Abstract
Cranial radiation therapy is commonly used in the treatment of childhood cancers. It is associated with cognitive impairments tentatively linked to the hippocampus, a neurogenic region of the brain important in memory function and learning. Hippocampal neurogenesis is positively regulated by voluntary exercise, which is also known to improve hippocampal-dependent cognitive functions. In this work, we irradiated the brains of C57/BL6 mice on postnatal day 9 and evaluated both the acute effects of irradiation and the effects of voluntary running on hippocampal neurogenesis and behavior 3 months after irradiation. Voluntary running significantly restored precursor cell and neurogenesis levels after a clinically relevant, moderate dose of irradiation. We also found that irradiation perturbed the structural integration of immature neurons in the hippocampus and that this was reversed by voluntary exercise. Furthermore, irradiation-induced behavior alterations observed in the open-field test were ameliorated. Together, these results clearly demonstrate the usefulness of physical exercise for functional and structural recovery from radiation-induced injury to the juvenile brain, and they suggest that exercise should be evaluated in rehabilitation therapy of childhood cancer survivors.
Keywords: exercise, juvenile, open field, radiotherapy, stem cell
Radiation therapy is an important treatment for primary or metastatic tumors located close to or within the central nervous system (CNS) in both adults and children. Improved survival rates and longevity of juvenile cancer patients now reveal debilitating late effects of radiation therapy for brain health (1–3), including significant attention deficits and learning problems in later life (4, 5).
The hippocampus is known to be important in memory function (6, 7). In addition, the lifelong birth of new granule neurons in the dentate gyrus (DG) of rodents and humans (8, 9) is assumed to be important for maintaining memory function. Even at low doses, irradiation reduces the number of proliferating cells in the DG of rodents by increased apoptotic cell death, indicating particular vulnerability of progenitor cells to ionizing radiation (10–14). In humans, radiotherapy severely decreases the numbers of immature neurons in the hippocampus (15). Irradiation-induced decreases in neurogenesis have been linked to significant hyperactivity and cognitive impairments (16–19). Voluntary running is known to increase cell proliferation and neurogenesis in the hippocampus of rodents (20–22), with concomitant improvements in cognitive function, spatial memory, and learning (23–25). Exercise also significantly alters the microenvironment of the hippocampus in rodents, with increased growth factor expression (26, 27) and synaptic plasticity (28, 29). In humans, exercise is known to improve cognitive function (30, 31) and to reduce the onset and progression of dementia, as recently reviewed (32). Furthermore, improved cognitive function has been correlated with the effects of exercise on neurogenesis in the hippocampus (33).
We hypothesized that voluntary running in adulthood after irradiation of the young brain would help to reduce the negative effects on neurogenesis and hippocampus-related behavior. We irradiated C57/BL6 mice on postnatal day 9 (P9) with a moderate, clinically relevant dose of 6 gray (Gy). At 9 weeks of age, mice were introduced to a running wheel, and after 4 weeks, behavior was tested in the open-field paradigm. Hippocampal progenitor activity was then analyzed histologically.
Results
Irradiation Dramatically Reduces Precursor Cell Proliferation in the Young Mouse Brain.
Mice (n = 4 per group) were subjected to 6-Gy irradiation or sham-irradiation at P9, and progenitor proliferation was analyzed at 36 h after irradiation [supporting information (SI) Fig. S1A]. A severe loss (85%) of proliferating cells in the DG was observed (Fig. S2A). This decrease in cell proliferation was observed throughout the entire hippocampal formation, confirming the deleterious effect of irradiation at this dose on progenitor proliferation in the developing brain (Fig. S2 B and C) (14, 34).
Voluntary Running Increases the Stem Cell Pool and Neurogenesis in the Dentate Gyrus of Irradiated Mice.
We evaluated the long-term effects of irradiation at P9 on adult neurogenesis. BrdU was injected 8 weeks after irradiation to label adult generated cells. In addition, we introduced running wheels to half of the animals to study compensatory effects that voluntary exercise may have after irradiation (n = 6 per group; Fig. S1B). We recorded running wheel activity and found no difference in the average daily running distance in irradiated mice compared with nonirradiated mice (data not shown).
