Abstract
Prokaryote–eukaryote interactions are ubiquitous and have important medical and environmental significance. Despite this, a paucity of data exists on the mechanisms and pathogenic consequences of bacterial–fungal encounters within a living host. We used the nematode Caenorhabditis elegans as a substitute host to study the interactions between two ecologically related and clinically troublesome pathogens, the prokaryote, Acinetobacter baumannii, and the eukaryote, Candida albicans. After co-infecting C. elegans with these organisms, we observed that A. baumannii inhibits filamentation, a key virulence determinant of C. albicans. This antagonistic, cross-kingdom interaction led to attenuated virulence of C. albicans, as determined by improved nematode survival when infected with both pathogens. In vitro coinfection assays in planktonic and biofilm environments supported the inhibitory effects of A. baumannii toward C. albicans, further showing a predilection of A. baumannii for C. albicans filaments. Interestingly, we demonstrate a likely evolutionary defense by C. albicans against A. baumannii, whereby C. albicans inhibits A. baumannii growth once a quorum develops. This counteroffensive is at least partly mediated by the C. albicans quorum-sensing molecule farnesol. We used the C. elegans–A. baumannii–C. albicans coinfection model to screen an A. baumannii mutant library, leading to the identification of several mutants attenuated in their inhibitory activity toward C. albicans. These findings present an extension to the current paradigm of studying monomicrobial pathogenesis in C. elegans and by use of genetic manipulation, provides a whole-animal model system to investigate the complex dynamics of a polymicrobial infection.
Keywords: A. baumannii, C. albicans, Acinetobacter, pathogenesis, biofilm
In nature, microorganisms exist within polymicrobial communities (1, 2), which abound with complex multispecies dynamics (3). These ecological interactions in general and prokaryote–eukaryote interactions in particular, are likely important for the evolution and maintenance of microbial virulence toward humans (4, 5). Moreover, humans are often co-infected or colonized with multiple pathogens (1, 6), whose interactions may determine the virulence potential of either organism. Despite the abundance of polymicrobial encounters within nature, there is a scarcity of in vivo models that explore the biological and pathological systems of interacting species. Significant challenges exist with reproducing polymicrobial interactions (6), and thus a facile, genetically tractable model system is desperately needed.
The soil-dwelling nematode Caenorhabditis elegans, has been successfully used as an alternative host in the study of host–pathogen interactions (7). Thus far, its use has been limited to the investigation of monomicrobial infections, including those caused by a wide range of bacteria and fungi (7). Importantly, microbial virulence determinants found to be relevant in pathogenesis toward C. elegans have also been found to be important in pathogenesis toward other hosts, including mammals (7). Recently, Candida albicans, the most common human fungal pathogen, with an attributable patient mortality reaching 40% in the face of invasive disease (8), was shown to cause a persistent lethal infection of the C. elegans intestinal tract (9). C. albicans infection leads to overwhelming intestinal proliferation and filamentation through the worm cuticle (9). The ability to form biofilm and undergo a morphological transition from yeast to a filamentous form, are critical virulence determinants of C. albicans toward mammals and C. elegans (9–13). Given the significance of this fungus to human health and its common cohabitation with other microbes, particularly bacteria (14, 15), we used C. elegans to identify and study interactions between C. albicans and various prokaryotic species.
This report shows that C. elegans can be effectively used to study the dynamics of a polymicrobial infection, more specifically that between a prokaryote and a eukaryote. We concentrated on the interaction between C. albicans and the emerging gram-negative pathogen, Acinetobacter baumannii. We describe an antagonistic relationship between these pathogens, whereby A. baumannii inhibits several key virulence determinants of C. albicans such as filamentation and biofilm formation. The observed A. baumannii–C. albicans interactions resulted in reduced C. albicans pathogenicity, as determined by reduced worm lethality when infected with both pathogens. However, illustrating the complexity of the interaction and a likely evolutionary defense process, C. albicans demonstrates growth-dependent antibacterial properties, which appear mediated by the quorum-sensing molecule farnesol. Finally, we describe the utility of using bacterial genetics and the C. elegans–A. baumannii–C. albicans model to identify potential underlying molecular mechanisms of the observed interaction. Our results extend the use of C. elegans in the study of polymicrobial pathogenesis and provide further evidence of the likely importance of crosskingdom interactions.
Results
A. baumannii and Other Bacteria Inhibit Filamentation of C. albicans in a C. elegans Coinfection Model.
When C. elegans glp-4; sek-1 nematodes are infected with C. albicans, and are exposed to a liquid environment, the majority of worms die with C. albicans filaments penetrating through the worm cuticle (9). C. albicans filamentation within C. elegans begins within 24 h of liquid-medium exposure and peaks by 72 h (data not shown). We used this C. elegans–C. albicans model to evaluate the interactions between a range of bacteria and C. albicans. Remarkably, when nematodes were infected sequentially with C. albicans followed by infection with the gram-negative bacteria A. baumannii or Pseudomonas aeruginosa, filamentation by C. albicans was significantly inhibited (Fig. 1A). In contrast, filamentation was minimally affected by the nonpathogenic Escherichia coli strains OP50 or HB101, or the gram-positive pathogens Enterococcus faecium or Staphylococcus aureus (Fig. 1A).
