Abstract
Single-nucleotide polymorphisms (SNPs) in the human ether-a-go-go-related gene 1, hERG1, are associated with cardiac arrhythmias. The Kv11.1 channels encoded by hERG1 are also essential for rhythmic excitability of the pituitary, where they are regulated by thyroid hormone through a signal transduction cascade involving the phosphatidylinositol 3-kinase (PI3K) and the Ser/Thr-directed protein phosphatase, PP5. Here, we show that the hERG1 polymorphism at codon 897, which is read as a Thr instead of a Lys, creates a phosphorylation site for the Akt protein kinase on the Kv11.1 channel protein. Consequently, hormonal signaling through the PI3K signaling cascade, which normally stimulates K897 channels through PP5-mediated dephosphorylation, inhibits T897 channels through Akt-mediated phosphorylation. Thus, hormonal regulation of Kv11.1 in humans with the T897 polymorphism is predicted to prolong the QT interval of cardiac myocytes. A systematic bioinformatics search for SNPs in human ion channel genes identified 15 additional candidates for such “phosphorylopathies,” which are predicted to create or destroy putative phosphorylation sites. Changes in protein phosphorylation might represent a general mechanism for the interaction of genetic variation and environment on human health.
Keywords: Kv11.1, LQT, Akt protein kinase, phosphatidylinositol 3-kinase, thyroid hormone
Human genetic variation alters individual susceptibility to disease, but the underlying mechanisms are still being elucidated. One class of human diseases, which is called channelopathies, has been shown to result from mutations that disrupt the assembly and function of ion channel proteins (1). For example, the human ether-a-go-go-related gene, hERG1 (KCNH2) (2), encodes Kv11.1 potassium channels that are essential for rhythmic excitability of cardiac muscle (3) and endocrine cells (4). Unlike other voltage-gated potassium channels, Kv11 channels have unique voltage-dependent kinetics, which make them more active at the end of the action potential (3). Thus, they control action potential duration in ventricular myocytes, which is measured clinically as the QT interval in the electrocardiogram. Long QT intervals (LQT) are associated with torsades de pointes and ventricular tachycardia, which increase the risk of fatal cardiac arrhythmias (5). SNPs in the hERG1 gene that reduce Kv11.1 activity are responsible for LQT2 in humans (6, 7). K897T is one of the most common polymorphisms in the human Kv11.1 channel protein, but its effects on channel function and QT interval are controversial. Two studies report longer QT intervals in women (8, 9), but other studies find no effect or a small decrease in QT interval (7). Similarly, biophysical studies of heterologously expressed hERG1 in dialyzed human embryonic fibroblasts have reported a wide range of modest effects on the voltage dependence of gating, but whether K897T impairs channel function by disrupting ion conduction or trafficking to the membrane is uncertain (9–12).
Recently, we discovered that a mutation in the CaV1.2 calcium channel, which had been associated with Timothy disorder (13), alters channel regulation by creating a new phosphorylation site on the channel (14). Therefore, we conducted a systematic bioinformatics search for common SNPs in human ion channel genes and identified 16 examples that are predicted to create or destroy phosphorylation sites in the channel proteins (Table 1). Here, we test one of those predictions, the hERG1 gene polymorphism that produces the T897 isoform of Kv11.1. We have shown (15, 16) that the activity of Kv11.1 channels is stimulated by thyroid hormone through a signaling cascade involving the Ser/Thr-directed protein phosphatase, PP5, which is activated by the Rac GTPase downstream of the phosphatidylinositol 3 kinase (PI3K). Now we show that the T897 polymorphism creates a phosphorylation site on the Kv11.1 channel protein for the Akt protein kinase, the cellular homologue of the Akt8 retrovirus transforming oncogene, which is also known as protein kinase B (17). Phosphorylation of Kv11.1 by Akt reverses the effect of hormonal regulation through the PI3K signaling cascade and inhibits Kv11.1 channel activity, which should increase cardiac action potential duration. The hormonal and phosphorylation dependence of this effect on the activity of T897 channels could provide an explanation for both the failure of some epidemiological studies to detect longer QT intervals in resting and/or fasting subjects with the T897 polymorphism, and the failure to observe this effect in previous biophysical studies that used conventional whole-cell recording through ruptured membrane patches on dialyzed cells.