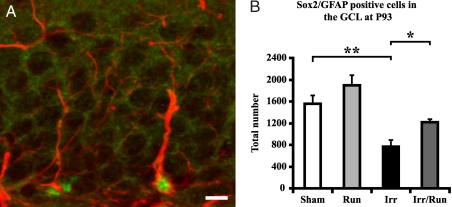
To assess changes in the stem cell pool, colabeling of glial–fibrillary acidic protein (GFAP) and the transcription factor Sox-2 was performed, which is indicative of radial glia-like stem cells (35, 36). A significant decrease in GFAP/Sox-2-positive cells was observed in irradiated animals compared with sham-irradiated controls (irr: 769 ± 120 vs. sham irr: 1,558 ± 150; Fig. 1 A and B; P < 0.01). Interestingly, voluntary running significantly increased the number of stem cells after irradiation (irr/run: 1,216 ± 51.6 vs. irr: 769 ± 120; P < 0.05; Fig. 1B). Similar results were found when Sox-2 was quantified as a single marker (Fig. S3).
Fig. 1.
Voluntary running increases the stem cell pool after irradiation. (A) Confocal microscopic analysis [GFAP-positive cells (red), Sox-2-positive cells (green)] revealed a significant decrease in the number of proliferative precursor cells in the DG of irradiated nonrunning compared with sham-irradiated nonrunning mice. (Scale bar, 10 μm.) (B) After voluntary running, there was a significant increase in the number of double-positive cells compared with irradiated nonrunning mice. Group means ± SEM are shown. *, P < 0.05; **, P < 0.01.
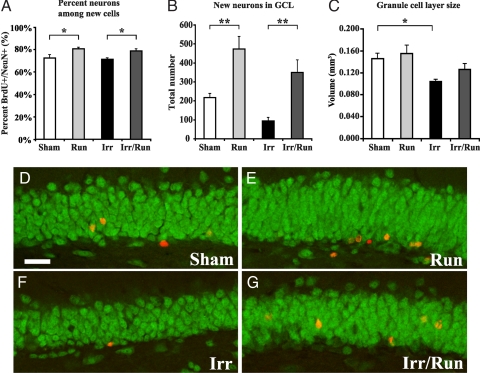
Neurogenesis was assessed by analysis of BrdU in combination with the neuronal marker NeuN. A significant increase in the percentage of new cells that became neurons in the sham-irradiated running mice compared with sham-irradiated nonrunning controls was observed (run: 81 ± 1.2% vs. sham: 72 ± 3.1%; Fig. 2A; P < 0.05). Although irradiation alone had no effect on the percentage of cells that became neurons (Fig. 2A), voluntary running after irradiation was able to increase the percentage of neurons among the newborn cells (irr/run: 79 ± 1.7% vs. irr: 71 ± 1.2%; Fig. 2A; P < 0.05). Using these percentages and the total number of stereologically counted BrdU-labeled cells (Fig. S4 A–D), we determined the level of neurogenesis in these mice. The total number of new neurons (BrdU+/NeuN+) significantly increased (117%) in sham-irradiated running compared with sham-irradiated nonrunning mice (Fig. 2 B and D–G; P < 0.01). In irradiated running mice, there was a significant increase (275%) in the number of newborn cells becoming neurons compared with irradiated nonrunning mice (Fig. 2 B and D–G; P < 0.01). At P93, we found a decrease in granule cell layer (GCL) volume in irradiated nonrunning mice compared with sham-irradiated nonrunning mice, demonstrating the long-lasting effects of irradiation on GCL volume (Fig. 2C; P < 0.05). However, there was no significant difference in GCL volume in the irradiated running mice compared with all other groups.
Fig. 2.
Voluntary running increases neurogenesis after irradiation. (A and D–G) Confocal microscopic analysis [BrdU-positive cells (red), NeuN-positive cells (green)] revealed a percentage increase in the number of newborn neurons in running and irradiated running mice compared with sham-irradiated nonrunning and irradiated nonrunning mice. (Scale bar, 20 μm.) (B) The total number of newly generated neurons in the GCL increased after voluntary running compared with control and irradiated animals. (C) Measurement of the GCL showed a significant decrease in volume in the irradiated animals compared with sham-irradiated nonrunning and sham-irradiated running animals. Group means ± SEM are shown. *, P < 0.05; **, P < 0.01.
Voluntary Running Rescues Cranial Irradiation-Induced Alterations in Doublecortin (DCX) Cell Formation and Orientation Within the DG.
