in the midbrain (1). Although the exact cause of PD is unclear, mitochondrial toxins such as 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) can selectively destroy dopaminergic neurons and cause clinical features similar to PD (2, 3). Moreover, mitochondrial respiratory dysfunction also occurs in sporadic PD (4). The most compelling evidence for a mitochondrial etiology of PD, however, derives from the study of genes mediating familial forms of the disease (4, 5). Mutations in PTEN-induced kinase 1 (Pink1; PARK6), which encodes a serine–threonine kinase localized to mitochondria, and parkin (PARK2), which encodes a RING finger-containing E3 ubiquitin ligase, have been found in recessively inherited and sporadic PD cases (6–9). Previously, we and others have reported that Drosophila pink1 and parkin function in the same genetic pathway, with pink1 acting upstream of parkin, to regulate mitochondrial integrity in testes, muscle, and dopaminergic neurons (10–12). Flies lacking pink1 or parkin function are viable and show muscle degeneration and TUNEL staining, indicative of cell death (10–13). Subsequent studies have shown that parkin can suppress mitochondrial defects caused by pink1 knockdown in cultured human cells (14), and mitochondrial dysfunction also occurs in PD cases with pink1 or parkin mutations (4). An understanding of how mutations in pink1 and parkin cause mitochondrial dysfunction may lead to the development of novel therapeutic agents for PD.
Mitochondria undergo dynamic changes in morphology through fusion and fission. Although these processes have been extensively studied in yeast, only recently have molecules regulating mitochondrial dynamics been identified in mammals (15–17). These include the homologous GTPases Mitofusin 1 (Mfn1) and Mitofusin 2 (Mfn2), which mediate fusion of the mitochondrial outer membrane, as well as Optic atrophy 1 (Opa1), a GTPase required for fusion of the inner membrane. Mitochondrial fission, conversely, requires Dynamin-related protein 1 (Drp1), which is also a GTPase (15–17) (Fig. 6). The Drosophila melanogaster genome encodes two homologues of Mfn, one being Fuzzy onion (Fzo), the first identified protein regulating mitochondrial dynamics in metazoans (18). The expression of Fzo is restricted to the testes, and mutations in fzo cause mitochondrial fusion defects in testes and male sterility (18). The second Drosophila Mfn homologue is a largely uncharacterized protein known as Mitochondrial assembly regulatory factor (Marf; CG3869), which is expressed in germline and somatic cells (19). The Drosophila genome also encodes single homologues of opa1 (20) and drp1 (21), both of which have been shown to function in mitochondrial dynamics in flies. Studies in yeast and mammals have demonstrated that defects in mitochondrial fission can be ameliorated by mutations in genes required for mitochondrial fusion and vice versa, indicating that a balance between fusion and fission is required to maintain proper mitochondrial morphology.
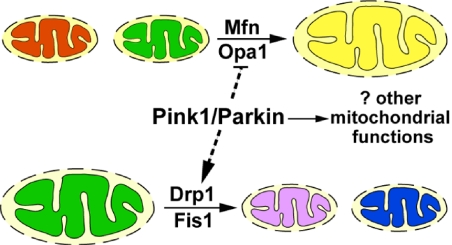
Fig. 6.
Interactions of pink1 and parkin with genes regulating mitochondrial fusion and fission. Mitochondrial fusion requires Mfn and Opa1, and mitochondrial fission requires Drp1 and Fis1. Pink1 and Parkin promote fission and/or inhibit fusion, either directly or indirectly (dashed lines). In addition, Pink1 and Parkin are unlikely to be components of the canonical pathways regulating mitochondrial dynamics. Rather, Pink1 and Parkin may regulate other mitochondrial functions that also impact mitochondrial integrity.
Careful studies of pink1 and parkin mutant phenotypes in testes (as detailed later) suggest the possibility that pink1 and parkin might regulate mitochondrial dynamics. To test this hypothesis, we examined genetic interactions between pink1 or parkin and genes required for mitochondrial fusion and fission in Drosophila. Our data suggest that the net action of the pink1/parkin pathway is to promote mitochondrial fission and/or inhibit fusion.
Results
pink1 and parkin Mutants Show Defects in Spermatogenesis Suggestive of Defects in Mitochondrial Fission, and Interact Genetically with fzo and drp1 in Testes.
