Abstract
DCC (Deleted in Colorectal Cancer) is a putative tumor suppressor whose expression is lost in numerous cancers and whose tumor suppressor activity appears to be dependent on its ability to trigger apoptosis when disengaged by its ligand netrin-1. In this sense, netrin-1 is a survival factor that controls tumorigenesis. However, netrin-1 is also the prototypical axon guidance cue and has been shown to orient many neurons or axons, especially commissural axons, during spinal cord development. Here we show that netrin-1 is not only an attractive cue for developing commissural axons but also promotes their survival. In primary neuronal culture, in mice or in chick embryos, netrin-1 inhibits the proapoptotic activity of DCC in developing commissural neurons. Thus, adequate commissural neurons navigation requires both the attractive activity of netrin-1 and the anti-apoptotic function of this cue.
Keywords: apoptosis, deleted in colorectal cancer, nervous system development, dependence receptor, axon guidance
The development of colonic carcinoma from normal colonic epithelium is associated with the mutation of a specific set of genes (1). Allelic deletions [loss of heterozygosity (LOH)] on chromosome 18q in 70% of primary colorectal tumors prompted the search for a tumor suppressor gene at that locus, leading to the cloning of a putative cell-surface receptor, DCC (Deleted in Colorectal Cancer) (2). DCC expression was then shown to be markedly reduced in 50% of colorectal tumors. Moreover, the loss of DCC expression is not restricted to colon carcinoma, but has been observed in many other tumors, suggesting that DCC is a tumor suppressor (3).
However, DCC is also the main component of a receptor complex that mediates the activity of the secreted axon-guidance molecule netrin-1 (4, 5). The key role of DCC and netrin-1 in mediating growth cone extension is supported by a large number of studies, such as the analysis of DCC and netrin-1 knock-out mice, which display the disorganization of many axonal tracts in the central nervous system (6).
The link between a tumor suppressor in colorectal cancer and a receptor mediating axon outgrowth and turning remained unclear until recently. We proposed that the tumor suppressor function could be explained by the fact that DCC is a dependence receptor (7). DCC is functionally related to other dependence receptors such as p75NTR, the common neurotrophin receptor, RET, Patched, UNC5H, neogenin, ALK, or TrkC (8–13). Such receptors create cellular states of dependence to their respective ligands by inducing apoptosis when unoccupied but inhibiting apoptosis in the presence of their ligands (12). We and others have shown that the in vitro expression of DCC induces apoptosis in the absence of netrin-1 (7, 14), while the presence of netrin-1 blocks this proapoptotic activity (15).
The fact that DCC displays such proapoptotic activity when unbound to its ligand has led to the hypothesis that DCC normally functions to kill tumor cells that grow in an inappropriate context (e.g., local growth in a setting of constant and limited netrin-1 concentration or growth at a secondary site where there is no netrin-1 expression). Accordingly, mice over-expressing netrin-1 in the gut show a marked decrease of cell death in the intestinal epithelium associated with an increased predisposition to develop colorectal tumors (16). Thus, DCC may be viewed as a tumor suppressor that controls tumorigenesis by regulating apoptosis (17, 18) and netrin-1 expression as a selective survival advantage for tumor growth and metastasis (19).
However, it is clear that the main role of netrin-1 in the developing CNS is not survival but axon guidance. The main defects of netrin-1 and DCC knock-out mice may, to a large extent, be attributed to a loss of the DCC/netrin-1 axon-guidance system. Yet, netrin-1 knock-out mice also display increased cell death in the developing brainstem (20). Thus, one may wonder whether netrin-1 in the developing nervous system also shows a survival activity that may participate to the neuronal navigation activity of this cue. Here we addressed this question in commissural neurons that are the prototype neurons guided by the netrin-1/DCC pair. We show that in primary cultures, commissural neurons die except if netrin-1 is provided and that apoptotic activity depends on DCC, as primary commissural neurons from DCC knock-out embryos survive in the absence of netrin-1. We also show enhanced apoptosis in developing mouse spinal cord from netrin-1 knock-out embryos. Moreover, we show that in developing chick neural tube, the DCC/netrin-1 pair acts as a pair dependence receptor/ligand as electroporation of DCC triggers cell death that is blocked by coelectroporation of netrin-1 while down-regulation of netrin-1 is associated with increased cell death.