Table 1.
Predicted phosphorylopathies in ion channel proteins
| Protein | Polymorph | Pphos, % | Kinase | Disease |
|---|---|---|---|---|
| Cav1.2 | S1545P | −60 | CAMKII | Not reported |
| Kv11.1 | K897T | +94 | AKT | LQT2 |
| Kv11.1 | R176W | −94 | PKA | LQT2 |
| Kv11.1 | T474I | −90 | PKA | LQT2 |
| Kv7.1 | G179S | +73 | GSK3 | LQT1 |
| Kv7.1 | Y184S | +80 | CAMKII | LQT1 |
| Kv7.1 | S566F | −76 | CAMKII | LQT1 |
| Kv7.1 | W392R | +75 | CAMKII | LQT1 |
| Kv7.1 | A525T | +67 | PKA | LQT1 |
| Kv7.1 | R583C | −91 | CAMKII | LQT1 |
| Kv7.2 | N780T | +99 | CAMKII | Not reported |
| Kir1.1 | S219R | −79 | PKA | Bartter Syndrome |
| Kir1.4 | T175I | −90 | CAMKII | Bartter Syndrome |
| Kir2.2 | S15L | −99 | GSK3 | Not reported |
| TRPC6 | P15S | +96 | CK1 | Glomerulosis |
| TRPC4 | P19S | +94 | CK2 | Not reported |
The polydom database (polydoms.cchmc.org) (36) was searched for nonsynonomous SNPs in human ion channel genes. In 16 cases from nine genes, the polymorphism is predicted by Netphos (www.cbs.dtu.dk/services/NetPhos) (37) to create or destroy kinase phosphorylation sites in domains that Uniprot (www.uniprot.org) predicts to be cytoplasmic. In many cases, the SNP is already known to be associated with the disease indicated.
Results
We studied recombinant human Kv11.1 channels under voltage clamp in metabolically intact Chinese hamster ovary (CHO) cells that had been transfected with plasmids encoding hERG1, TRbeta, and green fluorescent protein (GFP), as described in Methods. When cell-attached patches of membrane in symmetrical high potassium solutions are held at 0 mV, which corresponds to the plateau of the ventricular action potential, repolarization elicits robust currents, which deactivate with a time constant of ≈30 ms at −120 mV (Fig. 1A). Bath application of 100 nM thyroid hormone, 3–5-3′-triodothyronine (T3), rapidly increases currents produced by the K897 isoform of Kv11.1 (Fig. 1B), as we reported (16, 18). In every recording, successful isolation of Kv11.1 activity was confirmed at the end of the experiment with 5 μM E-4031, the class III antiarrhythmic methanesulfonanilide, which selectively blocks Kv11 channels (19).
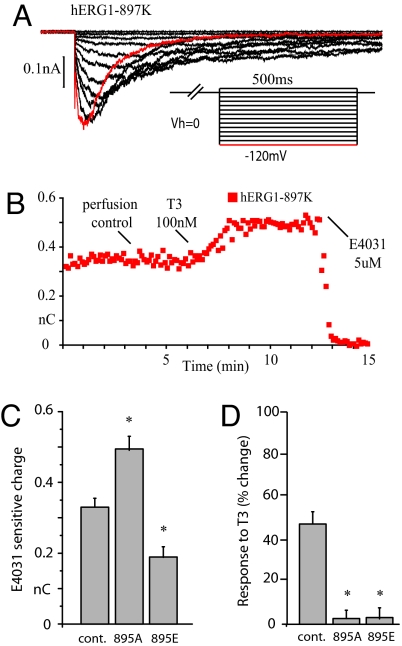
Fig. 1.