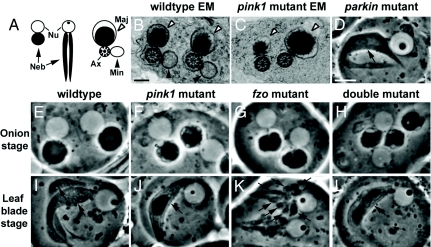
Both pink1- and parkin-null mutant adults show striking mitochondrial phenotypes in spermatids (10, 11, 13, 22). During spermatogenesis, stem-cell differentiation is followed by mitosis and meiosis with incomplete cytokinesis, creating syncytial cysts of 64 spermatids (23). Mitochondria undergo significant morphological changes throughout spermatid development. During the “onion stage,” the mitochondria in each spermatid aggregate adjacent to the nucleus and undergo fusion to form a large spherical structure called a nebenkern, which is composed of two intertwined mitochondria. Under phase-contrast microscopy, each spermatid can be identified as containing two giant, adjacent spherical structures: the phase-light nucleus, and the phase-dark nebenkern (Fig. 1 A and E). Subsequently, the spermatids begin to elongate, and the nebenkern unfurls to yield two mitochondrial derivatives (the “leaf blade stage”; Fig. 1 A and I). These structures are maintained throughout subsequent spermatid elongation such that a cross-section through the sperm tail reveals two mitochondrial derivatives, known as the major and minor, adjacent to the microtubule-based axoneme (Fig. 1 A and B) (23). pink1 mutant spermatids (Fig. 1 C, F, and J) showed vacuolated onion-stage nebenkerns, and, in subsequent stages, exhibited only one mitochondrial derivative rather than the normal two seen in WT spermatids (Fig. 1 B and I). Similar phenotypes have been observed in parkin mutant testes (Fig. 1D) (22). These results suggest that pink1 and parkin mutants might have defects in mitochondrial dynamics, with pink1 or parkin loss of function reducing mitochondrial fission and/or increasing fusion in spermatids.
Fig. 1.
pink1 and parkin mutants show phenotypes in spermatogenesis suggestive of defects in mitochondrial fission; pink1 genetically interacts with fzo in testes. (A) Schematic of an onion-staged (left) and a leaf-blade-staged (middle) spermatid showing the nucleus (Nu) and nebenkern (Neb, arrow), and a cross-section through a spermatid tail immediately before individualization (right). In these spermatid tails, an axoneme (Ax) is associated with two mitochondrial derivatives, the major (Maj, open arrowhead) and minor (Min, filled arrowhead). (B and C) EM images of WT (B) and pink1-mutant (C) spermatid tails show the presence of only the major mitochondrial derivative, but not the minor derivative in pink1 mutants. (D) A parkin-mutant spermatid during the leaf-blade stage, as with a similarly staged pink1-mutant spermatid (J), contains only one mitochondrial derivative compared with two seen in WT specimens (I). (E–L) Genetic interactions between pink1 and fzo. During the onion stage, pink1 mutants show vacuolations of the nebenkern (F), which are not seen in WT specimens (E). fzo1/Df(3R)P2O mutants have nebenkerns with irregular borders (G). Similar phenotypes are also seen in fzo1/fzo2 and fzo1-homozygous testes (data not shown). Double mutants removing both pink1 and fzo function exhibit nebenkerns that have smooth borders, yet are still vacuolated (H). (I) WT leaf-blade–staged spermatids have two mitochondrial derivatives. During the leaf-blade stage, fzo1/Df(3R)P2O spermatids show fragmented mitochondria (K). Double mutants with pink1 and fzo removed show a single mitochondrial derivative, the pink1 mutant-like phenotype (L). Note that the cell membranes encapsulating spermatids often contain multiple spermatids. This is a result of the disruption of cytoplasmic bridges connecting spermatids during sample preparation, and is not a phenotype. Genotypes: (B, E, and I) w/Y; (C, F, and J) w pink15 (G and K) w/Y; e fzo1/Df(3R)P2O; (H and L) w pink15/Y; e fzo1/Df(3R)P2O; Scale bars: 200 nm in B and C; 10 μm in D–L.