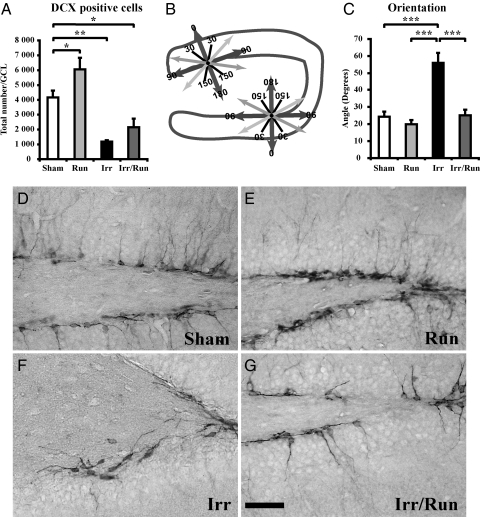
DCX is expressed in neuronal precursors and immature neurons (37–39). We found an increase in the number of DCX-positive cells in the sham-irradiated running animals compared with sham-irradiated nonrunning mice, confirming the strong effect that running has on hippocampal neurogenesis (Fig. 3A). As expected, irradiation decreased the number of DCX-positive cells in the irradiated nonrunning mice compared with sham-irradiated nonrunning mice (Fig. 3A; P < 0.01). These results highlight the detrimental effects of irradiation on immature cells committed toward a neuronal lineage. However, the apparent increase in DCX numbers in irradiated mice after running was not statistically significant (Fig. 3A). Double labeling of DCX with the proliferation marker phosphohistone H3 revealed very few double-positive cells (maximum of eight cells) in the DG (data not shown).
Fig. 3.
Quantification of neurogenesis by the detection of DCX-expressing cells in the DG. (A) There was an increase in the number of DCX-expressing cells after voluntary running and a significant decrease seen after irradiation, with a significant decrease in irradiated running compared with sham-irradiated nonrunning mice. (B) A mapping scheme was used to measure the orientation of 60 DCX-positive cells per animal in the SGZ (perfect process defined as 0°). (C) Analysis of DCX orientation revealed a significantly different angle in irradiated nonrunning animals compared with sham-irradiated nonrunning mice. This altered angle was reversed after voluntary running in irradiated animals. Group means ± SEM are shown. (Scale bar, 100 μm.) *, P < 0.05; **, P < 0.01; ***, P < 0.00.1 (D–G) Representative micrographs show the altered angle of the leading process on DCX-positive cells leading process after irradiation, which was reversed by voluntary running.
To discern whether irradiation and/or running altered the integration of immature cells into the DG, we analyzed the orientation of the leading apical process in DCX-positive cells, which later develops into the single dendritic tree of the mature granule cell (Fig. 3D). We used a compass system (see SI Methods) to determine the angle of the leading process in relation to the cells position along the subgranular zone (SGZ) border (Fig. 3B). We found that DCX-positive cells in sham-irradiated mice had an average leading process angle of 24 ± 3°, but after irradiation, the angle was significantly altered to 56 ± 5° (Fig. 3 C–G; P < 0.001). Interestingly, we found that the orientation of the lead process in the irradiated running mice was reversed to the sham-irradiated angle (25 ± 3°; Fig. 3 C–G).
Voluntary Running Ameliorates Irradiation-Induced Behavioral Changes.
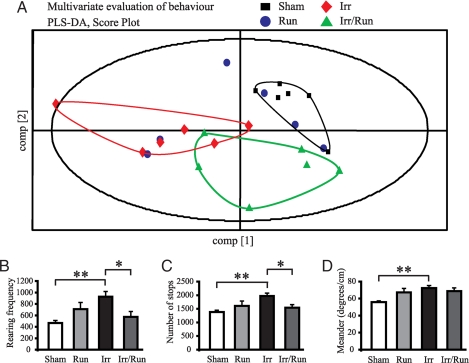
We used the open-field test, designed to measure alterations in a wide range of behavioral features, including locomotor behavior, spatial variability, and exploratory behaviors. We found that mice subjected to irradiation were clearly separated from sham-irradiated mice, indicating that these two groups of mice have different behavior patterns (Fig. 4A; each point represents an individual mouse). Irradiated mice subjected to exercise altered their behavioral pattern toward the direction of sham-irradiated mice, indicating that running alleviates irradiation-induced behavioral alterations. For example, irradiated mice revealed increased exploratory rearing frequency, increased numbers of stops, and increased meandering after irradiation (Fig. 4 B–D; P < 0.01). Furthermore, these irradiation-induced behavioral alterations, except meandering, were reversed by voluntary running (Fig. 4 B–C; P < 0.05).