Thyroid hormone stimulates hERG1–897K channels through dephosphorylation of T895. (A) hERG1 currents were elicited from cell-attached patches on CHO cells that were held at 0 mV in equimolar potassium by transiently repolarizing the patch to negative voltages for 500 ms at 5 s intervals, as shown in Inset. (B) The time course of the response to 100 nM thyroid hormone (T3) plotted as the total current integrated over the first 100 ms after the peak at −120 mV. All of the current is blocked by 5 μM E-4031. (C) Mutating Thr-895 to Ala to prevent dephosphorylation or to Glu to mimic phosphorylation alters basal current amplitude at −120 mV (D) T3 has no effect on current amplitude when T895 is mutated.
Although we had postulated that thyroid hormone stimulates Kv11.1 channels through protein dephosphorylation (15, 18), we had not identified the phosphorylation site on the channel protein. In rat pituitary cells, thyroid hormone antagonizes the action of thyrotropin releasing hormone (TRH), which inhibits the activity of ERG channels through a G13 and Rho-dependent GTPase cascade (18). The Rho GTPase signals primarily through protein Ser/Thr kinases (20), and Netphos identifies a recognition site for the Rho-stimulated protein kinase, PKN (21), at T895, which is two residues before the polymorphism at K897. When we mutated Thr 895 to Ala, which cannot be phosphorylated, the basal currents through A895 channels are 20% larger on average than the currents through T895 channels (Fig. 1C), mimicking the effect of thyroid hormone action. Conversely mutation of T895 to glutamic acid, to mimic irreversible phosphorylation of the protein at that site, reduced basal currents by 28% (Fig. 1C). Although the mutations of T895 produced opposite effects on basal currents, both mutations made the channels resistant to stimulation by thyroid hormone (Fig. 1D). These data are consistent with the hypothesis that the K897 isoform of Kv11.1 is a phosphoprotein with a consensus site for PKN at T895, which is dephosphorylated by thyroid hormone signaling through PP5 downstream of PI3K (15).
The consensus site for PKN (RxTxK) requires a basic amino acid at the +2 position after the phosphorylated residue. Thus, the K897T polymorphism is predicted to disrupt hormonal regulation of the channel. Although basal currents in metabolically intact CHO cells expressing the T897 variant (Fig. 2A) have very similar amplitudes, kinetics and voltage dependence when compared with those of the more common K897 variant (Fig. 1A), thyroid hormone had the opposite effect on the amplitude of the T897 variant. Thus, in contrast to the K897 variant, which is stimulated by thyroid hormone (Fig. 1B), the T897 variant is inhibited by thyroid hormone under identical conditions (Fig. 2B). This effect is not mediated by a separate biochemical pathway because it is blocked by 3 μM 1–850 (Fig. 2B), an antagonist of thyroid hormone receptors, which also blocks stimulation of the K897 variant (16).
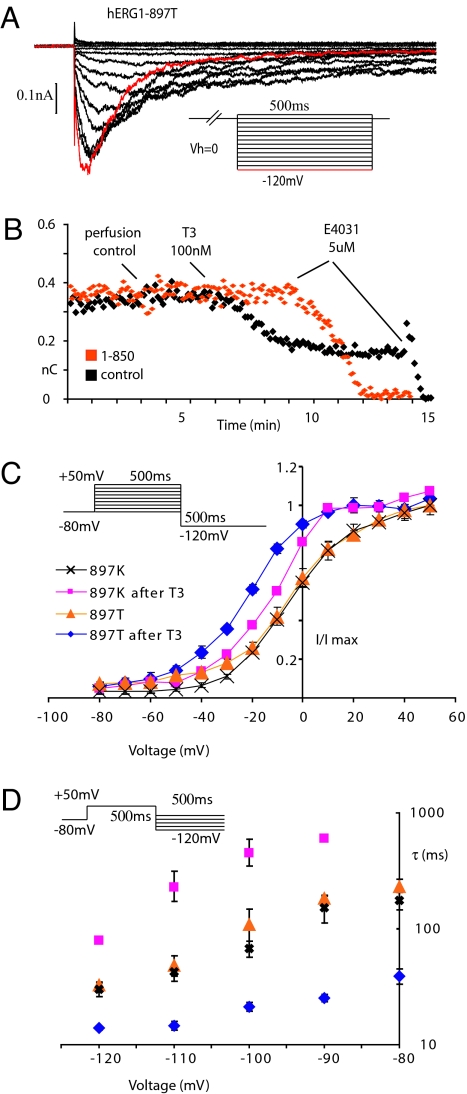
Fig. 2.