Results
Netrin-1 Acts as a Survival Factor for Commissural Neurons.
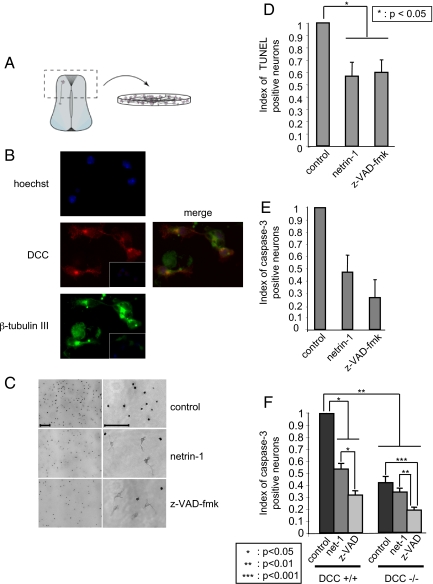
In the dorsal spinal cord, commissural axons that express DCC are attracted toward the ventral midline by floor-plate-derived netrin-1. To assess whether netrin-1 may also display survival activity toward these neurons, we cultured commissural neurons from E13 rat or E11.5 mouse embryos. Dorsal spinal cords were dissected out and dissociated and primary cells were then grown in neurobasal medium (Fig. 1A). The cells grown in these conditions were commissural neurons as they expressed the neuronal marker class III β-tubulin and DCC as shown by immunohistochemistry [Fig. 1B, specificity of the DCC antibody shown in supporting information (SI) Fig. S1]. When the rat commissural neuron cultures were analyzed 16 h after initial plating, about all of the cells were dead (data not shown and Fig. 1C). To confirm this observation, apoptosis was measured by counting TUNEL positive cells or by quantification of caspase activity. As shown in Fig. 1 C–E, commissural neurons grown in neurobasal medium died by apoptosis within the first 16 h. Interestingly, when the culture was performed in the presence of z-VAD-fmk, a general caspase and cell death inhibitor, 50% of the neurons survive after 16 h of culture and this effect was associated with a marked decrease in TUNEL- (Fig. 1 C and D) and active caspase-3 (Fig. 1E) -positive neurons. Netrin-1 was then added, instead of z-VAD-fmk, to the cultures and as shown in Fig. 1 C–E, netrin-1 presence increased cell survival and was associated with decreased number of TUNEL- and active caspase-3-positive cells to a similar extent than z-VAD-fmk (Fig. 1 C and E). Similar results were observed when mice commissural neurons instead of rat were cultured (data not shown and Fig. 1F). Thus, netrin-1 similarly to z-VAD-fmk enhances survival of commissural neurons.
Fig. 1.
