Abstract
Ubiquitin-dependent degradation is implicated in various cellular regulatory mechanisms. The SCFCdc4 (Skp1, Cullin/Cdc53, and the F-box protein Cdc4) complex is an ubiquitin ligase complex that acts as a regulator of cell cycle, signal transduction, and transcription. These regulatory mechanisms are not well defined because of the difficulty in identifying the interaction between ubiquitin ligases and their substrates. To identify substrates of the yeast SCFCdc4 ubiquitin ligase complex, we refined the yeast two-hybrid system to allow screening Cdc4-substrate interactions under conditions of substrate stabilization, and identified Swi5 as a substrate of the SCFCdc4 complex. Swi5 is the transcriptional activator of Sic1, the inhibitor of S phase cyclin-dependent kinases (CDKs). We showed that Swi5 is indeed ubiquitinated and degraded through the SCFCdc4 complex. Furthermore, the SCFCdc4-dependent degradation of Swi5 was required to terminate SIC1 transcription at early G1 phase, which ensured efficient entry into S phase: Hyperaccumulation of Sic1 was noted in cells expressing stabilized Swi5, and expression of stabilized Swi5 delayed S phase entry, which was dominantly suppressed by SIC1 deletion. These findings indicate that the SCFCdc4 complex regulates S phase entry not only through degradation of Sic1, but also through degradation of Swi5.
Keywords: cell cycle, proteolysis, ubiquitin
The importance of the ubiquitin/proteasome system in various cellular regulatory mechanisms has been established. Substrates are ubiquitinated by the enzymatic cascade of E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase) (1). E3s regulate the specificity of ubiquitination by directly binding to the substrates. The SCF (Skp1, Cullin/Cdc53 and the F-box proteins) ubiquitin ligase complexes belong to a class of E3s that are involved in the control of cell cycle, signal transduction, transcription, and development (2). Identification of substrates for SCF-dependent ubiquitination is of great importance for our understanding of these regulatory mechanisms. For example, the finding that SCFCdc4 (SCF complex with the F-box protein Cdc4) targets phosphorylated Sic1 for ubiquitin-dependent degradation revealed the molecular mechanism of S phase entry: Sic1 is the S phase CDK inhibitor in yeast and inhibits the initiation of S phase (3); SIC1 is expressed from late M phase until late G1 phase; Sic1 becomes phosphorylated at late G1 by G1-phase CDKs (G1 cyclin/Cdc28) and by Pcl1/Pho85, another CDK in yeast (3–8); phosphorylated Sic1 is then targeted for SCFCdc4-dependent ubiquitination, followed by degradation, an event ultimately leading to S-phase entry (3, 7, 8). However, progress in understanding cellular regulation by the SCF complexes has been limited because of the lack of convenient methods to identify substrates for ubiquitination.
The yeast two-hybrid system is one of the most widely used genetic methods for identifying protein–protein interactions (9). Various versions of this method have been described to expand its availability (10–14). Nevertheless, F-box protein-substrate interactions are still difficult to identify by the two-hybrid system. Such interactions may not be maintained to levels sufficient for their detection because of the ubiquitin-dependent degradation of substrates. We therefore reasoned that F-box protein-substrate interactions could be identified by designing a two-hybrid system to inhibit the degradation of substrates during screening.
We established a screening system for identification of F-box protein-substrate interactions, and identified a substrate of the SCFCdc4 complex; Swi5, a transcription factor known to control the expression of SIC1 cluster genes, such as SIC1, EGT2, CDC6, and RME1 (15). We showed that Swi5 is in fact a physiological substrate of the SCFCdc4 complex and provide evidence for the importance of the SCFCdc4-dependent degradation of Swi5 in proper progression into the S phase.
Results
Screening for Substrates of the SCFCdc4 Complex by a Two-Hybrid System Designed to Inhibit Ubiquitination of Substrates.
Although Sic1 is a substrate of the SCFCdc4 complex, their interaction could not be detected by the conventional two-hybrid system (Fig. 1A, line 1). We rationalized that SCFCdc4-Sic1 interaction is not maintained at a level sufficient for its detection because Sic1 could be targeted for degradation through two pathways. Sic1 fused with a transcription activation domain (AD-Sic1), could be targeted for ubiquitin-dependent degradation by (i) Cdc4 fused to a DNA binding domain (BD-Cdc4) (degradation pathway I via the SCFBD-Cdc4) or by (ii) Cdc4 originated from yeast genome (degradation pathway II via the SCFCdc4). We therefore designed a yeast two-hybrid system that inhibits these two-degradation pathways during screening. To inhibit degradation pathway I, we used as the bait a catalytically inactive mutant form of Cdc4—Cdc4-dF, which lacked the F-box motif but retained its substrate-binding domain. To inhibit degradation pathway II, we used the temperature-sensitive (ts) cdc4–1 mutant cells, which are defective in ubiquitination of substrates at their restriction temperature (37°C), as host cells for screening. We transformed cdc4–1 mutants with plasmids encoding BD-Cdc4-dF (Cdc4-dF fused with BD), AD-Sic1, and reporter LacZ. After formation of transformant colonies, the two-hybrid interaction was monitored at 37°C.