To explore this hypothesis, we searched for genetic interactions between pink1 and fzo, the fly Mfn homologue expressed exclusively in testes. Whereas an onion-staged nebenkern is composed of two giant intertwined mitochondria, the fusion defects in fzo-null mutants resulted in an onion-staged nebenkern composed of many small mitochondria. As a consequence of this, the borders of the nebenkern appeared irregular under phase-contrast microscopy (Fig. 1G) (18). When the nebenkern of fzo mutants unfurled at the leaf-blade stage, numerous small phase-dark mitochondria were seen adjacent to a single nucleus (Fig. 1K), rather than two mitochondrial derivatives seen in specimens (Fig. 1I). Double mutants with pink1 and fzo function removed exhibited nebenkerns that were still vacuolated, yet had smooth borders (Fig. 1H). Furthermore, leaf-blade spermatids of pink1/fzo double mutants showed a single elongated mitochondrial derivative (Fig. 1L). These results suggest that, in double mutants, fzo loss-of-function phenotypes are suppressed by pink1 loss of function, with double mutants showing pink1-like phenotypes. These results suggest a strong genetic interaction between pink1 and a fly homologue of mitofusin.
Next, we examined if pink1 and drp1 genetically interact in testes. Flies overexpressing drp1 specifically in testes (TMR-drp1) showed fzo-like phenotypes in a subset of nebenkerns [supporting information (SI) Fig. S1D]. Overexpression of drp1 in the pink1 mutant background resulted in suppression of the vacuolations in nebenkerns in a portion of the flies (Fig. S1 B–G). These results again implicate pink1 in promoting fission and/or inhibiting fusion.
A Balance Between Opposing Fusion and Fission Maintains Mitochondrial Morphology in Drosophila.
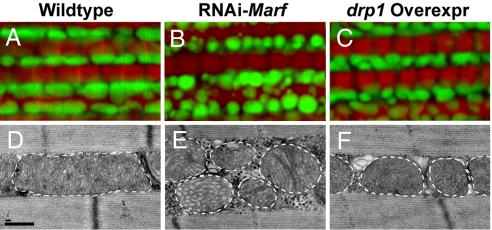
Drosophila adult indirect flight muscle (hereafter referred to as “muscle”) is an ideal system in which to study mitochondrial dynamics because it contains numerous large mitochondria that fill the spaces between bundles of well-organized muscle fibers, as visualized on transmission electron microscopy (EM) (Fig. 2D) (10). A similar pattern can be visualized in muscle by fluorescence microscopy by using a version of GFP (mitoGFP) that specifically localizes to mitochondria while simultaneously labeling muscle fibers with phalloidin, which binds to filamentous actin (Fig. 2A). The function of the putative Mfn homologue, Marf, has not been previously characterized. Drosophila Marf shows 47% amino acid identity and 65 to 67% similarity to two human Mfn homologues. As there are no mutations available in Marf, we generated two RNAi constructs targeted to two independent regions of the Marf transcript (the coding region and the untranslated region, respectively). These transgenes were used to carry out tissue-specific silencing using the UAS-Gal4 system (24), and both Marf RNAi transgenes gave identical phenotypes. Whereas ubiquitous knockdown of Marf using tubulin-Gal4 resulted in lethality, muscle-specific knockdown of Marf using either Mef2-Gal4 or 24B-Gal4 resulted in viable adults in which muscles showed mitochondrial fragmentation (i.e., smaller and rounder size), as visualized by mitoGFP and EM (Fig. 2 B and E). Abnormal cristae were also observed in these flies (Fig. 2E). These results indicate that Marf is a bona fide regulator of mitochondrial fusion in Drosophila. Similarly, muscle-specific knockdown of opa1 also resulted in mitochondrial fragmentation (Fig. 4B). Importantly, transgenic flies overexpressing drp1 specifically in muscle showed a similar, albeit weaker, phenotype of mitochondrial fragmentation (Fig. 2 C and F). In addition, overexpression of drp1 in the background of muscle-specific Marf knockdown resulted in lethality. Together, these data indicate that mitochondrial morphology in Drosophila, like that in yeast and mammals, is regulated by a balance between the activities of canonical regulators of mitochondrial fusion–fission dynamics.
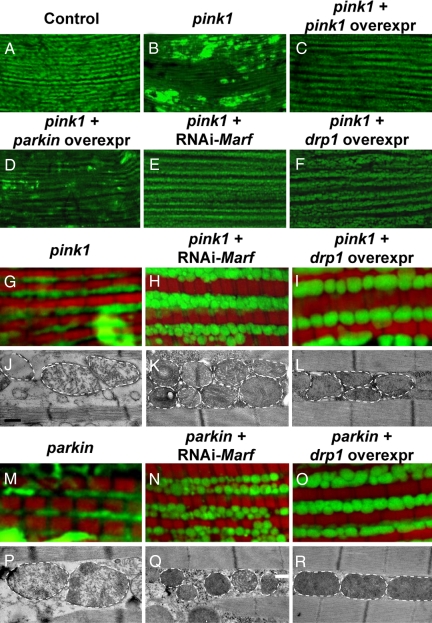
Fig. 2.