Netrin-1 is a survival factor for commissural neurons. (A) The dorsal spinal cord that contains the commissural neurons is represented. (B) The cultured cells are commissural neurons. Immunostainings using an anti-DCC antibody (A20) (central panel) and an anti-beta-tubulin III antibody (below panel) of commissural neurons from E13 rat embryos dorsal spinal cord culture. Merge signal between DCC and beta-tubulin signals is shown (Right) together with a hoechst. Insets, control staining without primary antibodies. (C) Representative photographs of commissural neurons cultured 16 h in the absence (control) or in the presence of netrin-1 (300 ng/ml) or of z-VAD-fmk (20 μM) and stained by TUNEL assay. Right panels are a magnification of left panels. (Scale bars, 50 μM.) (D and E) Histograms representing cell death quantified by numbering cells stained with TUNEL assay (D) or with anti-active caspase-3 (E). Index of cell stained with TUNEL or active caspase-3 is shown as the ratio between the number of cells stained in treated conditions (netrin-1 or z-VAD-fmk) to the number of cell stained in control condition. Standard deviations are indicated (n = 3). (F) Commissural neurons from E11.5 mice embryos were cultured 16 h in the presence or absence of netrin-1 (300 ng/ml) or z-VAD-fmk (20 μM). Cultures were from DCC+/+ or DCC-/- embryos as indicated. Histogram representing cell death measured by the number of cells stained with anti-active caspase-3. Index of cell stained with active caspase-3 is shown as the ratio between the number of cells stained in treated conditions (netrin-1 or z-VAD-fmk) to the number of cell stained in control condition. Standard deviations are indicated (n = 3). *, **, and ***, respectively, indicates a P < 0.05, P < 0.01, and P < 0.001, calculated using a two-sided Mann–Whitney test.
Netrin-1 Acts as a Survival Factor by Inhibiting DCC Proapoptotic Activity.
In tumor cells, netrin-1 has been shown to act as a survival factor inhibiting the proapoptotic activity of DCC or UNC5H receptors (7, 16, 19–21). To determine whether this involves a neurotrophic factor-like effect of netrin-1 (i.e., activation upon ligand binding of survival signaling pathways like PI3K or MAPK pathways) or occurs through the inhibition of DCC proapoptotic activity, we then compared commissural neuron survival in primary cultures from DCC mice mutant embryos. If netrin-1 behaved as a classic neurotrophic factor, one should expect the loss of DCC to be associated with a similar effect on survival instead of a loss of the ligand (i.e., the DCC-/- commissural neurons should die even in the presence of netrin-1 at a same level than DCC+/+ commissural neurons cultured in the absence of netrin-1). Alternatively, if netrin-1 were to act by inhibiting DCC-induced apoptosis, the survival of DCC-/- neurons should be higher than DCC+/+ ones in the absence of netrin-1. As shown in Fig. 1F, in the absence of netrin-1 treatment, commissural neurons from DCC+/+ embryos died and showed caspase activation, whereas DCC-/- commissural neurons showed increased survival and decreased caspase activation. Moreover, adding netrin-1 to DCC+/+ culture increased neuronal survival, while it failed to increase the survival of DCC-/- neurons. Taken together, these data demonstrate that netrin-1 is a survival factor that inhibits the proapoptotic activity of the DCC dependence receptor in spinal cord commissural neurons.
Netrin-1 Controls Cell Survival in the Developing Neural Tube.
The phenotypic analysis of netrin-1 knockout mice revealed profound defects in the organization of many axonal tracts, but no increase in cell death was noted (5). Yet, because the numeration of commissural neurons is not easy and was not done precisely, and because these mutants were hypomorphic mice rather than null mice, we then decided to assess whether netrin-1 inactivation is associated with increased cell death in the developing spinal cord. An increased caspase-3 activity was observed in spinal cord extracts from netrin-1-/- embryos compared with wild-type extracts (Fig. 2A). Interestingly, this increased caspase-3 activity is specific for the spinal cord as when a similar caspase activity measurement was performed in brain rather than spinal cord, no similar increase of caspase activity was observed in netrin-1 mutant mice. Moreover, this increased caspase activity is associated with an increased number of apoptotic (i.e., TUNEL-positive cells) in the spinal cord of E13.5 of netrin-1 mutant embryos (Fig. 2 B and C). These TUNEL-positive cells moreover colocalize with DCC staining (Fig. 2D), further supporting the view of commissural neurons death. Thus, netrin-1 appears to inhibit apoptotic cell death in the developing spinal cord and more specifically in commissural neurons.
Fig. 2.