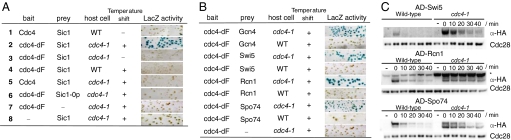
Fig. 1.
Detection of F-box protein-substrate interactions by the refined two-hybrid system. (A) The refined two-hybrid system allows the detection of Cdc4-dF-Sic1 interaction. By using the filter assay, we examined the expression of reporter LacZ in wild-type (WT) and cdc4–1 mutants harboring the indicated combinations of bait and prey constructs. (B) The refined two-hybrid system identified interactions of Cdc4-dF with Gcn4, Swi5, Rcn1, and Spo74. (C) Stability of AD- and HA-tagged proteins expressed from the GAL1 promoter after the addition of cycloheximide was compared in the indicated cells. Aliquots were taken at 10 min intervals and immunoblotted with anti-HA (12CA5) and anti-PSTAIRE antibodies. Cdc28 is shown as a loading control. (*, Nonspecific signals detected in this experiment.)
Strong expression of LacZ was observed by using our refined system lacking both ubiquitination pathways I and II (Fig. 1A, line 2). In contrast, LacZ expression was undetectable when either degradation pathway was active (Fig. 1A, lines 3–5). The interaction detected by our assay was relevant to biological function (Fig. 1A, line 6); it was abolished by sic1–0p mutations that inhibit phosphorylation by Cdc28 and Pho85 (6). Such phosphorylation enables Sic1-Cdc4 interaction required for ubiquitination of Sic1. As control, LacZ expression was detected neither in Cdc4-dF nor in Sic1 alone (Fig. 1A, lines 7 and 8). Together, our refined two-hybrid system, which was designed to inhibit the two-degradation pathways, clearly detected Cdc4-dF-Sic1 interaction.
Isolation of substrates of the SCFCdc4 complex was performed by following three screening steps. We first screened the yeast two-hybrid library. From ≈35,000 transformants, we identified 82 clones that consistently activated LacZ expression [see supporting information (SI) Text]. We next eliminated clones that produce false positives. We used two different assays with different specificities. The first assay was based on our criteria that Cdc4-dF-substrate interaction should be almost undetectable in wild-type cells (whose degradation pathway II is active). We transformed the potential positive plasmids into wild-type or cdc4–1 cells harboring the BD-Cdc4-dF construct and reporter LacZ gene, and selected 35 clones that did not activate LacZ expression in wild-type cells (examples of these clones are shown in Fig. 1B). In the second assay, we compared the half-lives of candidate proteins in wild-type and cdc4–1 cells by the pulse–chase experiments (SI Text), and selected five clones whose degradation depended on Cdc4 (examples are shown in Fig. 1C). In the final step, sequence analysis revealed that these clones encoded GCN4 (two clones), SWI5 (16), RCN1 (17, 18), and SPO74 (19). Gcn4 has been identified as a substrate of the SCFCdc4 complex (20). We focused our study on Swi5, because Swi5 is a transcription activator of SIC1 (15) and is reported to be stable in the G2-M phase when localized in the cytoplasm, but becomes destabilized after it moves into the nucleus in late M/G1 phase (21).
The validity of our strategy regarding another F-box protein was verified by using Grr1-dF (22), the F-box deleted mutant form of another F-box protein, Grr1 (Fig. S1).
Swi5 Is a Substrate of the SCFCdc4 Complex.
We confirmed the SCFCdc4-dependent degradation of Swi5 during the cell cycle. We used the GAL-CDC20 method for synchronous cultivation from metaphase (23). Synchrony through the cell cycle after the release was verified by analysis of cell cycle-regulated degradation of mitotic cyclin Clb2. Consistent with a previous report (21), most of Swi5-Myc in TK769 (GAL-CDC20 SWI5-Myc) cells was degraded after release from the metaphase arrest (Fig. 2A). In contrast, degradation of Swi5-Myc decreased markedly in cells containing a temperature-sensitive cdc4–1, cdc34–2, or cdc53–1 mutation (TK770, 771, or 772) at their restriction temperatures (Fig. 2A and Fig. S2a). CDC34 encodes the E2 enzyme of the SCFCdc4 complex. Cellular localization of Swi5-Myc confirmed the above results (Fig. 2B and Fig. S2b). Swi5-Myc was localized in the cytoplasm at metaphase as reported (21) (Fig. 2B). In wild-type cells (TK769), most of the immunofluorescence signals of Swi5-Myc rapidly decreased after release from the metaphase arrest (21) (Fig. 2B), such that <20% of cells showed faint signals of Swi5-Myc in nuclei at 20 min after the release. In contrast, in cdc4–1, cdc34–2, or cdc53–1 mutants (TK770, 771 or 772), Swi5-Myc moved into the nucleus within 20 min after release and remained in the nucleus during the time-course experiments (Fig. 2B and Fig. S2b). These findings clearly demonstrate that Swi5 is degraded in the nucleus in a SCFCdc4-dependent manner.