Fig. 1.
Prokaryote–eukaryote interactions within C. elegans. (A) Inhibition of the reference C. albicans strain DAY185 (CA) filamentation depended on the coinfecting bacterial genus: A. baumannii (ATCC strain no.19606) (AB), P. aeruginosa strain PA14 (PA), E. coli strain OP50 (EC), S. aureus (ATCC strain no. 29213) (SA) and E. faecium strain A6349 (EF). All bacteria inocula were 106 cfu/ml. (B) Live A. baumannii cells, in a dose-dependent manner, and filter-sterilized supernatant (SUP) from stationary phase growth (stat.) were able to inhibit C. albicans filamentation. Heat-killed (HK) cells had no effect. Light-field microscopy images show the inhibition of filamentation in the presence of A. baumannii (D and F) compared with C. albicans alone (C and E) (black arrow in D points to the nematode grinder organ). Fluorescent images of C. elegans after exposure to C. albicans strain MLR62, which constitutively expresses GFP (G and H), shows yeast cells within the mouth and pharynx of the nematode (white arrow in G); with filamentation protruding from the distended proximal gut (G). In contrast, in the presence of A. baumannii, reduced GFP signal was observed, and if C. albicans filaments were present, they were attenuated (H, white arrows point to sparse filaments). Fluorescent images of C. elegans infected with the C. albicans strain HGFP3, which expresses GFP in a hyphae-specific manner (I and J), shows that A. baumannii inhibits true C. albicans hyphae (J), with few hyphae observed within the protective confines of the worm cuticle (J). All images were taken at 24 h. Column bars represent the mean and error bars represent standard deviation (for all figures). Asterisks denote comparison with C. albicans strain DAY185 alone: ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05, by two-tailed t test. (Scale bars: C, D, and G, 50 μm; E, 20.35 μm; F, 21.89 μm; H, 45.84 μm; I, 13.85 μm; J, 21.89 μm.)
The ability of A. baumannii to inhibit C. albicans filamentation was especially interesting. A. baumannii is an emerging, multidrug-resistant bacterial pathogen, and like C. albicans, has a predilection for infecting immunocompromised, critically ill patients (16). Both microbes often share a common ecological niche within healthcare institutions, including bronchial airways, vascular and urinary catheters, and patient wounds (14–17). Given the increasing importance of both of these opportunistic pathogens in the morbidity and mortality of hospitalized patients (8, 16), we sought to define further their interactions within C. elegans. First, we assessed a range of Acinetobacter species, and found that the nonpathogenic species, Acinetobacter baylyi, and the unusual human pathogen, Acinetobacter lwoffii, inhibited C. albicans filamentation significantly less well than A. baumannii [supporting information (SI) Fig. S1]. Interestingly, Acinetobacter calcoaceticus, a common environmental organism (16), inhibited C. albicans filamentation to a similar extent as A. baumannii, which may be as a consequence of its evolutionary development with environmental fungi in nature, leading to the formation of inherent mechanisms for competitive survival. Notably, five other clinical A. baumannii strains also inhibited C. albicans filamentation (data not shown).
The observed A. baumannii–C. albicans interaction within C. elegans remained robust despite modifying the environment of the coinfection assay with factors that promote C. albicans filamentation. The A. baumannii-mediated inhibition of filamentation was not altered by bovine serum (at different concentrations up to 75%) (Sigma), filament-inducing spider medium (18), the quorum-sensing molecule tyrosol (Fluka) (4 μg/ml–200 μg/ml), which promotes filamentation (19), or by increased nutrients in the liquid medium for the assay [up to 80% brain–heart infusion (BHI)].
The degree of inhibition of C. albicans filamentation in C. elegans depended on the initial A. baumannii inoculum into the liquid medium of the assay, with measurable inhibition occurring with an inoculum as low as 102 CFU/ml (Fig. 1B). Washed, heat-killed A. baumannii cells at various densities (equivalent to 104–109 CFU/ml) caused no inhibition of C. albicans filamentation (Fig. 1B and data not shown), indicating the requirement for live cells or a secreted factor from A. baumannii. Confocal laser microscopy of the A. baumannii–C. albicans interaction within C. elegans showed the striking phenotypic differences between coinfection and C. albicans infection alone (Fig. 1 C–J). Notably, such differences were observed within 24 h after coinfection.
A Secretory Factor from A. baumannii Inhibits C. albicans Filamentation in C. elegans.