Netrin-1 as a survival factor in the developing mice spinal cord. (A) Spinal cord (or brain) from netrin1 +/+, netrin-1 -/- E11 or E13 embryos was dissected out and analyzed for global caspase-3 activity as described in the methods section. Standard deviation is indicated (n = 3). (B and C) TUNEL was performed on spinal cord of E13.5 netrin-1 -/- or +/- embryos. (B) representative TUNEL staining. Merge: enlargement of the colocalization signal located in the frame. (C) Quantification of the number of TUNEL-positive cells. (D) TUNEL + DCC immunostaining and the respective merge signal. CC, central canal. (Scale bars, 100 μM) In A and C error bars indicate SEM; * indicates a P < 0.05 calculated using a two-sided Mann–Whitney. In B and D arrows indicate apoptotic cells.
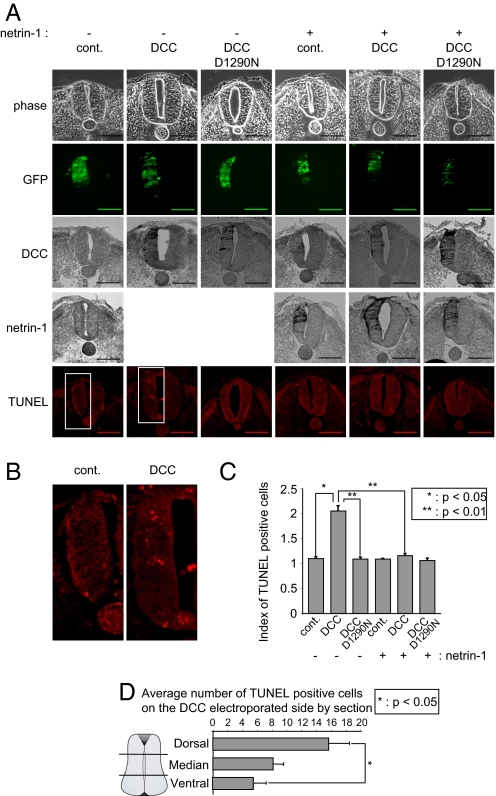
We then investigated whether the survival activity of netrin-1 could also be observed in developing chick neural tube. As a first approach, we analyzed whether forced expression of DCC in the developing chick neural tube triggered cell death as previously found for other dependence receptors (10, 11). HH10–11 embryos were electroporated in one side of the neural tube with DCC and GFP. As shown on Fig. 3 A–C, DCC electroporation was associated on the electroporated side with cell death induction as measured by TUNEL staining. This was not related to a toxic effect of the electroporation since an empty vector or a mutated DCC (DCC D1290N) unable to induce apoptosis in vitro (7) failed to trigger cell death in ovo (Fig. 3 A–C). Interestingly, this cell death induction appears to be mainly detected in the dorsal third of the neural tube (as opposed to the ventral two third of the neural tube) (Fig. 3D), thus supporting the view that (i) commissural neurons may be the target of DCC-induced apoptosis and/or (ii) that ectopic DCC expression is more effective in killing cells in the dorsal neural tube because endogenous netrin-1 is produced ventrally and may somehow inhibit DCC-induced apoptosis ventrally. Netrin-1 was then coelectroporated with DCC. The ectopic expression of netrin-1 fully inhibited the proapoptotic activity of DCC on the electroporated side (Fig. 3 A–C), thereby suggesting that the netrin/DCC ligand/dependence receptor couple is active during chick neural tube development.
Fig. 3.
DCC behaves as a dependence receptor in the developing chick neural tube. HH10–11 chick embryos were electroporated at the level of the dorsal neural tube with a mock plasmid (cont.), DCC construct (DCC), DCC D1290N mutant construct (DCC D1290N), or DCC and netrin-1 constructs (DCC+netrin-1) together with a GFP-encoding plasmid. (A) Representative phase, GFP, DCC (immunostaining with A20 antibody), netrin-1 (immunostaining with anti-c-myc, netrin-1 being c-myc-tagged), and TUNEL staining is shown. (Scale bars, 50 μM) (B) Enlargement of TUNEL staining of control vs. DCC electroporation. (C) Quantification of TUNEL immunostaining. Three to six embryos were analyzed for each condition. (D) Quantification of TUNEL immunostaining in the neural tube (Dorsal, Medium, and Ventral). In C and D, error bars indicate SEM; * indicates a P < 0.05 and ** indicates a P < 0.01 calculated using a two-sided Mann–Whitney test.