The hERG1 polymorphism, K897T, alters channel regulation by thyroid hormone. (A) hERG1 currents were elicited from cell-attached patches on CHO cells that were held at 0 mV in equimolar potassium by transiently repolarizing the patch to negative voltages for 500 ms at 5 s intervals, as shown in the Inset. (B) The time course of the response to 100 nM thyroid hormone (T3) plotted as the total current integrated over the first 100 ms after the peak. Black squares are control responses; orange squares are recorded in the presence of 3 μM 1–850, an antagonist of thyroid hormone receptors. In both cases all of the current is blocked by 5 μM E-4031. (C) Steady-state activation curve for hERG1–897K (n = 6) and hERG1–897T (n = 6) before and 5 min after 100 nM T3. I/Imax at −120 mV is plotted vs. the preceding depolarization. Points are means ± SEM although in many cases the symbols are larger than the error bars. (D) Voltage dependence of deactivation of hERG1–897T (n = 5) and hERG1–897K (n = 5) before and after 100 nM T3. The time constant of deactivation during the test pulse is plotted on a log scale vs. the amplitude of the test pulse. Points are means ± SEM although in many cases the symbols are larger than the error bars.
The effect of thyroid hormone on the kinetics of the two variants is analyzed more quantitatively in Fig. 2 C and D. In the absence of thyroid hormone, the voltage dependence of steady-state activation (Fig. 2C) and the kinetics of deactivation (Fig. 2D) are almost identical between the two variants, as predicted from the traces in Figs. 1A and 2A. Although thyroid hormone shifts the steady-state activation curve of both variants to more negative voltages (Fig. 2C), the hormone has opposite effects on the rate of deactivation (Fig. 2D). Thyroid hormone decreases the rate of deactivation of the K897 variant, which we demonstrated earlier with single channel recordings at −60 mV (16), by increasing the time constant at −120 mV from 30 ± 4.0 ms to 79 ± 4.5 ms (n = 5). In contrast, the hormone decreases the time constant of the T897 variant at −120 mV from 32 ± 2.6 ms to 14 ± 4.8 ms (n = 5). The net result is that the T897 variant deactivates approximately five times more rapidly than the K897 variant, which would result in smaller currents during repolarizing steps to negative voltages.