Muscle-specific knockdown of Marf and overexpression of drp1 results in abnormal mitochondrial morphology. MitoGFP- (green) and phalloidin-labeled (red) muscle (A–C) and EM images (D–F) from 1- to 2-day-old flies. Compared with control (A and D), both Marf knockdown (B and E) and drp1 overexpression (C and F) result in mitochondrial fragmentation, with Marf-knockdown flies also showing cristae irregularities and more severe mitochondrial fragmentation. The borders of mitochondria are marked with white dashed lines. Genotypes: (A and D) FM6/Y; Mef2-Gal4, UAS-mitoGFP/+; (B and E) w; UAS-RNAi-Marf/+; Mef2-Gal4, UAS-mitoGFP/+; (C and F) w; UAS-drp1/+; Mef2-Gal4, UAS-mitoGFP/+. Note that, as controls, Mef2-Gal4, UAS-mitoGFP flies show similar mitochondrial phenotypes in backgrounds of w/Y, FM6/Y, or w/Y; UAS-LacZ. Scale bars: 0.5 μm in D–F.
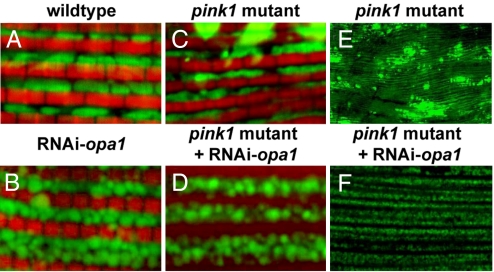
Fig. 4.
Muscle-specific opa1 knockdown results in mitochondrial fragmentation and suppression of mitochondrial defects observed in pink1 mutants. Muscle from 1- to 2-day-old flies labeled with mitoGFP (green) and phalloidin (red) at high magnification (A–D) or labeled with mitoGFP (green) at low magnification. Compared with control (A), opa1 knockdown results in smaller and rounder mitochondria (B), similar to what is observed in Marf knockdown (Fig. 2B). However, we note that the borders of opa1 knockdown mitochondria appear fuzzy, whereas those of Marf knockdown do not. Compared with pink1 mutants alone (C and E), opa1 knockdown in pink1 mutants displays striking rescue of mitochondrial morphology (D and F), Genotypes: (A) FM6/Y; Mef2-Gal4, UAS-mitoGFP/+; (B) w; UAS-RNAi-opa1/+; Mef2-Gal4, UAS-mitoGFP/+; (C and E) w pink15f/Y; Mef2-Gal4, UAS-mitoGFP/+; (D and F) w pink15 f/Y; Mef2-Gal4, UAS-mitoGFP/UAS-RNAi-opa1.
pink1 and parkin Genetically Interact with Components of the Mitochondrial Fission–Fusion Machinery in Muscle.
Indirect flight muscle from pink1 or parkin mutant adults showed severe defects in mitochondrial morphology, including swollen mitochondria with broken cristae, as observed under EM (Fig. 3 J and P) (10–13, 25). pink1 and parkin mutants also displayed weak mitoGFP signal compared with (Fig. 3 B, G, and M). In addition, large clumps of intense GFP signal, which appeared beyond the space between muscle fibers as demarcated by phalloidin staining, were also observed. These mitochondrial phenotypes in pink1 mutants could be completely suppressed by muscle-specific overexpression of pink1 (Fig. 3C) and partially rescued by overexpression of parkin (Fig. 3D).
Fig. 3.