We further analyzed whether down-regulation of endogenous netrin-1 produced by the ventral neural tube by a shRNA approach was associated to a DCC-dependent cell death. Netrin-1 shRNA effectively down-regulates chick netrin-1 (Fig. 4A and Fig. S2). We then electroporated netrin-1 shRNA at the level of the ventral neural tube and apoptosis was assessed by TUNEL staining (Fig. 4B). Electroporation of a netrin-1 shRNA but not a scramble shRNA was associated with enhanced neural tube cell death measured by TUNEL staining (Fig. 4 C and D) or active caspase-3 staining (Fig. S3). As expected, cell death induction was mainly observed in the dorsal third of the neural tube (Fig. 4D), demonstrating that netrin-1 is a survival factor that blocks DCC proapoptotic activity in chick neural tube.
Fig. 4.
Netrin-1 is a survival factor in the developing chick neural tube. (A) Effect of netrin-1 shRNA on chick netrin-1. HEK293-EBNA cell producing chick netrin-1 were transfected with netrin-1 shRNA and netrin-1 level was assessed by immunoblot. Actin is shown as loading control. (B–D) HH10–11 chick embryos were electroporated at the level of the ventral neural tube with a scramble shRNA plasmid (Scramble), or with a netrin-1 shRNA together with a GFP encoding plasmid. (B) Scheme representing the electroporation procedure. (C) Representative hoechst (Upper), GFP (Middle), and TUNEL (Lower) staining is shown. Arrows indicate the apoptotic cells located in the dorsal neural tube. (Scale bars, 50 μM) (D) Quantification of TUNEL immunostaining in the 1/3 dorsal versus 2/3 ventral neural tube. Five to eight embryos were analyzed for each condition. Error bars, SEM; * indicates a P < 0.05 and ** indicates a P < 0.01 calculated using a two-sided Mann–Whitney test.
Discussion
We show here that beside its well known function as a guidance cue, netrin-1 also acts a survival factor during nervous system development. Interestingly netrin-1 controls survival not like classic neurotrophic factors such as neurotrophins. Neurotrophic factors are usually believed to work by activation of survival signaling pathways within the cell including for example PI3K- or MAPK-dependent pathways (22). These positive signals enhance cell survival and the withdrawal of the neurotrophic factor in vitro or the lack/limitation of this factor in vivo is speculated to block these survival signals and consequently to trigger a death by default. In the case of netrin-1, the survival activity may not be based on activation of downstream survival signaling pathways (e.g., dependent on DCC) but on the inhibition of the proapoptotic activity of the DCC dependence receptor. It is then intriguing to note that NT-3, a classic neurotrophin, has been shown to behave like netrin-1 at least so far in primary culture, because TrkC was recently identified as a dependence receptor and as such directly implicated in the death of dorsal root ganglion neurons observed upon loss of NT-3 (13). Consequently, survival factors could probably be divided into two categories depending on their ability to generate a cell survival dependent state by activating survival pathways or on the opposite by inhibiting proapoptotic pathways.