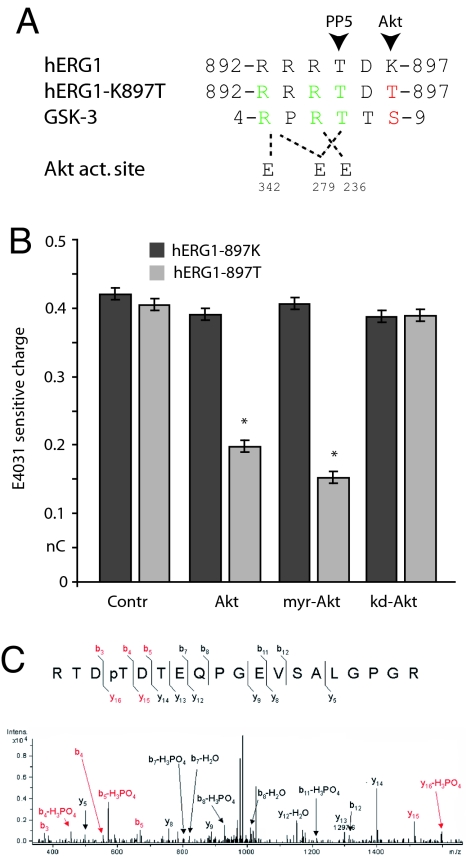
The Netphos database also predicts that when the Lys residue at 897 is changed to Thr by the hERG1 SNP (Fig. 3A), the surrounding sequence becomes a canonical phosphorylation site (RxRTxT/S) for Akt (22). Because Rho-dependent inhibition of Kv11.1 channel activity requires T895, we wondered whether phosphorylation by Akt at T897 could have the same effect. When we expressed human Akt constructs in metabolically intact CHO cells with Kv11.1 channel proteins, we observed a selective reduction of 49 ± 3% in the amplitude of hERG1–897T currents (n = 3, Fig. 3B). In contrast, Akt had no effect on the amplitude of the original hERG1–897K currents, and a catalytically inactive form of Akt (K179A) had no effect on basal currents from either of the channel constructs (Fig. 3B). Myristoylated Akt produced a larger reduction (62 ± 5%, n = 3) in basal hERG1–897T currents (Fig. 3B), presumably because myristoylation targeted more of the Akt to the plasma membrane. Thus, the activity of the T897 channels, but not of the more common K897 channels, appears to be inhibited by basal Akt activity in metabolically intact cells.
Fig. 3.
Thyroid hormone inhibits hERG1–897T through Akt. (A) Sequence alignment of the predicted Akt consensus site in hERG1–897K, hERG1–897T, and the glycogen synthase kinase, GSK-3. The Glu residues in the Akt active site, which coordinate substrate binding are indicated below. (B) Amplitude histograms of the basal hERG currents in cells expressing either hERG1–897K or hERG1–897T and the indicated human Akt construct: control (n = 6), wild type (Akt, n = 3), myristoylated (myr-Akt, n = 3), or kinase dead (kd-Akt, n = 3). (C) MS analysis of T897 phosphorylation. ESI-MS/MS spectrum of the doubly charged m/z 1033.0 ion. The m/z 1033.0 precursor ion corresponds in mass to residues 894–912 with the addition of a single phosphorylation. The extensive b- and y-series ions localize the site of phosphorylation to T897.
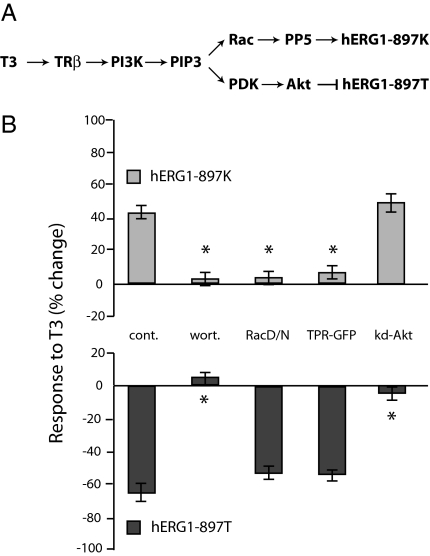
In principle, this effect of Akt on current amplitude could arise from a chronic effect of PI3K-dependent signaling on channel trafficking, as reported for other ion channels (23, 24), or it could result from acute effects of phosphorylation on channel activity. Therefore, we investigated how the K897T polymorphism altered the acute response of the channels to hormonal regulation (Fig. 4). First, we confirmed that the hERG1 channel protein is phosphorylated on T897 by mass spectrometry (Fig. 3C), as described in Methods. Then we tested whether inhibition of T897 channels by thyroid hormone, like the stimulation of the K897 channels (18), is blocked by inhibition of PI3K, which is upstream of both Akt and Rac (Fig. 4A). Preincubation of the cells for 10 min at 37°C with 50 nM wortmannin, an active site blocker of PI3K (25), completely eliminates the effects of thyroid hormone on both Kv11.1 variants (Fig. 4B). In contrast, inhibition of Rac GTPase signaling with the dominant negative mutant Rac 17N, or inhibition of PP5 with the dominant negative TPR domain (15), both selectively prevent stimulation of K897 channels (Fig. 4B). In contrast, neither construct prevented the inhibition of the T897 channels by thyroid hormone. Conversely, the catalytically inactive form of Akt (K179A), which has no effect on basal currents of either isoform (Fig. 4B), selectively prevents inhibition of T897 channels by T3 without reducing stimulation of K897 channels (Fig. 4B).