To determine the relative contribution of a bacterial secreted factor compared with live bacterial cells to the observed inhibition of C. albicans filamentation, we assessed the ability of A. baumannii culture filtrate, from different stages of growth, to inhibit C. albicans filamentation in C. elegans. We observed that filter-sterilized supernatant taken from A. baumannii grown to stationary phase inhibited C. albicans filamentation in C. elegans, however not to the level of live A. baumannii cells (Fig. 1B). Culture filtrate from exponential phase growth caused no inhibition (Fig. 1B). Supernatant from an environmental strain of A. baumannii was recently identified as having antifungal activity (20). Isomers of iturin A were identified as the active molecules. Thus, we tested pure iturin A (Sigma) in the C. elegans–C. albicans filamentation model and found that at concentrations up to 5 μM, no inhibition of filamentation was observed (data not shown), suggesting that antifungal entities are not limited to iturin A.
Given the growth-dependent activity of A. baumannii supernatant toward C. albicans, a further hypothesis was that a bacterial quorum-sensing molecule was responsible. Recently, the first of such molecules, a member of the LuxI family of autoinducer synthases (3-hydroxy-C12 homoserine lactone), was characterized from A. baumannii (21). After testing an A. baumannii mutant (abaI::Km) defective in production of this molecule and its isogenic parent strain (M2) in the C. elegans coinfection model, we observed a similar degree of inhibition of C. albicans filamentation (data not shown), suggesting that either acyl-homoserine lactone (AHL) molecules are not responsible for our observations or that a different AHL molecule is responsible. To assess the latter further, we tested a range of synthetic AHL molecules with varying carbon backbone lengths, including C4-, C6-, and 3-oxo-C12-homoserine lactone (Caymen Chemical) up to 200 μM, in the C. elegans–C. albicans infection model. No inhibition of C. albicans filamentation in C. elegans was observed (data not shown).
A. baumannii Attenuates C. albicans Pathogenicity in the C. elegans Coinfection Model.
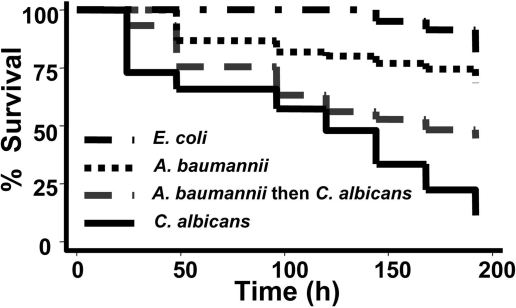
Given the importance of filamentation in the pathogenesis of C. albicans infection in mammals (10, 13) and C. elegans (9), we sought to assess the consequences of the observed A. baumannii–C. albicans interaction on the pathogenicity of C. albicans toward C. elegans. We hypothesized that, despite A. baumannii (22) (Fig. 2) and C. albicans (9) being able to kill C. elegans individually, a combined infection might lead to attenuated killing compared with C. albicans alone. We observed that when C. elegans were sequentially infected for 2 h with A. baumannii, followed by C. albicans on separate BHI agar plates, and then transferred into standard liquid medium, worm killing was significantly attenuated compared with that observed with C. albicans infection alone (P < 0.001) (Fig. 2). To remove uncertainty about whether the worm consumed C. albicans after A. baumannii exposure, we reversed the sequence of pathogen exposure. A similar attenuation in C. elegans killing was observed (P < 0.001) (data not shown).
Fig. 2.
A. baumannii attenuates the pathogenicity of C. albicans toward, C. elegans. The killing of C. elegans was significantly reduced when exposed to A. baumannii (ATCC strain no. 19606) and then C. albicans strain DAY185 (or vice versa) on solid medium before transfer to liquid medium, compared with exposure to C. albicans alone (P < 0.001). Nematodes feeding on E. coli OP50 were used as a control. Sixty to eighty nematodes were used per condition.