Fig. 2.
Swi5 is a physiological substrate of the SCFCdc4 complex. (A and B) Swi5 is stabilized and accumulates in nuclei of cells with functionally defective SCFCdc4 complex. Log phase cultures of TK769 (GAL-CDC20 SWI5-Myc) and TK770 (GAL-CDC20 SWI5-Myc cdc4–1) were arrested in metaphase in YPR medium. Cells were released from the metaphase arrest by the addition of galactose and further cultured at 30°C (TK769) or 37°C (TK770). Aliquots were taken at 20 min intervals for protein analysis (A) and for determination of subcellular localization (B). (A) Protein levels of Swi5-Myc, Clb2, and Cdc28 were analyzed by immunoblotting. (B) Swi5-Myc was visualized by anti-Myc antibody (9E10). DAPI (4′6-diamidino-2-phenylindole) was used to visualize nuclei. Numbers in parentheses represent the numbers of strains. (C) Swi5-ST8A-HH is stabilized in vivo. Cultures of TK713 (GAL-CDC20 SWI5-HH) or TK-714 (GAL-CDC20 SWI5-ST8A-HH) cells were processed as described in Fig. 2a at 30°C. Aliquots were taken at the time indicated and immunoblotted. (D) Cells arrested at G1 phase by α factor were treated with galactose for 20 min (to induce expression of Swi5-Myc or Swi5-ST8A-Myc) and then with cycloheximide. Aliquots were taken at the preselected time, and degradation of Swi5-Myc or Swi5-ST8A-Myc was monitored by immunoblotting. Bands were quantified, and Swi5-Myc signals were normalized against Cdc28 signals from the same blot probed with anti-PSTAIRE antibody. Values are shown as a percentage of the signal at 0 min time point: Swi5-Myc in wild-type cells (○) or in srb10Δ mutants (●); Swi5-ST8A-Myc in wild-type cells (□) or in srb10Δ mutants (■). (E) Ubiquitination of Swi5 in vivo. (Top) pdr5Δ mutants (lanes 1–10) or cdc4–1 pdr5Δ mutants (lanes 11 and 12) in the absence (lanes 1, 3, 5, 7, 9, and 11) or presence (lanes 2, 4, 6, 8, 10, and 12) of MG132, with the use of the following combinations of plasmids: YC33G (control plasmid) and pCUP-UbiHIS-MYC-RA (lanes 1 and 2); YC33G-SWI5-HA and pCUP-UbiHIS-MYC-RA (lanes 3, 4, 9, and 10); YC33G-SWI5-HA and pCUP-UbiMYC-RA (lanes 5 and 6); YC33G-SWI5-ST8A-HA and pCUP-UbiHIS-MYC-RA (lanes 7 and 8); and pYES2-SWI5-HA and pCUP-UbiHIS-MYC-RA (lanes 11 and 12). We used pYES2-SWI5-HA (a high-copy plasmid for expression of SWI5-HA from GAL1 promoter) because expression of Swi5-HA from YC33G-SWI5-HA was low in cdc4–1 pdr5Δ mutants. Extracts were incubated with Ni-NTA beads, and bead-bound proteins were immunoblotted with anti-HA (12CA5) antibody. (*, Nonspecific signals detected in this experiment.) (Middle and Bottom) Total extracts were analyzed by immunoblotting to examine the expression of Swi5-HA (Middle) and Cdc28 as loading control (Bottom).
We next examined whether phosphorylation of Swi5 is required for SCFCdc4-dependent degradation, because substrates of the SCFCdc4 complex require phosphorylation for their interaction with Cdc4 (2). Swi5 has been shown to be phosphorylated at multiple sites (21). We hypothesized that the phosphorylation sites required for the SCFCdc4-dependent degradation reside in the “inhibitory domain” of Swi5 (24). The domain is suggested to be a degron that targets Swi5 for degradation in nucleus and is required to inhibit the Swi5-dependent expression of HO (an endonuclease required for mating type exchange) in daughter cells (24). Unlike SIC1 cluster genes (whose transcription depends on Swi5), HO is expressed only in mother cells at late G1 phase. A very small fraction of Swi5 inherited in mother cells is sequestrated by mother cell-specific factors and prevents degradation. Deletion of the inhibitory domain allows HO expression even in daughter cells, because of the stabilization of Swi5 (24). There are eight potential sites (225SP, 231SP, 246SP, 250SP, 261SP, 300SP, 320TP, and 323TP) for proline-directed protein kinases, such as CDKs, in the inhibitory domain. To examine whether phosphorylation within the eight potential sites for phosphorylation is required for the SCFCdc4-dependent degradation of Swi5, we constructed a mutant form of Swi5; Swi5-ST8A, in which the eight residues in the inhibitory domain were substituted with nonphosphorylatable alanine residues. Mobility-shift assay of hexahistidine- and HA-tagged Swi5 (Swi5-HH) or Swi5-ST8A (Swi5-ST8A-HH) indicated that some of the eight residues were indeed phosphorylated in G1 (Fig. S3): (i) phosphorylated Swi5-HH was detected in G1 phase (Fig. S3 Right), and (ii) the fully phosphorylated form was absent in Swi5-ST8A-HH (Fig. S3 Left). Furthermore, we found that Swi5-ST8A failed to interact with Cdc4-dF by using our refined two-hybrid system (Fig. S4) and that Swi5-ST8A was stabilized even after the degradation of Clb2 (Fig. 2C). These results indicate that phosphorylation within the inhibitory domain is required for the SCFCdc4-dependent degradation of Swi5.