Fig. 4.
Thyroid hormone regulates different isoforms through different signaling branches downstream of PI3K. (A) Summary of signaling cascades downstream of phosphatidylinositol 3,4,5 Tris phosphate (PIP3). (B) Percentage change in hERG1 current in response 100 nM thyroid hormone (T3) for 3 min in control cells (cont, n = 6), cells pretreated with 50 nM wortmannin (wort, n = 5), or cells expressing dominant negative isoforms of the Rac GTPase (RacDN, n = 4), the PP5 protein phosphatase (TPR-GFP, n = 3) or a catalytically inactive form of Akt (K179A).
Discussion
The data presented here demonstrate that the human Kv11.1 channel encoded by the hERG1 gene is a phosphoprotein that is regulated by hormonal signaling through PI3K. Signaling through PI3K stimulates the most common K897 isoform of Kv11.1 through dephosphorylation of T895. However, substitution of a threonine for the lysine at 897 reverses the effect of PI3K signaling on channel activity by disrupting the putative PKN site at T895 and by creating a canonical Akt phosphorylation site on the channel protein at T897. When the T897 channels are phosphorylated by Akt, their activity is inhibited. Many hormones signal through PI3K in the heart (26), and both thyroid hormone and insulin, which signal through PI3K, decrease the QT interval of rodents expressing the K897 channels (27, 28). Conversely reducing Kv11.1 current in humans expressing the T897 channels is predicted to lengthen action potential duration and the corresponding QT interval. The hormonal- and phosphorylation dependence of this effect on the activity of T897 channels could provide an explanation for both the failure of some epidemiological studies to detect longer QT intervals in resting and/or fasting subjects with the T897 polymorphism, and the failure to observe this effect in previous biophysical studies that used conventional whole-cell recording through ruptured membrane patches on dialyzed cells.
The Kv11.1 channels also contribute to the regulation of β cell excitability in the pancreas (29), and insulin inhibits its own secretion by signaling through PI3K (30). Therefore, feedback inhibition of insulin secretion is predicted to be less effective in people with the K897T polymorphism, which might contribute to greater risk of developing insulin resistance. In other tissues, particularly the brain, two other hERG genes encode Kv11 channels with unusual voltage-dependent kinetics (31). It is noteworthy that the most common human variant of the hERG3 isoform already contains a consensus site (RRRKLS) for the Akt kinase at the homologous position in the channel protein. If the hERG3 isoform has similar effects on rhythmic neuronal activity as hERG1 has on cardiac myocytes and endocrine cells, then the results presented here predict that thyroid hormone and other neurotrophic factors that signal through PI3K, such as brain derived neurotrophic factor (32), could increase neuronal excitability and potentiate plasticity by reducing hERG3 activity.
Other ion channel diseases have also been proposed to result from aberrant phosphorylation, which interferes with channel function by altering kinase binding sites (33) or protein stability (34). We recently discovered that a mutation in the CACNA1C calcium channel gene, which is associated with Timothy disorder (13), alters channel gating by creating a new consensus site for the calmodulin-dependent protein kinase, CAMKII (14). When the channel is phosphorylated by CAMKII, each channel opening is 10 times longer on average, potentially leading to excitotoxicity. We have proposed the term “phosphorylopathy” to describe diseases that result from mutations that produce aberrant phosphorylation of ion channel proteins (14).