Both Marf knockdown and drp1 overexpression suppress mitochondrial phenotypes in pink1- and parkin-mutant muscle. MitoGFP-labeled muscle at low magnification (A–F), mitoGFP/phalloidin double-labeled muscle at higher magnification (G–I and M–O), and EM images (J–L and P–R) from 1- to 2-day-old flies. Both pink1 (B and G) and parkin (M) mutants show weakened mitoGFP labeling and clumps of intense mitoGFP signal. In pink1 mutants, these phenotypes can be completely suppressed by pink1 overexpression (C) and partially rescued by parkin overexpression (D). Moreover, pink1 and parkin phenotypes can also be suppressed by knockdown of Marf (E, H, and N) or by drp1 overexpression (F, I, and O). At the EM level, pink1 and parkin mutants show broken cristae (J and P), which can be suppressed by Marf knockdown (K and Q) or drp1 overexpression (L and R). However, pink1- and parkin-mutant flies with Marf knockdown (K and Q) still show significant mitochondrial fragmentation and cristae abnormalities reminiscent of those seen in Marf-knockdown flies alone (Fig. 2E). The borders of mitochondria are marked with white dashed lines. Genotypes: (A) FM6/Y; (B, G, and J) w pink15f/Y; Mef2-Gal4, UAS-mitoGFP/+; (C) w pink15 f/Y; Mef2-Gal4, UAS-pink1/+; UAS-mitoGFP/+; (D) w pink15 f/Y; UAS-parkin/+; Mef2-Gal4, UAS-mitoGFP/+; (E, H, and K) w pink15 f/Y; Mef2-Gal4, UAS-mitoGFP/UAS-RNAi-Marf; (F, I, and L) w pink15 f/Y; Mef2-Gal4, UAS-mitoGFP/UAS-drp1; (M and P) w/Y; UAS-mitoGFP/+; 24B-Gal4 park25/park25; (N and Q) w/Y; UAS-mitoGFP/UAS-RNAi-Marf; 24B-Gal4 park25/park25; (O and R) w/Y; UAS-mitoGFP/UAS-drp1; 24B-Gal4 park25/park25. Scale bars: 0.5 μm in J–L and P–R.
To test the hypothesis that pink1 and parkin regulate mitochondrial dynamics, we searched for genetic interactions between Marf/drp1 and pink1/parkin. If loss of pink1 or parkin function tips the fusion/fission balance toward fusion, we would expect silencing of Marf to suppress pink1/parkin mutant phenotypes. Consistent with this hypothesis, muscle-specific knockdown of Marf in the pink1 or parkin mutant background resulted in a significant rescue of pink1 and parkin mutant phenotypes: mitochondria were no longer elongated, and the intense accumulations of mitoGFP were no longer present (Fig. 3 E, H, and N). In addition, the broken cristae phenotypes observed in pink1 and parkin mutants were significantly suppressed (Fig. 3 K and Q). Interestingly, however, most of the mitochondria still appeared fragmented—the phenotype resulting from Marf knockdown (compare with Fig. 2 B and E). Similar suppression of mitochondrial defects seen in pink1 mutants could also be observed following muscle-specific knockdown of opa1 (Fig. 4 C–F). Importantly, overexpression of drp1 in the pink1/parkin mutant background also resulted in significant suppression of the pink1 or parkin mutant phenotypes, with mitochondria displaying drp1 overexpression-like phenotypes (Fig. 3 F, I, L, and R; compare with Fig. 2 C and F). These results suggest that pink1/parkin function to promote fission and/or inhibit mitochondrial fusion, and that Marf/drp1 is genetically epistatic to pink1/parkin.
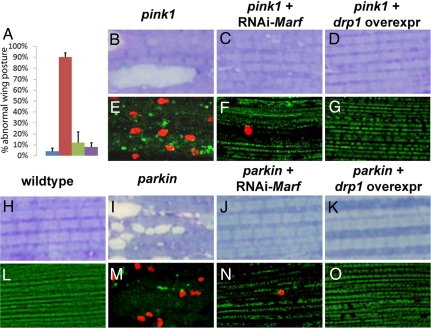
Next, we sought to determine if suppression of the mitochondrial morphological defects in pink and parkin mutants by drp1 overexpression and Marf knockdown was functionally significant. Both pink1- and parkin-null mutants showed wing posture defects associated with muscle degeneration as a result of extensive cell death (10–13). Remarkably, not only was normal wing posture restored in pink1 and parkin mutants by drp1 overexpression or Marf knockdown (Fig. 5A), but cell death (assayed by TUNEL-positive staining) and muscle degeneration (assayed by Toluidine blue staining) were also suppressed (Fig. 5 B–O). These data also suggest that apoptosis in pink1 and parkin mutant muscle is secondary to defects in mitochondrial dynamics.
Fig. 5.