Fig. 4.
Evaluation of behavior patterns in the open-field test. (A) Multivariate evaluation by partial least-square discriminate analysis. The animals in each group (n = 6) were tested in the open field for 3 consecutive days. The analysis was based on 108 behavioral variables and 4 dummy variables representing the treatment. The score plot for component 1 and 2 shows that the irradiated mice have a behavioral pattern that is clearly separated from sham-irradiated nonrunning animals. In relation to component 1, the irradiated mice with free access to the running wheels appear to have a behavioral pattern approaching that of sham-irradiated nonrunning mice (each point represents an animal). (B) After irradiation, there was an increase in exploratory behavior, represented by an increase in rearing frequency. (C) There was also an increase in the number of stops. These behavioral alterations were normalized by voluntary running after irradiation. (D) Irradiation also increased the movement path (meandering, degrees per cm) compared with sham-irradiated nonrunning animals. *, P < 0.05; **, P < 0.01.
Discussion
The present work demonstrates that voluntary physical exercise after cranial irradiation in the mouse enhances hippocampal neurogenesis. We were able to show normalized morphology of DCX-positive cells after exercise in irradiated animals. In addition, voluntary running improved long-term behavior after irradiation, highlighting the beneficial effects of running on both structure and function in the hippocampus after irradiation.
Prenatal stress decreases hippocampal neurogenesis and cognition into adulthood (40), indicating the importance of developmentally appropriate levels of neurogenesis for hippocampal function. Stimulation during the postnatal period, at least partly counteracts these deleterious effects. Moreover, exercise during pregnancy can up-regulate postnatal neurogenesis in the offspring at P9 (41). Voluntary exercise is also able to compensate for decreased levels of proliferation induced by prenatal ethanol exposure (42). In addition, an enriched environment is effective in boosting neuronal numbers and enhancing cognitive function after status epilepticus in P20 mice (43). Taken together, these studies demonstrate that early postnatal manipulations can have profound effects on proliferation, neurogenesis, cell survival, and cognition. We now demonstrate that the deleterious effects seen after irradiation of the young brain can be compensated by voluntary exercise later in life.
When we used the linear quadratic model (44) and an α/β ratio of 3 for late effects in normal brain tissue, the acute exposure of 6 Gy used in this work is equivalent to 12 Gy when delivered in 2-Gy fractions. Even higher doses are used to treat malignant brain tumors, up to 55 Gy to the actual tumor location, with various doses deposited in surrounding tissue. Children suffering from leukemia relapses are treated with 18 Gy whole-brain fractionated irradiation to prevent CNS involvement; we therefore believe the dose used is moderate and clinically relevant. A recent study that used a single dose of 4 Gy, with no treatment after irradiation, showed reversible effects of irradiation on neurogenesis in the DG in the adult brain (45). Although we did not observe a similar level of spontaneous recovery when we used 6 Gy, it is important to note that not all proliferating cells in our animals were ablated immediately after or up to 3 months after irradiation. However, we found a population of proliferative GFAP/Sox-2 positive, radial glia-like stem cells that were drastically reduced even 3 months after irradiation. In our irradiated animals, 4 weeks of voluntary running was sufficient to rescue this population of stem cells and to restore neurogenesis levels. These data suggest that voluntary running acted to stimulate the expansion of a residual endogenous stem cell population in the hippocampus after 6-Gy irradiation. A recent study suggests that social isolation (individually housed rats) delays the positive effects of running on progenitor proliferation (an increase was seen only after 48 days) (46). However, contrary to these findings, we observed a significant increase in precursor cell and mature neuronal numbers in individually housed mice with access to running wheels (for 28 days). These results indicate that social isolation of running animals in our mouse model is not necessarily detrimental to enhancing stem cell numbers and neuronal outcome.
Hippocampal neurogenesis can be regulated at multiple points, by regulating the number of cells generated and also by regulating the integration of DCX+ cells into the neuronal network (47). However, we found few DCX/phosphohistone H3 double-positive cells in all animals, suggesting that we see no expansion of the subpopulation of DCX-positive cells, as discussed elsewhere (48, 49). To assess further the effects of irradiation on immature neurons, we measured the orientation of the leading process from the DCX-positive cell body as an indicator of structural integration. We found a significantly altered angle after irradiation that was normalized after voluntary running. Structural integration is necessary for the continued presence and proper function of newly formed neurons in the hippocampus. Consequently, immature neurons are highly responsive and contribute quickly to the function of the DG (50–52), but they are also susceptible to developmental death (53, 54). After irradiation, the leading process from the majority of the DCX-positive cells did not extend correctly from the GCL into the molecular layer. This likely affects network integration and possibly contributes to altered behavior. If so, restoring functional integration of immature neurons in the hippocampus after irradiation by voluntary running may be an important mechanism whereby behavioral improvement was achieved.