We have used bioinformatics to identify 16 SNPs in human ion channel genes that are also predicted to create or destroy putative phosphorylation sites in nine different channel proteins (Table 1). Most of these SNPs are already known to be associated with increased risk of human disease. To qualify as a SNP in the Hapmap database, >1% of the population must have at least one allele with the polymorphism (35). Thus, our predictions potentially affect many people. However, the number of candidates depends strongly on the threshold one chooses for the probability that a specific protein kinase recognizes a specific sequence. We chose 65%, because that is the predicted probability for the CAMKII site in CACNA1C, which we already verified (14). Although the mutation in the CaCNA1C gene that is responsible for Timothy disorder is too rare to qualify as a SNP, >90% of all human genetic variation is estimated to occur as single nucleotide polymorphisms (35). Similar SNPs in genes encoding other classes of proteins could also produce many effects on human physiology and health by altering protein phosphorylation sites. Thus, changing the specificity of protein phosphorylation potentially provides a powerful mechanism linking genetic variation to environmental influences that impinge on cell physiology through cell signaling.
Methods
CHO cells (American Type Culture Collection) were grown in DMEM (high glucose) with 10% FBS. Cells grown to <80% confluence, so they remained spindle shaped, were trypsinized and plated on glass coverslips (Deutsche Spiegelglass, Carolina Biological). The cells were transfected 48 h later using Lipofectamine 2000 (Invitrogen) with independent plasmids encoding hERG1 (U04270), GFP (Clontech), and the human TRβ1 receptor (X04707). Mutations in hERG1 were introduced with the QuikChange XL site-directed mutagenesis kit (Stratagene) and confirmed by sequencing. Twelve to 24 hours after transfection, heat-polished 1.5 MΩ glass pipettes filled with 140 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM Hepes (pH 7.3) were used to make cell-attached patches on fluorescent cells that were bathed in 140 mM KCl, 0.1 mM CaCl2, 2 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.3). Only isolated spindle-shaped cells were selected for recording. High-resistance GΩ seals were obtained by releasing positive pressure on the pipette but without suction to ensure uniform patch size. The patches were voltage-clamped with a HEKA EPC-9 amplifier. Only patches with stable currents at −120 mV between 100 and 500 pA at the peak, no response to perfusion with control bath solution, and leak currents in E-4031 at the end of the experiment <10% of the control, were analyzed further. Current amplitudes at −120 mV were measured at the peak (pA) and by integrating total current (nC) during the first 100 ms after the peak. Values are reported as mean +/−SE. Differences between groups were evaluated with the Student's t test; P < 0.05 is indicated with an asterisk.
The human Akt protein kinase (NM_005163) construct in pcDNA3 with a CMV promoter was modified to produce a catalytically inactive form by mutating K179A (kd-Akt) and a myristoylated form (myr-Akt) by adding the sequence MGSSKSKPKDPSQRGGHM to the N terminus (John O'Bryan, University of Illinois, Chicago). For MS analysis, CHO cells in serum containing T3 were solubilized in a glycerol lysis buffer containing cocktails of proteinase (Complete Mini, EDTA-free, Roche) and phosphatase (Set II, Calbiochem) inhibitors. HA-tagged hERG1 was immunoprecipitated overnight using HA antibody (1:100, Covance) and resolved by SDS/PAGE. Bands corresponding to hERG1 were visualized with Simply Blue Safestain (Invitrogen), manually excised, and digested with trypsin (Promega) for 8 h in a Progest robotic digester from Genomic Solutions. Samples were lyophilized to dryness and then resuspended in 35 nl of 0.1% formic acid. NanoLC-ESI-MS/MS analyses were then performed using an Agilent 1100 nanoLC system on-line with an Agilent XCT Ultra ion trap mass spectrometer with the Chip Cube Interface. MS/MS data were processed and searched against the NCBInr database using the Spectrum Mill software suite from Agilent. Peptide identifications were validated manually.
Acknowledgments.