A. baumannii Affects the Viability of C. albicans in Vitro and Preferentially Associates with C. albicans Filaments.
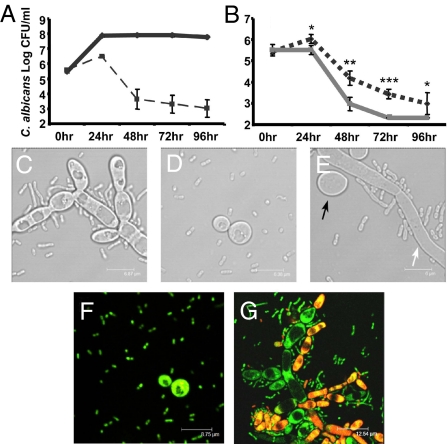
To provide supportive evidence for the observed anticandidal effects of A. baumannii in the C. elegans model, and to further define the interaction, we performed in vitro coinfection cultures under planktonic conditions. Using the reference C. albicans strain DAY185 (Table S1), we found that A. baumannii caused significant killing of C. albicans over a 96-h period (Fig. 3A). Given that the C. albicans DAY185 strain can exist in both yeast and filamentous forms, and our interest in assessing whether A. baumannii toxicity toward C. albicans has morphological specificity, we assessed the viability, in the same coinfection assay, of the constitutively filamentous C. albicans tup1 mutant (23) and the hyphal-defective mutant suv3, which remains in yeast form at 30°C (12). The C. albicans tup1 mutant was remarkably susceptible to A. baumannii (Fig. 3B). In comparison, the C. albicans suv3 mutant was also susceptible to A. baumannii but significantly less so than C. albicans tup1 (Fig. 3B), indicating a predilection of A. baumannii for killing C. albicans filaments. In addition, microscopy demonstrated that A. baumannii have greater cell–cell association with C. albicans filaments compared with the yeast form (Fig. 3 C–E). Further, when coinfection cultures with A. baumannii and the C. albicans tup1 and suv3 mutants were assessed by using the LIVE/DEAD staining system, whereby live cells stain green (SYTO9) and dead cells stain red (propidium iodide), killing of C. albicans filaments by A. baumannii was observed, whereas this was not evident for yeast-form cells at the same time point (Fig. 3 F and G). This morphological predilection of A. baumannii for the filamentous form of C. albicans was further confirmed by other C. albicans mutants, whereby the constitutively filamentous C. albicans nrg1 mutant (24) was killed significantly more than the C. albicans efg1 or tec1 mutants (11, 25) (Table S1), which are both in the yeast form at 30°C (P < 0.01 at 96 h time point) (data not shown).
Fig. 3.
A. baumannii has a predilection for killing C. albicans filaments in vitro. (A) The viability of C. albicans strain DAY185 (solid line) was significantly reduced when cocultured with A. baumannii (ATCC strain no. 19606) (dashed line). (B) A. baumannii caused more rapid killing of the constitutively filamentous C. albicans tup1 mutant (dotted line) compared with the hyphal-defective C. albicans mutant suv3 (gray solid line), which remains in yeast form at 30°C but can produce filaments at 37°C. In support, microscopy showed that A. baumannii cells have greater association with C. albicans filaments compared with the yeast form, tup1 (C), suv3 at 30°C (D), and suv3 at 37°C (E). The white arrow in E points to a C. albicans filament, whereas the black arrow points to a yeast cell. Cocultures with A. baumannii and C. albicans suv3 (F) and tup1 (G) mutants were stained with the LIVE/DEAD staining system. Viable cells stained green (SYTO9), whereas dead cells stained red (propidium iodide). Control cultures of C. albicans tup1 and suv3 mutants alone demonstrated green fluorescence (data not shown). All images were taken at 16–20 h. Asterisks denote comparison of Log CFU/ml between C. albicans tup1 and suv3 mutants when cocultured with A. baumannii. Results for the viability assays were derived from three independent experiments. (Scale bars: C, 6.87 μm; D, 6.35 μm; E, 6 μm; F, 8.75 μm; G, 12.54 μm.)
A. baumannii Inhibits C. albicans Biofilm Formation on an Abiotic Surface.
C. albicans biofilm communities are thought to be critical in its pathogenesis toward humans (11, 12) and are also involved in C. elegans killing (9). Given the importance of functional C. albicans filaments to biofilm integrity (12), we assessed the consequences of the A. baumannii–C. albicans interaction on C. albicans biofilm formation, using a silicone pad assay (12). When A. baumannii was introduced into the media of the biofilm assay, a significant dose-dependent inhibition of C. albicans biofilm formation was observed (Fig. 4A). Microscopy at the completion of the experiment showed that the sparse biofilm that formed was composed mainly of C. albicans cells that had yeast morphology (Fig. S2 A and B). Interestingly, C. albicans filaments in the mature biofilm were nonviable in the presence of A. baumannii, as determined by the LIVE/DEAD staining system (Fig. 4D). Of note, culture filtrate from A. baumannii also significantly inhibited the ability of C. albicans to form a biofilm, with the degree of inhibition being dependent on the A. baumannii growth phase in which the culture filtrate was taken (Fig. 4B). To assist in ruling out the possibility that the reduction of C. albicans biofilm on silicone pads was because of the depletion of some nutrient from the medium, we evaluated the C. albicans biofilm in the presence of less pathogenic species of Acinetobacter, including A. baylyi and A. lwoffii. These strains were unable to inhibit C. albicans biofilm formation (data not shown).
Fig. 4.
A. baumannii and C. albicans in an in vitro biofilm environment. (A) The degree of inhibition of C. albicans DAY185 (CA) biofilm formation on silicone pads depended on the initial A. baumannii (AB) inoculum. (B) A. baumannii culture filtrate, when used as the liquid medium for the assay, inhibited C. albicans biofilm formation, with the degree of inhibition depending on the bacterial growth phase (grown in spider media at 37°C) from which the supernatant (SUP) was derived (OD at 600 nm). When A. baumannii was introduced into the liquid medium of the biofilm assay after 8 h of C. albicans biofilm development (C), further biofilm growth was inhibited. However, when A. baumannii was inoculated after 24 h, the inhibitory effects were reduced, with evidence of further C. albicans biofilm growth (C). LIVE/DEAD staining of a mature (60 h) C. albicans biofilm in the presence of A. baumannii (D) showed viability only of yeast cells. (Scale bar: D, 17.49 μm.) Asterisks in A denote comparison of biofilm mass (mg) between C. albicans alone and in the presence of A. baumannii, and the asterisks in B denote comparison of biofilm mass between C. albicans alone in standard spider media compared with in A. baumannii supernatant as the media of the assay. Experiments were performed at least twice in triplicate.