While there is no doubt that netrin-1 serves as an attractive guidance cue for commissural neurons, we demonstrate here that netrin-1 also acts as a survival factor for these neurons. Such a dual of role for netrin-1 (axon guidance cue and survival factor) may collaborate to adequately attract axons or neurons. Indeed, the survival activity of netrin-1 may then be viewed as a mechanism to avoid wiring errors. It may be tempting to speculate that this survival activity is a mechanism that allows the elimination of commissural neurons that would have extended axons out of the guidance path. Neurons that would extend in regions devoid of netrin-1 would then have unbound DCC and consequently undergo apoptosis. Even though our present study supports a “yes” or “no” situation in which no netrin-1 means “commissural neuron death” and netrin-1 presence means “commissural neuron survival,” it can also be speculated that this regulatory mechanism may be local. Indeed, in regions of local netrin-1 absence, the axon or the growth cone would die in a mechanism that could morphologically resembles to a collapse but would mechanistically imply “local death” that includes DCC dependent caspase activation. Along this line, importance of caspase in axon turning has previously been reported (23) and our preliminary results suggest that addition of the general caspase inhibitor z-VAD-fmk in chick neural tube is associated with defects in commissural axon pathfinding that include aberrant projections (N. Rama, personal communication).
One would argue back that in such hypothesis, DCC mutant mice should not phenocopy commissural defects observed in netrin-1 mutant mice. However, while DCC inactivation should indeed be associated with more survival of commissural neurons, it is also associated with the loss of the “positive” function of DCC that mediates most of the guidance effect of netrin-1; such loss probably primes other accessory functions of netrin-1. Along this line, it is of interest to note that while the caspase cleavage site of DCC is required for DCC proapoptotic activity and is conserved in all mammalian species, it cannot be found in UNC40 of C. Elegans or Frazzled of D. melanogaster. Thus, it is tempting to speculate that the survival activity of such an important and conserved guidance cue among species has been acquired in complex organisms and is consequently a relatively late event in the evolution of these proteins. This may make sense given the greater plasticity of the mammalian and avian nervous systems, compared with those of the relatively simple hard-wired invertebrates.
Materials and Methods
Animals and Genotyping.
Pregnant OFA rat females were purchased from Charles River Laboratory. Netrin-1 and DCC mutant mice were described in ref. 24. Genotyping of DCC embryos was performed by PCR using 5′-GGCCATTGAGGTTCCTTT-3′, 5′-AAGACGACCACACGCGA GDCC-3′ and 5′-TCCTCGTGCTTTACGGTATC-3′ primers. Genotyping of netrin-1 embryos was realized by RT-PCR with 5′-TGACTGTAGGCACAACACGG-3′, 5′-GGCATTACCCAACAGCAAGT-3′ and 5′-GCCTCCCATCTCAACTCT-3′ primers.
Primary Cultures of Spinal Cord Commissural Neurons.
Commissural neurons were obtained from embryonic day 13 (E13) rat embryos or E11.5 mice embryos by dissecting out the dorsal spinal cord. The tissues were then dissociated by using 5 mg/ml trypsin and 0.1 mg/ml DNase I (Sigma) in Hanks' balanced salt solution (HBSS) without calcium or magnesium (Invitrogen). The dissociated cells that were obtained were plated on poly(L-lysine)-precoated coverslips at 0.5 × 105 cells per well on a 24-well plate. Commissural neurons were then cultured for 16 h in neurobasal medium containing B27 supplement. In some experiments, cells were treated with z-VAD-fmk (Biomol International; 20 μM).
Netrin-1 Protein and Plasmid Constructs.
Netrin-1, which is from Apotech (Axora) is a human Flag-tagged netrin-1 produced from CHO cells. For the in ovo chick electroporation, constructs were cloned in pCAGGS, which was also used as empty vector. Empty pCAGGS was a kind gift from R. Sadoul (25). pCAGGS-GFP has been obtained by cloning GFP into the EcoRI site of pCAGGS. pCAGGS-DCC and pCAGGS-DCC-D1290N have been respectively constructed by subcloning EcoRI-BglII fragments from pcDNA3-DCC and pcDNA3-DCC-D1290N into the corresponding sites of pCAGGS. pCAGGS-netrin-1 has been constructed by subcloning an XhoI-EcoRI fragment from pGNET1-myc into pCAGGS. Netrin-1 shRNA was generated using PSilencerTM1.0-U6siRNA Expression vector (Ambion) and the sh sequences sh1: G GCG CTG CCG CTT CAA CAT and sh2 : G GGT GCC CTT CCA GTT CTA. The Scramble sh sequence was: G TGG CCC CGA AGC CTT TAC.