Marf knockdown or drp1 overexpression in muscle results in functional rescue of pink1- and parkin-mutant phenotypes. (A) Abnormal wing posture seen in pink1 mutants (red) compared with WT specimens (blue) is significantly suppressed by Marf knockdown (green) or drp1 overexpression (purple) in muscle. The y axis denotes the percentile of flies showing “upheld” or “downheld” wings, an indication of muscle degeneration. Toluidine blue (B–D and H–K) or TUNEL/mitoGFP stainings (E–G and L–O) of muscles. Compared with WT specimens (H), muscles from pink1 and parkin mutants show vacuolations indicative of degeneration (B and I). These phenotypes can be suppressed by Marf knockdown (C and J) or drp1 overexpression (D and K). WT muscle does not show any TUNEL-positive cell death (L), whereas pink1 and parkin mutants show prominent TUNEL-positive staining (red; E and M). These phenotypes are suppressed by expression of RNAi-Marf (F and N) or drp1 (G and O). Genotypes are as shown in Fig. 3.
pink1 Mutant Phenotypes Are Distinct from Those of Mitochondrial Dynamics Genes.
Because our results indicate that the pink1/parkin pathway promotes mitochondrial fission and/or inhibits fusion, we sought to determine if Pink1 and Parkin serve as essential components of the Drp1-dependent mitochondrial fission machinery. If this were the case, we would expect pink1 and drp1 mutants to show similar phenotypes. drp1 mutants were largely lethal, but rare escapers emerged. Muscles from drp1 mutant fly escapers showed elongated mitochondria, but largely homogeneous mitoGFP signals, and no TUNEL-positive staining (Fig. S2 E and F). These observations stand in contrast to those associated with loss of pink1 or parkin (Figs. 3 B, G, and M; Fig. 5 E and M). drp1 mutants also showed phenotypes distinct from those of pink1 and parkin mutants in testes. Onion-staged nebenkerns of drp1 mutants were large, bizarrely shaped blobs that also contained irregular-shaped phase-light materials distinct from the phase-light nucleus (Fig. S2D). Finally, although some onion-stage nebenkerns from flies overexpressing drp1 showed nebenkerns with irregular borders reminiscent of the fzo mutant phenotype, overexpression of pink1 did not affect nebenkern structure (data not shown). Together, these data suggest that the mitochondrial phenotypes associated with alterations of pink1 and parkin are distinct from those of drp1 mutants, supporting the idea that pink1 and parkin are not essential components of the canonical fission machinery controlled by drp1.
To further test this hypothesis, we sought to determine if loss of drp1 function could enhance the pink1 mutant phenotype. Interestingly, we were unable to recover any pink1 mutant flies that were heterozygous for each of three independent drp1-null or drp1-strong hypomorphic alleles under normal culturing conditions, whereas we had no difficulty recovering pink1 mutant or drp1 heterozygous flies alone (Fig. S2A). The lethality prohibited us from examining the mitochondria of these animals. However, this striking synthetic lethal interaction between a pink1-null allele and a modest reduction in drp1 function suggests that the phenotype resulting from a complete lack of pink1 function can be further enhanced through reduction of drp1 function. Collectively, these results suggest that pink1 does not strictly function in a linear pathway to only regulate drp1.
Discussion
In yeast and mammals, mitochondrial morphology is maintained by a dynamic balance between fusion and fission. In Drosophila, although the functions of drp1 and opa1 in regulating mitochondrial morphology are known, the role of the main mfn homologue, Marf, was largely uncharacterized. Herein we show that Marf knockdown in muscle results in significant mitochondrial fragmentation and abnormal morphology of cristae, thereby indicating that Marf is a bona fide pro-fusion molecule. As would be expected for a dynamic opposing action between mitochondrial fusion and fission, overexpression of drp1 leads to similar mitochondrial fragmentation.
Previously, we and others have shown that flies lacking pink1 or parkin function show similar mitochondrial phenotypes in the male germline, indirect flight muscle, and dopaminergic neurons (10–12). In these settings, pink1 and parkin function in a common genetic pathway to regulate mitochondrial integrity and function (10–12). In this report, we have established that pink1 and parkin mutants also show similar genetic interactions with molecules involved in mitochondrial dynamics. Specifically, muscle-specific Marf or opa1 knockdown or drp1 overexpression results in significant rescue of mitochondrial morphology phenotypes, and suppression of muscle cell death and degeneration in pink1 and/or parkin mutants. Furthermore, in testes, pink1 also genetically interacts with the testes-specific mfn homologue fzo. In this case, however, whereas loss of pink1 function strongly suppresses fzo mutant phenotypes, the pink1 mutant phenotype is not strongly suppressed. Because Marf is also expressed in testes (19), and may have partially redundant functions with fzo, it remains possible that removal of both Marf and fzo may result in rescue of the pink1 testes phenotype. These results are consistent with those of a recent report (26). Collectively, data from our work and Poole et al. provide compelling evidence that the function of the pink1/parkin pathway is to promote mitochondrial fission and/or inhibit fusion in Drosophila (Fig. 6).