We found behavioral changes in irradiated mice that are to some extent indicative of hippocampal damage (55, 56). Although in some instances, cranial irradiation causes limited impairment of some hippocampal-dependent learning and memory tasks (11, 16, 18, 57), cranial irradiation can also lead to behavioral changes similar to those seen in attention deficit—hyperactivity disorder (ADHD) (58, 59). Furthermore, cranial irradiation causes learning disabilities in children, suggested to be caused by poor attention and deficient working memory rather than a low intellectual level (60). The increased activity observed after irradiation was ameliorated by voluntary running, and these mice approached a behavioral pattern similar to the nonirradiated animals. Thus, physical exercise induced both qualitative and quantitative changes in hippocampal structure and function and concomitant behavioral improvement of ADHD-like symptoms.
In the P9 mouse, the hippocampus is still undergoing development, corresponding in humans to <3 years of age. It may be assumed that the young developing brain, with a higher level of neurogenesis, is more plastic and could more easily compensate for morphological damage. Experimental and clinical evidence, however, points in the opposite direction (58). Cognitive and endocrine late effects after radiation therapy are much more severe in young children, so this treatment modality would not be considered for brain tumor patients younger than 4 years of age, except for palliative purposes. Negative consequences on cognitive function will get worse with time, such that the differences between the treated children and their peers will grow bigger year by year (61). In this context, our results are encouraging, indicating that irradiation-induced damage even in the young brain can to some degree be compensated for through later therapeutic intervention, such as physical exercise.
In conclusion, this work demonstrates that voluntary exercise restores the radiation-injured hippocampal stem cell pool, increases neurogenesis, and alters immature neuronal cell orientation after a moderate dose of irradiation. From a clinical perspective, our work indicates that the functional deficits observed in pediatric patients after radiation therapy are not irreversible and may be amenable to treatment; therefore, exercise should be evaluated in rehabilitation therapy of children after radiotherapy to the brain. We believe it is important that a small number of stem cells survive the radiation treatment to participate in the restructuring process. Selective shielding of the hippocampus or careful fractionation of the irradiation dose could contribute to improving the long-term prognosis and efficacy of rehabilitation therapies, including physical exercise.
Methods
For a full description of all materials and methods, see SI Methods.
Animals.
Adult, male C57/BL6 mice pups (Charles River Breeding Laboratories) were used for all experiments. Animals were housed at a constant temperature (24°C) with a relative humidity of 50–60%. A 12-h dark/light cycle was maintained with lights on from 19:00 to 7:00 with food and water available ad libitum. All procedures described were conducted in accordance with and after approval from the Swedish Animal Welfare Agency (Gothenburg animal ethics application no. 407-2004).
Irradiation Procedure.
Mouse pups aged 9 days (P9) were anesthetized and sham-treated or exposed to bilateral cranial irradiation by using a linear accelerator (Varian Clinac 600 CD; Radiation Oncology Systems) as described in ref. 34. A total dose of 6 Gy was given to each mouse. After irradiation, the pups were returned to their biological dams.
Statistical Analysis.
Values are expressed as mean ± SEM. Data were analyzed by using a relevant ANOVA followed by post hoc comparisons with a Newman–Keuls test to determine differences between groups. Open-field data were evaluated as described in ref. 62. In addition, group comparisons for individual variables were analyzed with a nonparametric one-way ANOVA (Kruskal–Wallis) followed by Mann–Whitney U test for significance.
Supplementary Material
Acknowledgments.
We thank the Center for Mouse Physiology and Bio-Imaging for use of equipment; Rita Grandèr, Ann-Marie Alborn, and Birgit Linder for valuable technical assistance; and Christi Kuhn for important input and discussion. This work was supported by the Swedish Research Council, the Swedish Childhood Cancer Foundation, the King Gustav V Jubilee Clinic Research Foundation (JK-fonden), The Frimurare Barnhus Foundation, Swedish governmental grants to scientists working in health care, the Wilhelm and Martina Lundgren Foundation, Edit Jacobssons Fund, the Söderberg Foundation, Adlerbertska Forskningsstiftelsen, MS-förenings Forsknings-och Byggnadsfonder, Stiflesen Goljesminne, the Swedish Brain Foundation, and the Göteborg Medical Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711128105/DCSupplemental.