Chick Electroporation.
Fertilized chicken eggs were obtained from a local supplier (Morizeau. EARL, Danger, France) and incubated at 38°C. Neural tube electroporation and cell death assay were perfomed as previously described (11). The various pCAGGS plasmids (1 μg/μl) were introduced into stage HH10–11chick embryos by in ovo electroporation using CUY-21 electroporator and CUY-610 standard electrode (NEPA gene) as previously done (11). We routinely carried out electroporation with 3 pulses of 50-ms duration at 20 V. Green fluorescent protein (GFP) expression vector (0.5 μg/μl pCAGGS-GFP) was coelectroporated to check the electroporation efficiency. Chick embryos were collected 24 h after electroporation. Loss of function of netrin-1 was induced by in ovo shRNA at stage HH10–11 chick embryos. Embryos were injected with a solution containing shRNA plasmid (2.5 μg/μl) mixed with GFP expression vector (0.5 μg/μl). Then embryos were electroporated with specifically designed electrodes. These electrodes allowed us to fit the anode under the embryo and to lay the cathode on the embryo with 3-mm spacing. We carried out electroporation with 4 pulses of 50-ms duration at 20 V. The embryos were collected 24 h after electroporation.
Cell Death Assays.
Apoptosis was monitored by measuring caspase activation either by counting cells stained with anti-active caspase-3 or with TUNEL technique, or by measuring caspase activity in cell lysate as described before (13, 26). Briefly, immunostaining with anti-active caspase-3 was performed on cells fixed with 4% paraformaldehyde in PBS for 30 min, and permeabilized with 0.2% Triton X-100 in PBS for 30 min. The endogenous peroxidase activity was inhibited by preincubation in 5% H2O2 in PBS for 30 min. First antibody incubation was performed by using anti-active caspase-3 antibody (Cell Signaling Technology, Beverly, MA) followed by biotin-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch). The peroxidase activity was then provided by a biotin/avidin/peroxidase complex (ABC kit, Vector Laboratories), and staining was performed using 3,3′-diaminobenzidine chromogen. The percentage of caspase-3-stained cells was calculated by comparing the number of active caspase-3-stained cells with the total number of cells present on a given surface. An index is presented as the ratio between the percentage of stained cells in the treated population and the percentage obtained in the non-treated population. Caspase-3 activity was also measured by using the Caspase-3 Fluorometric Assay Kit from Gentaur. This assay utilizes the DEVD-AFC substrate. The activity was determined according to the manufacturer's instructions, and caspase activation is presented as the ratio between the caspase activity of the spinal cords of mutant embryos and that measured in spinal cords of wild-type mice. Apoptosis was also analyzed by counting cells stained by the TUNEL technique according to the manufacturer's instructions (Roche Diagnostics). Fixation, permeabilization, and staining were performed as for caspase-3 staining except that the antibody reaction was replaced by cell incubation for 1 h at 37°C with terminal deoxynucleotidyltransferase and biotin-dUTP. For Figs. 2, 3, and 4, TUNEL on sections was performed as above except that it was revealed with streptavidine coupled Cy-3 (Jackson ImmunoResearch).
Cell Death Quantification.
For the mouse spinal cord, serial section 20-μm sections were performed and TUNEL-positive cells located in the spinal cord were counted blinded on 200 μm. For the electroporation of DCC and the coelectroporation of DCC+netrin-1, the index corresponds to the number of TUNEL-positive cells on the electroporated side versus the non-electroporated side. For the DCC electroporation experiment, on each slice the spinal cord was divided into three parts: dorsal, median, and ventral via the use of computer analysis (Axovision software, Zeiss). The number of TUNEL-positive cells was counted blinded and an average calculated for each part. For the electroporation of shRNA, chick spinal cord was divided in two parts: 1/3 dorsal and 2/3 ventral using the Axovision software. On each section, the number of TUNEL-positive cells was counted blinded on each part and an average was calculated. In each case, the counting was done in blind by two independent experimenters.