We furthermore explored a kinase that is involved in the degradation of Swi5. Transcription factors such as Gcn4 and Hac1 are also degraded via the SCFCdc4 complex, and such degradation depends on phosphorylation by one of the CDK—Srb10 (25, 26), a component of the SRB mediator complex in the RNA polymerase II holoenzyme (27). We therefore tested whether Srb10 is involved in the SCFCdc4-dependent degradation of Swi5 (Fig. 2D). Pulse–chase experiments showed that Swi5 was rapidly degraded in wild-type cells arrested in G1 phase by α factor, with a half-life of ≈10 min. The srb10Δ mutation moderately stabilized Swi5. The half-life of Swi5 was extended to ≈20 min in srb10 mutants, indicating the involvement of Srb10 in the degradation of Swi5. However, Swi5-ST8A was highly stabilized in wild-type cells, with a half-life of >40 min. This stability was not changed in srb10Δ mutants (Fig. 2D), suggesting that Srb10 targets Swi5 for degradation through phosphorylation of the inhibitory domain. Considered together, these results strongly suggest the involvement of Srb10 in the SCFCdc4-dependent degradation of Swi5. However, another protein kinase(s) could also be involved in the degradation of Swi5 because Swi5-ST8A in G1-arrested wild-type cells was more effectively stabilized than Swi5 in G1-arrested srb10Δ mutants (Fig. 2D).
Finally, we examined the SCFCdc4-dependent ubiquitination of Swi5 in vivo. To ensure the ubiquitination of Swi5, we transformed pdr5Δ mutants, which are permeable to the proteasome inhibitor MG132, with YC33G-SWI5-HA (the low-copy plasmid for expression of Swi5-HA from GAL1 promoter) and pCUP-UbiHIS-MYC-RA (the plasmid for expression of a hexahistidine- and MYC-tagged K48R, G76A mutant ubiquitin from CUP1 promoter) (28). Because Swi5 becomes unstable in G1 phase, ubiquitination was examined in cells arrested in G1 phase by α factor. Fig. 2E clearly shows the ubiquitination of Swi5 in vivo, and that treatment with MG132 increased the fraction of ubiquitinated Swi5-HA (Fig. 2E, lanes 3, 4, 9, and 10), indicating that ubiquitinated Swi5 is degraded by proteasome. Swi5 ubiquitination in vivo depended on the function of Cdc4 and on the phosphorylation of Swi5: Ubiquitination of Swi5-HA was markedly decreased in cdc4–1 pdr5Δ double mutants (Fig. 2E, lanes 11 and 12), and ubiquitination of Swi5-ST8A was also diminished (Fig. 2E, lanes 7 and 8). Ubiquitinated Swi5-HA was detectable neither in cells expressing UbiHIS-MYC-RA alone (Fig. 2E, lanes 1 and 2) nor in cells expressing Swi5-HA in combination with the mutant ubiquitin that was not tagged with hexahistidine (UbiMYC-RA) (Fig. 2E, lanes 5 and 6). Taken together, our findings demonstrate that Swi5 is a physiological substrate of the SCFCdc4 complex.
Degradation of Swi5 Contributes to Proper Entry into S Phase.
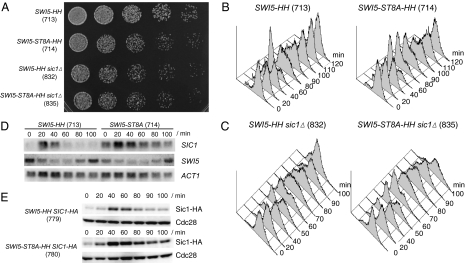
We tested the effect of the ubiquitin-dependent degradation of Swi5 on cell growth. The GAL-CDC20 SWI5-ST8A-HH cells (TK714) were viable but exhibited slow growth, as judged by the rate of colony formation (Fig. 3A). They also showed a marked replication delay (Fig. 3B). In the majority of GAL-CDC20 SWI5-HH cells (TK713), DNA replication started 60–80 min after release from the metaphase arrest, whereas replication of GAL-CDC20 SWI5-ST8A-HH cells (TK714) started at 80–100 min. The S-phase delay was dominantly suppressed by deletion of SIC1; the kinetics of S phase entry of GAL-CDC20 SWI5-ST8A-HH sic1Δ cells (TK835) was similar to that of the GAL-CDC20 SWI5-HH sic1Δ cells (TK832) (Fig. 3C). Furthermore, SIC1 deletion suppressed the slow growth of GAL-CDC20 SWI5-ST8A-HH cells; GAL-CDC20 SWI5-HH sic1Δ and GAL-CDC20 SWI5-ST8A-HH sic1Δ cells showed similar growth rate judged by colony formation (Fig. 3A). These results suggest that stabilized Swi5 delays S-phase entry through SIC1 and consequently causes slow growth.