References
- 1.Lannering B, Marky I, Lundberg A, Olsson E. Long-term sequelae after pediatric brain tumors: Their effect on disability and quality of life. Med Pediatr Oncol. 1990;18:304–310. doi: 10.1002/mpo.2950180410. [DOI] [PubMed] [Google Scholar]
- 2.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 3.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 4.Correa DD, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 5.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 6.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;2:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 11.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 12.Mizumatsu S, et al. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 13.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda H, et al. Irradiation-induced progenitor cell death in the developing brain is resistant to erythropoietin treatment and caspase inhibition. Cell Death Differ. 2004;11:1166–1178. doi: 10.1038/sj.cdd.4401472. [DOI] [PubMed] [Google Scholar]
- 15.Monje ML, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 16.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 17.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 18.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Altman J. Morphological and behavioral markers of environmentally induced retardation of brain development: An animal model. Environ Health Perspect. 1987;74:153–168. doi: 10.1289/ehp.8774153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 21.Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005;93:2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- 22.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 23.Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BJ, et al. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- 25.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 26.Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 27.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 28.Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 30.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 31.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 32.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 33.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda A, et al. Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem. 2005;92:569–584. doi: 10.1111/j.1471-4159.2004.02894.x. [DOI] [PubMed] [Google Scholar]
- 35.Steiner B, et al. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- 36.Steiner B, et al. Type 2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 37.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 38.Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson JG, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 40.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bick-Sander A, Steiner B, Wolf SA, Babu H, Kempermann G. Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc Natl Acad Sci USA. 2006;103:3852–3857. doi: 10.1073/pnas.0502644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redila VA, et al. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- 43.Faverjon S, et al. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology. 2002;59:1356–1364. doi: 10.1212/01.wnl.0000033588.59005.55. [DOI] [PubMed] [Google Scholar]
- 44.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 45.Ben Abdallah NM, Slomianka L, Lipp HP. Reversible effect of x-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus. 2007;17:1230–1240. doi: 10.1002/hipo.20358. [DOI] [PubMed] [Google Scholar]
- 46.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plumpe T, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77–91. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- 49.Walker TL, Yasuda T, Adams DJ, Bartlett PF. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 2007;27:3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doetsch F, Hen R. Young and excitable: The function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Deisseroth K, et al. Excitation–neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 52.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 54.Kuhn HG, et al. Increased generation of granule cells in adult Bcl-2-overexpressing mice: A role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 55.Mickley GA, Ferguson JL, Mulvihill MA, Nemeth TJ. Progressive behavioral changes during the maturation of rats with early radiation-induced hypoplasia of fascia dentata granule cells. Neurotoxicol Teratol. 1989;11:385–393. doi: 10.1016/0892-0362(89)90012-3. [DOI] [PubMed] [Google Scholar]
- 56.Mickley GA, Ferguson JL, Nemeth TJ, Mulvihill MA, Alderks CE. Spontaneous perseverative turning in rats with radiation-induced hippocampal damage. Behav Neurosci. 1989;103:722–730. doi: 10.1037//0735-7044.103.4.722. [DOI] [PubMed] [Google Scholar]
- 57.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 58.Highfield DA, Hu D, Amsel A. Alleviation of x-irradiation-based deficit in memory-based learning by d-amphetamine: Suggestions for attention deficit-hyperactivity disorder. Proc Natl Acad Sci USA. 1998;95:5785–5788. doi: 10.1073/pnas.95.10.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mickley GA, Ferguson JL, Nemeth TJ. Serial injections of MK 801 (dizocilpine) in neonatal rats reduce behavioral deficits associated with x-ray-induced hippocampal granule cell hypoplasia. Pharmacol Biochem Behav. 1992;43:785–793. doi: 10.1016/0091-3057(92)90409-9. [DOI] [PubMed] [Google Scholar]
- 60.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 61.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 62.Nilsson M, Markinhuhta KR, Carlsson ML. Differential effects of classical neuroleptics and a newer generation antipsychotics on the MK-801-induced behavioural primitivization in mouse. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:521–530. doi: 10.1016/j.pnpbp.2005.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.