Although the net action of the pink1/parkin pathway is to promote fission and/or inhibit fusion, it seems unlikely that Pink1 and Parkin are core components of the fission–fusion machinery. First, loss of function of key regulators of the mitochondrial dynamics machinery (Marf, opa1, drp1) causes lethality, whereas pink1- and parkin-null mutants are viable. Second, pink1 and parkin mutants show distinct phenotypes from drp1 mutants in both muscle and testes, and pink1 overexpression in testes results in different phenotypes from those caused by loss of fzo function or drp1 overexpression. In addition, as we have shown, pink1 and parkin mutants show synthetic lethality with a heterozygous mutation in drp1 (26). Because a modest reduction in drp1 levels can further worsen the phenotype as a result of compete absence of pink1 or parkin function, it seems unlikely that the pink1/parkin pathway acts in a strict linear pathway to regulate the mitochondrial dynamics machinery, at least for drp1. One likely possibility is that the pink1/parkin pathway regulates additional aspects of mitochondrial function that also impact mitochondrial morphology (Fig. 6).
How might Pink1 and Parkin regulate mitochondrial dynamics at the mechanistic level? Most literature suggests that Pink1 is present in the mitochondrial intermembrane space and may be anchored to the inner membrane of the mitochondrion (27, 28), although a cytosolic localization of Pink1 has also been noted (29). Parkin, on the other hand, has largely been found located in the cytosol and endoplasmic reticulum (30). As for molecules mediating mitochondrial dynamics, Mfn is a membrane-spanning protein with domains exposed to the intermembrane space and cytosol (31, 32). Drp1 is localized to the outer membrane (33, 34), and in yeast, Drp1 localization to the outer membrane is facilitated by another pro-fission molecule, Fis1 (35). The role of Fis1 in mammals, however, is less clear, and it remains to be seen if Fis1 is involved in regulating fission in Drosophila. Based on the subcellular localization of these molecules, it is possible that Pink1 may directly phosphorylate Mfn and/or Opa1 to inhibit fusion, or phosphorylate Drp1 or Fis1 to promote fission. Alternatively, Parkin may act on Drp1 and/or Fis1 via non-degradative ubiquitination to facilitate mitochondrial localization of Drp1, or exert its function on Marf via degradative ubiquitination. As pink1 acts upstream of parkin, it is possible that the interface between the pink1/parkin pathway and the mitochondrial dynamics machinery occurs at the level of Parkin. Alternatively, both Pink1 and Parkin could be involved, i.e., with Pink1 acting, directly or indirectly, on the fusion machinery, and Parkin acting on the fission machinery, or vise versa. In any case, our studies suggest that manipulation of mitochondrial dynamics may provide a novel therapeutic target for PD.
Our results and those of Poole et al. suggest a need to investigate whether patients with PD manifest defects in mitochondrial dynamics. Interestingly, defects in mitochondrial morphology have been reported in mice overexpressing α-Synuclein (4). Dominant mutations or increased genetic dosage of α-Synuclein cause inherited forms of PD (36, 37), and α-Synuclein is a major component of Lewy bodies, the characteristic intracytoplasmic inclusions seen in most PD cases, including sporadic cases (1). Thus, it will be interesting to determine whether mitochondrial defects resulting from α-Synuclein overexpression are also mediated by defects in mitochondrial dynamics.
Materials and Methods
Molecular Biology.
To silence Marf, two independent regions in the Marf transcript (coding region and UTR) were independently targeted using a microRNA-based technology (38, 39). To silence opa1, the coding region of opa1 transcript was targeted. PCR products of these microRNA precursors were cloned into pUASt. To generate UAS-drp1 and TMR-drp1, the drp1 cDNA (EST clone from Drosophila Genome Research Center, AT04516), was subcloned into each vector. All cloned PCR products were confirmed by DNA sequencing.