Fig. 3.
SCFCdc4-dependent degradation of Swi5 contributes to proper progression into S phase. (A) Serial 3-fold dilutions of TK713 (GAL-CDC20 SWI5-HH), TK714 (GAL-CDC20 SWI5-ST8A-HH), TK832 (GAL-CDC20 SWI5-HH sic1Δ), and TK835 (GAL-CDC20 SWI5-ST8A-HH sic1Δ) were spotted on YPRG plates (containing raffinose and galactose as carbon sources) and incubated at 30°C for 38 h. (B) Arrest of metaphase in TK713 and TK714 cells was released by the addition of galactose, and aliquots were taken at the indicated time points for FACS analysis. (C) Arrest of metaphase in TK832 and TK835 cells was released by the addition of galactose, and aliquots were taken at the indicated time points for FACS analysis. (D) Degradation of Swi5 is required to terminate SIC1 transcription. TK713 (GAL-CDC20 SWI5-HH) or TK714 (GAL-CDC20 SWI5-ST8A-HH) cells were grown as described in Fig. 2A. Total RNA was extracted and analyzed with SWI5, SIC1, and ACT1 (for loading control) probes as described in ref. 18. (E) Sic1 hyperaccumulated in cells expressing Swi5-ST8A. Arrest of metaphase in TK779 (GAL-CDC20 SWI5-HH SIC1-HA) and TK780 (GAL-CDC20 SWI5-ST8A-HH SIC1-HA) cells was released by the addition of galactose. Aliquots were taken at the indicated time points, and the protein level of Sic1-HA was compared by immunoblotting. Cdc28 is shown as a loading control. Numbers in parentheses represent the numbers of strains.
SCFCdc4-Dependent Degradation of Swi5 Terminates SIC1 Transcription in Early G1 and Allows Efficient Degradation of Sic1 at Late G1.
We hypothesized that stabilized Swi5 results in continuous expression of Sic1, which antagonizes the degradation of Sic1 at late G1 and delays S-phase entry. To test this possibility, we first examined the effect of the degradation of Swi5 on the Swi5-dependent transcription of SIC1. GAL-CDC20 SWI5-HH (TK713) and GAL-CDC20 SWI5-ST8A-HH (TK714) cells released from metaphase arrest were harvested at 20 min intervals, and Northern blot analyses were performed (Fig. 3D). SIC1 transcription reached a peak level at 20 min after release in both cells. However, the peak level of SIC1 transcription increased markedly in GAL-CDC20 SWI5-ST8A-HH (TK714) cells. In addition, transcription of SIC1 continued during the time course (at least until 100 min after release) in GAL-CDC20 SWI5-ST8A-HH (TK714) cells, whereas it disappeared suddenly as cells entered G1 phase in GAL-CDC20 SWI5-HH (TK713) cells. The ST8A mutation did not change the profile of SWI5 mRNA (Fig. 3C), indicating that the increased and prolonged transcription of SIC1 by ST8A mutation was not because of up-regulation of SWI5 transcription. Similarly, Swi5-dependent transcription of SIC1 was not terminated in cdc4–1 GAL-CDC20 (TK770) and cdc53–1 GAL-CDC20 (TK772) cells (Fig. S5). These results demonstrate that degradation of Swi5 via the SCFCdc4 complex is required for down-regulation of SIC1 transcription at early G1 phase.
We also compared the level of Sic1 protein. To detect Sic1, we replaced SIC1 at its genomic locus in TK-713 and TK714 with SIC1-HA. The resultant GAL-CDC20 SWI5-HH SIC1-HA (TK779) and GAL-CDC20 SWI5-ST8A-HH SIC1-HA (TK780) cells were cultured synchronously from metaphase. As expected, Sic1 protein hyperaccumulated in GAL-CDC20 SWI5-ST8A-HH SIC1-HA cells (Fig. 3E). This hyperaccumulation was associated with a delay in S-phase entry. Most of the TK779 cells entered S phase 80 min after the release when Sic1 protein level decreased; in contrast, most of the TK780 were still in G1 phase at the same time point when Sic1 protein level was still high (Fig. 3E and Fig. S6). We also ruled out the possibility that Swi5-ST8A inhibits degradation of Sic1 (Fig. S7); the addition of cycloheximide (an inhibitor of protein synthesis), rapidly decreased the Sic1-HA protein level in both cells. Furthermore, the kinetics of degradation of Sic1-HA after the addition of cycloheximide in cells expressing SWI5-ST8A was similar to that in cells expressing SWI5. These results indicate that increased and continuous expression of Sic1 by stabilized Swi5-ST8A allows hyperaccumulation of Sic1 and in turn antagonizes Sic1 degradation at late G1 phase, which results in S-phase delay. We conclude that the SCFCdc4-dependent degradation of Swi5 is required to terminate SIC1 transcription in early G1, which ensures proper progression into the S phase.