To determine the effect of A. baumannii on an already formed C. albicans biofilm, we inoculated the biofilm environment with live A. baumannii cells at different stages of C. albicans biofilm development. We observed that when A. baumannii was inoculated at a cell density of 106 CFU/ml up to 8 h after C. albicans biofilm development, the ability of further C. albicans biofilm formation was inhibited (Fig. 4C and data not shown). Unexpectedly, we noticed that there was a time point beyond which the effect of A. baumannii cells toward C. albicans decreased. More specifically, when A. baumannii was introduced into the C. albicans biofilm assay after 24 h of C. albicans biofilm formation, not only was it less able to inhibit further biofilm development (Fig. 4C) but interestingly, the growth of the bacteria appeared restricted.
The Complex Interplay Between Competing Pathogens: The Eukaryotic Quorum-Sensing Molecule, Farnesol, Inhibits the Growth of A. baumannii.
To further explore the counteroffensive by C. albicans toward A. baumannii in a biofilm environment, we assessed the supernatant from the liquid medium of a C. albicans biofilm at different stages of development for its activity against A. baumannii. We observed that the antibacterial activity of the C. albicans supernatant increased as biofilm development matured. More specifically, significant A. baumannii growth inhibition (1.85 Log CFU/ml) was seen when grown in supernatant from an 18-h C. albicans biofilm. (P < 0.01) (Fig. S3A). Given the growth-dependent characteristics of these findings, we hypothesized that C. albicans quorum-sensing molecules may be responsible for the inhibitory activity against A. baumannii. Farnesol appeared an ideal candidate for this finding as it is produced in parallel to C. albicans cell growth, its activity increases during the later stages of biofilm development (26), and it has been described to have antibacterial activity (27). Indeed, we showed that pure farnesol (Sigma) caused a significant inhibition of A. baumannii growth (Fig. S3B), of similar magnitude to that seen with C. albicans supernatant. To further confirm these findings we assessed the supernatant from a mature biofilm of a C. albicans mutant defective in farnesol production (C. albicans KWN2) and compared its effect on A. baumannii growth to the supernatant of its parent strain (C. albicans SN152) and a reconstituted strain (C. albicans KWN4) (28). Supernatant from C. albicans KWN2 caused no inhibition of A. baumannii growth compared with A. baumannii grown in fresh medium, whereas C. albicans SN152 and KWN4 caused a subtle yet significant reduction in A. baumannii growth (0.59 and 0.51 Log CFU/ml reduction, respectively, P < 0.01 for both). These data confirm that the eukaryotic quorum-sensing molecule, farnesol, has crosskingdom inhibitory effects on the prokaryotic organism, A. baumannii.
A. baumannii Mutants with Attenuated Virulence toward C. albicans Identified by Using a C. elegans–A. baumannii–C. albicans Screen.
To determine whether the C. elegans–A. baumannii–C. albicans coinfection model can be used to explore the molecular mechanisms of this prokaryote–eukaryote interaction, we extended our model to allow for analysis in 96-well plates of ∼600 random A. baumannii MAR2xT7 transposon mutants. After performing confirmatory assays, five mutants were identified that had significantly less ability to inhibit C. albicans filamentation in C. elegans. All five mutants had similar growth kinetics to the parent strain (data not shown). The insertion site of one of these mutants was identifiable using gene sequence homology to the gacS-like sensor kinase gene, which is part of a highly conserved two-component regulatory system (GacS sensor kinase/GacA response regulator) important for a diverse array of virulence functions in other gram-negative bacteria (29, 30). Interestingly, for many gram-negative bacteria, the gacS/gacA two-component system has been shown to control the synthesis of secretory products, including secondary metabolites with antimicrobial activity (29). Further, a P. aeruginosa gacA mutant, which is attenuated in virulence toward mammals (30), was found to be delayed in its inhibitory effect toward C. albicans (4). Apart from causing significantly less inhibition of C. albicans filamentation in C. elegans, the A. baumannii gacS-like sensor kinase mutant was also attenuated in its ability to kill the C. albicans DAY185 strain in vitro (Fig. S4). With regard to the insertion sites of the other four mutants, one was identified within a gene coding for a hypothetical protein and the last three mutants were not identifiable.