We are grateful to Peter Smutko for assistance in completing the database search and to John O'Bryan for Akt constructs. This work was supported by the National Institutes of Health Intramural Research Program at National Institute of Environmental Health Sciences through Grant Z01-ES080043.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- 2.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz JR, Bauer CK. Functions of erg K+ channels in excitable cells. J Cell Mol Med. 2004;8:22–30. doi: 10.1111/j.1582-4934.2004.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer AJ, et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–337. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 6.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 7.Newton-Cheh C, et al. Common genetic variation in KCNH2 is associated with QT interval duration: The Framingham Heart Study. Circulation. 2007;116:1128–1136. doi: 10.1161/CIRCULATIONAHA.107.710780. [DOI] [PubMed] [Google Scholar]
- 8.Pietila E, et al. Association between HERG K897T polymorphism and QT interval in middle-aged Finnish women. J Am Coll Cardiol. 2002;40:511–514. doi: 10.1016/s0735-1097(02)01979-4. [DOI] [PubMed] [Google Scholar]
- 9.Paavonen KJ, et al. Functional characterization of the common amino acid 897 polymorphism of the cardiac potassium channel KCNH2 (HERG) Cardiovasc Res. 2003;59:603–611. doi: 10.1016/s0008-6363(03)00458-9. [DOI] [PubMed] [Google Scholar]
- 10.Bezzina CR, et al. A common polymorphism in KCNH2 (HERG) hastens cardiac repolarization. Cardiovasc Res. 2003;59:27–36. doi: 10.1016/s0008-6363(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 11.Anson BD, et al. Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol. 2004;286:H2434–41. doi: 10.1152/ajpheart.00891.2003. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CL, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 13.Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Erxleben C, et al. Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices. Proc Natl Acad Sci USA. 2006;103:3932–3937. doi: 10.1073/pnas.0511322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile S, et al. Rac GTPase signaling through the PP5 protein phosphatase. Proc Natl Acad Sci USA. 2006;103:5202–5206. doi: 10.1073/pnas.0600080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storey NM, et al. Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci USA. 2006;103:5197–5201. doi: 10.1073/pnas.0600089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Storey NM, O'Bryan JP, Armstrong DL. Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr Biol. 2002;12:27–33. doi: 10.1016/s0960-9822(01)00625-x. [DOI] [PubMed] [Google Scholar]
- 19.Herzberg IM, Trudeau MC, Robertson GA. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J Physiol. 1998;511:3–14. doi: 10.1111/j.1469-7793.1998.003bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao ZS, Manser E. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J. 2005;386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J Biochem (Tokyo) 2003;133:17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 23.Viard P, et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 24.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 25.Walker EH, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 26.Oudit GY, et al. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Pazos-Moura C, et al. Cardiac dysfunction caused by myocardium-specific expression of a mutant thyroid hormone receptor. Circ Res. 2000;86:700–706. doi: 10.1161/01.res.86.6.700. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, et al. Restoring depressed HERG K+ channel function as a mechanism for insulin treatment of abnormal QT prolongation and associated arrhythmias in diabetic rabbits. Am J Physiol. 2006;291:H1446–H1455. doi: 10.1152/ajpheart.01356.2005. [DOI] [PubMed] [Google Scholar]
- 29.Rosati B, et al. Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: The role of HERG K(+) channels in firing and release. FASEB J. 2000;14:2601–2610. doi: 10.1096/fj.00-0077com. [DOI] [PubMed] [Google Scholar]
- 30.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi W, et al. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 33.Marx SO, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 34.de Mattia F, et al. Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2–R254L explains dominant nephrogenic diabetes insipidus. J Am Soc Nephrol. 2005;16:2872–2880. doi: 10.1681/ASN.2005010104. [DOI] [PubMed] [Google Scholar]
- 35.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jegga AG, Gowrisankar S, Chen J, Aronow BJ. PolyDoms: A whole genome database for the identification of non-synonymous coding SNPs with the potential to impact disease. Nucleic Acids Res. 2007;35:D700–D706. doi: 10.1093/nar/gkl826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]