Discussion
In the present study, we established the refined two-hybrid system to identify substrates of the SCFCdc4 complex and identified Swi5. To allow screening of Cdc4-substrate interactions under conditions of substrate stabilization, we introduced two modifications: (i) the use of the temperature-sensitive cdc4 mutants as host cells and (ii) the use of a catalytically inactive form of Cdc4, which lacks the F-box motif but retains the substrate-binding domain as bait. The use of Cdc4-dF as bait alone was unsuccessful for identification of Cdc4-dF-substrate interaction (Fig. 1A, line 4). Expression of Cdc4-dF was viable and thus did not cause dominant negative phenotype in Cdc4-dependent degradation of substrates. Therefore, substrates might be cleared by degradation via Cdc4 originated in yeast, which consequently impedes Cdc4-dF-substrate interaction. To remedy this, we used the temperature-sensitive cdc4–1 mutants as host cells, in combination with the use of Cdc4-dF as bait (Fig. 1A, line 2). A similar approach was also effective in detecting interaction between Grr1-dF (22) and Cln2 (Fig. S1), suggesting that our strategy can be applied for identifying substrates of the SCF complexes. To extend our technique to human proteins, it is necessary to phosphorylate substrates in yeast because phosphorylation is required for SCF complex-dependent degradation of substrates. Such difficulty can be overcome by ectopic expression of human kinases. For example, conditional expression of tyrosine kinase v-src enables the identification of substrates of tyrosine phosphatase ζ by the yeast two-hybrid system (13). Another version of our method is the use of proteasome mutants instead of cdc4–1 mutants. Treatment of cells with MG132, the proteasomal inhibitor, could also be valuable to detect F-box protein-substrate interaction in mammalian cells, where no ts mutant is readily available.
We demonstrated that the SCFCdc4 complex targets Swi5 for ubiquitin-dependent degradation, which results in termination of SIC1 transcription. Swi5-dependent transcription is activated by the mitotic exit network (MEN) pathway that controls mitotic exit (21, 29). Swi5 is retained in the cytoplasm by Clb/Cdc28-dependent phosphorylation, whereas it enters the nucleus after dephosphorylation by Cdc14 protein phosphatase (a component of the MEN pathway) and activates transcription of Swi5-dependent genes. Therefore, the transcription activity of Swi5 is under cell cycle control. The MEN pathway and the SCFCdc4 complex confine Swi5-dependent transcription within a narrow window in early G1 phase.
The SCFCdc4 complex plays a crucial role in regulating S-phase entry through ubiquitin-dependent degradation of Sic1 (3). In this study, we demonstrated that SCFCdc4 regulates S phase entry not only through degradation of Sic1, but also through degradation of Swi5. SCFCdc4-dependent degradation of Swi5 was required to terminate SIC1 transcription at early G1 phase, thus ensuring efficient degradation of Sic1 and proper progression into S phase (Fig. 3, Figs. S6 and S7). Consequently, cells expressing stabilized Swi5-ST8A, which is deficient in SCFCdc4-dependent degradation, delayed S-phase entry (Fig. 3B). Thus, the SCFCdc4 complex regulates S-phase entry through at least two hierarchies (Fig. 4): (i) degradation of Sic1 at late G1 phase and (ii) degradation of Swi5 at early G1 phase. Degradation of Sic1 is strictly required for S-phase entry (3), whereas that of Swi5 ensures efficient entry into S phase. Degradation of Swi5 at early G1 phase may reduce the risk of battle between synthesis and degradation of Sic1 at late G1 phase, leading to tilt toward entry into S phase.
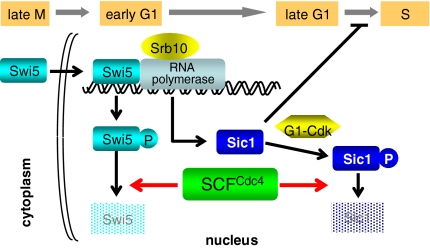
Fig. 4.
Schematic diagram of regulation of S-phase entry by the SCFCdc4 complex. SCFCdc4 complex regulates S-phase entry by degrading not only Sic1 at late G1 phase but also Swi5 at early G1. Sic1 degradation is strictly required for S-phase entry, whereas Swi5 degradation is required to proper progression into S phase through termination of SIC1 transcription at early G1 phase. Phosphorylation ensures timing of degradation of Swi5 and Sic1. Srb10 triggers degradation of Swi5 at early G1, whereas G1-CDKs trigger degradation of Sic1 at late G1.