Discussion
Given the abundance of polymicrobial encounters in nature, and the paucity of knowledge about the pathogenic consequences and molecular details of these interactions, we developed a facile in vivo whole animal model that can be effectively used to study pathogen–pathogen interactions. Using this model system, we found that important virulence traits of C. albicans, such as biofilm and filament formation, are targets of A. baumannii. Interestingly, A. baumannii cells have a greater affinity and toxicity toward C. albicans filaments compared with the yeast form. Remarkably, although bacteria inhibit C. albicans biofilm formation, when allowed to develop, the growth of A. baumannii is inhibited. This likely evolutionary defense system by C. albicans is at least partly because of the release of the quorum-sensing molecule farnesol. Moreover, although A. baumannii and C. albicans can independently kill C. elegans, when nematodes are infected with both pathogens they survive significantly longer compared with C. albicans infection alone. Finally, in a “proof of concept” study, we screened A. baumannii mutants and identified those with reduced toxicity toward C. albicans.
Thus far, there is a scarcity of realistic in vivo models that exist to study pathogen–pathogen interactions (6). The C. elegans model, which has thus far been used to study monomicrobial pathogenesis (7), provides many advantages for the study of multispecies dynamics, including genetic tractability, ease of handling and simplicity of equipment, short reproductive cycle, translucent body that enables microscopic visualization of internal events, and absence of ethical considerations associated with mammalian models. Also, for the study of the prokaryote–eukaryote interactions described herein, two relatively unambiguous assay endpoints are used: C. albicans filamentation and worm survival. In addition, the ability to genetically manipulate both the host and the pathogen provides an efficient system to study the molecular mechanisms of pathogen–pathogen interactions and host responses to polymicrobial infections. Such models will help advance our understanding of microbial pathogenesis within a realistic environment of coexisting microbes.
Our results show that A. baumannii has profound anticandidal properties, with a predilection for the filamentous form of C. albicans. Filamentation has been shown to be an important virulence determinant in C. albicans (10, 13), and thus our findings of reduced C. albicans pathogenicity toward C. elegans when co-infected with A. baumannii, are understandable. Recently, our group demonstrated that C. elegans killing was reduced when infected with C. albicans mutants defective in hyphae formation (9), thus highlighting the relevance of filamentation in the C. elegans infection model. However, the reduction in nematode killing by C. albicans in the presence of A. baumannii is likely because of a broader effect on C. albicans, including the toxicity of A. baumannii to the yeast-form cells as well. After C. elegans consumes C. albicans, a persistent gut infection ensues, initially composed of yeast-form cells. These cells proliferate and cause marked gut distension followed by a morphological transition to the filamentous form, eventually leading to worm death (9). Given the observed effects of A. baumannii on the viability of C. albicans yeast and filamentous forms, it is reasonable to assume that A. baumannii slows or reduces the degree of C. albicans proliferation in the worm gut, thus reducing worm lethality. Also, it is possible, however not assessed in this study, that an augmented or altered host immune response with polymicrobial infection may favor worm survival.
The cause of the antagonistic interaction between A. baumannii and C. albicans appears multifactorial. It is clear that a bacterial secretory factor, whose production increases from late exponential growth phase onwards, plays a significant role. However, inhibition of C. albicans filamentation and biofilm formation was more pronounced with live A. baumannii cells compared to supernatant taken from stationary phase growth, suggesting that cellular interaction may also contribute. Also, microscopy demonstrated marked cell–cell association of A. baumannii with the filamentous cells of C. albicans, further raising the question of direct cellular toxicity. Recently, Smith et al. performed whole genome sequencing of a reference strain of A. baumannii (American Type Culture Collection [ATCC] strain no. 17978) and identified eight genes homologous to the Legionella/Coxiella Type IV secretion apparatus (22). This secretion system is capable of exporting virulence factors across the membranes of gram-negative bacteria, often to eukaryotic cell targets (31). Thus, this type of system may be used by A. baumannii for direct toxicity toward C. albicans. Also, the close cellular association of A. baumannii to C. albicans that we observed likely optimizes the potency of a secreted factor with antifungal activity.
Other factors that may be contributing to the observed antagonistic interaction between A. baumannii and C. albicans include changes in environmental pH, which can impair the ability of C. albicans to form filaments (32) and nutritional competition. We observed that A. baumannii can produce a mildly alkalotic environment under the conditions of the C. elegans coinfection assay but that this was not sufficient to cause appreciable inhibition of C. albicans filamentation (data not shown). With regard to nutrient depletion, we observed that C. albicans filamentation in the presence of A. baumannii remained significantly inhibited despite frequent (6 hly) replenishment of the liquid media with fresh BHI medium during the C. elegans coinfection filamentation assay. Moreover, the inhibition of filamentation occurred rapidly, before one would expect the depletion of nutrients to occur, and A. baumannii showed a cellular predilection for C. albicans filaments, suggesting an interaction beyond just nutrient deprivation.