Drosophila Genetics and Strains.
fzo1, fzo2, and fzo-deficiency (Df(3R)P2O) flies (18) were obtained from Margaret Fuller; drp11 and drp12 flies (21), from Patrik Verstreken and Hugo Bellen; and Mef2-Gal4 from Leo Pallanck. pink15 (10) and parkin25 (13) were previously described. drp1KG03185, UAS-mitoGFP, and 24B-Gal4 flies were obtained from the Bloomington Drosophila Stock Center. For experiments involving transgenic flies, multiple independent fly lines were generated (Rainbow Transgenic Flies) and tested for each transgene.
Phase-Contrast, Confocal, and Electron Microscopy.
For light microscopic analysis of the male germline, testes were dissected from recently eclosed males, squashed in PBS buffer, and imaged using an Olympus BX51 microscope equipped with phase-contrast optics. For analysis of muscle, notums of 1- to 2-day-old adult flies were dissected, fixed in 4% paraformaldehyde, and stained with phalloidin, and indirect muscle fibers were isolated and imaged by a Zeiss LSM5 confocal microscope. For transmission EM, testes and muscle were dissected, fixed in paraformaldehyde/glutaraldehyde, postfixed in osmium tetroxide, dehydrated, and embedded in Epon. Tissue sections 1.5 μm thick were stained with Toluidine Blue. Sections 80 nm thick were stained with uranyl acetate and lead citrate and examined using a JEOL 100C transmission electron microscope (UCLA Brain Research Institute EM Facility). TUNEL assays were carried out using the In Situ Cell Death Detection Kit from Roche.
Supplementary Material
Acknowledgments.
We thank G. Mohiddin Lone for generating one construct; Margaret Fuller, Hugo Bellen, and Leo Pallanck for fly stocks; Frank Laski for his microtome; Bruce Hay for comments on the manuscript; and the Guo lab members for discussions. This work was supported by the a National Institute of Health NRSA predoctoral fellowship (to M.W.D.), National Institute of Health Grants K02 and R01 (to M.G.), the Alfred P. Sloan Foundation, American Parkinson's Disease Association, Glenn Family Foundation, and the McKnight Endowment Fund for Neuroscience (to M.G.).
Note.
While this article was in review, Yang et al. published a report (Proc Natl Acad Sci USA 105:7070–7075) suggesting that pink1 interacts with drp1, fis1, and opa1, findings that are consistent with this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803998105/DCSupplemental.
References
- 1.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Langston JW, et al. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 3.Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson's disease. Neuroscientist. 2002;8:192–197. doi: 10.1177/1073858402008003004. [DOI] [PubMed] [Google Scholar]
- 4.Dodson MW, Guo M. Pink1, parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease. Curr Opin Neurobiol. 2007;17:331–337. doi: 10.1016/j.conb.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med. 2006;12:521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 7.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 8.Bonifati V, et al. Early-onset parkinsonism associated with PINK1 mutations: Frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- 9.Valente EM, et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 10.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 11.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene JC, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exner N, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 16.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 17.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 18.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 19.Hwa JJ, et al. Differential expression of the Drosophila mitofusin genes fuzzy onions (fzo) and dmfn. Mech Dev. 2002;116:213–216. doi: 10.1016/s0925-4773(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 20.Yarosh W, et al. The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment. PLoS Genet. 2008;4:e6. doi: 10.1371/journal.pgen.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Riparbelli MG, Callaini G. The Drosophila parkin homologue is required for normal mitochondrial dynamics during spermiogenesis. Dev Biol. 2006;303:108–120. doi: 10.1016/j.ydbio.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Fuller MT. Spermatogenesis. In: Martinez-Arias A, Bate M, editors. The Development of Drosophila Melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1993. pp. 71–147. [Google Scholar]
- 24.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 25.Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 26.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestri L, et al. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi S, et al. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- 29.Beilina A, et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci USA. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimura H, et al. Immunohistochemical and subcellular localization of Parkin protein: Absence of protein in autosomal recessive juvenile parkinsonism patients. Ann Neurol. 1999;45:668–672. doi: 10.1002/1531-8249(199905)45:5<668::aid-ana19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Rapaport D, et al. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J Biol Chem. 1998;273:20150–20155. doi: 10.1074/jbc.273.32.20150. [DOI] [PubMed] [Google Scholar]
- 32.Rojo M, et al. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 33.Bleazard W, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank S, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 35.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polymeropoulos MH, et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 37.Singleton AB, et al. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 39.Ganguly A, Feldman RM, Guo M. Ubiquilin antagonizes presenilin and promotes neurodegeneration in Drosophila. Hum Mol Genet. 2008;17:293–302. doi: 10.1093/hmg/ddm305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.