Immunohistolocalisation.
DCC and β-tubulin III immunostaining was realized on mouse commissural neurons fixed and permeabilized as described. Anti-DCC antibody (DCC-A20) (Santa Cruz) and anti-β-tubulin III (Tuj-1) (AbCam) were detected with Alexa488-Donkey anti-goat antibody (Invitrogen) and cyanin 3 donkey anti-mouse antibody (Jackson ImmunoResearch), respectively. The coverslips were mounted in fluoromount (EMS) and sections photographed with a Zeiss Axiovert 200 microscope. To perform DCC immunostainings on mouse spinal cord, sections were fixed and permeabilized as described above for TUNEL assay. Anti-DCC antibody (DCC-A20; Santa Cruz) was detected with Alexa488-Donkey anti-Goat (Invitrogen). Sections were treated with Hoechst (Sigma) and were mounted as described above. DCC and netrin-1 immunostaining on chick spinal cords was performed with anti-DCC antibody (DCC-A20; Santa Cruz) and anti-c-myc antibody (Sigma), respectively, which were detected with biotinylated-Donkey anti-Goat (Jackson ImmunoResearch) and biotinated-Donkey-anti-mouse (Jackson ImmunoResearch), respectively. The peroxidase activity was revealed as described.
Supplementary Material
Acknowledgments.
We thank C.Guix for her excellent technical help, H. Bilak for text correction, and L. Pays for helpful discussion. This work was supported by institutional funds from the Centre National de la Recherche Scientifique and by grants from the Ligue Nationale Contre le Cancer, the National Institute of Health (NIH) and the ANR. CF was supported by a doctoral fellowship from the ARC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803645105/DCSupplemental.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990;247:49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- 3.Mehlen P, Fearon ER Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22:3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Keino-Masu K, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 5.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 6.Fazeli A, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 7.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 8.Rabizadeh S, et al. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 9.Bordeaux MC, et al. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 2000;19:4056–4063. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga E, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- 12.Mehlen P, Thibert C. Dependence receptors: between life and death. Cell Mol Life Sci. 2004;61:1854–1866. doi: 10.1007/s00018-004-3467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauszig-Delamasure S, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci USA. 2007;104:13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YQ, et al. Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene. 1999;18:2747–2754. doi: 10.1038/sj.onc.1202629. [DOI] [PubMed] [Google Scholar]
- 15.Shin SK, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133:1849–1857. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazelin L, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 17.Mehlen P, Llambi F. Role of netrin-1 and netrin-1 dependence receptors in colorectal cancers. Br J Cancer. 2005;93:1–6. doi: 10.1038/sj.bjc.6602656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grady WM. Making the case for DCC and UNC5C as tumor-suppressor genes in the colon. Gastroenterology. 2007;133:2045–2049. doi: 10.1053/j.gastro.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Fitamant J, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20:2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernet A, et al. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 23.Campbell DS, Holt CE. Apoptotic Pathway and MAPKs Differentially Regulate Chemotropic Responses of Retinal Growth Cones. Neuron. 2003;37:939–952. doi: 10.1016/s0896-6273(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 24.Forcet C, et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 25.Mahul-Mellier AL, Hemming FJ, Blot B, Fraboulet S, Sadoul R. Alix, making a link between apoptosis-linked gene-2, the endosomal sorting complexes required for transport, and neuronal death in vivo. J Neurosci. 2006;26:542–549. doi: 10.1523/JNEUROSCI.3069-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furne C, Corset V, Herincs Z, Cahuzac N, Hueber AO, Mehlen P. The dependence receptor DCC requires lipid raft localization for cell death signaling. Proc Natl Acad Sci USA. 2006;103:4128–4133. doi: 10.1073/pnas.0507864103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.