The mechanism that controls the timing of the degradation of Swi5 and that of Sic1 is likely to be specified by phosphorylation. Our results indicated that degradation of Swi5 requires Srb10, a CDK and component of the Srb10/mediator module of the RNA polymerase II holoenzyme (Fig. 2D). Srb10-dependent phosphorylation also triggers the SCFCdc4-dependent degradation of the transcriptional activators, Gcn4 and Hac1 (25, 26). It was postulated that Srb10 targets promoter-bound Gcn4 and Hac1 for the SCFCdc4-dependent degradation and controls how long any single activator remains bound to the promoter. A similar transcription-dependent mechanism can converge on Swi5 degradation in the early G1 phase, given that Swi5-dependent transcription is activated from late M phase. As a result, the majority of the Swi5 protein is degraded in early G1 phase before START (24), the cell cycle point in G1 phase that commits irreversible entry into cell cycle. However, Srb10 does not fully account for the rapid degradation of Swi5; Swi5-ST8A in wild-type cells was more effectively stabilized than Swi5 in srb10 mutants (Fig. 2D), indicating that phosphorylation of the degron might be sustained by Srb10 and by an unidentified kinase in a parallel manner. On the other hand, degradation of Sic1 requires phosphorylation by G1-CDKs (Cln1/2-Cdc28 and Pcl1-Pho85 kinases) at late G1 phase (3–8). The cyclin subunits of G1 CDKs, CLN1/2 and PCL1, are transcribed by the SBF (Swi4/6) factor after START (30, 31), and then activate phosphorylation of Sic1 at late G1 phase. Our model is illustrated schematically in Fig. 4.
The existence of another degradation pathway that does not require the phosphodegron cannot be ruled out. Though Swi5 became stabilized significantly by mutations within the inhibitory domain or mutations in CDC4, CDC53, or CDC34, Swi5 was still degraded in those cells. At present, the nature of Swi5 degradation pathway other than the SCFCdc4 complex is unclear.
It has been established that transcription during a single stage of the cell cycle is terminated by repressors (or repressor activities) that are expressed by the following stages of the cell cycle (15, 32, 33). Our results suggest that degradation of transcriptional activators can be an alternative mechanism for terminating cell cycle-dependent gene expression. The transcriptional coactivator Ndd1 (34), which is responsible for transcription of mitotic cyclin, Clb2, is also degraded at mitotic exit (35). Degradation of Ndd1 contributes to the termination of CLB2 transcription, and degradation of Clb2 is required for mitotic exit. Degradation of transcriptional activators could assist in keeping the cell cycle forward.
In summary, we described a screening method for isolating Cdc4-substrate interaction. We identified Swi5, Rcn1, and Spo74 as substrates of the SCFCdc4 complex. We demonstrated that Swi5 degradation is important for efficient turnover of Sic1, which promotes completion of G1 phase and subsequent progression into S phase. Further analysis and identification of substrates of the SCF complexes could provide insights into precise molecular mechanisms governing cell cycle and other cellular functions.
Materials and Methods
Yeast Strain and Growth Conditions.
Table S1 lists all yeast strains used in the present study. The strains were constructed and grown by using standard protocols (36).
Chemicals.
Cycloheximide (Sigma) was added to cultures at a final concentration of 50 μg/ml. MG132 (Peptide Institute) was added at a final concentration of 50 μM. The α factor (Peptide Institute) was added to cultures at a final concentration of 10 μg/ml.
Supplementary Material
Acknowledgments.