The culture filtrate from A. baumannii grown to stationary phase significantly inhibited C. albicans filamentation and biofilm formation. Given the growth-dependent properties of this antifungal activity, a quorum-sensing molecule may be a likely etiological candidate. However, we were unable to demonstrate this by using an A. baumannii mutant defective in the production of 3-hydroxy-C12 homoserine lactone (abaI::Km) (21) and a range of synthetic AHL molecules with varying carbon-length backbones. The production of another cell-density-dependent compound is also a possibility, as has been shown for an environmental strain of Acinetobacter toward phytopathogenic fungi, which produced iturin A (20). In our study, iturin A was not effective at inhibiting C. albicans filamentation in C. elegans up to a concentration of 5 μM. Given the therapeutic potential of our findings, further work to identify the active compound in A. baumannii supernatant is ongoing.
Remarkably, we observed a counteroffensive by C. albicans toward A. baumannii within the complex environment of a mature biofilm. We identified that a secretory factor with antibacterial activity was being released from C. albicans toward the later stages of biofilm development. Through use of purified farnesol and a C. albicans mutant defective in farnesol production (28), we confirmed that this eukaryotic quorum-sensing molecule, which represses hyphae formation, was at least partly responsible for the observed antibacterial effect against A. baumannii. Interestingly, farnesol is a molecule with a 12-carbon backbone chain length, similar to certain bacterial quorum-sensing molecules (32). Such crosskingdom targets of extracellular signaling illustrates the diversity of these molecules and highlights their potential in uncovering novel therapeutic targets for clinically problematic pathogens.
In conclusion, this report extends the current paradigm of studying monomicrobial pathogenesis in the genetically tractable, whole animal model system, C. elegans. The evolution of synergistic, symbiotic, or antagonistic interactions between diverse organisms in nature or the clinical environment, especially those between prokaryotes and eukaryotes, is likely important for their pathogenesis toward a range of hosts, including humans. The exploitation of the likely evolutionary defense mechanisms used by competing microbes may provide critical insights into novel therapeutic targets, which are desperately needed for pathogens such as A. baumannii and C. albicans.
Experimental Procedures
Bacterial and Fungal Strains.
Unless specified otherwise, bacterial and fungal cultures were grown overnight in Luria–Bertani (LB) broth at 37°C and yeast peptone dextrose (YPD) (Difco) broth at 30°C in a rollerdrum, respectively. The genotypes and other characteristics of C. albicans strains used in this study are reported in SI Text and Table S1. Heat-killed A. baumannii were produced by incubating cells in a heat block at 80°C for 90 min.
C. elegans Strains.
C. elegans glp-4; sek-1 nematodes were used for all experiments because of the untoward effects of using wild-type C. elegans strains in liquid assay experiments (see SI Text). The glp-4; sek-1 nematodes were propagated on E. coli strains OP50 or HB101 by using established procedures (9).
C. elegans Coinfection Assay for Filamentation.
The methodology used for the C. elegans–C. albicans liquid medium assay was as described previously (9), with some modification. Young adult nematodes were allowed to feed on lawns of C. albicans on solid BHI media (Difco), containing kanamycin (45 μg/ml), ampicillin (100 μg/ml), and streptomycin (100 μg/ml), for 4 h at 25°C (preinfection). The worms were then washed with sterile M9 minimal media and pipetted (approximately 60 to 80 worms per well) into wells of a six-well microtiter dish (Corning) containing 2 ml of liquid media (80% M9 and 20% BHI). Bacteria from an overnight culture were directly inoculated into the liquid media immediately before the preinfected worms were included. Plates were incubated at 25°C and were examined daily for the number of worms with penetrative filamentation by using a Nikon SMZ645 dissecting microscope. Filamentation was defined as any breach in the worm cuticle by filamentous cells as seen at X50 magnification.
Differences in worm filamentation on day 5 were compared by the Student's t test. For this and all subsequent statistical comparisons, a P value of <0.05 was considered statistically significant. Qualitative assessment of C. albicans filamentation was performed by confocal laser microscopy (TCS NT; LeicaMicrosystems). All experiments were performed at least twice.
C. elegans Coinfection Assay for Survival.
Nematodes were preinfected with both organisms sequentially for 2 h on solid medium (BHI) before being transferred into liquid medium (M9:BHI as described). To remove excess bacterial or fungal cells from the worm cuticle, nematodes were briefly washed with M9 media between exposures to each organism. Worm death was monitored daily, and time to death was calculated by using the Kaplan–Meier method, with differences calculated by using the log-rank test (STATA 6).
In Vitro Coinfection Cultures in a Planktonic Environment.
Coinfection cultures were performed in 2 ml of LB broth at 30°C in a rollerdrum. YPD plates containing kanamycin (45 μg/ml) and LB plates containing fluconazole (32 μg/ml) were used to determine C. albicans and A. baumannii CFUs, respectively. Results were obtained from three independent experiments. The viability of C. albicans in the presence of A. baumannii was also assessed by using the BacLight LIVE/DEAD staining system according to the manufacturer's protocol (Molecular Probes) (32).
In Vitro Biofilm Assay on Silicone Pads.
The effect of A. baumannii on C. albicans biofilm formation was determined by using a silicone pad assay as described previously (11, 12) and detailed in SI Text. To assess the effects of Acinetobacter on C. albicans biofilm formation, bacterial cells were introduced into the biofilm medium at different time points. Silicone pads exposed to Acinetobacter only were used as a control. Results were obtained from at least two independent experiments performed in triplicate.
Development of the A. baumannii MAR2xT7 Mutant Library.
MAR2xT7 insertions were generated by introducing pMAR2xT7 into a gentamicin-susceptible A. baumannii clinical strain A9844 from E. coli MC4100 in six separate tripartite matings as described previously (34), with slight modification (SI Text). Formal species identification of A9844 was performed before mutagenesis (SI Text).
Screening Using the C. elegans–A. baumannii–C. albicans Model.
An A. baumannii library consisting of approximately 600 MAR2xT7 mutants was screened for their ability to inhibit C. albicans filamentation in C. elegans. First, A. baumannii mutants were replicated into 96-well microtiter plates containing fresh LB media with 15 μg/ml of gentamicin and allowed to grow overnight at 37°C. Nematodes were then preinfected with C. albicans and were then pipetted into each well of the A. baumannii preinoculated 96-well microtiter plates (approximately 30 to 40 worms per well). The percentage of worms with filamentation was assessed on day 5. Mutants that allowed ≥30% of the C. albicans preinfected worms to filament were tested two more times by using the standard assay. Transposon insertion sites were identified by using a nested PCR as described previously (34).
Supplementary Material
Acknowledgments.
We thank Tara Thurber and team from the Massachusetts General Hospital Genetics CORE laboratory for use of the Hudson RapidPick robot and Gerald Fink for the C. albicans nrg1 mutant. Support was provided by National Institutes of Health K08 Award AI63084 and R01 Award AI075286 (to E.M.), a New Scholar Award in Global Infectious Diseases from the Ellison Medical Foundation (to E.M.), and a University of Queensland Postgraduate Scholarship Award (to A.Y.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805048105/DCSupplemental.
References
- 1.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci USA. 1999;96:14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman DK, Banfield JF. Geomicrobiology: How molecular-scale interactions underpin biogeochemical systems. Science. 2002;296:1071–1077. doi: 10.1126/science.1010716. [DOI] [PubMed] [Google Scholar]
- 3.Hansen SK, Rainey PB, Haagensen JA, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445:533–536. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- 4.Hogan DA, Kolter R. Pseudomonas-Candida interactions: An ecological role for virulence factors. Science. 2002;296:2229–2232. doi: 10.1126/science.1070784. [DOI] [PubMed] [Google Scholar]
- 5.Wang LH, et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004;51:903–912. doi: 10.1046/j.1365-2958.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- 6.Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mylonakis E, Casadevall A, Ausubel FM. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 2007;3:e101. doi: 10.1371/journal.ppat.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudlaugsson O, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 9.Breger J, et al. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo HJ, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 11.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–1155. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Richard ML, Nobile CJ, Bruno VM, Mitchell AP. Candida albicans biofilm-defective mutants. Eukaryot Cell. 2005;4:1493–1502. doi: 10.1128/EC.4.8.1493-1502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal VD, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: The emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chim H, Tan BH, Song C. Five-year review of infections in a burn intensive care unit: High incidence of Acinetobacter baumannii in a tropical climate. Burns. 2007;33:1008–1014. doi: 10.1016/j.burns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Fujita M, Feng Q, Clardy J, Fink GR. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci USA. 2004;101:5048–5052. doi: 10.1073/pnas.0401416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CH, et al. Study of the antifungal activity of Acinetobacter baumannii LCH001 in vitro and identification of its antifungal components. Appl Microbiol Biotechnol. 2007;76:459–466. doi: 10.1007/s00253-007-1010-0. [DOI] [PubMed] [Google Scholar]
- 21.Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MG, et al. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 24.Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–4761. doi: 10.1093/emboj/20.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 26.Hornby JM, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue Y, et al. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett. 2004;237:325–331. doi: 10.1016/j.femsle.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Navarathna DH, et al. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect Immun. 2007;75:1609–1618. doi: 10.1128/IAI.01182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeb S, Haas D. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact. 2001;14:1351–1363. doi: 10.1094/MPMI.2001.14.12.1351. [DOI] [PubMed] [Google Scholar]
- 30.Rahme LG, et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 31.Yeo HJ, Waksman G. Unveiling molecular scaffolds of the type IV secretion system. J Bacteriol. 2004;186:1919–1926. doi: 10.1128/JB.186.7.1919-1926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004;54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 33.Staab JF, Bahn YS, Sundstrom P. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology. 2003;149:2977–2986. doi: 10.1099/mic.0.26445-0. [DOI] [PubMed] [Google Scholar]
- 34.Liberati NT, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.