We thank Mike Tyers, Xiaojing Tang, Avelino Bueno, and Yoshiko Kikuchi for providing various plasmids and strains; Rich Roberts for the encouragement at the initial stage of this work; Hideo Hirokawa for the continuous encouragement; Hiroshi Handa and Yoichiro Kamimura for the useful discussion; and Satomi Yamamoto for technical assistance. This work was supported by grants from RIKEN; the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and the Sumitomo Foundation (T.K.) T.K. was partly supported by PRESTO, Japan Science and Technology Corporation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806253105/DCSupplemental.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Willems A, Schwab M, Tyers M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Schwob E, Böhm T, Mendenhall MD, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 4.Verma R, et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science. 1997;278:455–460. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- 5.Nishizawa M, Kawasumi M, Fujino M, Toh-e A. Phosphorylation of Sic1, a cyclin-dependent kinase (Cdk) inhibitor, by Cdk including Pho85 kinase is required for its prompt degradation. Mol Biol Cell. 1998;9:2393–2405. doi: 10.1091/mbc.9.9.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash P, et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 7.Skowyra D, et al. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 8.Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 9.Fields S, Song OK. A novel genetic system to detect protein–protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 10.Aronheim A, et al. Isolation of an AP-1 repressor by a novel method for detecting protein–protein interactions. Mol Cell Biol. 1997;17:3094–3102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stagljar I, Korostensky C, Johnsson N, Heesen ST. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada S, et al. A cloning method for caspase substrates that uses the yeast two-hybrid system: Cloning of the antiapoptotic gene gelsolin. Proc Natl Acad Sci USA. 1998;95:8532–8537. doi: 10.1073/pnas.95.15.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawachi H, Fujikawa A, Maeda N, Noda M. Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase zeta/beta by the yeast substrate-trapping system. Proc Natl Acad Sci USA. 2001;98:6593–6598. doi: 10.1073/pnas.041608698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo D, et al. A tethered catalysis, two-hybrid system to identify protein–protein interactions requiring post-translational modifications. Nat Biotechnol. 2004;22:888–892. doi: 10.1038/nbt985. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberg C, Reed SI. Cell cycle-dependent transcription in yeast: Promoters, transcription factors, and transcriptomes. Oncogene. 2005;24:2746–2755. doi: 10.1038/sj.onc.1208606. [DOI] [PubMed] [Google Scholar]
- 16.Nasmyth K, Seddon A, Ammerer G. Cell cycle regulation of SW15 is required for mother-cell-specific HO transcription in yeast. Cell. 1987;49:549–558. doi: 10.1016/0092-8674(87)90457-0. [DOI] [PubMed] [Google Scholar]
- 17.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 18.Kishi T, Ikeda A, Nagao R, Koyama N. The SCFCdc4 ubiquitin ligase regulates calcineurin signaling through degradation of phosphorylated Rcn1, an inhibitor of calcineurin. Proc Natl Acad Sci USA. 2007;104:17418–17423. doi: 10.1073/pnas.0704951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickas ME, Schwartz C, Neiman AM. Ady4p and Spo74p are components of the meiotic spindle pole body that promote growth of the prospore membrane in Saccharomyces cerevisiae. Eukaryotic Cell. 2003;2:431–435. doi: 10.1128/EC.2.3.431-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meimoun A, et al. Degradation of the transcription factor Gcn4 requires the kinase Pho85 and the SCF(CDC4) ubiquitin-ligase complex. Mol Biol Cell. 2000;11:915–927. doi: 10.1091/mbc.11.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moll T, et al. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991;66:743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- 22.Kishi T, Yamao F. An essential function of Grr1 for the degradation of Cln2 is to act as a binding core that links Cln2 to Skp1. J Cell Sci. 1998;111:3655–3661. doi: 10.1242/jcs.111.24.3655. [DOI] [PubMed] [Google Scholar]
- 23.Yeong FM, Lim HH, Padmashree CG, Surana U. Exit from mitosis in budding yeast: Biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol Cell. 2000;5:501–511. doi: 10.1016/s1097-2765(00)80444-x. [DOI] [PubMed] [Google Scholar]
- 24.Tebb G, Moll T, Dowzer C, Nasmyth K. SWI5 instability may be necessary but is not sufficient for asymmetric HO expression in yeast. Genes Dev. 1993;7:517–528. doi: 10.1101/gad.7.3.517. [DOI] [PubMed] [Google Scholar]
- 25.Chi Y, et al. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001;15:1078–1092. doi: 10.1101/gad.867501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal B, et al. SCFCdc4-mediated degradation of the Hac1p transcription factor regulates the unfolded protein response in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:426–440. doi: 10.1091/mbc.E06-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers VE, Young RA. RNA polymerase II holoenzymes and subcomplexes. J Biol Chem. 1998;273:27757–27760. doi: 10.1074/jbc.273.43.27757. [DOI] [PubMed] [Google Scholar]
- 28.Willems AR, et al. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 29.Visintin R, et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 30.Nasmyth K, Dirick L. The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast. Cell. 1991;66:995–1013. doi: 10.1016/0092-8674(91)90444-4. [DOI] [PubMed] [Google Scholar]
- 31.Ogasa J, Andrews BJ, Herskowitz I. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell. 1991;66:1015–1026. doi: 10.1016/0092-8674(91)90445-5. [DOI] [PubMed] [Google Scholar]
- 32.Simon I, et al. Serial regulation of transcriptional regulators in the yeast cell cycle. Cell. 2001;106:697–708. doi: 10.1016/s0092-8674(01)00494-9. [DOI] [PubMed] [Google Scholar]
- 33.Breeden LL. Periodic transcription: A cycle within a cycle. Curr Biol. 2003;13:R31–R38. doi: 10.1016/s0960-9822(02)01386-6. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds D, et al. Recruitment of Thr 319-phosphorylated Ndd1p to the FHA domain of Fkh2p requires Clb kinase activity: A mechanism for CLB cluster gene activation. Genes Dev. 2003;17:1789–1802. doi: 10.1101/gad.1074103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loy CJ, Lydall D, Surana U. NDD1, a high-dosage suppressor of cdc28–1N, is essential for expression of a subset of late-S-phase-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:3312–3327. doi: 10.1128/mcb.19.5.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ausubel FM, et al. Current Protocol in Molecular Biology. New York: Wiley; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.